Introduction
Lung cancer is the most prevalent type of cancer,
accounting for >26% of all cancer-associated mortalities
(1). Mortality among lung cancer
patients is primarily caused by metastasis; therefore, it is
essential to elucidate the mechanisms involved in lung cancer
metastasis in order to determine novel therapeutic targets. Cancer
metastasis is a complex process in which cancer cells migrate from
the primary tumor and invade into the surrounding tissues (2). Epithelial-mesenchymal transition
(EMT) provides a novel basis for understanding the progression and
metastasis of cancer (3–5). During EMT, polarized epithelial cells
lose their epithelial characteristics and are converted to motile
mesenchymal-like cells (6).
The number of studies regarding the critical roles
of micro (mi)RNAs in EMT is increasing (7). miRNAs are a class of small noncoding
RNAs that bind to areas in the 3′-untranslated region (UTR) of
target mRNAs (8), resulting in
either mRNA degradation or translational repression (9). Previous studies have identified a
variety of miRNAs that have important roles in cancer progression,
particularly in tumor invasion and metastasis (10).
Downregulation of miR-145 was reported to occur in
various types of human cancer (11–13),
in which it exerts its function in a cell-specific manner (14–16).
Yin et al (17)
demonstrated that miR-145 suppressed human lung
adenocarcinoma-initiating cell proliferation, which resulted in the
inhibition of lung cancer development. However, the role of miR-145
in lung cancer metastasis and its potential mechanisms of action
remain to be elucidated. The present study aimed to investigate the
effects of miR-145 on metastasis and EMT in A549 human lung
adenocarcinoma cells. In addition, the underlying mechanisms by
which miR-145 regulates EMT were examined.
Materials and methods
Reagents and antibodies
The pRL-TK Renilla luciferase reporter
vector, pmirGLO luciferase reporter vector and Dual-Luciferase
Reporter Assay system were purchased from Promega Corporation
(Madison, WI, USA). The following antibodies: Rabbit polyclonal
Oct4 (sc-9081), mouse monoclonal Wnt3a (sc-136163), rabbit
polyclonal β-catenin (sc-7199), mouse monoclonal GAPDH (sc-365062),
horseradish peroxidase (HRP)-conjugated goat anti-mouse
immunoglobulin (IgG) and HRP-conjugated goat anti-rabbit IgG were
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Rabbit polyclonal vimentin antibody (ab92547) was obtained from
Abcam (Cambridge, MA, USA). Rabbit polyclonal N-cadherin (SAB,
21474), rabbit polyclonal E-cadherin (SAB, 21473) and rabbit
polyclonal pan-cytokeratin (SAB, 23032) were obtained from
Signalway Antibody Inc. (College Park, MD, USA). The miR-145 mimic,
miR-145 inhibitor and miR-145 negative control (NC) were
synthesized by Biomics Biotechnology Co., Ltd (Jiangsu, China).
Cell culture
Human lung cancer cell lines, SPC-A-1, A549,
LTEP-a-2, SK-LU-1 and GLC-82, as well as the normal human bronchial
epithelial (HBE) cell line were purchased from the American Type
Culture Collection (Manassas, VA, USA) and maintained in Dulbecco’s
modified Eagle’s medium (Gibco-BRL, Grand Island, NY, USA)
containing 10% fetal bovine serum (FBS; Gbico-BRL) at 37°C in a
humidified atmosphere with 5% CO2.
Tissues
Tumor and adjacent normal lung tissue specimens were
collected from 10 patients with non-small-cell lung cancer in
Jiangxi Provincial Chest Hospital (Jiangxi, China). This study was
approved by the Ethics Committee of Jiangxi Provincial Chest
Hospital. All patients provided written informed consent in
compliance with the code of ethics of the Declaration of Helsinki.
None of the patients enrolled in the present study had received
chemotherapy or radiotherapy prior to surgery. The tumor and
adjacent normal tissue samples were frozen in liquid nitrogen
immediately following surgery and stored at −80°C until further
use.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from tissue samples or cells
using TRIzol® (Invitrogen Life Technologies, Carlsbad,
CA, USA), miR-145 was isolated using an miRNeasy Mini kit (Qiagen,
Limburg, Netherlands) according to the manufacturer’s instructions.
Approximately 5 μg RNA was able to be isolated from 1 mg tissue or
1×106 cells. Complementary DNA (cDNA) was synthesized
using a First Strand cDNA Synthesis kit (Fermentas, Vilnius,
Lithuania) using 1 μg RNA template. The primers were as follows:
miR-145 forward, 5′-GTCCAGTTTTCCCAGGAATCCCT-3′ and reverse,
5′-TGGTGTCGTGGAGTCG-3′; U6 forward, 5′-GCTTCGGCAGCACATATACTAAAAT-3′
and reverse, 5′-CGCTTCACGAATTTGCGTGTCAT-3′. qPCR was performed
using a SYBR-Green PCR kit (Applied Biosystems, Foster City, CA,
USA) in a 7300 Sequence Detection system (Applied Biosystems). The
PCR conditions consisted of 5 min of initial denaturation at 95°C,
40 cycles of denaturation at 95°C for 10 sec, annealing at 60°C for
20 sec and elongation at 72°C for 10 sec. The relative quantities
of each mRNA were calculated using the comparative CT methods.
Western blot analysis
Tissues and cells were lysed using a total protein
extraction kit (Promab, Inc., Hunan, China). Protein concentrations
were determined using the bicinchoninic acid kit (Beyotime,
Shanghai, China). A total of 50 μg protein was separated using 10%
SDS-PAGE and blotted onto nitrocellulose membranes (Millipore,
Billerica, MA, USA). Following blocking in 5% non-fat milk
overnight at 4°C, the membranes were incubated with the primary
antibodies for 1 h at room temperature, followed by incubation with
the secondary antibodies for 1 h at room temperature. Detection was
performed using an enhanced chemiluminescence kit (Pierce
Biotechnolgy, Inc., Rockford, IL, USA). GAPDH was used as a loading
control.
Transfection
Transfection was performed using Lipofectamine™ 2000
(Invitrogen Life Technologies) according to the manufacturer’s
instructions. Briefly, 4×104 cells were seeded into
six-well plates and incubated at 37°C with 5% CO2 for 24
h. Prior to transfection, 5 μl Lipofectamine™ 2000 was diluted in
250 μl Opti-MEM® I (Gibco-BRL) and incubated for 5 min.
The miR-145 mimic or inhibitor was diluted in 250 μl
Opti-MEM® I to a final concentration of 2 μM. The two
solutions were mixed gently and incubated at room temperature for
20 min. The lipid-DNA complexes were added to each well and cells
were incubated at 37°C. Sequences for the miR-145 mimic were:
Sense, 5′-GUCCAGUUUUCCCAGGAAUCCCU-3′ and antisense, 5′-GGAUUCCUG
GGAAAACUGGACUU-3′. Sequences for the miR-145 inhibitor were:
antisense, 5′-AGGGAUUCCUGGGAAAACUG GAC-3′. Following 6 h, cells
were incubated with fresh medium and maintained in the cultures for
a minimum of 48 h; cells were then harvested for analysis. To
knockdown Oct4 in A549 cells, small hairpin(sh)RNAs for Oct4 were
constructed and the DNA sequences were cloned into pGPU6/GFP/Neo
vectors (Invitrogen Life Technologies). The most effective shRNA
sequences were: sense, 5′-CACCGGGTAGGT
TATTTCTAGAAGTTTCAAGAGAACTTCTAGAAATAACC TACCCTTTTTTG-3′ and
antisense, 5′-GATCCAAAAAAG GGTAGGTTATTTCTAGAAGTTCTCTTGAAACTTCTAG
TAGAAATAACCTACCC-3′. The knockdown of Oct4 in A549 cells was
generated by transfection with 2 μg shRNA-Oct4.
Luciferase assay
Reporter plasmids containing 3′UTR Oct4
(pmirGLO-Oct4) were co-transfected with miR-NC or miR-145 mimic
into A549 cells. pRL-TK Renilla luciferase reporter vector
was used as an internal control in each assay. Firefly and
Renilla luciferase activities were measured using the
Dual-Luciferase Reporter Assay system. Results were expressed as
firefly luciferase activity normalized to Renilla luciferase
activity.
Transwell-matrigel invasion assay
Cell invasion assays were performed using Transwell
inserts pre-coated with Matrigel® (BD Biosciences,
Franklin Lakes, NJ, USA). A549 cells were harvested and resuspended
in serum-free medium in order to obtain a density of
5×104 cells/ml. A total of 1 ml cell suspension was
added to the upper chambers, the lower chamber was filled with 1 ml
cell medium containing 10% FBS. Following incubation at 37°C for 12
h, non-invaded cells were removed using a cotton swab, the invaded
cells were fixed using 95% ethanol for 15 min and then stained with
hematoxylin for 10 min. The invaded cells were counted under a
light microscope (TS100; Nikon, Tokyo, Japan).
Adhesion assay
Fibronectin (Sigma-Aldrich, St. Louis, MO, USA) was
used to coat 96-well plates and the plates were left for 2 h. The
wells were then blocked with 1% bovine serum albumin in
phosphate-buffered saline (PBS; Maixin, Fuzhou, China) for a
further 2 h. Cells were suspended at a final concentration of
3×105 cells/ml in serum-free medium prior to seeding
into the wells. Following incubation for 2 h, the wells were washed
with PBS to remove non-adherent cells, and then fixed in
paraformaldehyde (Xinchenghuagong, Inc., Guangzhou, China). The
number of adherent cells was determined using the colorimetric MTT
assay (MTT reagent; Sigma-Aldrich) according to the manufacturer’s
instructions.
Statistical analysis
Values are presented as the mean ± standard
deviation of at least three independent experiments. Statistical
analysis was performed using SPSS 19.0 statistical software (IBM,
Armonk, NY, USA). Student’s t-test was used to evaluate differences
between groups. P<0.05 was considered to indicate a
statistically significant difference between values.
Results
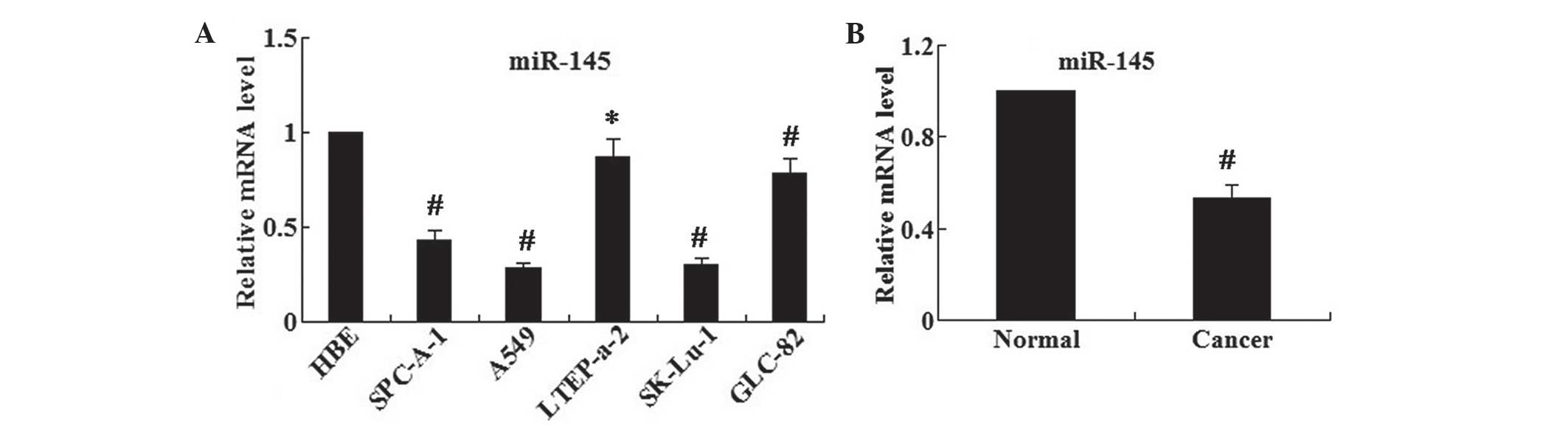
miR-145 is decreased in human lung cancer
cell lines and human lung cancer tissues
The relative mRNA levels of miR-145 in a series of
human lung cancer cell lines, SPC-A-1, A549, LTEP-a-2, SK-LU-1 and
GLC-82, as well as the normal human bronchial epithelial (HBE)
cells were examined using RT-qPCR. The results demonstrated that
the relative mRNA expression levels of miR-145 in human lung cancer
cell lines were significantly reduced compared with those of the
normal HBE cells (all P<0.05 except LTEP-a-2 P<0.01)
(Fig. 1A). In addition, A549 cells
demonstrated the lowest mRNA expression levels of miR-145.
Furthermore, the expression levels of miR-145 in human lung cancer
and adjacent normal tissues were examined (Fig. 1B); it was revealed that the
expression levels of miR-145 were significantly decreased in the
lung cancer tissues compared with those in the adjacent normal
tissues (P<0.01).
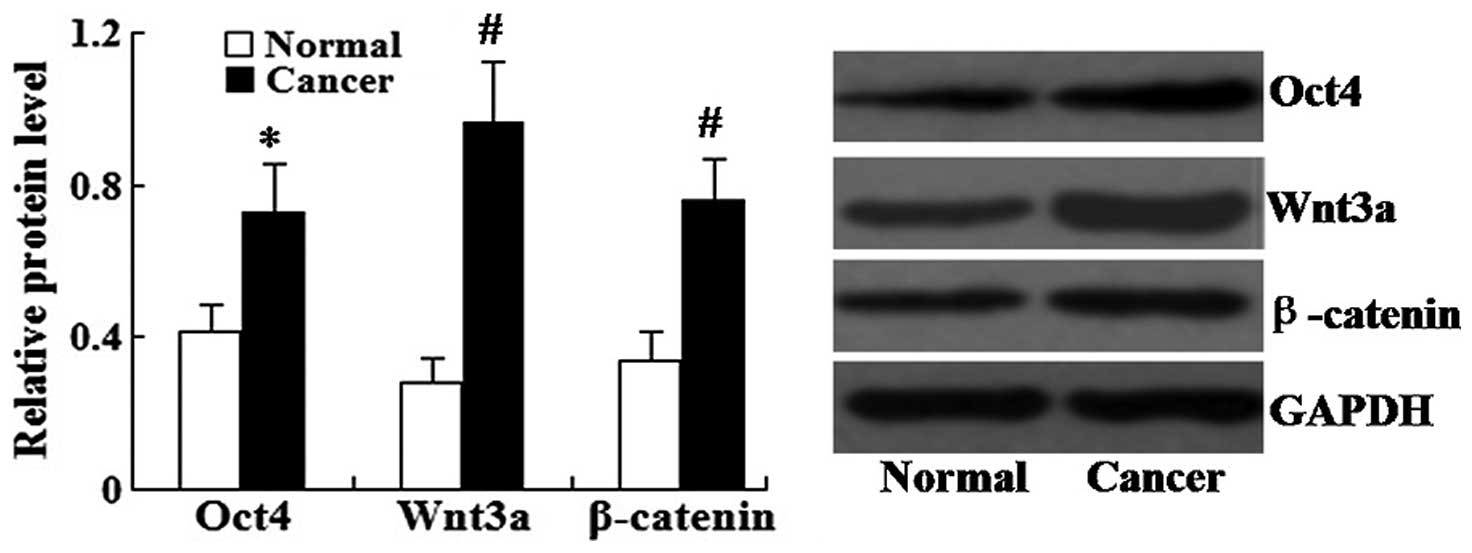
Relative protein expression of Oct4,
Wnt3a and β-catenin is increased in human lung cancer tissues
Western blot analysis was used to determine the
relative protein expression levels of Oct4, Wnt3a and β-catenin in
human lung cancer tissues. As shown in Fig. 2, the relative protein levels of
Oct4, Wnt3a and β-catenin in the lung cancer tissues were all
significantly increased compared with those in the adjacent normal
tissues (P<0.05 for Oct-4 and P<0.01 for Wnt3a and
β-catenin).
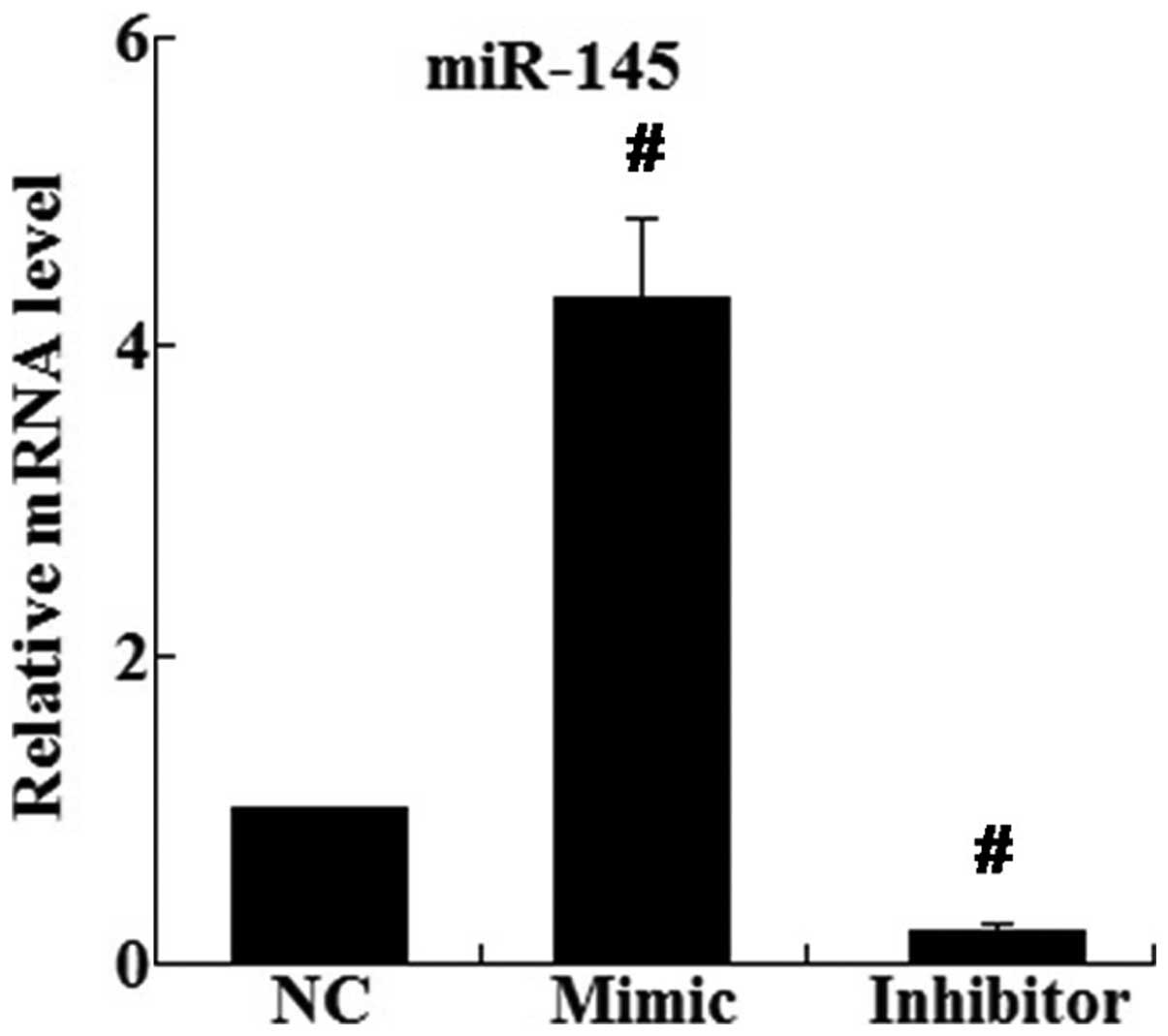
Restoration of miR-145 inhibits the
metastasis and EMT of A549 cells
miR-145 mimic and inhibitor were transfected into
A549 cells. As shown in Fig. 3,
RT-qPCR demonstrated that the relative mRNA expression of miR-145
in the mimic group was significantly increased 4.31-fold compared
with that of the control group (P<0.01); however, miR-145 in the
inhibitor group was significantly decreased to 22.01% of the
control group (P<0.01) (Fig.
3).
Following transfection of the miR-145 mimic into
A549 cells, cell invasion and adhesion assays were performed in
order to determine the metastasis and EMT of the transfected cells.
The results of the invasion assay revealed that the miR-145 mimic
significantly inhibited cell invasion; the number of invaded cells
in the miR-145 mimic group was markedly decreased compared with
that of the control group (21±4 and 36±6, respectively; P<0.05).
Cell adhesion assays demonstrated that following transfection with
the miR-145 mimic, the adhesion activity of A549 cells was reduced
to 51.01% of the untransfected control (P<0.05, data not
shown).
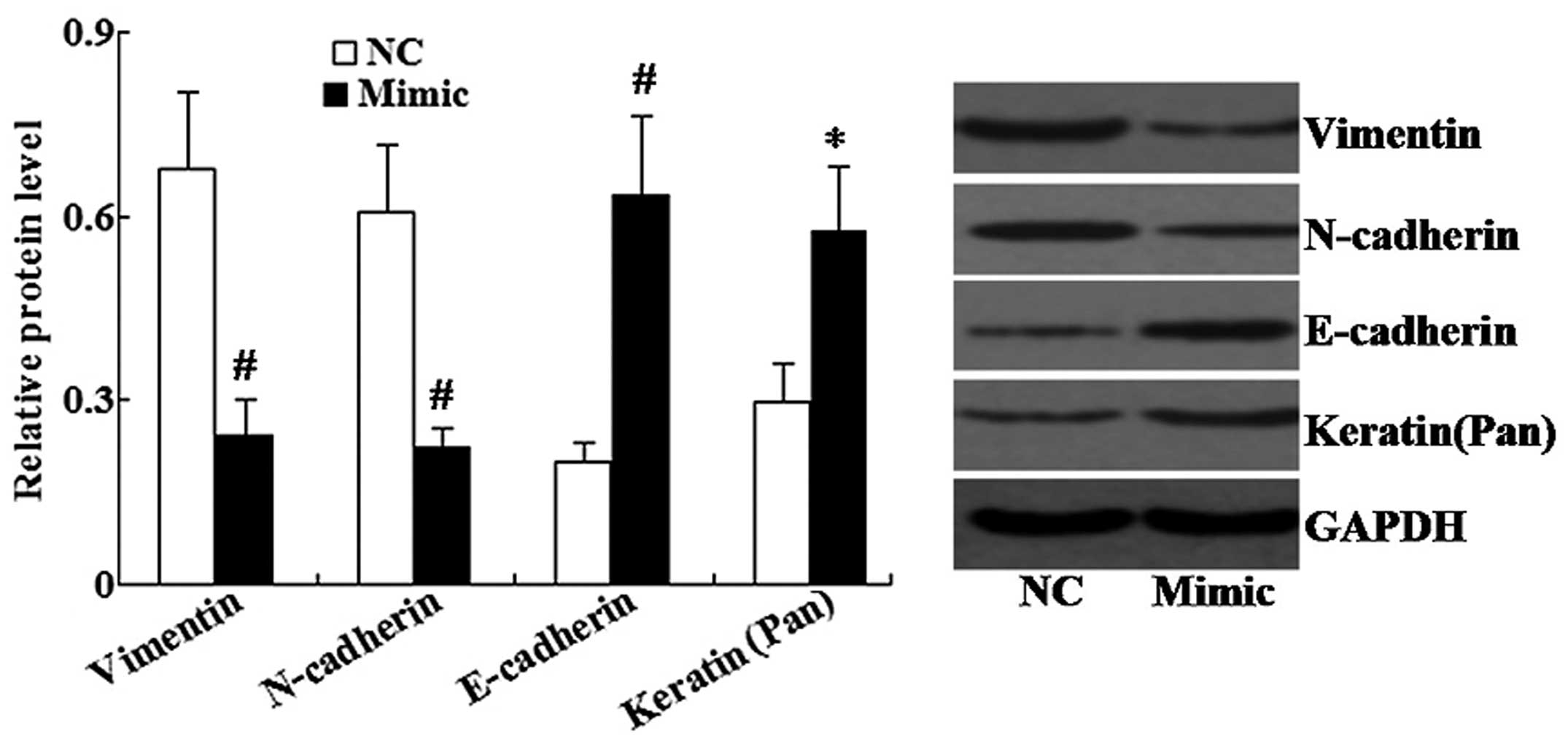
Western blot analysis was used to determine the
protein expression levels of mesenchymal cell markers (Vimentin and
N-cadherin) and epithelial cell markers (E-cadherin and Keratin) in
A549 cells. As shown in Fig. 4,
the miR-145 mimic significantly increased the expression of
E-cadherin and Keratin, and decreased the expression of Vimentin
and N-cadherin compared with that of the control group.
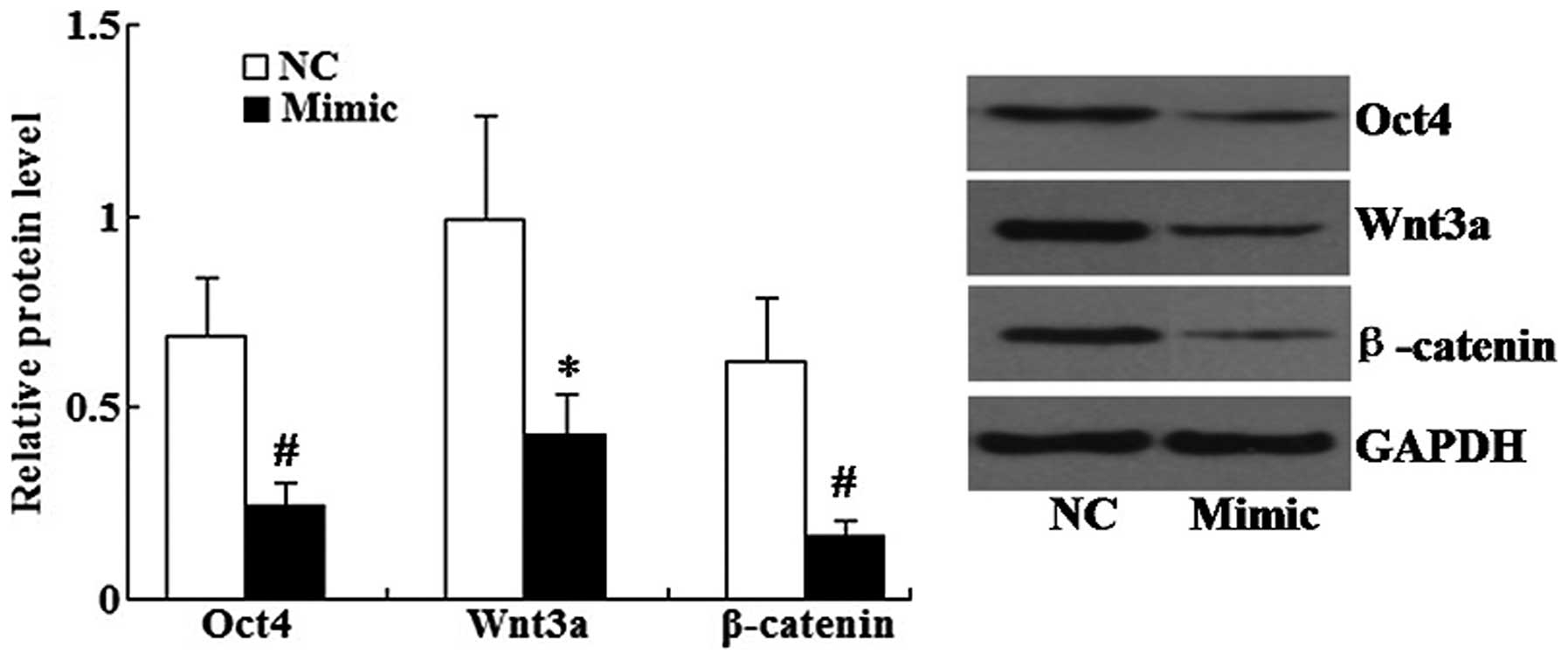
miR-145 restoration downregulates the
expression of Oct4, Wnt3a and β-catenin in A549 cells
Following transfection of the miR-145 mimic into
A548 cells, western blot analysis revealed that the relative
protein levels of Oct4, Wnt3a and β-catenin were all significantly
decreased in the miR-145 mimic group compared with those of the
control group (Fig. 5).
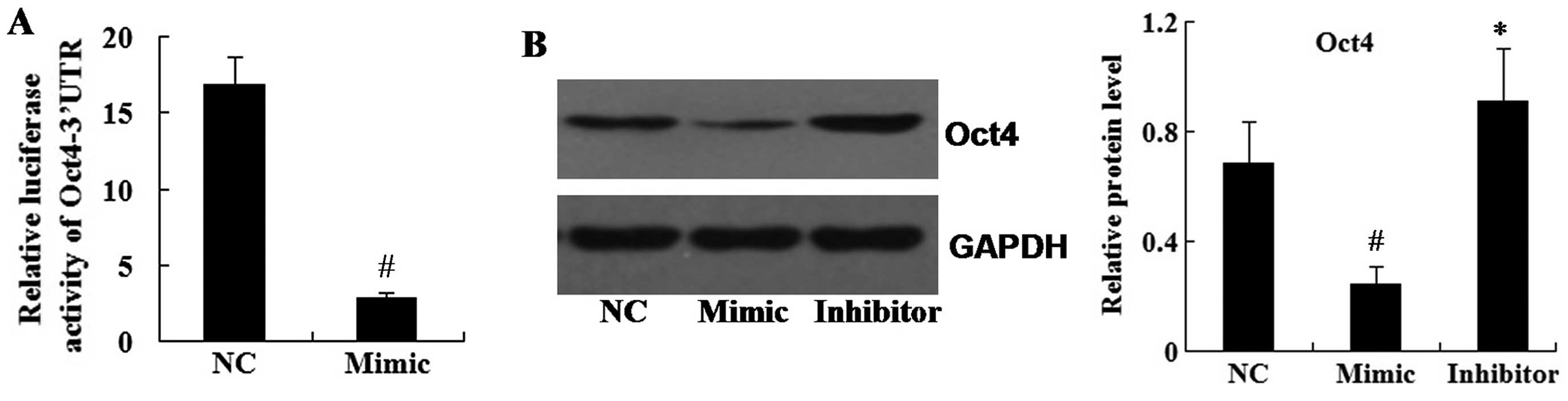
Oct4 is a direct target of miR-145
Luciferase assays revealed that the translational
activity of the luciferase-expressing plasmid containing the 3′UTR
of Oct4 was significantly supressed in the miR-145 mimic group
compared with that of the control group (Fig. 6A).
Furthermore, in order to determine whether miR-145
affected the expression of Oct4, A549 cells were transfected with
the miR-145 mimic and the miR-145 inhibitor. Western blot analysis
revealed that Oct4 protein expression was significantly decreased
in the miR-145 mimic group compared with that of the control group,
while Oct4 was significantly increased in the miR-145 inhibitor
group (Fig. 6B).
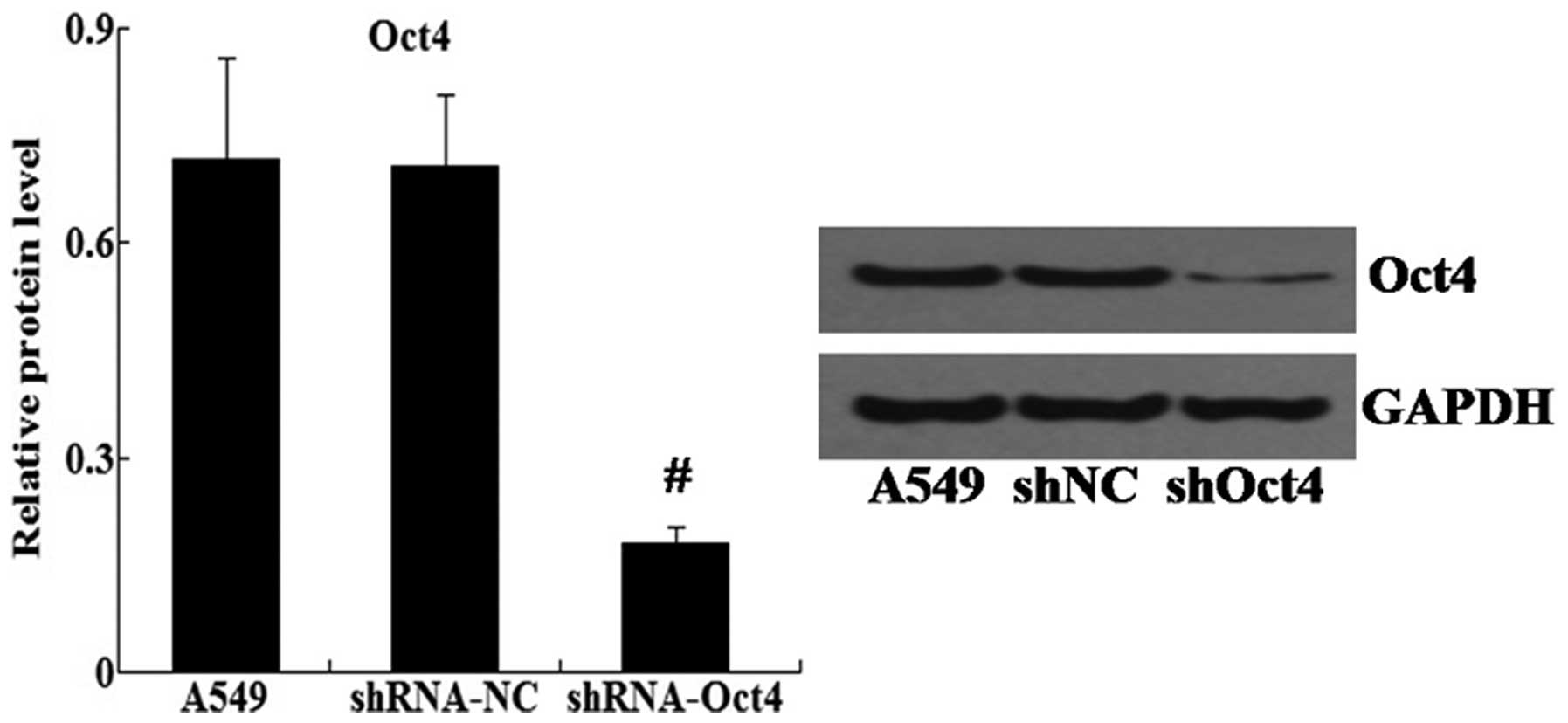
Knockdown of Oct4 inhibits metastasis and
EMT of A549 cells
In order to knockdown the expression of Oct4, small
hairpin (sh)RNA-Oct4 was transfected into A549 cells. Western blot
analysis revealed that the relative protein expression levels of
Oct4 were significantly decreased in the shRNA-Oct4 group compared
with those of the shRNA-NC group (Fig.
7).
Subsequently, the effect of Oct4 on metastasis and
EMT in A549 cells was evaluated. Cell invasion assays revealed that
following transfection with shRNA-Oct4, the number of invaded cells
was significantly decreased compared with that of the control group
(21±3 and 37±5, respectively; P<0.05). In addition, cell
adhesion assay revealed that the adhesion activity of A549 cells
was markedly decreased in the group transfected with shRNA-Oct4 to
48.91% of the negative control group (P<0.01) (data not
shown).
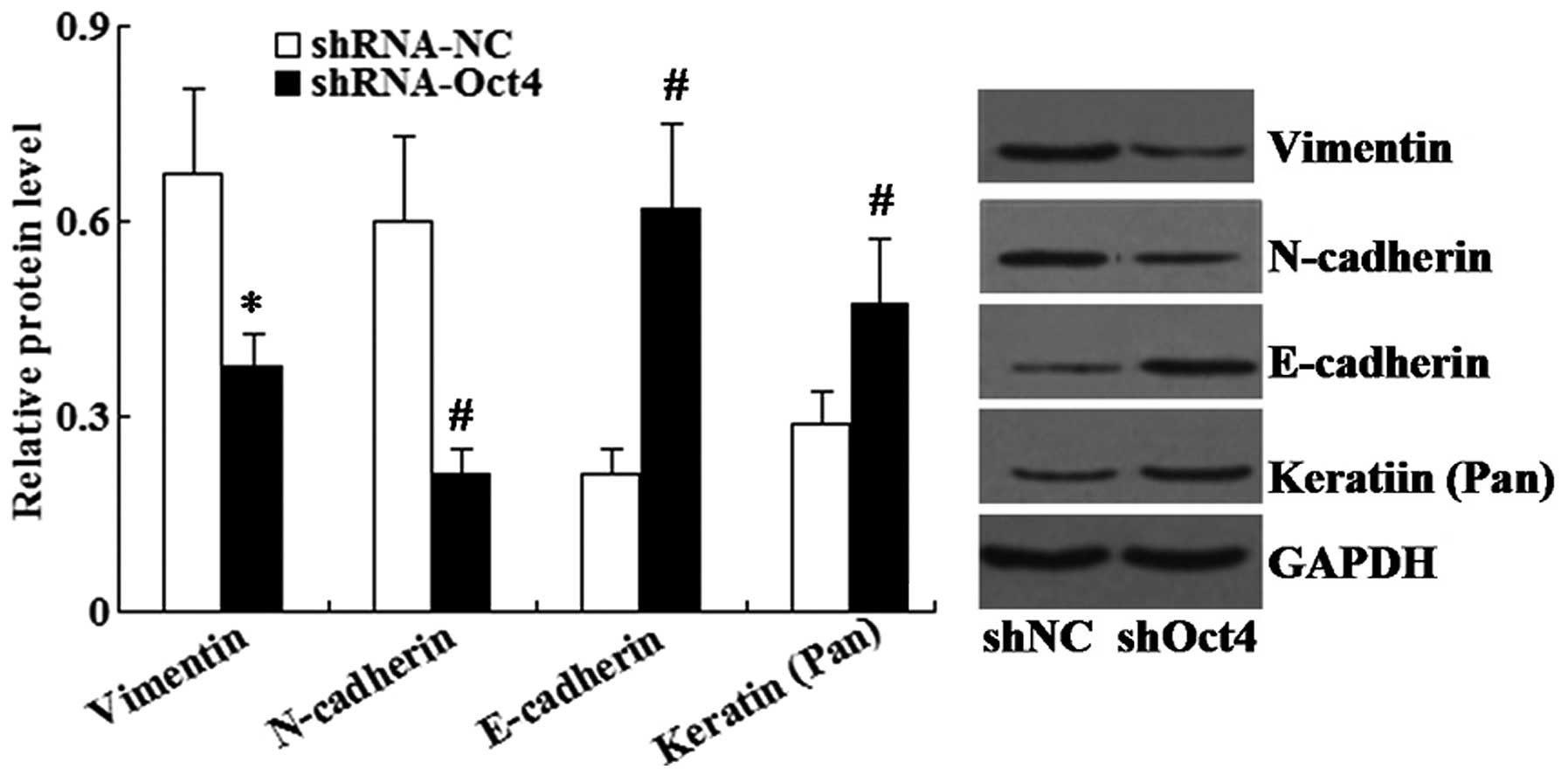
Furthermore, western blot analysis demonstrated that
the expression of epithelial cell markers (E-cadherin and Keratin)
were significantly increased in the shRNA-Oct4 group compared to
that of the negative control group, whereas the expression of
mesenchymal cell markers (Vimentin and N-cadherin) was
significantly decreased (Fig.
8).
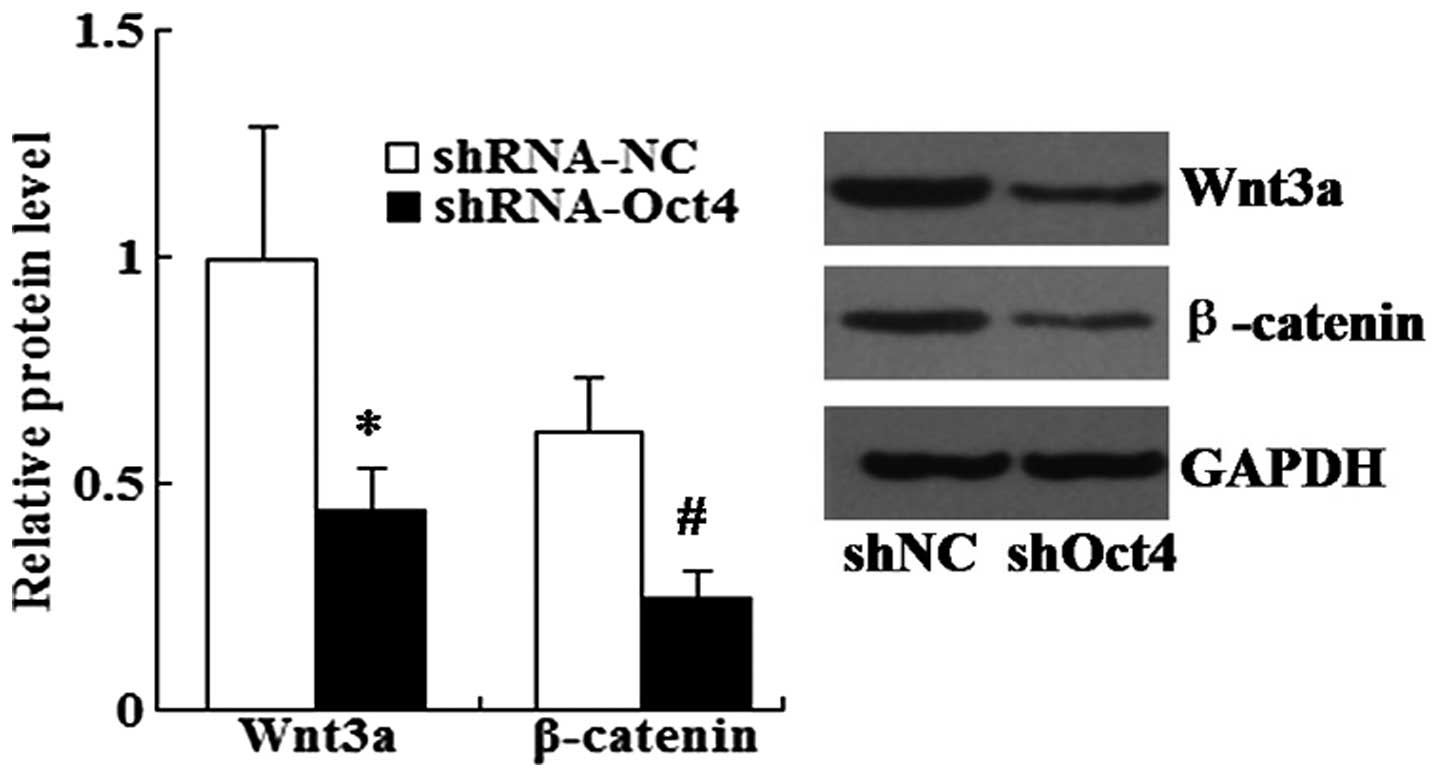
Knockdown of Oct4 downregulates the
expression of Wnt3a and β-catenin in A549 cells
Western blot analysis was performed in order to
examine the effects of Oct4 on the protein expression levels of
Wnt3a and β-catenin. As shown in Fig.
9, the expression levels of Wnt3a and β-catenin were
significantly decreased in shRNA-Oct4-transfected cells compared
with those of the shRNA-NC-transfected cells.
Discussion
Downregulation of miR-145 has been observed in
various types of human cancer (11–13),
and studies have indicated that the role of miR-145 in metastasis
may be cell type-specific (14–16).
The results of the present study revealed that miR-145 was
downregulated in human lung cancer tissues as well as in a series
of human lung cancer cell lines; this therefore indicated that
miR-145 acted as an anti-oncogene in tumor development.
Furthermore, an miR-145 mimic was transfected into A549 cells,
which revealed that miR-145 restoration inhibited the invasive and
adhesive abilities of A549 cells. These results therefore suggested
that miR-145 had an inhibitory role in the regulation of lung
cancer metastasis.
EMT is a process in which epithelial cells lose
their morphology and gene expression patterns, including the
molecular markers E-cadherin, α-catenin and γ-catenin, as well as
obtain mesenchymal cell morphology and gene expression
characteristics, including the molecular markers N-cadherin,
Vimentin and α-smooth muscle actin. EMT has been implicated in
numerous pathological conditions. It exhibits a crucial role in
promoting tumor progression and metastasis (18). Notably, EMT is reversible and
mesenchymal-epithelial transition (MET) is the reverse process of
EMT (19). MET is an important
early event in somatic cell reprogramming (20). Therefore, studies have focused on
elucidating the mechanisms in EMT regulation and identifying novel
strategies for MET induction (19,21).
Certain miRNAs have been found to suppress EMT in cancer cells;
however, the effect of miR-145 on EMT of lung cancer cells remains
to be elucidated. The present study revealed that miR-145
restoration led to induced MET, as characterised by the altered
expression of mesenchymal cell markers Vimentin and N-cadherin, as
well as epithelial cell markers E-cadherin and Keratin in lung
cancer cells.
Furthermore, the present study aimed to investigate
the mechanisms underlying the role of miR-145 in the regulation of
EMT. Oct4 is an octamer motif-binding transcription factor that
belongs to the Pit-Oct-Unc family (22). It was proposed that Oct4 acts as a
multi-functional factor in cancer and stem cell biology; Oct4 is a
key regulator that induces somatic cell pluripotency (23,24).
In addition, Oct4 has been shown to exhibit an oncogenic effect in
several types of cancers (25–29).
In the present study, luciferase assays and western blot analysis
revealed that Oct4 was a directly downregulated by miR-145.
Furthermore, the knockdown of Oct4 expression in lung cancer cells
in the present study, significantly inhibited cell invasion and
adhesion as well as induced MET. These results therefore indicated
that miR-145 regulated EMT through targeting Oct4 in lung cancer
cells.
The Wnt/β-catenin signaling pathway has been
reported to exhibit important roles in regulating the cellular
processes involved in development, differentiation, proliferation
and adult tissue homeostasis (30). Wnt/β-catenin signaling is
controlled at multiple levels; however, aberrant Wnt/β-catenin
signaling has been shown to be involved in the pathogenesis of
multiple tumors and other disease states (31,32).
Previous studies have demonstrated that Wnt/β-catenin signaling had
important roles in the acquisition of an EMT phenotype and cancer
metastasis (33–35). In addition Wnt/β-catenin signaling
was reported to be regulated by Oct4 in embryonic stem cells
(36). The results of the present
study demonstrated that following knockdown of Oct4 in lung cancer
cells, Wnt3a and β-catenin expression was downregulated.
In conclusion, the present study demonstrated, for
the first time, that miR-145 inhibited EMT in lung cancer cells
through targeting the Oct4-mediated Wnt/β-catenin signaling
pathway. In view of the inhibitory role of miR-145 in lung cancer
metastasis, these results may provide a potential novel approach
for lung cancer therapy.
References
|
1
|
Jemal A, Siegel R, Xu J, et al: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nguyen DX, Bos PD and Massagué J:
Metastasis: from dissemination to organ-specific colonization. Nat
Rev Cancer. 9:274–284. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bates RC and Mercurio AM: The
epithelial-mesenchymal transition (EMT) and colorectal cancer
progression. Cancer Biol Ther. 4:365–370. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brabletz T, Hlubek F, Spaderna S, et al:
Invasion and metastasis in colorectal cancer:
epithelial-mesenchymal transition, mesenchymal-epithelial
transition, stem cells and beta-catenin. Cells Tissues Organs.
179:56–65. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bracken CP, Gregory PA, Khew-Goodall Y and
Goodall GJ: The role of microRNAs in metastasis and
epithelial-mesenchymal transition. Cell Mol Life Sci. 66:1682–1699.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Humphreys DT, Westman BJ, Martin DI and
Preiss T: MicroRNAs control translation initiation by inhibiting
eukaryotic initiation factor 4E/cap and poly(A) tail function. Proc
Natl Acad Sci USA. 102:16961–16966. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nicoloso MS, Spizzo R, Shimizu M, et al:
MicroRNAs - the micro steering wheel of tumour metastases. Nat Rev
Cancer. 9:293–302. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yanaihara N, Caplen N, Bowman E, et al:
Unique microRNA molecular profiles in lung cancer diagnosis and
prognosis. Cancer Cell. 9:189–198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sempere LF, Christensen M, Silahtaroglu A,
et al: Altered microRNA expression confined to specific epithelial
cell subpopulations in breast cancer. Cancer Res. 67:11612–11620.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schepeler T, Reinert JT, Ostenfeld MS, et
al: Diagnostic and prognostic microRNAs in stage II colon cancer.
Cancer Res. 68:6416–6424. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sachdeva M and Mo YY: MicroRNA-145
suppresses cell invasion and metastasis by directly targeting mucin
1. Cancer Res. 70:378–387. 2010. View Article : Google Scholar :
|
|
15
|
Wang L, Tang H, Thayanithy V, et al: Gene
networks and microRNAs implicated in aggressive prostate cancer.
Cancer Res. 69:9490–9497. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Arndt GM, Dossey L, Cullen LM, et al:
Characterization of global microRNA expression reveals oncogenic
potential of miR-145 in metastatic colorectal cancer. BMC Cancer.
9:3742009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yin R, Zhang S, Wu Y, et al: microRNA-145
suppresses lung adenocarcinoma-initiating cell proliferation by
targeting OCT4. Oncol Rep. 25:1747–1754. 2011.PubMed/NCBI
|
|
18
|
Thiery JP, Acloque H, Huang RY, et al:
Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lim J and Thiery JP:
Epithelial-mesenchymal transitions: insights from development.
Development. 139:3471–3486. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Samavarchi-Tehrani P, Golipour A, David L,
et al: Functional genomics reveals a BMP-driven
mesenchymal-to-epithelial transition in the initiation of somatic
cell reprogramming. Cell Stem Cell. 7:64–77. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang J and Ma L: MicroRNA control of
epithelial-mesenchymal transition and metastasis. Cancer Metastasis
Rev. 31:653–662. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Scholer H, Ruppert S, Suzuki N, Chowdhury
K and Gruss P: New type of POU domain in germ line-specific protein
Oct4. Nature. 344:435–439. 1990. View
Article : Google Scholar
|
|
23
|
Nichols J, Zevnik B, Anastassiadis K, et
al: Formation of pluripotent stemcells in the mammalian embryo
depends on the POU transcription factor Oct4. Cell. 95:379–391.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
de Jong J and Looijenga LH: Stem cell
marker OCT3/4 in tumor biology and germ cell tumor diagnostics:
history and future. Crit Rev Oncog. 12:171–203. 2006. View Article : Google Scholar
|
|
25
|
Kim RJ and Nam JS: OCT4 expression
enhances features of cancer stem cells in a mouse model of breast
cancer. Lab Anim Res. 27:147–152. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Y, Zhang X, Wang X, et al:
Inhibition of LDH-A by lentivirus-mediated small interfering RNA
suppresses intestinal-type gastric cancer tumorigenicity through
the downregulation of Oct4. Cancer Lett. 321:45–54. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen Z, Xu WR, Qian H, et al: Oct4, a
novel marker for human gastric cancer. J Surg Oncol. 99:414–419.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guo Y, Liu S, Wang P, et al: Expression
profile of embryonic stem cell-associated genes Oct4, Sox2 and
Nanog in human gliomas. Histopathology. 59:763–775. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Iida H, Suzuki M, Goitsuka R and Ueno H:
Hypoxia induces CD133 expression in human lung cancer cells by
up-regulation of OCT3/4 and SOX2. Int J Oncol. 40:71–79. 2012.
|
|
30
|
Chien AJ, Conrad WH and Moon RT: A Wnt
survival guide: from flies to human disease. J Invest Dermatol.
129:1614–1627. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chien AJ and Moon RT: WNTS and WNT
receptors as therapeutic tools and targets in human disease
processes. Front Biosci. 12:448–457. 2007. View Article : Google Scholar
|
|
32
|
Moon RT, Kohn AD, De Ferrari GV and Kaykas
A: WNT and beta-catenin signalling: diseases and therapies. Nat Rev
Genet. 5:691–701. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fu Y, Zheng S, An N, et al: β-catenin as a
potential key target for tumor suppression. Int J Cancer.
129:1541–1551. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Valenta T, Hausmann G and Basler K: The
many faces and functions of β-catenin. EMBO J. 31:2714–2736. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Anson M, Crain-Denoyelle AM, Baud V, et
al: Oncogenic β-catenin triggers an inflammatory response that
determines the aggressiveness of hepatocellular carcinoma in mice.
J Clin Invest. 122:586–599. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Davidson KC, Adams AM, Goodson JM, et al:
Wnt/β-catenin signaling promotes differentiation, not self-renewal,
of human embryonic stem cells and is repressed by Oct4. Proc Natl
Acad Sci USA. 109:4485–4490. 2012. View Article : Google Scholar
|