Introduction
Hemostasis is a physiological host defense mechanism
focused on the arrest of bleeding following vascular injury. It
preserves vascular integrity and prevents excessive blood loss.
Arrest of bleeding requires rapid formation of hemostatic plugs at
sites of vascular injury to prevent exsanguination. This depends on
a complex series of regulatory pathways that activate the platelets
and the coagulation system. By contrast, excessive activation of
the coagulation system may lead to thrombosis (1).
Total hip arthroplasty (THA) and total knee
arthroplasty (TKA) have been performed with increasing frequency in
previous years. In patients undergoing THA or TKA, different
patterns of altered venous hemodynamics and hypercoagulability have
been identified, thus the rate of distal deep venous thrombosis
(DVT) has increased (2). In
addition, the risk of venous thrombosis embolism (VTE) in THA and
TKA is among the highest for all surgical procedures (3). VTE can be treated, however when left
untreated it may be fatal (4).
Thrombosis and hemostasis are dependent upon the
involvement of von Willebrand factor (VWF) and the involvement of
VWF is a result of competition between the biosynthesis of large
VWF multimers and their degradation by A disintegrin and
metalloproteinase with thrombospondin motif, member 13 (ADAMTS13)
(5). ADAMTS13 is a member of a
family of proteases termed ADAMTSs (6,7).
ADAMTS13 has been identified as a VWF cleaving protease, which
cleaves between the Tyr1605 and Met1606 residues in the central A2
domain of VWF (8–10) to inhibit the activity of VWF. VWF
is a carrier protein for factor VIII (FVIII) and once bound to
platelets and the extracellular matrix may promote platelet
aggregation or platelet adhesion to areas of vascular damage
(5). Several studies have found
that FVIII and VWF levels were independently associated with venous
thromboembolism and suggested that VWF may make a significant
contribution to the risk of venous thrombosis, independent of FVIII
(11,12).
In the present study, blood samples were collected
from patients who underwent a THA or TKA and the changes of
procoagulant/fibrinolytic factors were analyzed, particularly VWF
and ADAMTS13.
Patients and methods
Patients
This study conformed to the ethical guidelines of
the 2004 Declaration of Helsinki and was approved by the
institutional Ethics Committee at the First Affiliated Hospital of
Soochow University, (Suzhou, China). Between June 2012 and March
2013, a total of 93 patients (58 females and 35 males; age, 44–84
years; mean age, 59.5 years) who underwent a THA or TKA were
enrolled in the present study after written informed consent had
been provided. The exclusion criterion was a history of abnormal
bleeding.
Collection and processing of blood
samples
Blood samples were obtained on the day of surgery
and the mornings of the first, second, third, fifth and seventh
postoperative days (PODs). The blood samples were collected by
venipuncture into plastic tubes containing 1/9th volume 0.109 mol/l
buffered trisodium citrate. For the analysis of prothrombin time
(PT; %), activated partial thromboplastin time (APTT), thrombin
time (TT), fibrinogen level, ADAMTS13 level and VWF level, platelet
poor plasma was obtained by centrifugation at 2,500 × g for 15 min
at 4°C and stored at −80°C until further analysis. The normal human
plasma pool was composed of the plasma of 25 normal individuals
with different ABO blood groups.
Coagulation assessment
PT, APTT, TT, antithrombin III and plasma fibrinogen
were measured on a Sysmex® CA500 system (Sysmex
Corporation, Chuo-ku, Japan). PT was measured using the
Thromborel® S reagent (Siemens Healthcare Diagnostics,
Marburg, Germany), PT international normalized ratio was calculated
as the reaction time of the sample (sec) divided by the reaction
time of the normal plasma (sec). APTT was measured using the
Dade® Actin® Activated Cephaloplastin reagent
(Siemens Healthcare Diagnostics). TT was measured using the Test
Thrombin reagent (Siemens Healthcare Diagnostics). Antithrombin III
was measured using the Berchrom® Antitrombina III
reagent (Siemens Healthcare Diagnostics). D-dimer was measured on a
STA-R fully automated coagulation analyzer with the corresponding
reagents (Diagnostica Stago, Asnieres, France).
Plasma ADAMTS13 antigen
determination
Plasma ADAMTS13 antigen was determined by western
blotting using known concentrations of a recombinant ADAMTS13,
which was provided by Jiangsu Institute of Hematology (Jiangsu,
China), as a reference (13). Each
sample was subjected to an 8% SDS-PAGE together with a serial
dilution of recombinant ADAMTS13 (from 0.5–2 μM) and then analyzed
by western blotting with a 1:3,000 dilution of anti-human ADAMTS13
rabbit polyclonal antibody (ab28274; Abcam, Cambridge, UK) in 0.1%
Tween-20 in phosphate-buffered saline (PBS). Chemiluminescence was
detected with SuperSignal West Pico (Thermo Scientific, Rockford,
IL, USA). Proteins were quantified with the calibration curve of
recombinant ADAMTS13, determined by densitometry with ImageJ
software 1.46r (National Institutes of Health, Bethesda, MA,
USA).
Cleavage of fluorescence resonance energy
transfer substrate VWF73 by plasma ADAMTS13
Fluorescence resonance energy transfer substrate
VWF73 (FRETS-VWF73) was provided by Dr Xinglong Zheng (The
Children’s Hospital of Philadelphia, Philadelphia, PA, USA).
FRETS-VWF73 is a chemically synthesized fluorogenic peptide,
containing the 73-amino-acid residues between D1596 and R1668 in
the A2 domain of VWF (14). The
normal human plasma (NHP) from 25 normal individuals with different
ABO blood groups was mixed as the standard plasma. The blood
samples were diluted in a pH 6.0 buffer containing 5 mmol/l
Bis-Tris, 25 mmol/l CaCl2 and 0.005% Tween-20. The NHP
was diluted at the ratio of 1:12.5, 1:25, 1:50, 1:100, 1:200 and
1:400, as standard. A total of 50 μl of the diluted samples was
added to an enzyme immunoassay/radioimmunoassay plate (Costar,
Corning, NY, USA) and then 50 μl of fluorescein-labeled VWF73 was
added to each well as the substrate and mixed well. After 5 min,
the absorbance of each sample was read at 485/530 nm every 2 min 30
times with a Varioskan™ Flash Multimode Reader (Thermo Fisher
Scientific Inc., Vantaa, Finland)
VWF:Ag enzyme-linked immunosorbent assay
(ELISA)
VWF:Ag was measured by an ELISA with paired
antibodies against human VWF, SZ29 and SZ34 from Jiangsu Institute
of Hematology (Suzhou, China) (15). Microtiter plates (96 well) were
coated overnight at 4°C with 100 μl/well of monoclonal anti-VWF
antibody SZ-29 (1:1,000; Jiangsu Institute of Hematology) (15) in coating buffer (0.05 M
carbonate/bicarbonate buffer, pH 9.6). The plates were washed three
times with 0.1% Tween-20 in PBS (PBST) and blocked overnight at 4°C
with 2% (w/v) bovine serum albumin (BSA) in PBST, followed by
washing with PBST. Standard plasma pool dilutions (1:20, 1:50,
1:100, 1:200, 1:500 and 1:1,000) and diluted test samples, all in
0.2% BSA-PBST, were added in duplicate to the wells and incubated
for 2 h at 37°C. Following washing, the samples were incubated with
an anti-human VWF mouse monoclonal antibody SZ-34 labeled with
horseradish peroxidase (HRP; 1:6,000 in 0.2% BSA-PBST) obtained
from the Jiangsu Institute of Hematology (15) for 2 h at 37°C and then detected by
tetramethyl benzidine (TMB; Thermo Scientific) reaction and
absorbance measurement as described previously (16).
VWF: RCo-ELISA
Microtiter wells (Nunc-immunoplate; Nunc AS,
Kamstrup, Denmark) were coated with 100 μl of mAb SZ-151 at 10
μg/ml in coating buffer and incubated overnight at 4°C. The wells
were washed with PBST three times and then blocked with 2% BSA-PBST
for 2 h at 37°C. Subsequently, the wells were incubated with TBST
(0.025 M Tris-buffered saline with 0.1% Tween-20, pH 7.4)
containing 4 μg/ml recombinant fragment of platelet glycoprotein
Ibα (rfGPIbα) for 2 h at 37°C. Following washing the plates six
times with TBST, the mixed-plasma pool as standard (diluted from
1:50 to 1:3,200) and test samples (diluted 1:50) containing 760
μg/ml ristocetin (Sigma, St. Louis, MO, USA) were added to the
plates with captured rfGPIbα and incubated for 2 h at 37°C.
Following washing, bound VWF was incubated with HRP-conjugated
rabbit anti-human VWF antibody (P0226; Dako-Cytomation, Glostrup,
Denmark) for 1 h at 37°C and was detected with TMB. The reaction
was stopped with 3 M H2SO4 and the absorbance
of each sample was read at 450 nm, as previously described
(16).
Statistical analysis
All results are expressed as the mean ± standard
deviation. Statistical significance was analyzed using the
Friedman’s test (a repeated measures analysis of variance for
nonparametric data). P<0.05 was considered to indicate a
statistically significant difference. Statistical calculations were
performed using Prism5 (GraphPad Software, La Jolla, CA, USA).
Results
Patient complications
Of the 97 patients primarily enrolled in the present
study, four were excluded from the analysis due to the requirement
for an intraoperative transfusion of fresh frozen plasma. A total
of 51 patients underwent THA and 42 had a TKA. During the 7 day
postoperative observation period, no apparent clinical
thromboembolic complications were recorded. The demographic data
are summarized in Table I.
 | Table IDemographic data. |
Table I
Demographic data.
| Characteristic | Variable |
|---|
| Female | 58 (62.36%) |
| Male | 35 (37.63%) |
| Age (years) | 59.5 (44–84) |
| Total hip
arthroplasty | 51 (54.83%) |
| Total knee
arthroplasty | 42 (45.16%) |
PT, APTT and TT levels
PT, APTT and TT were measured on a Sysmex CA500
system. The reference ranges were: PT, 10.8–13.6 sec; PT-INR,
0.800–1.500 sec; APTT, 23.0–37.0 sec; and TT, 14.0–21.0 sec,
respectively. The results of the PT, APTT and TT are presented in
Fig. 1. The mean PT, APTT and TT
remained within reference limits throughout the entire observation
period.
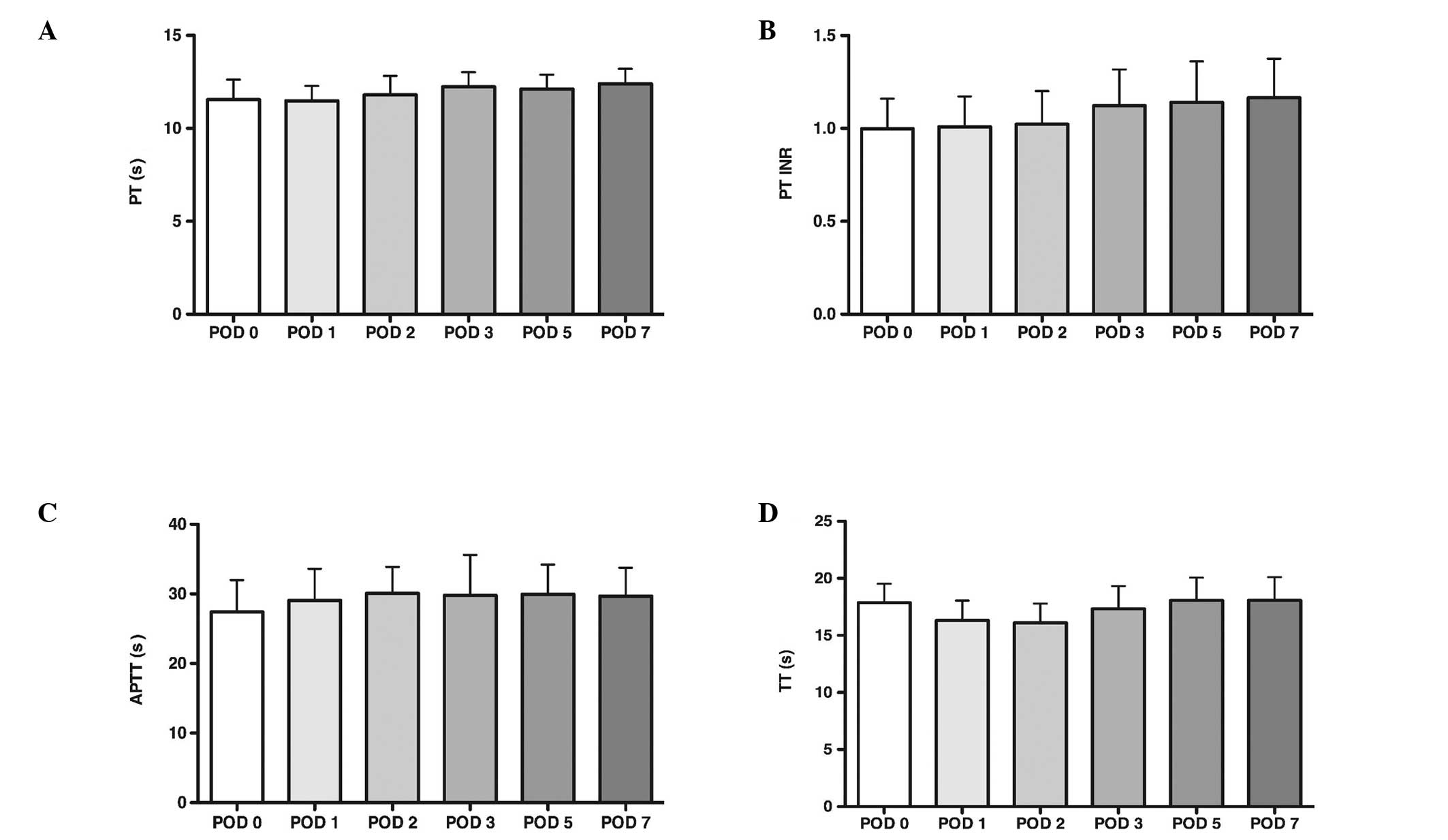 | Figure 1PT, APTT and TT levels. (A) PT level.
(B) The PT-INR was obtained by conversion of the prothrombin ratio
into internationally comparable values using the International
Sensitivity Index. The prothrombin international normalized ratio
was obtained by divinding the reaction time of the sample by the
reaction time of the normal plasma pool. (C) APTT level. (D) TT
level. (A), (C) and (D) were measured on a Sysmex® CA500
system (Sysmex® Corporation, Chuo-ku, Japan). The mean
PT, APTT and TT levels remained within reference limits throughout
the entire observation period. The results are expressed as the
mean ± standard deviation; *P<0.05, compared with
POD0. PT, prothrombin time; APTT, activated partial thromboplastin
time; TT, thrombin time; POD, post-operative day; INR,
international normalized ratio. |
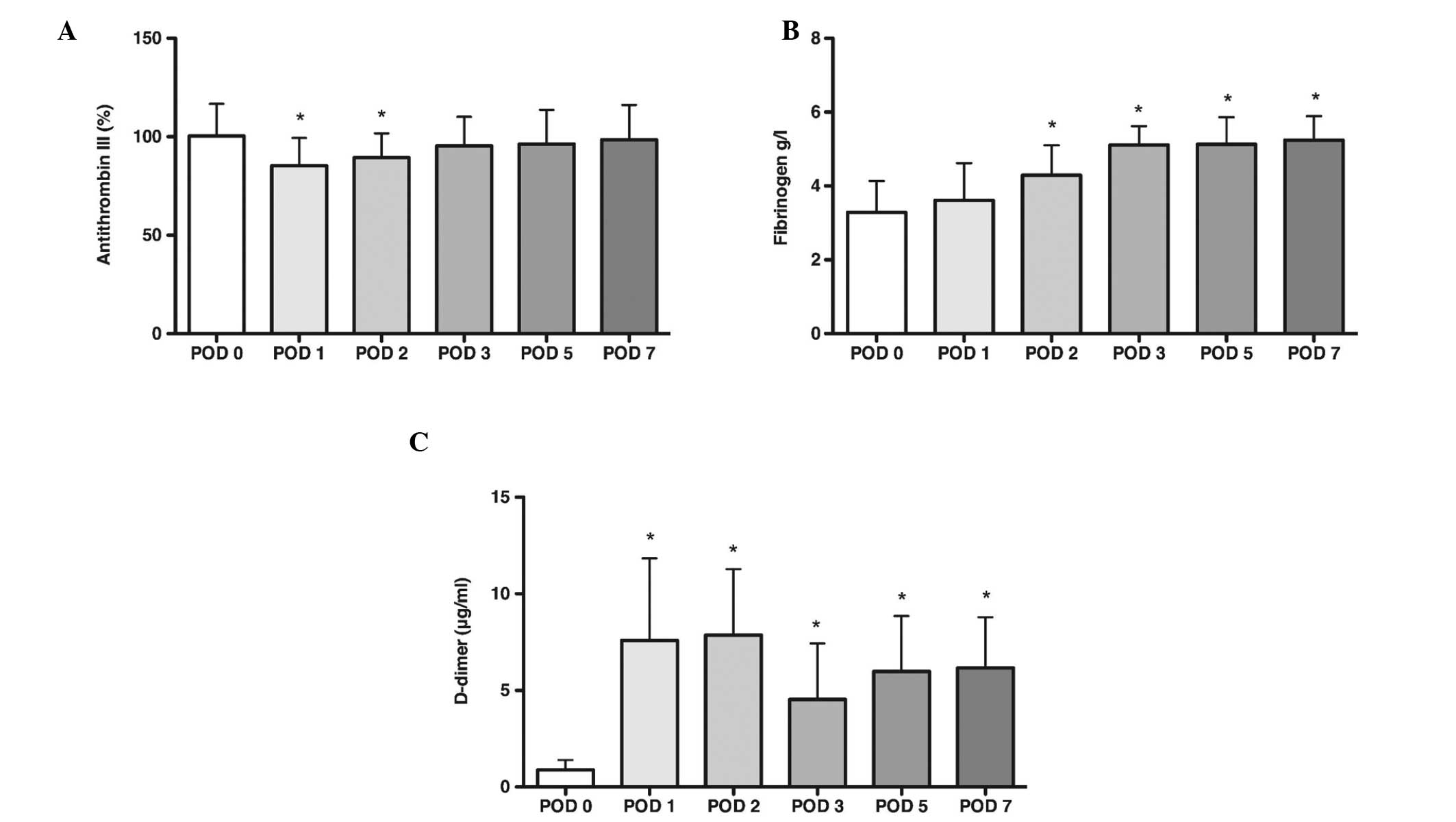
Antithrombin III, plasma fibrinogen and
D-dimer levels
Antithrombin III and plasma fibrinogen were measured
with a Sysmex CA500 system. D-dimer was measured on a STA-R fully
automated coagulation analyzer. The reference ranges are as
follows: Antithrombin III, 70–125%; fibrinogen, 2.000–5.000 g/l;
and D-dimer, 0.01–0.5 μg/ml. The results for antithrombin III,
plasma fibrinogen and D-dimer are presented in Fig. 2. The mean level of antithrombin III
was observed to be significantly below preoperative values on POD1
and POD2 (P<0.05), however it remained within the normal range
and returned to preoperative values by POD3. On POD1, D-dimer was
observed to increase significantly above preoperative levels. From
POD2, the mean level of fibrinogen also increased significantly.
During POD7 D-dimer and fibrinogen levels remained raised
(P<0.05).
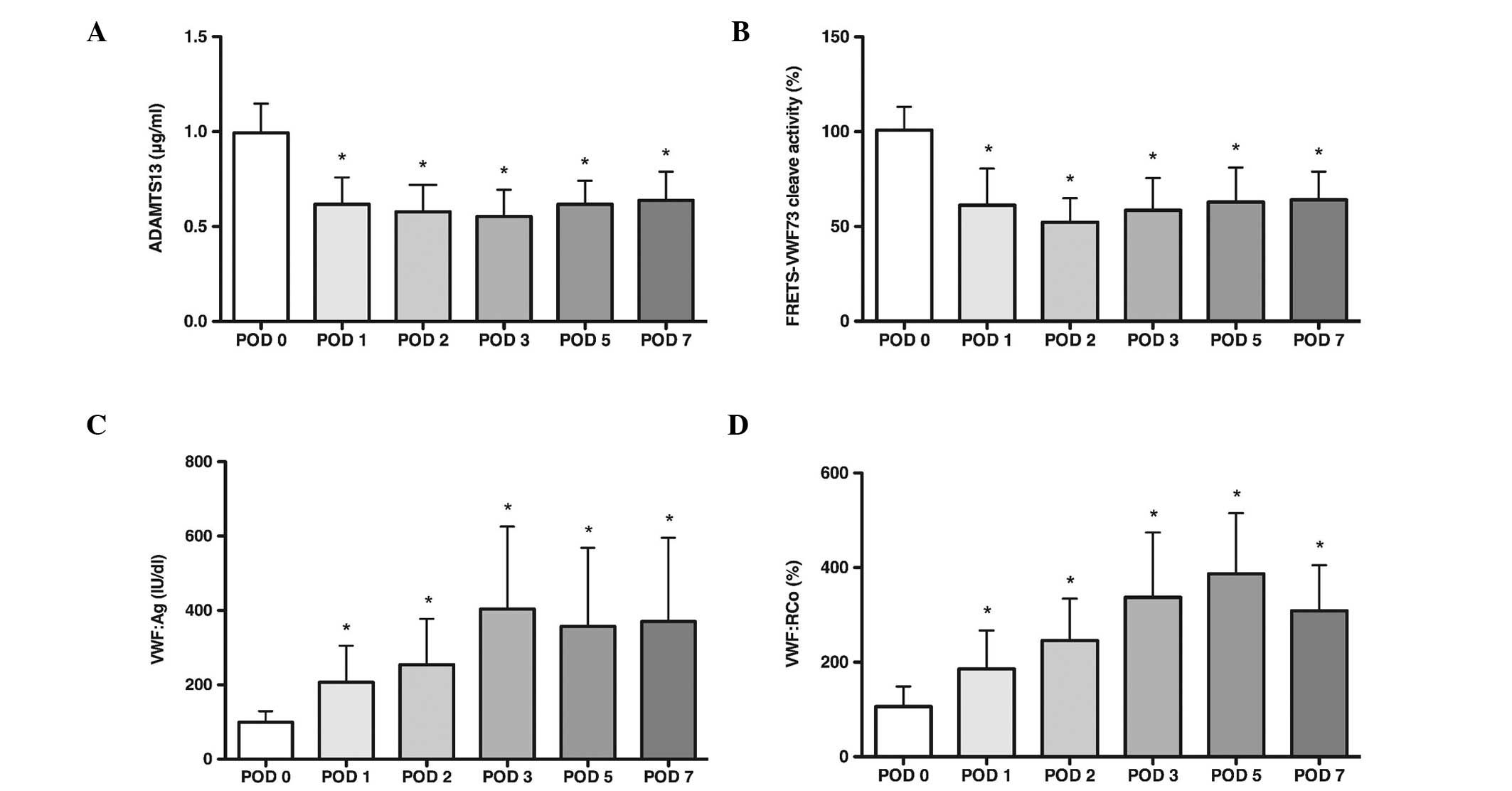
ADAMTS13 and VWF
The results of the ADAMTS13 and VWF are presented in
Fig. 3. To examine the
concentration of plasma ADAMTS13, equivalent fractions of each
sample were subjected to an 8% SDS-PAGE followed by western blot
analysis. The result indicates that the mean level of plasma
ADAMTS13 decreased continuously from POD1 compared with the
preoperative level (P<0.05). The FRETS-VWF73 assay was used to
validate the proteolytic activity of the ADAMTS13. VWF73 is a
peptide fragment from the VWF A2 domain that contains the
proteolytic site of ADAMTS13. The plasma ADAMTS13 activity was
defined as the value divided by that of the normal human plasma
diluted at 1:25 and multiplied by 100. The mean level of plasma
ADAMTS13 proteolytic activity also decreased from POD1 (P<0.05).
VWF antigen and activity were measured with ELISA as described
previously (16). The present
results demonstrated that the VWF antigen and activity were
increased significantly above preoperative levels (P<0.05) as
the plasma ADAMTS13 and its proteolytic activity decreased.
Discussion
A total of 93 patients were assessed, who underwent
either a THA or a TKA The present study demonstrated that no
significant changes were observed for PT, APTT and TT; the mean
level of antithrombin III was found to be significantly below
preoperative values on POD1 and POD2 and the fibrinogen and D-dimer
levels were increased continuously during the 7 days following
surgery. The levels of VWF antigen and activity were increased
significantly above preoperative levels, and the plasma ADAMTS13
and its proteolytic activity decreased continuously from POD1 to
POD7.
A study of 252 patients who underwent a total joint
arthroplasty revealed that the prevalence of DVT was 32% (16%
distal, 16% proximal) following THA and 66% (50% distal, 16%
proximal) following TKA, without routine chemical or mechanical
prophylaxis (17). A prospective
epidemiological study was performed in 19 centers across Asia, in
patients undergoing elective THA, TKA or hip fracture surgery (HFS)
without pharmacological thromboprophylaxis. The results revealed
that total DVT and proximal DVT rates were highest in TKA patients
(58.1 and 17.1%, respectively), followed by HFS patients (42.0 and
7.2%, respectively) and THA patients (25.6 and 5.8%, respectively).
DVT was more frequent in female patients aged at least 65 years.
The rate of venographic thrombosis in the absence of
thromboprophylaxis following major joint surgery in Asian patients
is similar to that previously reported in patients in Western
countries (18).
There is evidence that, during surgery, dysfunction
of the coagulation activation system may be responsible for
hemorrhage. In addition, anesthetic techniques have also been
observed to affect hemorheological and hemostasiological parameters
(19). These clinically
significant hemorheological and hemostasiological alterations
include: Hyperreagibility of platelets with increased aggregation
and adhesion tendency; changes in fibrinogen, albumin and globulin
concentrations, which affect viscosity and erythrocyte aggregation
and impairment of deformability; increase in clotting factors and
disturbance of fibrinolysis (20–22).
Patients undergoing surgical procedures have been subject to
investigation, in which thrombin activation parameters, clotting
factors, fibrinolysis and thrombelastographic changes were examined
in order to determine whether hypercoagulation occurs (23–27).
The present study revealed that no significant changes were
observed for PT, APTT and TT; the mean level of antithrombin III
was found to be significantly below preoperative values on POD1 and
POD2, and the fibrinogen and D-dimer levels were increased
continuously during the 7 days following surgery. These results are
consistent with previous studies (23,27).
It was also observed that the levels of VWF antigen and activity
were increased significantly above preoperative levels as the
plasma ADAMTS13 and its proteolytic activity decreased continuously
from POD1 to POD7. By contrast, following surgery, a number of
secretagogues, including thrombin, fibrin, histamine, the C5b-9
complement complex and several inflammatory cytokines triggered the
secretion of VWF from its storage site in the Weibel-Palade body.
While constitutively secreted multimers of VWF are of a relatively
small size, the multimers stored within the Weibel-Palade body are
the largest, most biologically potent form (28). By contrast, as the proteolytic
activity of ADAMTS13 was impaired, the feedback proteolysis of VWF
by ADAMTS-13 was decreased. The ultra large VWF in the plasma,
which was secreted from the Weibel-Palade body, and its activity
increased.
A population-based patient-control study was
undertaken to elucidate the roles of the ABO blood group, VWF and
clotting factor VIII in the process of DVT (11). Using univariate analysis, it was
identified that blood group, VWF concentration and factor VIII
concentration were all associated with DVT. The present study also
suggested that the thrombosis risk associated with high VWF was
mediated through factor VIII (11). Another study with samples obtained
from 19,237 adults demonstrated that Factor VIII and VWF were
linearly associated with increased risk of venous thromboembolism
and suggested that VWF may make a significant contribution to the
risk of venous thrombosis, independent of FVIII (12). VTE is a serious complication
following major orthopedic surgery and ~10% of mortality in
hospitals is attributed to PE. There are several strategies to
prevent this, including pharmacological and mechanical approaches
(29). As high VWF is an
independent risk factor of venous thrombosis, inhibiting the
activity of VWF may be a novel form of prophylaxis to reduce
postoperative DVT and PE, such as a monoclonal antibody SZ123,
which was demonstrated to prevent in vivo thrombus formation
by inhibiting VWF A3-collagen and VWF A1-platelet interactions
(30).
In conclusion, the plasma ADAMTS13 and its
proteolytic activity decreased as the level of VWF antigen and
activity were increased significantly above preoperative levels
following surgery. It is expected that the ADAMTS13 and VWF levels
may be potentially useful markers in predicting thrombotic
complications following arthroplasty and inhibiting the activity of
VWF may be a novel prophylaxis to reduce postoperative DVT and
PE.
Acknowledgements
The authors would like to thank Dr X. Long Zheng for
providing FRETS-VWF73. The abstract of the present study was
presented at the 34th SICOT Orthopaedic World Congress,
Hyderabad, India, October 17–19, 2013. The present study was
supported by the Natural Science Foundation of Jiangsu Province
(no. BK20140285) and the Jiangsu Provincial Special Program of
Medical Science (no. BL2012004).
References
|
1
|
Anderson JA, Lim W and Weitz JI: Genetics
of coagulation: what the cardiologist needs to know. Can J Cardiol.
29:75–88. 2013. View Article : Google Scholar
|
|
2
|
Fisher WD: Impact of venous
thromboembolism on clinical management and therapy after hip and
knee arthroplasty. Can J Surg. 54:344–351. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Falck-Ytter Y, Francis CW, Johanson NA, et
al; American College of Chest Physicians. Prevention of VTE in
orthopedic surgery patients: Antithrombotic Therapy and Prevention
of Thrombosis, 9th ed: American College of Chest Physicians
Evidence-Based Clinical Practice Guidelines. Chest. 141(2 Suppl):
e278S–e325S. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Barrack RL: Current guidelines for total
joint VTE prophylaxis: dawn of a new day. J Bone Joint Surg Br.
94:3–7. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sadler JE: von Willebrand factor: two
sides of a coin. J Thromb Haemost. 3:1702–1709. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zheng X, Chung D, Takayama TK, Majerus EM,
Sadler JE and Fujikawa K: Structure of von Willebrand
factor-cleaving protease (ADAMTS13), a metalloprotease involved in
thrombotic thrombocytopenic purpura. J Biol Chem. 276:41059–41063.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Porter S, Clark IM, Kevorkian L and
Edwards DR: The ADAMTS metalloproteinases. Biochem J. 386:15–27.
2005. View Article : Google Scholar :
|
|
8
|
Tsai HM: Physiologic cleavage of von
Willebrand factor by a plasma protease is dependent on its
conformation and requires calcium ion. Blood. 87:4235–4244.
1996.PubMed/NCBI
|
|
9
|
Furlan M, Robles R and Lämmle B: Partial
purification and characterization of a protease from human plasma
cleaving von Willebrand factor to fragments produced by in vivo
proteolysis. Blood. 87:4223–4234. 1996.PubMed/NCBI
|
|
10
|
Fujikawa K, Suzuki H, McMullen B and Chung
D: Purification of human von Willebrand factor-cleaving protease
and its identification as a new member of the metalloproteinase
family. Blood. 98:1662–1666. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Koster T, Blann AD, Briet E, Vandenbroucke
JP and Rosendaal FR: Role of clotting factor VIII in effect of von
Willebrand factor on occurrence of deep-vein thrombosis. Lancet.
345:152–155. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tsai AW, Cushman M, Rosamond WD, et al:
Coagulation factors, inflammation markers, and venous
thromboembolism: the longitudinal investigation of thromboembolism
etiology (LITE). Am J Med. 113:636–642. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang AY, Liu F, Ma ZN, Dong NZ, Zhang JY
and Ruan CG: Research on the C-terminal domain of ADAMTS13
regulates its cleaving activity. Zhonghua Xue Ye Xue Za Zhi.
31:830–834. 2010.(In Chinese).
|
|
14
|
Kokame K, Nobe Y, Kokubo Y, Okayama A and
Miyata T: FRETS-VWF73, a first fluorogenic substrate for ADAMTS13
assay. Br J Haematol. 129:93–100. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ruan CG, Xi XD and Gu JM: Studies on
monoclonal antibodies to human von Willebrand factor. Zhonghua Nei
Ke Za Zhi. 25:547–550. 1986.(In Chinese).
|
|
16
|
Zhao Y, Gu Y, Ji S, Yang J, Yu Z and Ruan
C: Development of an ELISA method for testing VWF ristocetin
cofactor activity with improved sensitivity and reliability in the
diagnosis of von Willebrand disease. Eur J Haematol. 88:439–445.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Clarke MT, Green JS, Harper WM and Gregg
PJ: Screening for deep-venous thrombosis after hip and knee
replacement without prophylaxis. J Bone Joint Surg Br. 79:787–791.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Piovella F, Wang CJ, Lu H, et al:
Deep-vein thrombosis rates after major orthopedic surgery in Asia.
An epidemiological study based on postoperative screening with
centrally adjudicated bilateral venography. J Thromb Haemost.
3:2664–2670. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Spirin VA, Khomenko NM and Vasil’kov VG:
Hemostasis system in patients with bile duct diseases undergoing
surgery under peridural anesthesia and neuroleptanalgesia.
Anesteziol Reanimatol. 55–57. 1982.(In Russian).
|
|
20
|
Bergqvist D: Assessment of the risk and
the prophylaxis of venous thromboembolism in surgical patients.
Pathophysiol Haemost Thromb. 33:358–361. 2003. View Article : Google Scholar
|
|
21
|
Müller R and Musikić P: Hemorheology in
surgery - a review. Angiology. 38:581–592. 1987. View Article : Google Scholar
|
|
22
|
Picker SM: In-vitro assessment of platelet
function. Transfus Apher Sci. 44:305–319. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lindberg F, Rasmussen I, Siegbahn A and
Bergqvist D: Coagulation activation after laparoscopic
cholecystectomy in spite of thromboembolism prophylaxis. Surg
Endosc. 14:858–861. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
El Kady N, Khedr H, Yosry M and El Mekawi
S: Perioperative assessment of coagulation in paediatric
neurosurgical patients using thromboelastography. Eur J
Anaesthesiol. 26:293–297. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schietroma M, Carlei F, Mownah A, et al:
Changes in the blood coagulation, fibrinolysis, and cytokine
profile during laparoscopic and open cholecystectomy. Surg Endosc.
18:1090–1096. 2004.PubMed/NCBI
|
|
26
|
Goobie SM, Soriano SG, Zurakowski D,
McGowan FX and Rockoff MA: Hemostatic changes in pediatric
neurosurgical patients as evaluated by thrombelastograph. Anesth
Analg. 93:887–892. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lison S, Weiss G, Spannagl M and Heindl B:
Postoperative changes in procoagulant factors after major surgery.
Blood Coagul Fibrinolysis. 22:190–196. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sadler JE: von Willebrand factor assembly
and secretion. J Thromb Haemost. 7(Suppl 1): 24–27. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Geerts WH, Pineo GF, Heit JA, et al:
Prevention of venous thromboembolism: the Seventh ACCP conference
on antithrombotic and thrombolytic therapy. Chest. 126:338S–400S.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao YM, Jiang M, Ji SD, et al: Anti-human
VWF monoclonal antibody SZ-123 prevents arterial thrombus formation
by inhibiting VWF-collagen and VWF-platelet interactions in Rhesus
monkeys. Biochem Pharmacol. 85:945–953. 2013. View Article : Google Scholar : PubMed/NCBI
|