Introduction
Rheumatoid arthritis (RA) is a synovial disease
characterized by chronic inflammation of the joints culminating in
joint destruction. Proliferative fibroblast-like synoviocytes
(FLSs) have crucial roles in joint inflammation and bone damage, as
they produce large quantities of pro-inflammatory mediators,
including interleukin (IL)-1, IL-6, tumor necrosis factor-α
(TNF-α), matrix metalloproteinases (MMPs) and prostaglandin E2
(1). These mediators bind to
specific receptors, causing gene transcription, and form
complicated signaling interactions that contribute to the
progression of inflammatory arthritis, including leukocyte
infiltration, cytokine network formation and cartilage catabolism
elevation (2). The manner in which
rheumatoid arthritis is treated has changed markedly with the
introduction of anti-tumor necrosis factor (anti-TNF) biologics;
however, a number of patients still have less than adequate control
of their disease, even with these therapeutic regimens involving
disease-modifying anti-rheumatic drugs and currently available
biologics (3). Thus, there is a
requirement for safer and more effective anti-arthritic strategies
for long-term use.
Tumor necrosis factor receptor (TNFR)-associated
factor (TRAF) 6 transduces signals from several members of the TNFR
superfamily and the TLR/IL-1R family to activate the transcription
factors nuclear factor (NF)-κB and activator protein (AP)-1
(4). It has been also shown that
TRAF6 is required for NF-κB activation (5). TRAF6 has been shown to be critically
involved in regulating inflammatory response signaling pathways
involving adaptive immunity, innate immunity and bone metabolism
(6). As TRAF6 regulates
inflammation and links immunity to bone metabolism, the aim of the
current study was to explore the role of TRAF6 in the development
of the collagen-induced arthritis (CIA) model and the migration of
human RA-FLSs.
Materials and methods
Mouse collagen-induced arthritis and
evaluation
Sixteen 10-week-old male DBA/1 mice were purchased
from the Chinese Academy of Sciences (Shanghai, China) and were
maintained under a controlled temperature (22°C) and a 12-h
light/dark cycle (light, 0700–1900 h), with access to a water and a
standard diet ad libitum. Mice were immunized with Freund’s
adjuvant (Sigma-Aldrich, St. Louis, MO, USA)) and 100 μg bovine
type II collagen (Chondrex, Redmond, WA, USA) at the base of the
tail on day 0 and day 21 as previously described (7,8).
Animal experimental protocols were approved by the Animal Committee
of Zaozhuang Municipal Hospital (Zaozhuang, China). Clinical
arthritic scoring was performed every three days as follows
(9): 0, normal; 1, mild; 2,
moderate; and 3, maximal redness and swelling. The maximum score
per paw was 3 with a total maximum score of 12 per mouse.
TRAF6 inhibition in vivo and in
vitro
Small interfering RNA (siRNA) against TRAF6
(siTRAF6) and control siRNA were obtained from Santa Cruz
Biotechnology Inc. (Santa Cruz, CA, USA). The siTRAF6 was delivered
in vivo using in vivo-jet polyethylene imine (PEI,
Polyplus-transfection, Qbiogene, CA, USA) according to the
manufacturer’s instructions. In brief, siTRAF6 and PEI were mixed
and dissolved in 400 μl 5% glucose for 20 min for intraperitoneal
injection at room temperature, and the mixture was administered
three times daily, three days a week over three weeks. Mixtures
containing control siRNA was used as control.
In vitro, TRAF6 inhibition was achieved with
an anti-TRAF6 monoclonal antibody (anti-TRAF6mAb; Santa Cruz
Biotechnology, Inc.). FLSs were seeded in 6-well plates at
1×106 cells/well.
Histopathology
The mouse hind limbs were fixed in 4%
paraformaldehyde, decalcified and embedded in paraffin. Serial 4-μm
sections were cut and stained with hematoxylin and eosin (H&E).
Sections were analyzed microscopically for the extent of
inflammation and bone erosion as previously reported (10). The histological scores were
determined as follows: Score 0, no signs of inflammation; 1, mild
inflammation without cartilage destruction; 2–4, increasing degrees
of inflammatory cell infiltration and cartilage/bone
destruction.
Human RA patients
Twenty newly diagnosed active RA patients, who had
not received any treatment, who fulfilled the 2010 American College
of Rheumatology/European League Against Rheumatism criteria for RA
(11), were recruited from the
Zaozhuang Municipal Hospital (Zaozhuang, China). Active disease was
defined as a Disease Activity Score 28-joint assessment (DAS28) of
>5.1 (12). This study was
performed with the approval of the Ethics Committee of Zaozhuang
Municipal Hospital (Zaozhuang, China) in accordance with the
Helsinki Declaration. All patients provided informed consent.
Cell isolation and culture
RA-FLSs were obtained from the synovium during knee
joint arthroscopy. FLSs were isolated from synovial tissues by
enzymatic digestion as previously described (13). RA-FLSs were grown in Dulbecco’s
modified Eagle’s medium (DMEM; Thermo Fisher Scientific, Waltham,
MA, USA) containing 10% fetal bovine serum (Thermo Fisher
Scientific) in a humidified incubator at 37°C.
Western blot analysis
Total protein was extracted from the joints and FLSs
using an radioimmunoprecipitation assay buffer (Sigma-Aldrich). The
protein concentration was measured with a DC Protein assay
(Bio-Rad, Hercules, CA, USA). Proteins were separated using 10%
SDS-PAGE, and then transferred to a nitrocellulose membrane
(Millipore, Billerica, MA, USA). After blocking in 5% skimmed milk,
the membranes were incubated with rabbit polyclonal anti-TRAF6
antibody (1:1,000; sc-7221; Santa Cruz Biotechnology, Inc.) or
rabbit polyclonal anti-GAPDH (internal control; 1:2,000; sc-25778;
Santa Cruz Biotechnology, Inc.) overnight. Thereafter, the
membranes were rinsed in blocking solution and incubated for 1 h
with a secondary mouse anti-rabbit antibody conjugated to
horseradish peroxidase (1:2,000; sc-53804; Santa Cruz
Biotechnology, Inc.). Bands were visualized using an acridan-based
substrate detection system (ECL; Millipore).
ELISA
At the end of experiment, animals were sacrificed
under 40 mg/kg body weight pentobarbitol administered
intraperitoneally anesthetic and sera were obtainedby cardiac
puncture. The anti-collagen II antibodies (anti-CII)ELISAs
(Chondrex, USA) and MMP-1, MMP-3 and MMP-9 ELISAs (R&D Systems)
were performed according to the manufacturer’s instructions.
Determination of MMP-1, MMP-3 and MMP-9
in the supernatants
Cells were seeded at a density of
1×106/ml and pretreated with anti-TRAF6mAb for 24 h,
followed by a 72-h stimulation with human IL-1β (50 ng/ml,
eBioscience, San Diego, CA, USA). Cell supernatants were
centrifuged at 1,500 × g for 10 min to avoid any cell debris prior
to ELISA analysis. The levels of MMP-1, MMP-3 and MMP-9 were
determined by Human MMP-3 and MMP-9 Quantikine ELISA kits (R&D
Systems, Shanghai, China) according to the manufacturer’s
instructions.
Cell migration and invasion
FLSs were seeded at a density of 4,000 cells/well in
a 96-well plate. After treatment with the anti-TRAF6mAb and IL-1β,
the cells were observed under a ECLIPSE 80i fluorescent microscope
(Nikon, Melville, NJ, USA). In order to assay cell migration, cells
were seeded on 24-well plates coated with diluted gelatin
(Sigma-Aldrich) at a density of 5×105. Once the cells
reached 80% confluence, they were wounded by dragging a plastic
pipette tip across the cell monolayer. Cells were then incubated in
DMEM with 1% serum. Five fields of vision were selected at random
and the migrated distances of cells were measured under a CarlZeiss
LSM710 light microscope (Carl Zeiss, Oberkochen, Germany).
Experiments were repeated a minimum of three times. Wounded
monolayers were washed with phosphate-buffered saline (Thermo
Fisher Scientific) to remove detached cells. The ability of FLSs to
close the wounded space was used to assess their migration ability.
Transwell migration assays were performed using a 24-well Boyden
chamber (8.0 μm; BD Biosciences, Franklin Lakes, NJ, USA) according
to the manufacturer’s instructions. In brief, FLSs were treated
with anti-TRAF6mAb and cultured for 24 h. Cells were then harvested
by trypsinization, washed and resuspended in serum-free media at a
density of 2×104 cells/well. A 100 μl cell suspension
was placed onto the upper and the lower chambers of the Transwell,
which were filled with 500 μl media containing serum as an adhesive
substrate. Cells were incubated at 37°C under 5% CO2 for
24 h and then non-migrating cells on the upper side of the membrane
were removed with a cotton swab. Migrating cells on the lower side
of the membrane were fixed with 4% methanol for 20 min and stained
with 0.1% crystal violet for 1 min. Photomicrographs (Carl Zeiss)
of five random fields were obtained, and cells were counted to
calculate the average number of cells that migrated. For the in
vitro invasion assay, similar experiments were performed using
inserts coated with a Matrigel baSDent membrane matrix (Thermo
Fisher Scientific). The Matrigel (BD Biosciences) was diluted in
serum-free cold media and placed into upper chambers of a 24-well
Transwell plate and incubated at 37°C for 1 h. Cells were
resuspended with serum-free media at a density of 5×104
cells/well and incubated for 48 h to evaluate cell migration.
Statistical analyses
All data are expressed as the mean ± standard
deviation. An unpaired t-test (two-tailed) was applied. Statistical
analyses were performed using the SPSS software version 17.0 (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
TRAF6 expression is elevated in CIA
joints and human RA-FLSs
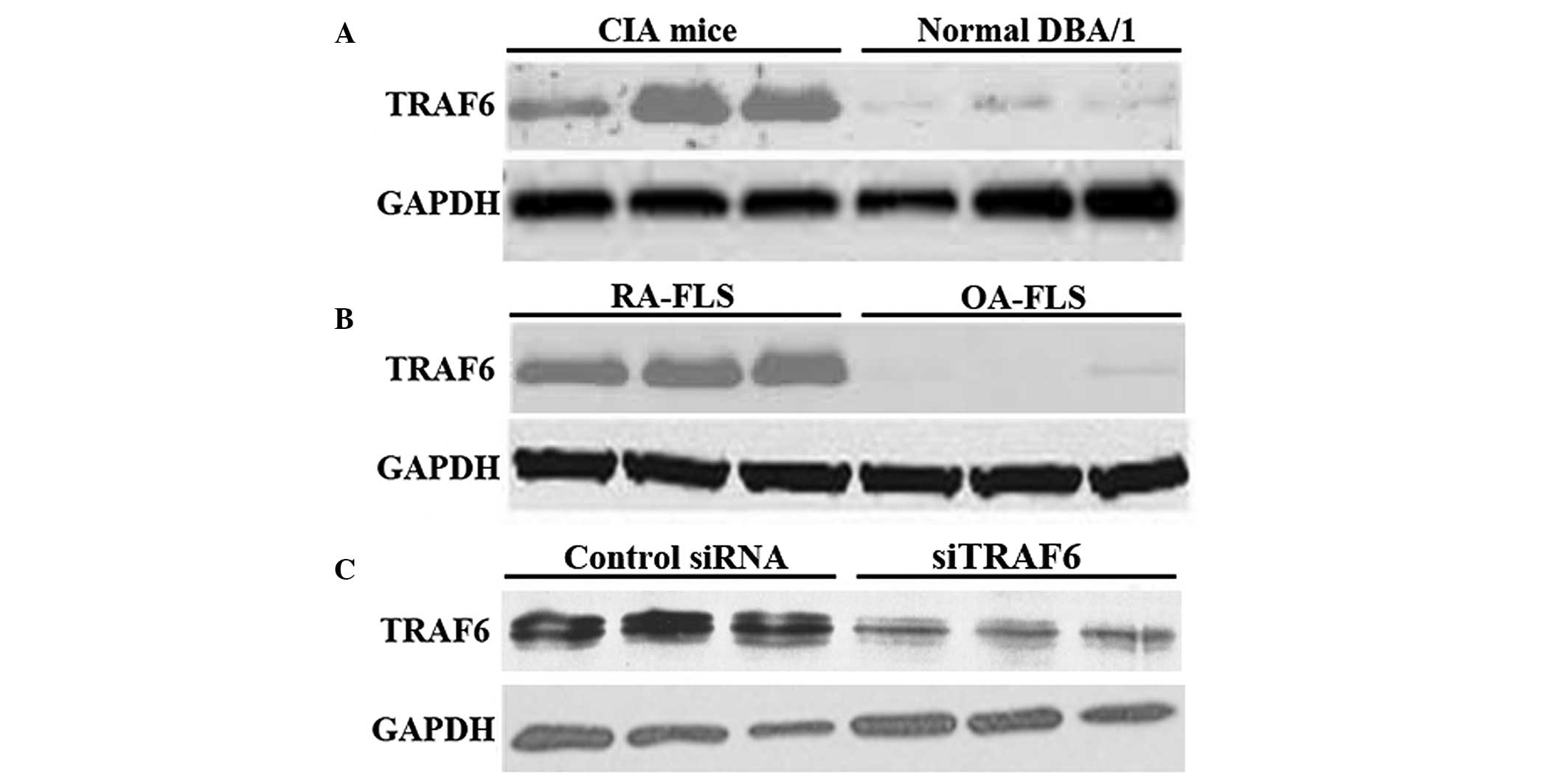
The expression level of TRAF6 was detected by
western blot analysis. The expression level of TRAF6 was
upregulated in the joints of CIA mice compared with that of the
normal DBA/1 mice (Fig. 1A).
Consistently, the protein expression of TRAF6 was upregulated in
human RA-FLSs (Fig. 1B).
Furthermore, western blot analysis confirmed that TRAF6 was knocked
down in CIA mice by in vivo siTRAF6 (Fig. 1C). These results suggest that
aberrant TRAF6 expression may participate in the pathogenesis of
RA.
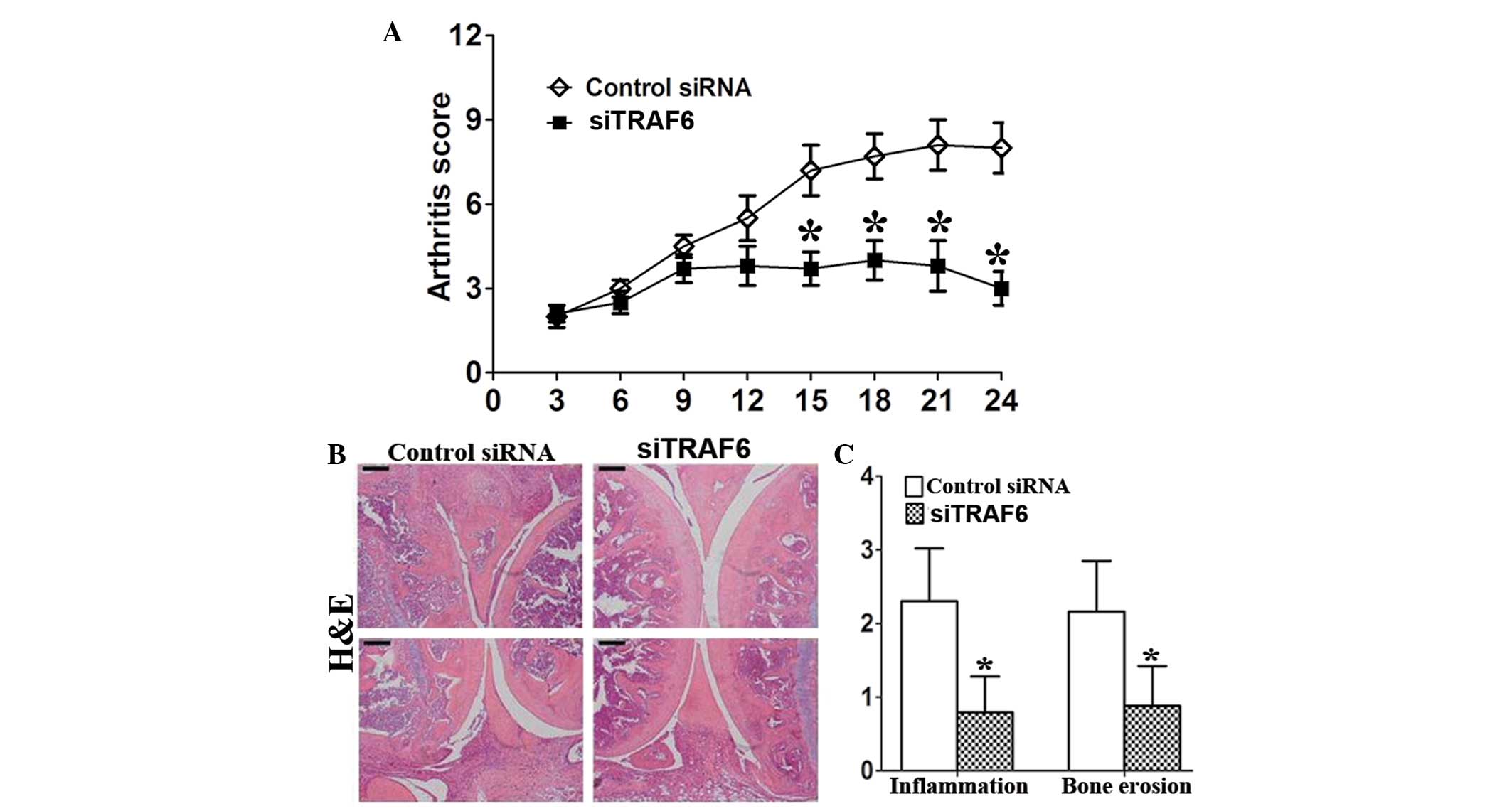
TRAF6 knockdown reduces arthritis and
histological damage in CIA mice
Treatment with siTRAF6 started on day 27 after first
immunization, when arthritis was present in all mice. TRAF6
knockdown reduced the arthritis score of CIA mice (Fig. 2A). Consistently, the results showed
that the histopathological damage, including inflammatory cell
infiltration and bone destruction, was significantly attenuated in
siTRAF6-treated CIA mice compared with that of the control
siRNA-treated CIA mice (Fig. 2B and
C).
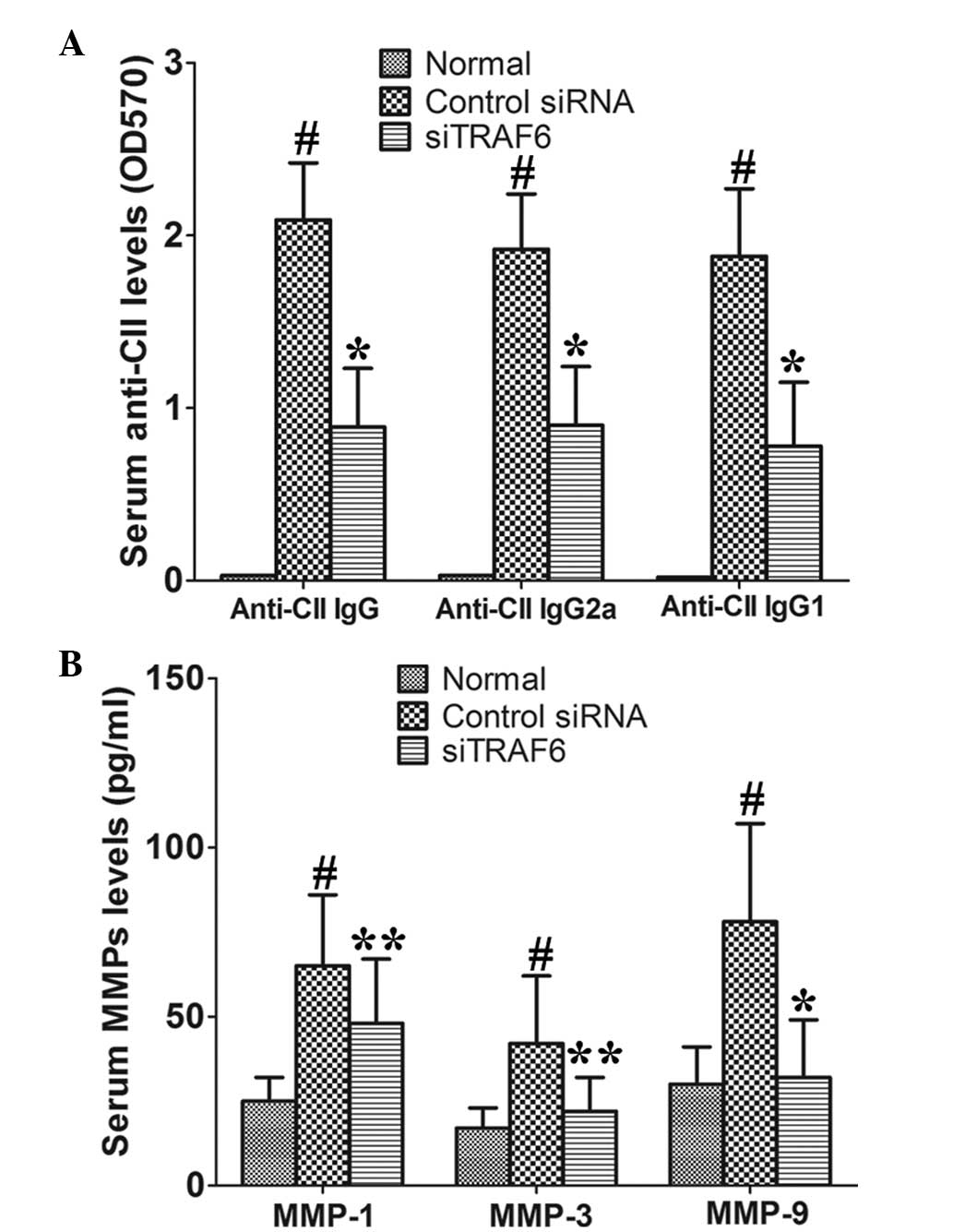
Serum anti-CII antibodies and MMPs are
reduced by siTRAF6 in CIA mice
In vivo TRAF6 knockdown significantly reduced
serum anti-CII IgG, IgG1 and IgG2a as compared with those of the
control siRNA-treated CIA mice (Fig.
3A). In addition, the data showed that siTRAF6 inhibited the
production of serum MMP-1, MMP-3 and MMP-9 as compared with that of
the control siRNA-treated CIA mice (Fig. 3B). These results indicate that
TRAF6 may be involved in the development of CIA.
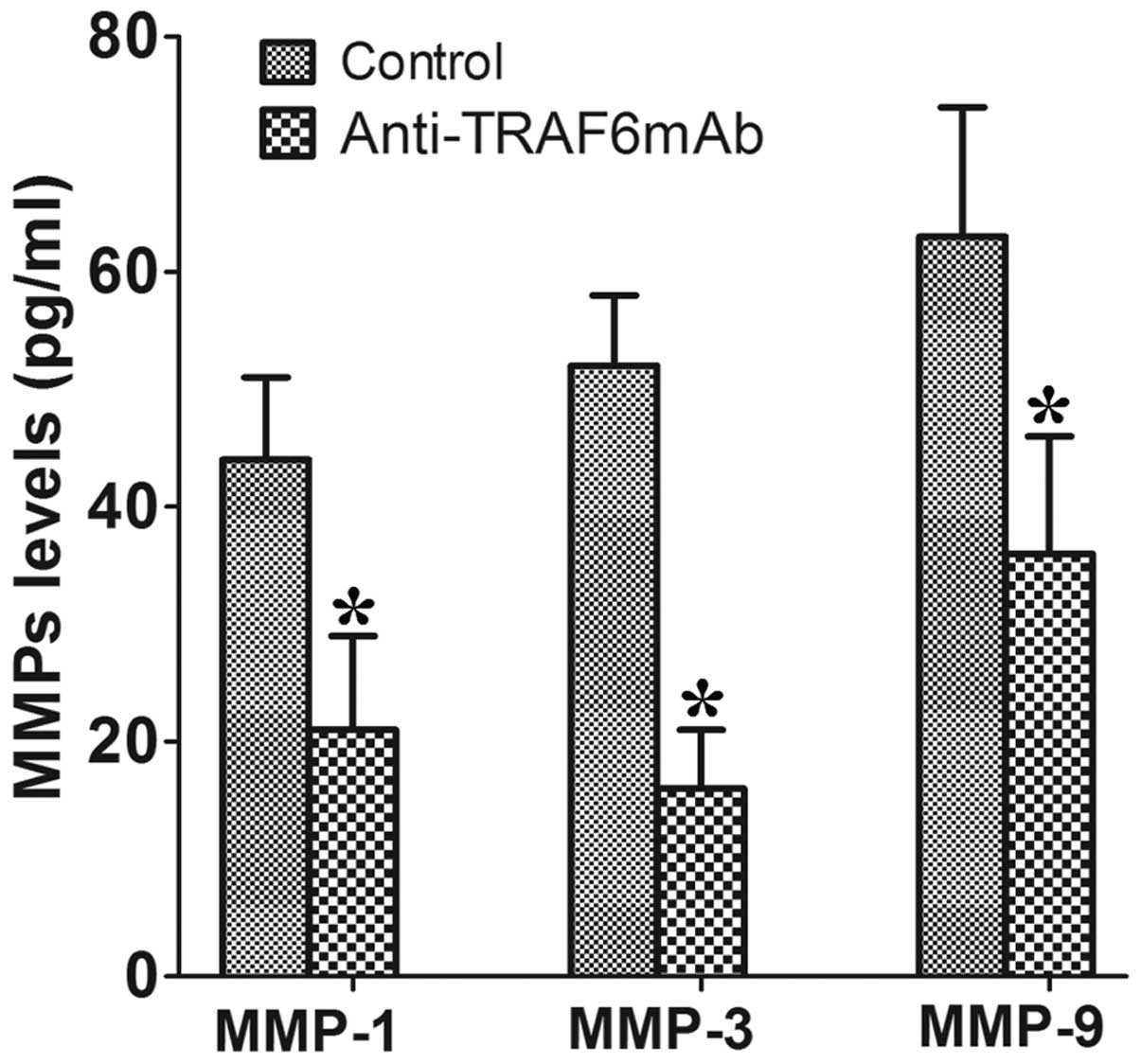
Anti-TRAF6mAb reduces the secretion of
MMP-1, MMP-3 and MMP-9 by human RA-FLSs
To investigate whether TRAF6 affects the human
RA-FLSs in a similar way, the RA-FLSs were pretreated with
anti-TRAF6mAb for 24 h, stimulated with human IL-1β (50 ng/ml) for
72 h and supernatants were then collected. The results showed that
anti-TRAF6mAb reduced the secretion of MMP-1, MMP-3 and MMP-9 by
human RA-FLSs in the supernatants as compared with those of the
control (Fig. 4).
Anti-TRAF6mAb restricts the migration of
human RA-FLSs
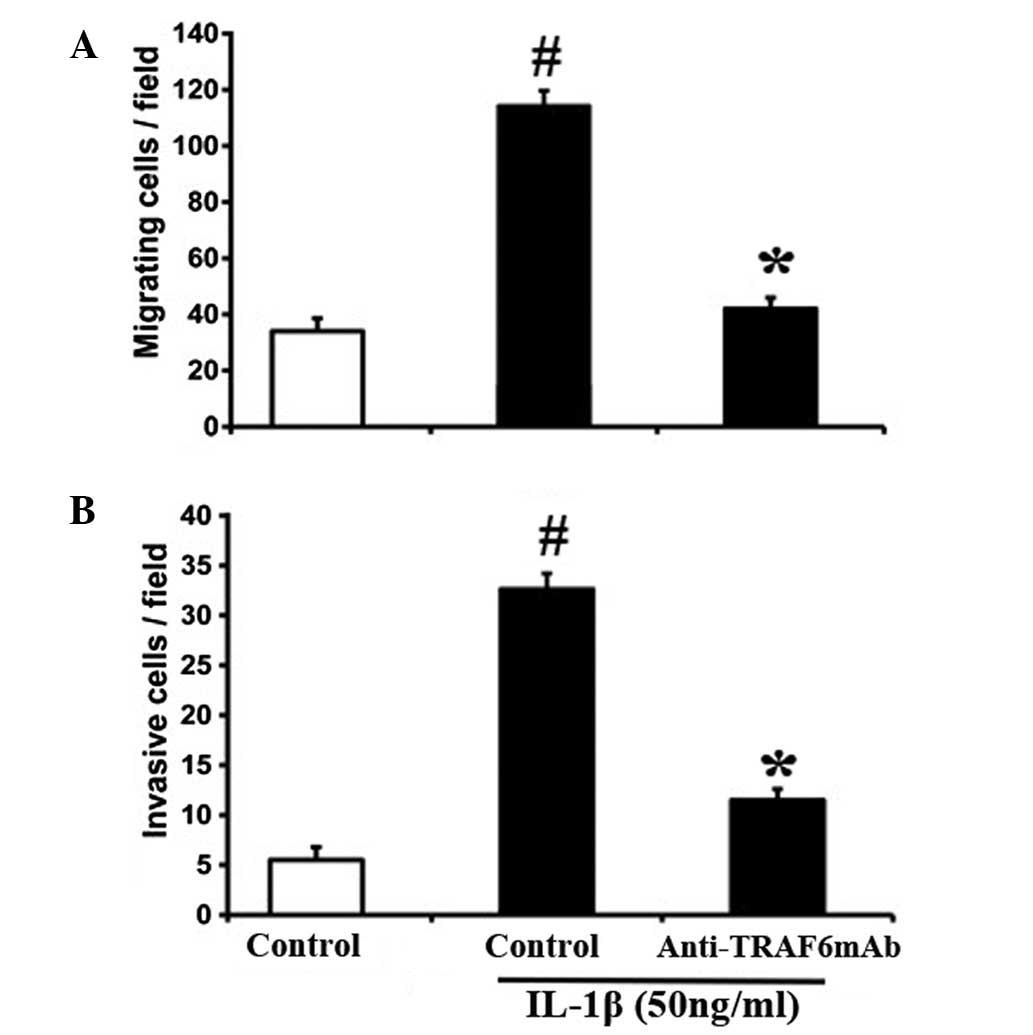
MMPs interact with multiple chemokines and are
critically involved in the FLS migration of RA associated with RA
progression (14–16). The effect of TRAF6 on the migration
and invasion of FLSs was investigated. Treatment with anti-TRAF6mAb
markedly inhibited the IL-1β-induced migration and invasion of
RA-FLSs (Fig. 5). These results
indicate that TRAF6 may promote the migratory behavior of human
RA-FLSs by potentiating the production of MMPs.
Discussion
A previous study suggested that FLSs may have the
capacity to migrate from joint to joint, potentially explaining the
evolution of extensive cartilage and bone deterioration and human
RA (17). Recent evidence
indicates that the protein TRAF6 is involved in the regulation of
autoimmune diseases, including RA and systemic lupus erythmatosus
(18,19). However, the role of TRAF6 in RA
remains to be investigated. Thus, this study was conducted to
explore the potential role of TRAF6 in CIA and human RA-FLSs. In
the present study, it was identified that TRAF6 expression was
elevated in CIA joints and human RA-FLSs as compared with those in
the controls. TRAF6 was knocked down in CIA mice by in vivo
siTRAF6. In vivo siTRAF6 treatment reduced the arthritis
score and bone destruction. In addition, the production of
antibodies and MMPs was reduced by siTRAF6. IL-1β-induced RA-FLS
migration and invasion were significantly inhibited by
anti-TRAF6mAb in vitro. These data suggest that TRAF6 may
promote migratory behavior of FLSs in RA. Inhibition of TRAF6 may
be a potential therapeutic target for human RA.
TRAF6 has recently been shown to be essential for
maintenance of regulatory T cells (Tregs) that suppress
Th2 type autoimmunity (20).
Impaired Treg function has been shown to be associated
with human autoimmune diseases, including RA (21,22).
Additionally, TRAF6 has been shown to establish innate immune
responses by activating NF-κB (23) and NF-κB activation is widely
accepted as a key mediator in the development of RA (24). Previous data have shown that the
accumulation of TRAF6-TAK1 complexes and their activation promote
osteoclast differentiation (25).
TRAF6 is a key mediator in the IL-1β-mediated signaling pathway,
consequently upregulating its downstream inflammatory events
(26). IL-1β is one of the key
regulators in the FLS activation in the pathogenesis of RA
(27). In the current study, the
results revealed that arthritis was attenuated by siTRAF6 in CIA
mice, as demonstrated by decreased serum anti-CII, MMP-1, MMP-3 and
MMP-9 and reduced histological damage.
Furthermore, the role of TRAF6 in IL-1β-induced MMP
expression was investigated in RA-FLSs. Blockade of TRAF6 inhibited
IL-1β-induced production of MMP-1, MMP-3 and MMP-9. These data
suggest that TRAF6 may activate FLSs from RA patients, resulting in
the elevated expression of MMP-1, MMP-3 and MMP-9. In addition, the
data revealed that anti-TRAF6mAb significantly inhibited the
IL-1β-induced migration and invasion of RA-FLSs.
In conclusion, the results of the present study
showed that TRAF6 knockdown reduces the arthritis score and joint
destruction via modulating FLS migration and invasion and MMP
expression by FLSs. Thus, TRAF6 inhibition may be a potential
therapeutic target for RA.
References
|
1
|
Stanford SM, Maestre MF, Campbell AM,
Bartok B, Kiosses WB, Boyle DL, Arnett HA, Mustelin T, Firestein GS
and Bottini N: Protein tyrosine phosphatase expression profile of
rheumatoid arthritis fibroblast-like synoviocytes: a novel role of
SH2 domain-containing phosphatase 2 as a modulator of invasion and
survival. Arthritis Rheum. 65:1171–1180. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kapoor M, Martel-Pelletier J, Lajeunesse
D, Pelletier JP and Fahmi H: Role of proinflammatory cytokines in
the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 7:33–42.
2011. View Article : Google Scholar
|
|
3
|
Paula FS and Alves JD: Non-tumor necrosis
factor-based biologic therapies for rheumatoid arthritis: present,
future, and insights into pathogenesis. Biologics. 8:1–12.
2014.
|
|
4
|
Inoue J, Gohda J and Akiyama T:
Characteristics and biological functions of TRAF6. Adv Exp Med
Biol. 597:72–79. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun L, Deng L, Ea CK, Xia ZP and Chen ZJ:
The TRAF6 ubiquitin ligase and TAK1 kinase mediate IKK activation
by BCL10 and MALT1 in T lymphocytes. Mol Cell. 14:289–301. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chung JY, Lu M, Yin Q, Lin SC and Wu H:
Molecular basis for the unique specificity of TRAF6. Adv Exp Med
Biol. 597:122–130. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee HS, Ka SO, Lee SM, Lee SI, Park JW and
Park BH: Overexpression of sirtuin 6 suppresses inflammatory
responses and bone destruction in mice with collagen-induced
arthritis. Arthritis Rheum. 65:1776–1785. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nam EJ, Kang JH, Sung S, Sa KH, Kim KH,
Seo JS, Kim JH, Han SW, Kim IS and Kang YM: A matrix
metalloproteinase 1-cleavable composite peptide derived from
transforming growth factor β-inducible gene h3 potently inhibits
collagen-induced arthritis. Arthritis Rheum. 65:1753–1763. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tarrant TK, Liu P, Rampersad RR, Esserman
D, Rothlein LR, Timoshchenko RG, McGinnis MW, Fitzhugh DJ, Patel DD
and Fong AM: Decreased Th17 and antigen-specific humoral responses
in CX3 CR1-deficient mice in the collagen- induced
arthritis model. Arthritis Rheum. 64:1379–1387. 2012. View Article : Google Scholar
|
|
10
|
Nishikawa M, Myoui A, Tomita T, Takahi K,
Nampei A and Yoshikawa H: Prevention of the onset and progression
of collagen-induced arthritis in rats by the potent p38
mitogen-activated protein kinase inhibitor FR167653. Arthritis
Rheum. 48:2670–2681. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Aletaha D, Neogi T, Silman AJ, et al: 2010
Rheumatoid arthritis classification criteria: an American College
of rheumatology/european league against rheumatism collaborative
initiative. Arthritis Rheum. 62:2569–2581. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dale J, Purves D, McConnachie A, McInnes I
and Porter D: Tightening up? Impact of musculoskeletal ultrasound
disease activity assessment on early rheumatoid arthritis patients
treated using a treat to target strategy. Arthritis Care Res
(Hoboken). 66:19–26. 2014. View Article : Google Scholar
|
|
13
|
Yoshioka Y, Kozawa E, Urakawa H, Arai E,
Futamura N, Zhuo L, Kimata K, Ishiguro N and Nishida Y: Suppression
of hyaluronan synthesis alleviates inflammatory responses in murine
arthritis and in human rheumatoid fibroblasts. Arthritis Rheum.
65:1160–1170. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee A, Qiao Y, Grigoriev G, Chen J,
Park-Min KH, Park SH, Ivashkiv LB and Kalliolias GD: Tumor necrosis
factor α induces sustained signaling and a prolonged and
unremitting inflammatory response in rheumatoid arthritis
fibroblasts. Arthritis Rheum. 65:928–938. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Laragione T, Brenner M, Sherry B and Gulko
PS: CXCL10 and its receptor CXCR3 regulate synovial fibroblast
invasion in rheumatoid arthritis. Arthritis Rheum. 63:3274–3283.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
García-Vicuña R, Gómez-Gaviro MV,
Domínguez-Luis MJ, Pec MK, González-Alvaro I, Alvaro-Gracia JM and
Díaz-González F: CC and CXC chemokine receptors mediate migration,
proliferation, and matrix metalloproteinase production by
fibroblast-like synoviocytes from rheumatoid arthritis patients.
Arthritis Rheum. 50:3866–3877. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cooles FA and Isaacs JD: Pathophysiology
of rheumatoid arthritis. Curr Opin Rheumatol. 23:233–240. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Muto G, Kotani H, Kondo T, Morita R,
Tsuruta S, Kobayashi T, Luche H, Fehling HJ, Walsh M, Choi Y and
Yoshimura A: TRAF6 is essential for maintenance of regulatory T
cells that suppress Th2 type autoimmunity. PLoS One. 8:e746392013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Namjou B, Choi CB, Harley IT and
Alarcón-Riquelme ME; BIOLUPUS Network. Kelly JA, Glenn SB, et al:
Evaluation of TRAF6 in a large multiancestral lupus cohort.
Arthritis Rheum. 64:1960–1969. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Muto G, Kotani H, Kondo T, Morita R,
Tsuruta S, Kobayashi T, Luche H, Fehling HJ, Walsh M, Choi Y and
Yoshimura A: TRAF6 is essential for maintenance of regulatory T
cells that suppress Th2 type autoimmunity. PLoS One. 8:e746392013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park JS, Lim MA, Cho ML, Ryu JG, Moon YM,
Jhun JY, Byun JK, Kim EK, Hwang SY, Ju JH, Kwok SK and Kim HY: p53
controls autoimmune arthritis via STAT-mediated regulation of the
Th17 cell/Treg cell balance in mice. Arthritis Rheum. 65:949–959.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moon YM, Lee J, Lee SY, Her YM, Ryu JG,
Kim EK, Son HJ, Kwok SK, Ju JH, Yang CW, Park SH, Kim HY and Cho
ML: Gene-associated retinoid-interferon- induced mortality 19
(GRIM-19) attenuates autoimmune arthritis by regulation of Th17 and
Treg cells. Arthritis Rheum. 18:2013.
|
|
23
|
Konno H, Yamamoto T, Yamazaki K, Gohda J,
Akiyama T, Semba K, Goto H, Kato A, Yujiri T, Imai T, et al: TRAF6
establishes innate immune responses by activating NF-kappaB and
IRF7 upon sensing cytosolic viral RNA and DNA. PLoS One.
4:e56742009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bamborough P, Morse MA and Ray KP:
Targeting IKKβ for the treatment of rheumatoid arthritis. Drug News
Perspect. 23:483–490. 2010.PubMed/NCBI
|
|
25
|
Wei ZF, Tong B, Xia YF, Lu Q, Chou GX,
Wang ZT and Dai Y: Norisoboldine suppresses osteoclast
differentiation through preventing the accumulation of TRAF6-TAK1
complexes and activation of MAPKs/NF-κB/c-Fos/NFATc1 pathways. PLoS
One. 8:e591712013. View Article : Google Scholar
|
|
26
|
Lee HJ, Jang SH, Kim H, Yoon JH and Chung
KC: PINK1 stimulates interleukin-1β-mediated inflammatory signaling
via the positive regulation of TRAF6 and TAK1. Cell Mol Life Sci.
69:3301–3315. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Maruotti N, Grano M, Colucci S, d’Onofrio
F and Cantatore FP: Osteoclastogenesis and arthritis. Clin Exp Med.
11:137–145. 2011. View Article : Google Scholar
|