Introduction
Gastric cancer is one of the most common
malignancies in China (1).
Increasing numbers of people succumb to gastric cancer each year,
and gastric cancer accounts for ~25% of all cancer-associated
mortality (2). Furthermore, the
number of new patients diagnosed with gastric cancer is increasing
annually (2,3), and it is therefore considered a
serious threat to public health. Gastric cancer may occur at any
age, with the majority of patients aged between 40 and 60 years old
(3). Currently, the cause of
gastric cancer is unknown and may be associated with numerous
factors, such as diet, lifestyle, environmental factors, genetic
predisposition, mental factors and Helicobacter pylori
infection (4). Comprehensive
therapy including chemotherapy is currently the main method used
for treatment of advanced gastric cancer; however, chemotherapeutic
drugs may not only kill tumor cells, but also damage normal tissue
cells. Therefore, the overall survival of gastric cancer patients
has not significantly improved (5). To maximize the effects of
cytotoxicity to malignant tumor cells, research has recently
focused on pharmaceutical drugs with the lowest normal cell
toxicity (3,6).
Allicin is an organic sulfur compound from the bulbs
of Allium sativum, which is also present in onions and other
Alliaceae plants. Allicin has strong antibacterial and
anti-inflammatory effects, and may inhibit the growth of or kill
various bacteria, fungi and viruses (2). A previous epidemiological study
demonstrated the antitumor activity of allicin (1), and allicin has been shown to directly
kill tumor cells, inhibit tumor cell proliferation and induce
apoptosis (7). Previous studies
have shown that allicin may significantly induce apoptosis in LNCaP
prostate cancer cells, mouse melanoma cells, and SGC-790l human
gastric adenocarcinoma cells (7,8). In
addition, allicin may stimulate the immune system to release more
active factors, which could inhibit the growth of tumor cells
through enhancement of its anticancer effects (3,7). As
compared with traditional chemotherapy drugs, in the clinical
treatment of tumors allicin not only has no toxic effects on the
body, but may also stimulate the body to release various cytokines
and enhance immune resistance (5,9).
The process by which allicin induces apoptosis of
tumor cells is precisely regulated, and numerous proteins are
involved. The present study aimed to explore the specific
mechanisms of the pro-apoptotic effects of allicin on the
proliferation and apoptosis of the MGC-803 gastric carcinoma cell
line, which may provide information on the clinical application of
allicin for the treatment of gastric cancer.
Materials and methods
Human samples and cell lines
The MGC-803, BGC-823 and SGC-7901 human gastric
carcinoma cell lines were purchased from the American Type Tissue
Culture Collection (Manassas, VA, USA). The cells were cultured in
Dulbecco’s modified Eagle’s medium (DMEM)/F12 (Hyclone
Laboratories, Inc., Logan, UT, USA), supplemented with 10% fetal
bovine serum (Hyclone Laboratories, Inc.), 100 U/ml penicillin and
streptomycin (Sigma-Aldrich, St. Louis, MO, USA), in
25-cm2 culture flasks at 37°C in a humidified atmosphere
containing 5% CO2.
Cell viability assay
Cell viability was determined using a colorimetric
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay (Sigma-Aldrich). In order to determine the impact of allicin
on the MGC-803, BGC-823 and SGC-7901 human gastric carcinoma cell
lines, the cells were cultured to ~70% confluence and starved in
serum-free DMEM (Life Technologies, Grand Island, NY, USA)
overnight. The three cell lines were then incubated with 0.1, 1 and
10 μg/ml allicin for 48 h. Following treatment with allicin
(Sigma-Aldrich) for disease prevention, the cells were cultured in
fresh medium including 0.5 mg/ml MTT, for 4 h. Dimethyl sulfoxide
(Sigma-Aldrich) was then added to the wells to dissolve the blue
formazan product, and the optical density of the samples was
determined spectrophotometrically (Cary 4000; Agilent Technologies,
Novato, CA, USA) at a wavelength of 550 nm. To determine whether
the effects of allicin were time-dependent, the cells were
preincubated with 1 μg/ml allicin for 12, 24 or 48 h and cell
viability was determined using the same method as previously
described. Each experiment was independently performed at least
three times.
Hoechst 33258 staining
The MGC-803, BGC-823 and SGC-7901 human gastric
carcinoma cells (1×105 cells per well) were cultured in
six-well tissue culture plates. Once they had reached 70–80%
confluence, the cells were incubated for 16 h in serum-free DMEM.
Following the incubation, 1 μg/ml allicin was added to the fresh
media and the cells were incubated for a further 48 h. Following
treatment with allicin, the media was removed, and the cells were
rinsed three times with cold phosphate-buffered saline (PBS) and
fixed with 4% formaldehyde (Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd., Beijing, China) in PBS for 20 min at room
temperature. The cells were then washed three times with cold PBS
and stained with Hoechst 33258 (10 μg/ml; Sigma-Aldrich) for 5 min.
After staining, the cells were further washed with cold PBS and
examined under a fluorescence microscope (Agilent 1200; Agilent
Technologies).
Western blot analysis
The three cell lines, MGC-803, BGC-823 and SGC-7901
were used for western blot analysis. The cells were initially lysed
using radioimmunoprecipitation assay buffer [Beijing Solarbio
Science & Technology Co., Ltd., Beijing, China; 50 mM Tris/HCl,
pH 7.4, 150 mM NaCl 1% (v/v) NP-40, 0.1% (w/v) SDS] containing 1%
(v/v) PMSF (Beijing Solarbio Science & Technology Co., Ltd.),
0.3% (v/v) protease inhibitor (Sigma-Aldrich) and 0.1% (v/v)
phosphorylated proteinase inhibitor (Sigma-Aldrich). The lysates
were then centrifuged at 4,000xg at 4°C for 15 min, in order to
collect the supernatants. To quantify the relative concentration of
the total protein samples, a Bicinchoninic Acid Protein Assay kit
(Pierce Biotechnology, Inc., Rockford, IL, USA) was used. Equal
quantities of protein (15 μg) were separated on a 10% SDS-PAGE gel,
and then transferred onto polyvinylidene fluoride (PVDF) membranes
(General Electric Company, New York City, NY, USA) at 300 mA for 2
h. In order to block non-specific binding proteins, the PVDF
membranes were blocked using 8% (w/v) milk in Tris-buffered saline
with Tween®, for 2 h at room temperature. The membranes
were then incubated with primary antibodies (1:1,000; Cell
Signaling Technology, Inc., Danvers, MA, USA) targeting β-actin
(#4967; rabbit polyclonal synthetic peptide), cleaved caspase 3
(#9661; rabbit polyclonal synthetic peptide), p–p38 (#9212; rabbit
polyclonal synthetic peptide), caspase-3 (#9661; rabbit polyclonal
synthetic peptide), Bax (#2774; rabbit polyclonal synthetic
peptide), Bcl (#2872; rabbit polyclonal synthetic peptide) and p38
(#9212, rabbit polyclonal synthetic peptide) overnight at 4°C. The
membranes were washed four times with PBS-Tween® (5
min/time), and then incubated with horseradish
peroxidase-conjugated goat anti-rabbit immunoglobulin G (Abmart,
Inc., Shanghai, China; 1:5,000 dilution), for 2 h at room
temperature. Following the incubation, the membranes were washed
four times (5 min/time). The blots were visualized using an
Enhanced Chemiluminescence substrate (EMD Millipore, Billerica, MA,
USA), according to the manufacturer’s instructions. The relative
protein expression levels were then determined and quantified using
Bandscan 4.30 (Glyko Biomedical, Novato, CA, USA). In order to
quantify changes to protein expression, the target protein was
normalized against β-actin.
Apoptosis assay
To detect the effects of allicin on the rate of
apoptosis of MGC-803 cells BGC-823 and SGC-7901, the cells that had
reached 50–60% confluence were treated with 0.1, 1 or 10 μg/ml
allicin for 48 h. Following the treatment with allicin, the cells
were washed three times with 1X PBS. The Annexin V-fluorescein
isothiocyanate (FITC)-propidium iodide (PI) Apoptosis kit
(Invitrogen Life Technologies, Carlsbad, CA, USA) was then used to
determine the rate of apoptosis by flow cytometry. This assay
employs fluorescein-labeled Annexin V in conjunction with PI, to
detect cells undergoing apoptosis. Briefly, the cells were washed
three times with 1X PBS and suspended at 2–3×106
cells/ml in 1X Annexin V Binding Buffer (10 mM HEPES/NaOH, pH 7.4;
140 mM NaCl and 2.5 mM CaCl2). Annexin V-FITC and PI
Buffer were then added to the cells, which were incubated at room
temperature for 15 min in the dark. The untreated cells were used
as an internal control. Following incubation, the cells were
filtered using a filter screen and the cells were analyzed by flow
cytometry (BD FACSVerse™, BD Biosciences, Franklin Lakes, NJ, USA)
within 1 h of staining using the FL1 (FITC) and FL3 (PI) lines.
Statistical analysis
The data are expressed as the mean ± standard error
of the mean. The number of independent experiments is represented
by ‘n’. Multiple comparisons were performed using one-way analysis
of variance, followed by Tukey’s multiple-comparison test.
Statistical analyses were performed using PASW Statistic 18 (IBM,
Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
MGC-803, BGC-823 and SGC-7901 cell
viability is affected by allicin in a dose- and time-dependent
manner
To investigate the impact of allicin on cell
viability, the MGC-803, BGC-823 and SGC-7901 human gastric
carcinoma cells were treated with 0.1, 1 and 10 μg/ml allicin, for
48 h. Cell viability was then analyzed by an MTT assay. The
viability of the MGC-803, BGC-823 and SGC-7901 cells was
significantly reduced by ~30%, when the concentration of allicin
was increased from 0 to 10 μg/ml (Fig.
1A). Based on these results, 1 mg/ml allicin was selected for
use in follow-up studies. Furthermore, the MGC-803, BGC-823 and
SGC-7901 cells were also treated with 1 μg/ml allicin for 12, 24
and 48 h, and the corresponding cell viability was determined by
MTT. The viability of the MGC-803, BGC-823 and SGC-7901 cells was
reduced by ~11 and 16%, at 24 and 48 h, respectively (Fig. 1B). These results suggest that the
viability of human gastric carcinoma cells is significantly reduced
in response to treatment with allicin in a dose- and time-dependent
manner.
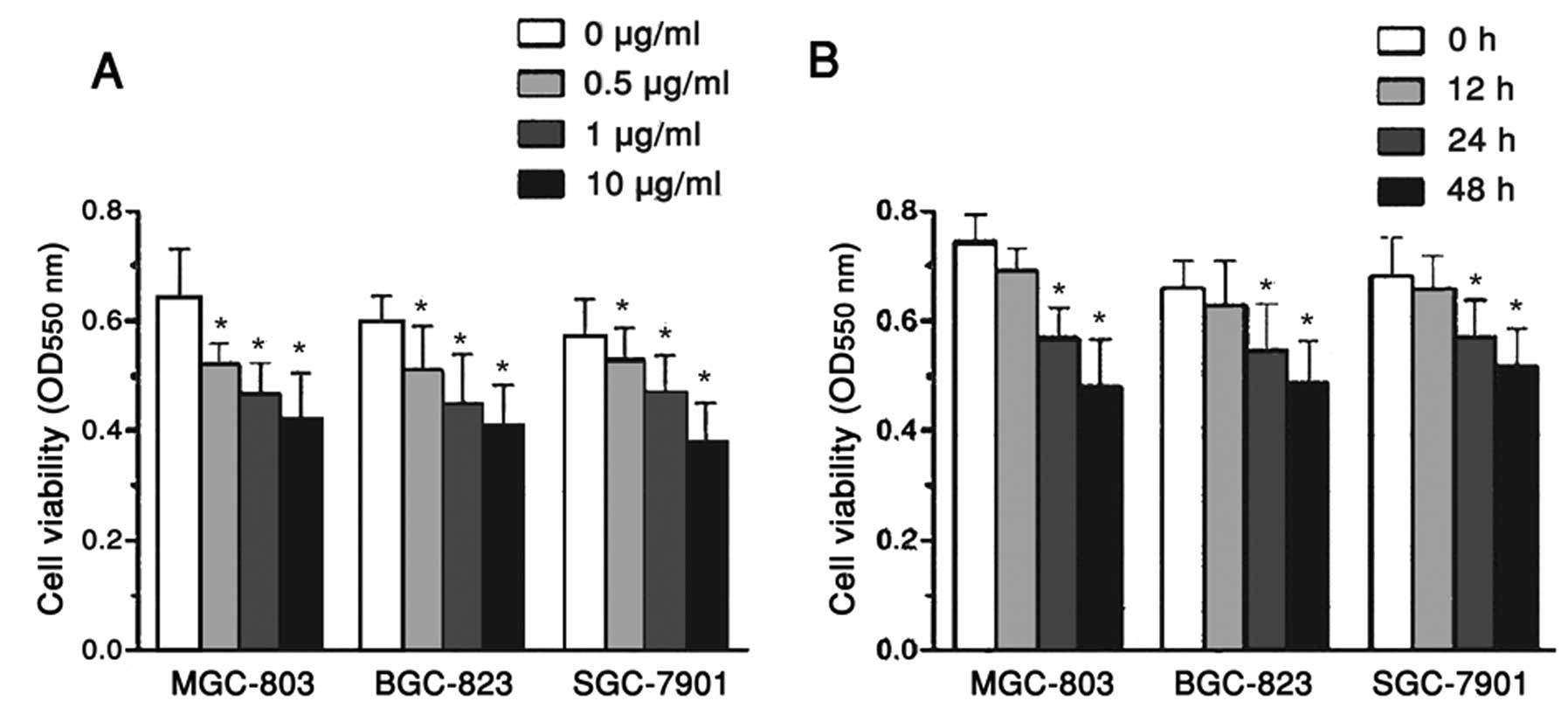 | Figure 1MGC-803, BGC-823 and SGC-7901 human
gastric carcinoma cell viability was affected by treatment with
allicin in a dose- and time-dependent manner. (A) MGC-803, BGC-823
and SGC-7901 cells were exposed to 0.1, 1, and 10 μg/ml allicin for
48 h. (B) MGC-803, BGC-823 and SGC-7901 cells were treated with 1
μg/ml allicin for 12, 24 and 48 h. Cell viability was determined by
a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
assay, and the optical density (OD) of the cells was measured at
550 nm using a spectrophotometer. Data are represented as the mean
± standard error of the mean, n=6 independent experiments.
*P<0.05 versus control. |
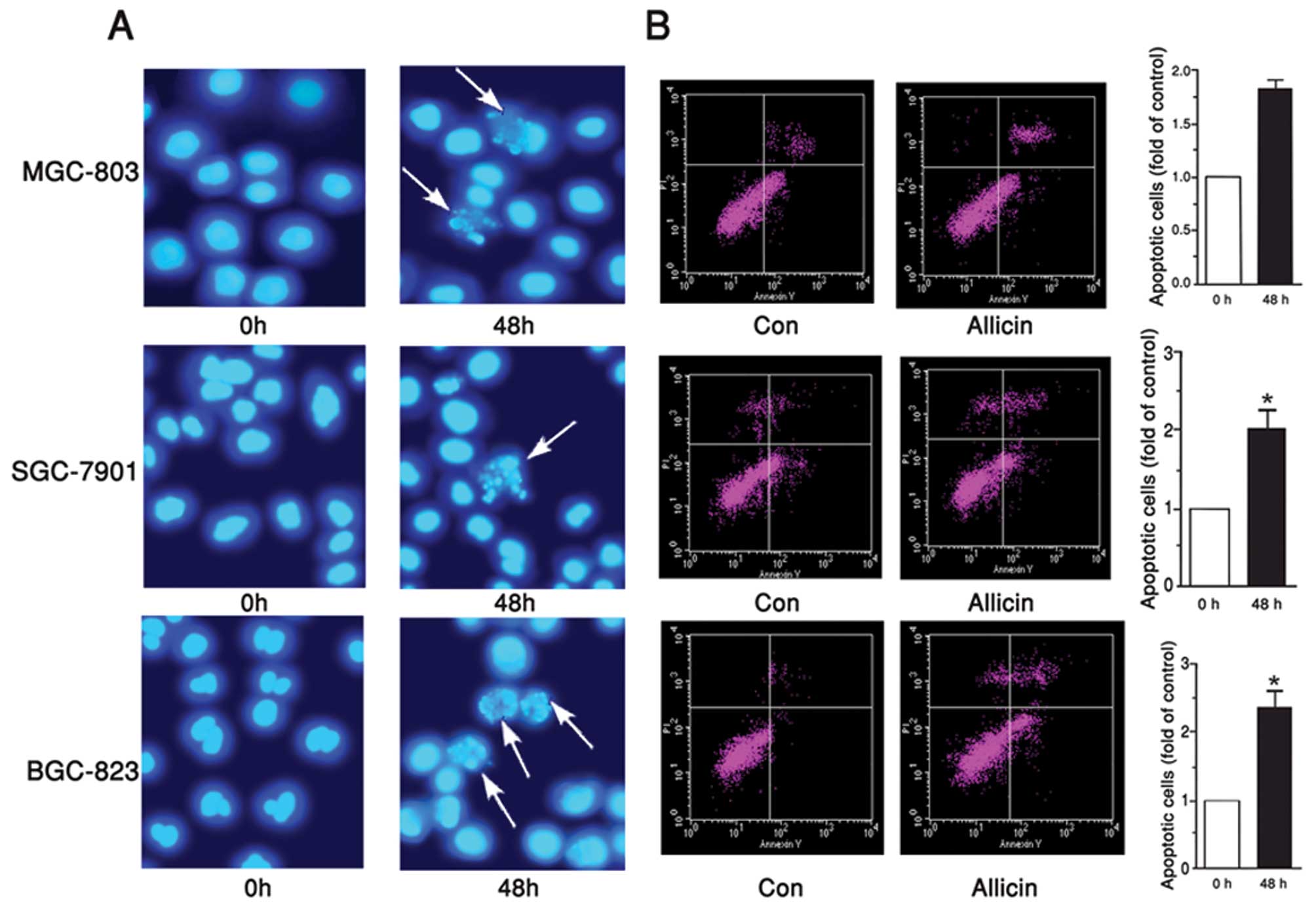
Allicin induces apoptosis of gastric
carcinoma cells
Since MGC-803, BGC-823 and SGC-7901 cell viability
was significantly inhibited by allicin, the present study examined
the correlation between the apoptosis of MGC-803, BGC-823 and
SGC-7901 cells, and allicin. Hoechst staining is one of the most
common methods used to directly detect cell apoptosis, since it
provides vivid morphological results. In the present study,
MGC-803, BGC-823 and SGC-7901 cells were treated with 1 μg/ml
allicin for 48 h, and then stained with Hoechst 33258. Enhanced
cell apoptosis was detected in the cells, in response to incubation
with allicin (Fig. 2A). To
quantify the rate of apoptosis, an Annexin V-FITC-PI kit was used.
Flow cytometry revealed that the number of apoptotic MGC-803,
BGC-823 and SGC-7901 cells was increased in response to treatment
with allicin, by 87, 100 and 132%, as compared with the control
group, respectively (Fig. 2B).
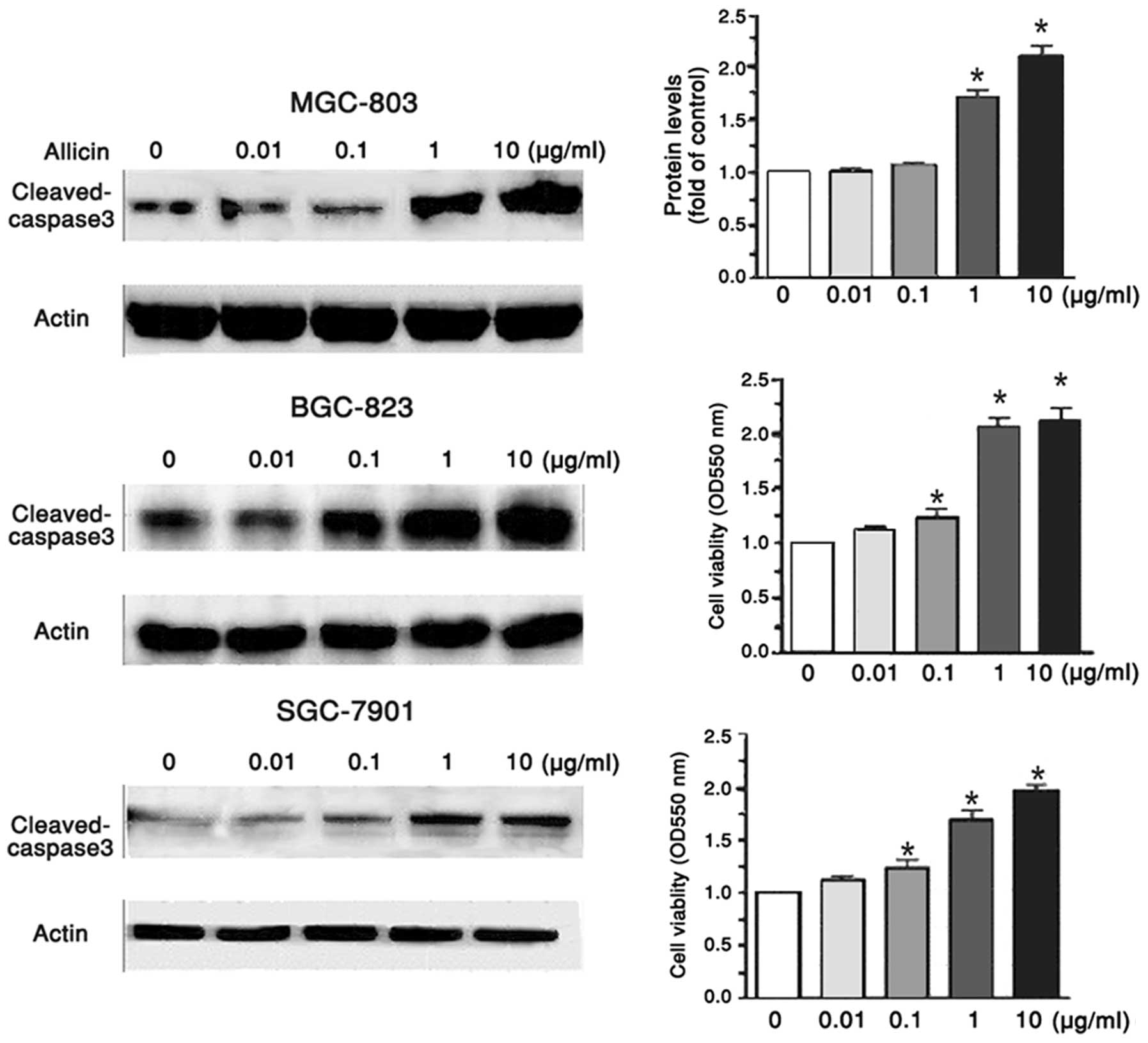
Cleaved caspase 3 regulates
allicin-induced apoptosis of gastric carcinoma cells
To further explore the effects of allicin on the
apoptosis of MGC-803, BGC-823 and SGC-7901 human gastric carcinoma
cells, the present study determined the altered expression levels
of apoptosis-associated proteins by western blotting. When the
MGC-803, BGC-823 and SGC-7901 cells were treated with 0.01, 0.1, 1
and 10 μg/ml allicin for 48 h, the protein expression levels of
cleaved caspase 3 were increased in a dose-dependent manner
(Fig. 3). Caspase 3 is an inactive
zymogen within the body; however, during apoptosis, caspase 3 may
be hydrolyzed into an active enzyme (10). Once the expression levels of
cleaved caspase 3 are enhanced, cell apoptosis signaling may be
activated and protein degradation may occur. The results of the
present study indicate that allicin may induce apoptosis of
MGC-803, BGC-823 and SGC-7901 cells mainly by enhancing the
expression of cleaved caspase 3. From these data, it may be
hypothesized that allicin induces the expression of p38, which
activates caspase 3, thereby triggering the caspase cascade
reaction, inhibiting cell growth and promoting apoptosis.
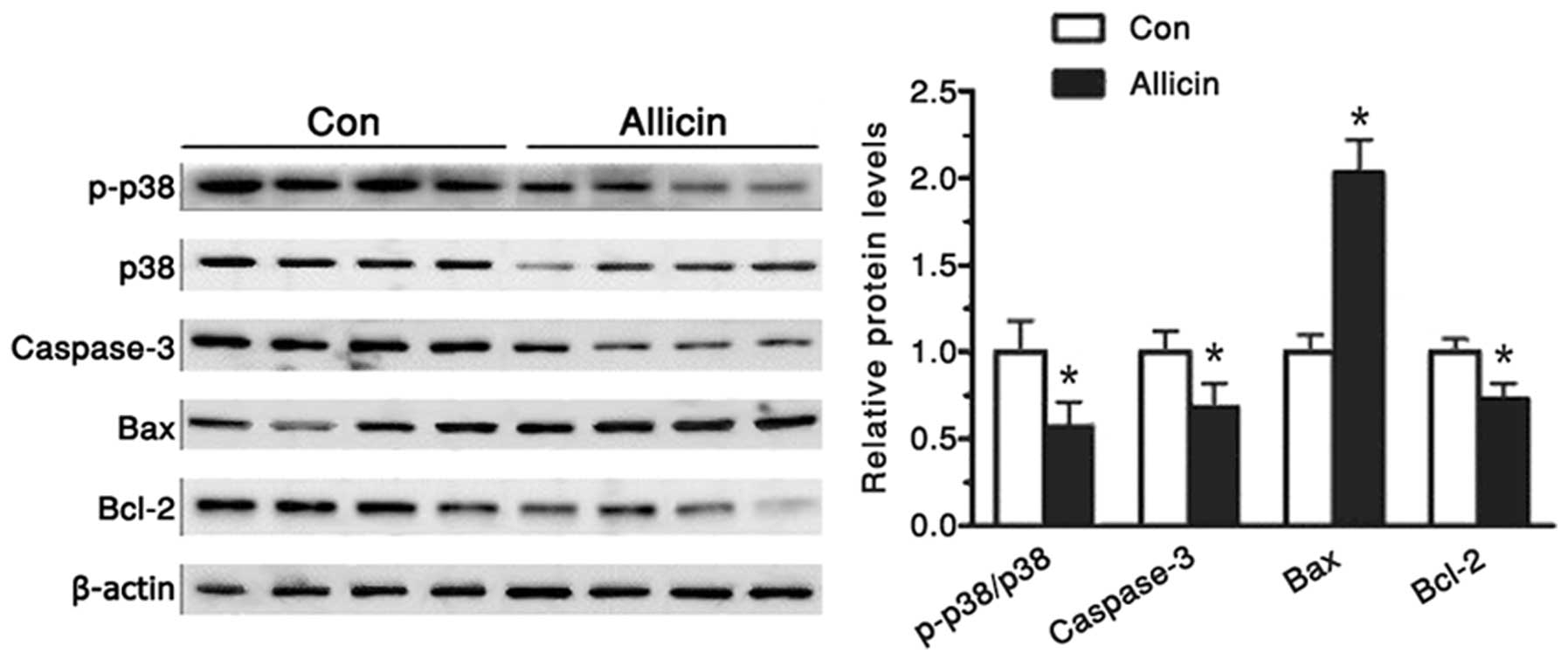
Increased p38 expression levels promote
caspase 3 activation
The p38 mitogen-activated protein kinase (MAPK)
signal transduction pathway is an important branch of the MAPK
pathway. It has an important role in numerous physiological and
pathological processes, including inflammation, stress, apoptosis,
cell cycle and growth (11,12).
A previous study suggested that it may also be a potential target
for tumor treatment (13). Studies
have shown that p38 activation leads to increased expression of
cleaved caspase 3 and promotes apoptosis (3,14).
In the present study, the protein expression levels of p38 were
gradually enhanced in the MGC-803 cells, in response to treatment
with 1 μg/ml allicin for 48 h (Fig.
4). Whereas, the protein expression levels of phospho-p38 and
caspase 3 were decreased (Fig. 4).
The present study also aimed to determine the expression levels of
apoptosis-associated proteins, Bcl-2 and Bax. When the cells were
treated with allicin, the protein expression levels of Bax were
increased nearly one-fold, whereas the protein expression levels of
Bcl-2 level were decreased >35%.
Discussion
Garlic is the bulb of Allium plants. The main
bioactive substance in garlic is considered to be the sulfur
compound, which is also known as allicin
(C6H10S3) (15). A previous study showed that allicin
may possess antibacterial and anti-inflammatory effects (16). Furthermore, it has also been shown
to inhibit tumor growth, this finding has been demonstrated by
clinical and experimental statistics (17). The results of the present study
confirm that allicin can inhibit the growth of cancer cells in
vitro; however, the underlying mechanism remains unclear
(18). Previous research has shown
that allicin can induce the apoptosis of cancer cells through the
activation of caspase 3, caspase 8 and caspase 9 (14). Notably, as an antitumor drug,
allicin exhibits little toxicity towards normal cells and tissues
(18). Therefore the present study
focused on the effects of allicin on human gastric carcinoma cells,
in order to elucidate its potential mechanism.
The MGC-803 cell line is an immortal cell line
derived from human gastric carcinoma, which was widely applied in
the present gastric cancer study. Furthermore, two other human
gastric cancer cell lines, BGC-823 and SGC-7901 were also used. The
MGC-803, BGC-823 and SGC-7901 cells were initially treated with
different concentrations of allicin. According to an MTT assay, the
rate of cell proliferation was inhibited, thus suggesting that
tumor growth was significantly inhibited. Furthermore, when the
MGC-803, BGC-823 and SGC-7901 cells were treated with 1 μg/ml
allicin for 12, 24 and 48 h, the rate of cell proliferation was
also reduced. These results suggest that the proliferation of human
gastric carcinoma cells was inhibited by allicin in a dose- and
time-dependent manner. In addition, Hoechst staining demonstrated
increased apoptosis of the MGC-803 cells following treatment with 1
μg/ml allicin for 48 h. There are two main classic cell apoptosis
signaling pathways (19): The
death receptor pathway, which is mediated by the Fas/FasL pathway;
and the mitochondrial pathway, which initiates downstream caspase
cascade activation. These two pathways mediate apoptosis through
the common caspase pathway. Caspase 3 is a member of the caspase
family, which has a key role in apoptosis (20). The present study analyzed the
expression levels of apoptosis-associated proteins by western
blotting. In response to an increased concentration of allicin, the
intracellular protein expression levels of cleaved caspase 3 were
significantly increased in the MGC-803 cells, thus suggesting an
increased rate of cell apoptosis.
Previous research has shown that the activation of
intracellular p38 MAPK is often accompanied by activation of
caspase 3, which then induces the apoptotic caspase cascade
(14,21). Therefore, the present study further
analyzed the protein expression levels of p38 MAPK in MGC-803
cells. In response to treatment with an increased concentration of
allicin, the intracellular protein expression levels of p38 MAPK
were increased. Furthermore, the rate of cell apoptosis was
determined by flow cytometry, using an Annexin V-FITC-PI kit. Based
on the flow cytometry results, the rate of apoptosis was increased,
when the concentration of allicin was increased from 0.1 to 10
μg/ml. These results suggest that the p38 MAPK/caspase 3 pathway
has an important role in the effects of allicin on gastric cancer
cell apoptosis.
In conclusion, the results of the present study
demonstrate that allicin can inhibit the proliferation and induce
apoptosis of human gastric cancer cells. The underlying mechanisms
may involve activation of the p38 MAPK signaling pathway and
hydroxylation of caspase 3. As compared with other chemotherapeutic
drugs, allicin is characterized by low levels of toxicity and few
side effects. As a promising chemotherapeutic drug, the elucidation
of allicin’s molecular mechanism will have significant effects in
tumor prevention and treatment
References
|
1
|
Zhang W, Ha M, Gong Y, Xu Y, Dong N and
Yuan Y: Allicin induces apoptosis in gastric cancer cells through
activation of both extrinsic and intrinsic pathways. Oncol Rep.
24:1585–1592. 2010.PubMed/NCBI
|
|
2
|
Ha MW and Yuan Y: Allicin induced cell
cycle arrest in human gastric cancer cell lines. Zhonghua Zhong Liu
Za Zhi. 26:585–589. 2004.(In Chinese).
|
|
3
|
Park SY, Cho SJ, Kwon HC, Lee KR, Rhee DK
and Pyo S: Caspase-independent cell death by allicin in human
epithelial carcinoma cells: involvement of PKA. Cancer Lett.
224:123–132. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang YW, Eom SY, Yim DH, et al:
Evaluation of the relationship between dietary factors,
CagA-positive Helicobacter pylori infection, and RUNX3 promoter
hypermethylation in gastric cancer tissue. World J Gastroenterol.
19:1778–1787. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sato T, Kikuchi Y, Saito T, Hirano S and
Kouzuma T: Results of chemotherapy using new anti-cancer drugs
since S-1 for advanced or recurrent gastric cancer in our
institute. Gan To Kagaku Ryoho. 34:1819–1825. 2007.(In Japanese).
PubMed/NCBI
|
|
6
|
Tyagi G, Pradhan S, Srivastava T and
Mehrotra R: Nucleic acid binding properties of allicin:
spectroscopic analysis and estimation of anti-tumor potential.
Biochim Biophys Acta. 1840:350–356. 2014. View Article : Google Scholar
|
|
7
|
Hirsch K, Danilenko M, Giat J, et al:
Effect of purified allicin, the major ingredient of freshly crushed
garlic, on cancer cell proliferation. Nutr Cancer. 38:245–254.
2000. View Article : Google Scholar
|
|
8
|
Chu YL, Ho CT, Chung JG, Rajasekaran R and
Sheen LY: Allicin induces p53-mediated autophagy in Hep G2 human
liver cancer cells. J Agric Food Chem. 60:8363–8371. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Osman M, Adnan A, Salmah Bakar N and
Alashkham F: Allicin has significant effect on autoimmune
anti-islet cell antibodies in type 1 diabetic rats. Pol J Pathol.
63:248–254. 2012. View Article : Google Scholar
|
|
10
|
Liang X, Yang Y, Deng C, et al: The
variation of Caspase3 activity in tanshinone induced NB4 cells
apoptosis. Sichuan Da Xue Xue Bao Yi Xue Ban. 34:549–551. 2003.(In
Chinese). PubMed/NCBI
|
|
11
|
Guo L, Dong F, Hou Y, Cai W, Zhou X, Huang
AL, Yang M, Allen TD and Liu J: Dihydroartemisinin inhibits
vascular endothelial growth factor-induced endothelial cell
migration by a p38 mitogen-activated protein kinase-independent
pathway. Exp Ther Med. 8:1707–1712. 2014.PubMed/NCBI
|
|
12
|
Feidantsis K, Pörtner HO, Markou T, Lazou
A and Michaelidis B: Involvement of p38 MAPK in the induction of
Hsp70 during acute thermal stress in red blood cells of the
gilthead sea bream, Sparus aurata. J Exp Zool A Ecol Genet Physiol.
317:303–310. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Franks HA, Wang Q, Lax SJ, et al: Novel
function for the p38-MK2 signalling pathway in circulating CD1c+
(BDCA-1+) myeloid dendritic cells from healthy donors and advanced
cancer patients; inhibition of p38 enhances IL-12 whilst
suppressing IL-10. Int J Cancer. 34:575–586. 2013.
|
|
14
|
Jameel NM, Thirunavukkarasu C, Wu T,
Watkins SC, Friedman SL and Gandhi CR: p38-MAPK- and
caspase-3-mediated superoxide-induced apoptosis of rat hepatic
stellate cells: reversal by retinoic acid. J Cell Physiol.
218:157–166. 2009. View Article : Google Scholar
|
|
15
|
Andualem B: Combined antibacterial
activity of stingless bee (Apis mellipodae) honey and garlic
(Allium sativum) extracts against standard and clinical pathogenic
bacteria. Asian Pac J Trop Biomed. 3:725–731. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mozaffari-Khosravi H, Hesabgar HA, Owlia
MB, Hadinedoushan H, Barzegar K and Fllahzadeh MH: The effect of
garlic tablet on pro-inflammatory cytokines in postmenopausal
osteoporotic women: a randomized controlled clinical trial. J Diet
Suppl. 9:262–271. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li L, Sun T, Tian J, Yang K, Yi K and
Zhang P: Garlic in clinical practice: an evidence-based overview.
Crit Rev Food Sci Nutr. 53:670–681. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pittler MH and Ernst E: Clinical
effectiveness of garlic (Allium sativum). Mol Nutr Food Res.
51:1382–1385. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou Y, Wang Q, Evers BM and Chung DH:
Signal transduction pathways involved in oxidative stress-induced
intestinal epithelial cell apoptosis. Pediatr Res. 58:1192–1197.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Simpson KL, Cawthorne C, Zhou C, et al: A
caspase-3 ‘death-switch’ in colorectal cancer cells for induced and
synchronous tumor apoptosis in vitro and in vivo facilitates the
development of minimally invasive cell death biomarkers. Cell Death
Dis. 4:e6132013. View Article : Google Scholar
|
|
21
|
Boosani CS, Nalabothula N, Munugalavadla
V, et al: FAK and p38-MAP kinase-dependent activation of apoptosis
and caspase-3 in retinal endothelial cells by alpha1 (IV) NC1.
Invest Ophthalmol Vis Sci. 50:4567–4575. 2009. View Article : Google Scholar : PubMed/NCBI
|