Introduction
The therapeutic efficacy of allogeneic bone marrow
transplantation (allo-BMT) for the treatment of a variety of
neoplastic diseases, including hematological malignancies, relies
upon the graft-versus-tumor (GVT) effects that eliminate residual
malignant cells. To date, acute graft-versus-host disease (GVHD)
remains the most severe complication of allo-BMT, which limits its
application and efficacy (1,2).
Therefore, minimizing the incidence and severity of GVHD without
sacrificing critical GVT activity remains a major clinical
challenge for bone marrow transplantation (BMT). Therapeutic
approaches developed to attenuate GVHD have focused on the
production of immunosuppressive agents and the ex vivo
removal of donor T cells from bone marrow grafts. However, T-cell
depletion is associated with increased rates of engraftment
failure, sustained immunosuppression and leukemia relapse.
Therefore, more effective therapies are required in order to reduce
the incidence and severity of GVHD and improve the survival rate of
transplant recipients. Strategies for the reliable induction of a
robust, permanent state of immunological tolerance may markedly
improve the prospects of allograft recipients. Strategies for
inducing immunologic tolerance in hematopoietic stem cell
transplantation may be able to decrease or prevent the incidence of
GVHD (3–5). Acute GVHD fundamentally depends upon
donor T-cell interaction with antigen-presenting cells (APC) and
their subsequent activation, proliferation and differentiation, a
process which occurs during the second step of the afferent
phase.
Dendritic cells (DCs) represent a heterogeneous
population of professional APCs. DCs have crucial functions in the
initiation and regulation of immune responses and are additionally
involved in mediating the induction and maintenance of immune
tolerance. The ability of DCs to initiate immune responses or
induce immune tolerance is dependent on their maturation status.
DCs are altered immunophenotypically and functionally during
maturation. Normal immature DCs (imDCs) that lack co-stimulatory
molecules, including CD80 and CD86, exhibit tolerance-inducing
activities, but demonstrate immunogenicity following maturation
(6,7). However, the clinical applications of
normal imDCs may be unsuitable for the treatment of
immunopathogenic diseases, as they may mature under inflammatory
conditions (8). Therefore, the
prevention of DC maturation or the maintenance of imDC in their
immature state is required for the induction of long-term immune
tolerance in vivo (9,10).
Tumor necrosis factor α (TNF-α), which is a potent pro-inflammatory
cytokine produced by stimulated monocytes, macrophages, activated T
cells and DCs, is a critical mediator of alloreactive responses
(11). The development of DCs from
progenitor cells to mature DCs that possess potential immune
response functions requires multiple cytokines, including TNF-α
(12,13). TNF-α is important in DC
differentiation and maturation. Inhibition of TNF-α function may
arrest DCs in an immature state, prolonging their tolerogenic
potential (14,15). TNF-α binds to two independent cell
surface receptors, type 1 (p55) and type 2 (p75); TNF receptor 1
(TNFR1) is the major signal transducer (16). Soluble TNFR1 (sTNFR1) and sTNFR2,
which are formed by shedding the extracellular domain of TNFR, are
effective in blocking and neutralizing TNF-α (17,18).
However, whether or not the blockade of TNF-α by sTNFR1 is able to
inhibit the maturation of DCs and potentiate imDC tolerogenicity
remains to be elucidated. In a previous study by our group, a
recombinant lentiviral vector expressing sTNFR1 was constructed and
successfully used to transfect imDCs, which maintained a long-term
immature status. The immunological characteristics of the sTNFR1
gene-modified imDCs were additionally analyzed (19). In the present study, mouse leukemia
models were established in order to investigate whether
sTNFR1-induced tolerogenic imDCs were able to reduce the effects of
acute GVHD following allo-BMT whilst maintaining
graft-versus-leukemia (GVL) responses to provide a novel approach
for potential therapeutic application.
Materials and methods
Animals and cell strains
Female C57BL/6 mice (H-2b) were used as
recipients and male BALB/c mice (H-2d) were used as
donors. All animals, aged eight to 12 weeks, weighing 18 to 22 g,
were provided by the Laboratory Animal Centre of Yangzhou
University (Yangzhou, China). Mice were reared in cages under a 12
h light/dark cycle at a constant temperature (25 ± 2°C) and 50–60%
relative humidity. Mice were administered sterile water containing
250 mg/l erythromycin (Xi’an Lijun Pharmaceutical Co., Ltd., Xi’an,
China) and 320 mg/l gentamicin (Chengdu Haitong Pharmaceutical Co.,
Ltd., Chengdu, China) for seven days prior to transplantation and
were transferred to a fume cupboard following transplantation. The
food and pads were sterilized. The lymphoma cell-strain EL4 was
purchased from the Cell Bank of Shanghai Institute of Cell Biology
(Shanghai, China) and was used as the T-cell leukemia/lymphoma cell
line in C57BL/6 mice. The present study was performed in accordance
with the recommendations in the Guide for the Care and Use of
Laboratory Animals of the National Institutes of Health. The
experimental animal protocol was reviewed and approved by the
Institutional Animal Care and Use Committee of the Affiliated
Hospital of Xuzhou Medical College (Xuzhou, China).
Separation of bone marrow and spleen
cells
The bone marrow mononuclear cell suspension from
BALB/c mice was collected under sterile conditions (20) and adjusted to a cell concentration
of 5×107 cells/ml. BALB/c mouse spleens were harvested
to prepare a spleen cell suspension. Mononuclear cells were
obtained using lymphocyte isolation solution (Shenzhen Dakewe
Biotech Co., Ltd., Shenzhen, China) and the cell concentration was
adjusted to 5×107 cells/ml. Cell viability was >95%,
as detected by trypan blue staining.
Construction of lentivirus
Reconstruction, identification and packaging of
lentiviral vectors were performed as described previously (19). The correctly ligated recombinant
plasmid was named pXZ208-sTNFRl-IRES-enhanced green fluorescence
protein (eGFP) (pXZ9-sTNFRl) using liposome
LipofectamineTM reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA). Plasmids pXZ9-sTNFRl, pXZ9, ΔNRF and envelope
protein plasmid VSVG (all obtained from the Laboratory of
Hematology, The Affiliated Hospital of Xuzhou Medical College,
Xuzhou, China) were co-transfected into 293 FT cells (Laboratory of
Hematology, Affiliated Hospital of Xuzhou Medical College) in the
exponential growth phase. At 24 and 48 h following transfection,
eGFP expression was observed under an IX71 fluorescence microscope
(Olympus Optical Co., Ltd., Tokyo, Japan). At 48, 72 and 96 h
following transfection, the virus containing the supernatant was
collected, condensed, filtered and preserved at −80°C for further
use.
imDC culture and transfection
Bone marrow suspension was collected from BALB/c
mice, placed in six-well culture plates at 1×106 cells
per well and incubated in RPMI-1640 (Gibco-BRL, Invitrogen Life
Technologies, Carlsbad, CA, USA) containing 20 μg/l
granulocyte-macrophage colony-stimulating factor (GM-CSF) and 10
μg/l interleukin-4 (IL-4) (PeproTech, Inc., Rocky Hill, NJ, USA) in
a 5% CO2 incubator at 37°C. Cell growth was monitored
using a CKX41 inverted phase contrast microscope (Olympus Optical
Co., Ltd.). Following 48 h of culture, the culture media and any
freely floating cells were discarded and the attached cells were
cultured in fresh RPMI-1640 medium (Gibco-BRL, Invitrogen Life
Technologies) containing cytokines at the same concentration for
five days. Then 10 g/l exogenous lipopolysaccharide was used to
stimulate the cells, followed by culture until day 7. Cells were
harvested, incubated with CD16/CD32 monoclonal antibodies
(BioLegend Inc., London, UK) at 4°C for 30 min to block Fc
receptors and subsequently incubated with anti-mouse fluorescein
isothiocyanate (FITC)-CD40, CD80, phycoerythrin (PE)-CD86, CD11c
and FITC-I-A/I-E monoclonal antibodies (BioLegend, Inc., London,
UK) for 30 min. Phenotyping was performed using flow cytometry
(FACSCalibur flow cytometer; Becton, Dickinson and Company, East
Rutherford, NJ, USA). The imDCs cultured for five days were
collected, placed in fresh culture medium containing GM-CSF and
IL-4 and subsequently cultured with 5 ml pXZ9-sTNFR1 or pXZ9 virus
(empty vector control) to generate sTNFR1-imDC or pXZ9-imDCs,
followed by polybrene (Sigma-Aldrich Inc., Seelze, Germany) to
achieve a concentration of 8 mg/l. Following viral infection for 96
h, cells were harvested and eGFP expression was observed using a
fluorescence microscope. Viral infection efficiency was determined
using flow cytometry. DC surface markers were analyzed using flow
cytometry. Gene transcription of sTNFR1 in target cells was
assessed using reverse transcription polymerase chain reaction
(RT-PCR). The sTNFR1 protein levels in cell supernatants were
determined by western blot analysis (19).
EL4 leukemia/lymphoma model and
grouping
On the day of transplantation, donor mice underwent
X-ray total body irradiation (TBI) using a linear accelerator (7.5
Gy in total at a rate of 0.5 Gy/min). Transplantation was performed
4 h following irradiation. Mice were randomly assigned to five
groups (n=10 per group), excluding those used for chimera analysis
and cytokine determination: i) TBI alone, infused with 0.3
ml normal saline by the caudal vein; ii) leukemia model
group, infused with isogeneic 5×106 spleen cells,
5×106 bone marrow cells and 5×103 EL4 cells
by the caudal vein; iii) allo-BMT group, infused with donor
mice 5×106 spleen cells, 5×106 bone marrow
cells and 5×103 EL4 cells by the caudal vein (data for
the GVHD model were excluded because the GVHD model was common
(4,20) and a GVL effect was observed);
iv) pXZ9-imDC group, infused with 5×106 spleen
cells from donor mice, 5×106 bone marrow cells,
5×103 EL4 cells and 5×106 pXZ9-imDCs by the
caudal vein; and v) sTNFR1-imDC group, infused with donor
mice 5×106 spleen cells, 5×106 bone marrow
cells, 5×103 EL4 cells and 5×106 sTNFR1-imDCs
by the caudal vein.
Observation parameters
Food intake and behavior were assessed daily
following transplantation. Survival duration was recorded to
calculate the survival rate. Peripheral blood cells were quantified
regularly and blood film was examined to evaluate hematogenesis. A
white blood cell count of <0.5×109/l represented
transplantation failure, whereas a count of
>1.0×109/l represented hematopoietic recovery. GVHD
was diagnosed in mice exhibiting a white blood cell count of
>10×109/l, lethargy, reduced activity, weight loss,
hunched back, ruffled fur and diarrhea. GVHD severity was evaluated
based on weight loss, activity, posture, ruffled fur and presence
of dermal lesions (for a total score of 10) (21). The GVL effect was evaluated
according to mouse survival duration, survival rate, mixed chimera
formation and peripheral blood examination. Leukemia-induced
mortality was identified by a white blood cell count of
>20×109/l, enlarged liver and spleen and a large
number of leukemia cells in the peripheral blood. In contrast to
leukemia- and GVHD-induced mortalities, mortality caused by
hematopoietic depression-induced infection and hemorrhaging within
two weeks of transplantation was defined as
transplantation-associated mortality.
Histopathological examination
The liver, spleen, small intestine and skin were
collected from moribund mice with GVHD, or recipients sacrificed at
>30 d, in each group. Tissues were sliced, fixed with 100 g/l
formaldehyde (Shanghai Haling Biological Science and Technology
Co., Ltd., Shanghai, China), embedded in paraffin, and stained with
hematoxylin and eosin (Boster Biological Engineering Co., Ltd.,
Wuhan, China). Sections were observed with an SZ61 optical
microscopy (Olympus Optical Co., Ltd.) to detect pathological GVHD
changes and leukemia cell infiltration (22,23).
Peripheral blood cytokine determination
following transplantation
Blood was harvested from the eyeballs of recipient
mice in the allo-BMT, pXZ9-imDC and sTNFR1-imDC groups at 0, 7, 14,
21 and 28 d following transplantation and stored at −80°C. IL-4 and
interferon (IFN)-γ concentrations in the serum were determined
using ELISA kits (Genetimes Technology Co., Ltd., Shanghai,
China).
Chimera detection
Bone marrow cells (1×106) were harvested
from recipient mice surviving >30 d, incubated with PE-labelled
anti-H-2Kb monoclonal antibody (BD Pharmingen, San Diego, CA, USA)
for 20 min in the dark, treated with hemolysin for 5 min in the
dark and washed with phosphate-buffered saline. The percentage of
cells from donor mice was determined using flow cytometry and
allogeneic chimeras were detected using Cellquest software version
3 (BD Biosciences, Franklin Lakes, NJ, USA).
Statistical analysis
Values are expressed as the mean ± standard
deviation and analyzed using SPSS 16.0 software (SPSS, Inc.,
Chicage, IL, USA) with one-way analysis of variance. Paired
comparisons among groups were conducted using the q-test. Survival
curves were calculated using the Kaplan-Meier method and compared
using the log-rank test. The leukemia incidence was determined
using the χ2 test. A value of P<0.05 was considered
to indicate a statistically significant difference between
values.
Results
Lentiviral infection of imDCs
Mouse bone marrow mononuclear cells adhered
following 24 h of culture. Cell colonies were observed for 2–3 d
and gradually increased in number and size. Following 5 d of
culture, variously sized protuberant burr formed on the surface of
certain cells, and single floating DCs were observed. DCs at 5 d
were positive for CD11c and negative for CD40, CD86, CD80 and major
histocompatibility complex (MHC) II. Following 6–8 d of culture,
various sizes of protuberant burrs formed on the surface of certain
cells and singular floating cells displaying typical DC appearance
were observed. At 7 d following lipopolysaccharide stimulation,
imDCs or pXZ9-imDCs expressed MHC II, CD40, CD80 and CD86,
indicating mature DC status. By contrast, MHC II, CD40, CD80 and
CD86 expression remained unchanged in the sTNFR1-imDC group.
sTNFR1- or pXZ9-packaged recombinant lentiviruses were used to
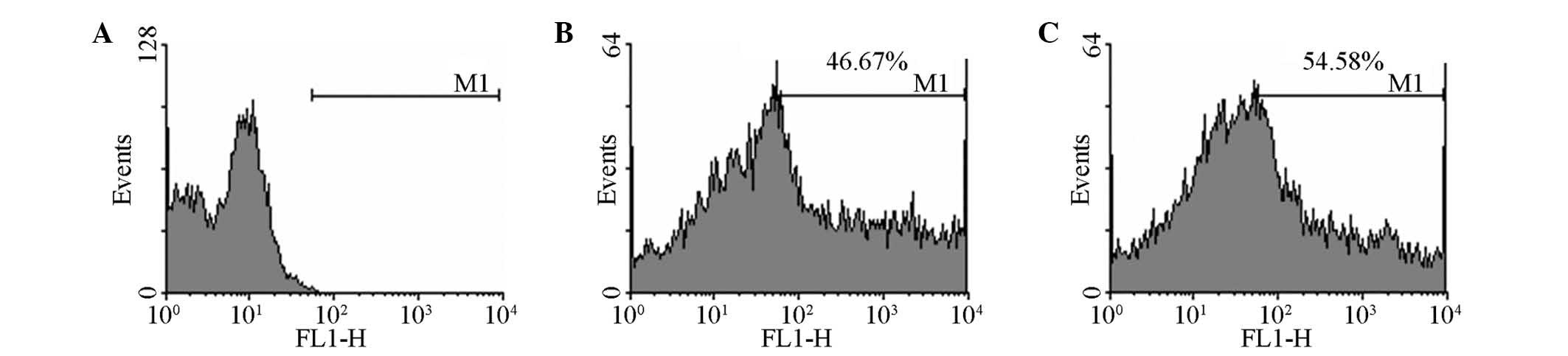
infect imDCs. Fluorescence microscopy observation detected eGFP
expression in half of the cells, which was consistent with the flow
cytometry results (Fig. 1). RT-PCR
indicated a specific band that was 368 bp in size in imDCs injected
with lentivirus carrying the sTNFR1 gene, but not in that of the
control group. Western blot analysis detected sTNFR1 protein in the
supernatant of lentivirus-infected imDCs, but not in that of the
control group.
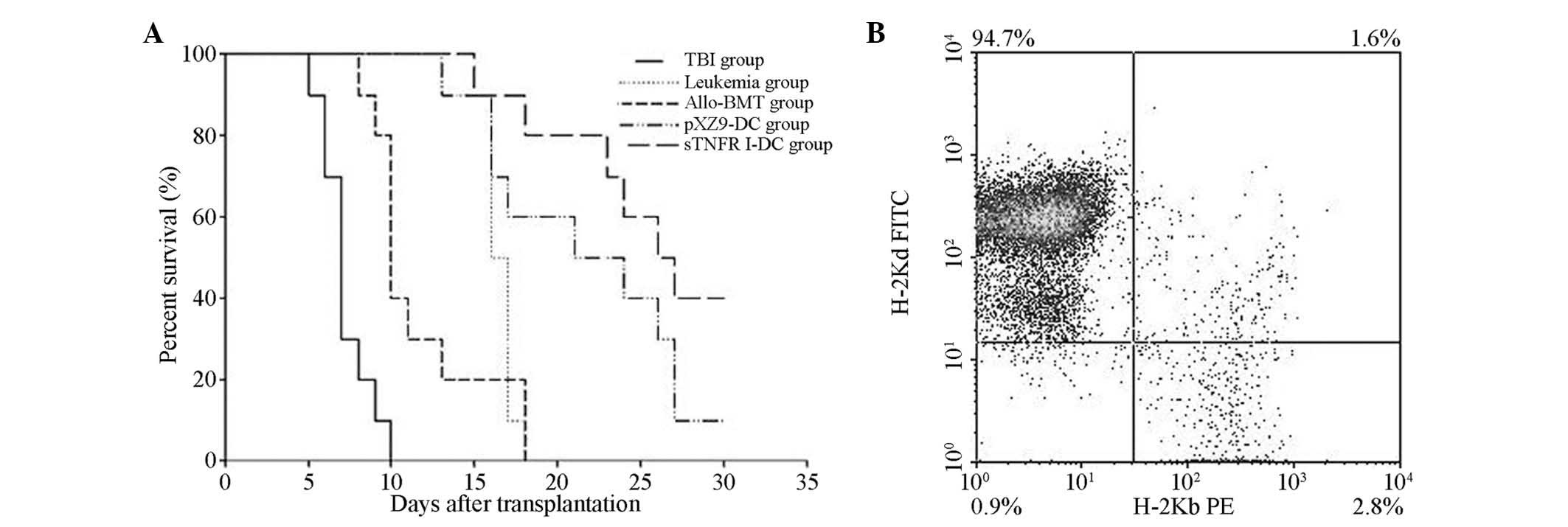
Survival of mice following
transplantation
Mice from the TBI alone group died between six and
ten days following transplantation. The average survival duration
was 7.8±1.3 d. Mice from the leukemia model group succumbed to
leukemia 13–18 d following transplantation. The average survival
duration was 16.60±0.97 d. The first mice from the allo-BMT group
died eight days following transplantation and all mice had died by
day 18. The majority of the mice in the pXZ9-imDC group died within
21 d, although 10% of the mice survived for >30 d. The average
survival duration was 21.70±5.80 d, which was significantly
prolonged in comparison with the survival duration of the allo-BMT
group (P<0.05). Mice in the sTNFR1-imDC group first died at day
15 and 40% of the mice survived up to 30 d. The average survival
duration was 25.80±5.20 d, which was significantly prolonged in
comparison with the survival duration of the allo-BMT group
(P<0.05) and was also longer than that of the pXZ9-imDC group
(P>0.05; Fig. 2A). Flow
cytometric analysis of bone marrow cells from mice surviving for
>30 d in the allo-BMT, pXZ9-imDC and sTNFR1-imDC groups revealed
that 95–100% of cells were H-2d positive, demonstrating
complete donor chimerism (Fig.
2B).
GVHD incidence
Acute GVHD clinical manifestations, including
lethargy, reduced activity, weight loss, hunched back, ruffled fur,
diarrhea and hair loss, were observed in the allo-BMT, pXZ9-imDC
and sTNFR1-imDC groups. Symptoms were most severe in the allo-BMT
group, with a clinical score of 6.8±1.2. The GVHD scores in the
pXZ9-imDC and sTNFR1-imDC groups were 5.5±1.1 and 4.2±1.0,
respectively. These values were significantly lower than scores in
the allo-BMT and pXZ9-imDC groups (P<0.05) (Fig. 3A). Pathological examination
indicated that the GVHD pathological grade of the skin, liver and
small intestine was III–IV in the allo-BMT group; II–III in the
pXZ9-imDC group and 0–I in the sTNFR1-imDC group (0–I, mild;
II–III, moderate; III–IV, severe; Fig.
3B).
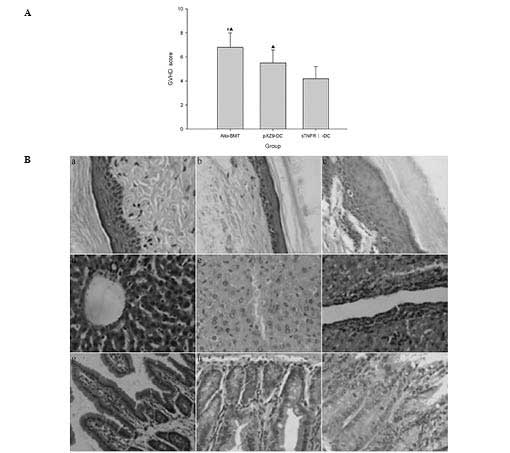 | Figure 3Clinical score of GVHD and
histopathological evidence in the liver, small intestine and skin
of mice following transplantation. (A) Average total GVHD score of
groups at serial time-points following transplantation. (B)
Histopathological evidence in liver, small intestine and skin of
mice following transplantation. (a, d and g) Skin, liver and small
intestines of mice which exhibited no evidence of GVHD in the
sTNFR1-imDC group; (b, e and h) skin, liver and small intestines of
mice with moderate GVHD in the pXZ9-imDC group; (c, f and i) skin,
liver and small intestines of mice with severe GVHD in the allo-BMT
group. (#P<0.05 vs. pXZ9-imDC group;
▲P<0.05 vs. sTNFR1-imDC group, hematoxylin and eosin
staining; magnification, ×400). GVHD, graft-versus-host disease;
allo-BMT, allogeneic bone marrow transplantation; imDC, immature
dendritic cell; sTNFR1, soluble tumour necrosis factor receptor
1. |
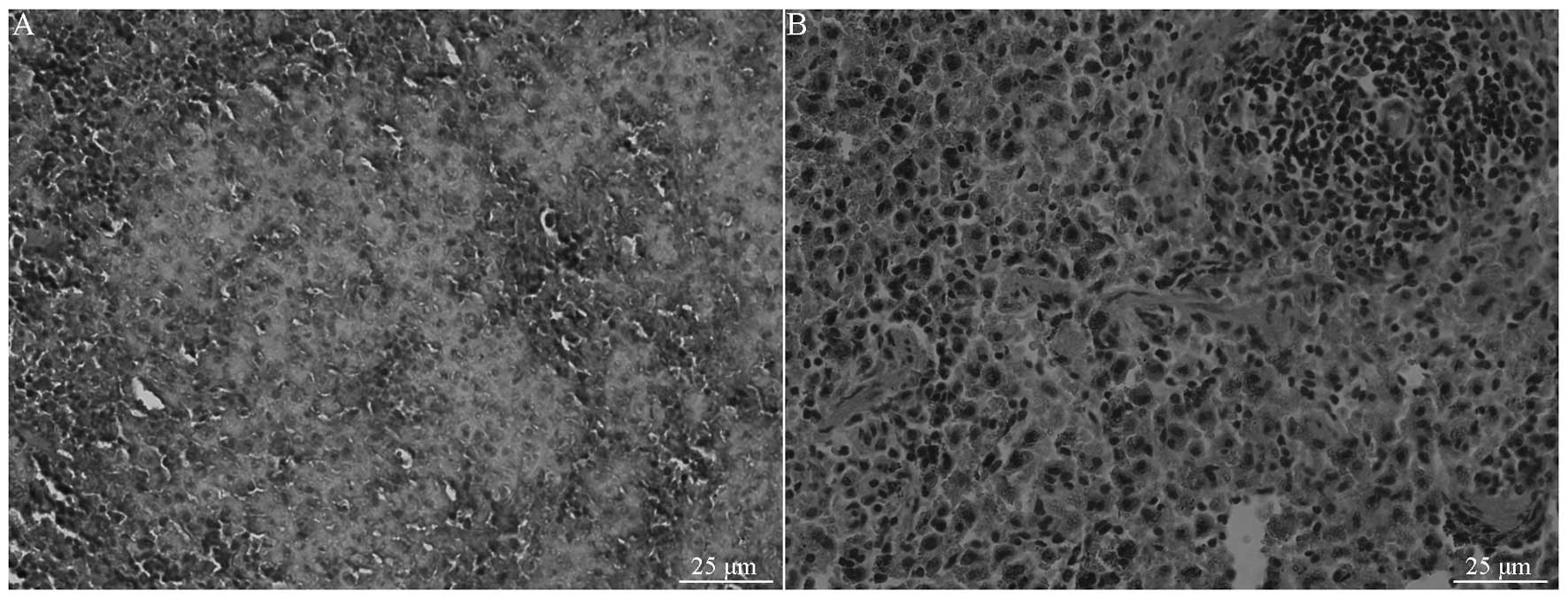
Leukemia incidence
Mice in the leukemia model group all succumbed to
leukemia within 18 d. Histopathological examination revealed
infiltration by a large number of leukemia cells (Fig. 4), with a 20×109/l to
24×109/l white blood cell count in peripheral blood. The
incidence of leukemia in the allo-BMT, pXZ9-imDC and sTNFR1-imDC
groups was 10, 20 and 10%, respectively. No statistically
significant difference was observed between the groups
(P>0.05).
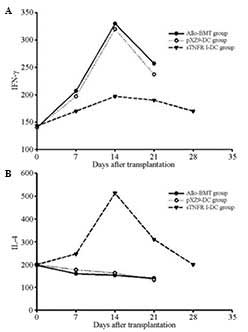
Cytokine expression alterations in mice
following transplantation
The serum IFN-γ concentration peaked at 14 d in the
allo-BMT, pXZ9-imDC and sTNFR1-imDC groups and subsequently
decreased. It was significantly reduced in the sTNFR1-imDC group
compared with that of the allo-BMT and pXZ9-imDC groups
(P<0.05). The serum IFN-γ concentration was also reduced in the
pXZ9-imDC group compared with that of the allo-BMT group
(P<0.05) (Fig. 5A). The levels
of IL-4 in the serum were reduced in the allo-BMT and pXZ9-imDC
groups, but gradually increased in the sTNFR1-imDC group, peaking
at 14 d. There was significant difference in IL-4 levels between
the sTNFR1-imDC and allo-BMT groups, and between the sTNFR1-imDC
and pXZ9-imDC groups (P<0.05). No significant difference was
detected between the allo-BMT and pXZ9-imDC group (P>0.05;
Fig. 5B).
Discussion
At present, allo-BMT is regarded to be the most
effective treatment for numerous malignant hematologic diseases,
including leukemia, lymphoma and myelodysplastic syndrome (24,25).
The GVL effect exerted by donor T cells contributes to the efficacy
of allo-BMT (26). However, GVHD
is a life-threatening condition and over the past several decades,
no existing method has been able to completely separate GVHD and
GVL. Reducing the incidence and severity of GVHD whilst retaining
the GVT effects is a crucial step in improving the overall
effectiveness of allo-BMT against hematological malignancies
(27–29). Several studies have indicated that
the use of tolerogenic DCs or of freshly-isolated, regulatory T
cells for the control of GVHD does not affect the activity of GVT
against leukemic cells and lymphomas (30–32).
Conventional therapies have previously targeted T cells; however,
immunostimulatory DCs are critical to the pathogenesis of GVHD and
also have tolerogenic properties (33). One therapeutic approach that has
emerged is the use of imDCs for the induction of peripheral immune
tolerance in allograft recipients. Administration of donor-provided
imDCs or manipulation of imDCs using immunosuppressive cytokines,
including IL-10 and TGF-β, significantly prolonged allograft
survival in non-immunosuppressed transplant recipients (7,34).
Studies have indicated that intervention in DCs from recipients,
donors or a third party may induce immunologic tolerance, which has
an important function in the regulation of GVHD and GVL (35). The present study, in agreement with
other studies, has demonstrated that unlike mature DCs which
express high levels of MHC II and co-stimulatory molecules on their
surface and induce immune responses, imDCs were deficient in
co-stimulatory molecules, inhibited T-cell responses and induced
tolerance. Moreover, further studies have suggested that imDCs
expressing sTNFR1 were resistant to maturation induced by
lipopolysaccharide stimulation and retained low levels of MHC II
and co-stimulatory markers characteristic of imDCs. In the present
study, donor imDCs were selectively manipulated by transfection
with the sTNFR1 gene to inhibit the maturation and
activation of DCs. In leukemia mouse-models of allo-BMT,
sTNFR1-modified imDCs induced long-lasting immune tolerance and
ameliorated GVHD without affecting the GVL response.
The C57BL/6-derived T-cell leukemia/lymphoma cell
line EL4 was used for leukemia modelling, as previously described
(36), with minor modification.
EL4 cells share histocompatibility antigens with normal C57BL/6
mice and the variation in the numbers of EL4 leukemia cells and
normal target cells in the host, as well as tissue compensation,
enable attenuation of GVHD and retention of GVL (36,37).
The GVL effect observed in this model depends on the alloreactivity
of donor T cells which recognize various MHC molecules expressed on
the surface of EL4 cells. In a previous study by our group, an EL4
H-2b leukemia model was constructed (20,38)
with a success rate of 100%. The EL4 cells exhibited marked
invasiveness and the leukemia model was rapid and stable with
extensive infiltration into the liver and spleen. In addition to
bone marrow transplantation, EL4 leukemia cells from C57BL/6 mice
were infused, and mice in the leukemia group succumbed to leukemia
within 18 d following transplantation. Anti-leukemia effects of
allo-BMT were observed in the allo-BMT, pXZ9-imDC and sTNFR1-imDC
groups. Few mice developed leukemia in these three groups and the
survival rate and average survival duration were prolonged in the
pXZ9-imDC and sTNFR1-imDC groups compared with those of the
allo-BMT group. Furthermore, the survival rate was prolonged in the
sTNFR1-imDC group compared with that of the pXZ9-imDC group,
suggesting that allo-BMT GVL effects were retained following
pXZ9-imDC and sTNFR1-imDC infusions. Clinical scores were higher
and GVHD was more severe in the allo-BMT group compared with the
pXZ9-imDC and sTNFR1-imDC groups. The sTNFR1-imDC group produced
stable chimeras and exhibited significantly fewer symptoms and a
prolonged survival rate. The imDC infusion alone was able to
attenuate GVHD. However, as the imDCs gradually matured due to
antigen stimulation, the expression of co-stimulatory molecules
increased, antigen presentation was enhanced and tolerance was
reduced, which suppressed the ability of imDCs to inhibit GVHD.
However, sTNFR1-imDCs attenuated GVHD and improved survival rates
whilst maintaining imDC properties.
Developments in immunology and genetics have
improved the understanding of the immunologic mechanisms of
allo-hemopoietic stem cell transplantation. Recent evidence has
indicated that GVHD is a process where T cells within donor
transplants recognize ‘foreign’ recipient histocompatibility
antigens, resulting in endogenous cytokine release and tissue
over-reactivity. Consequently, T cells attack tissues expressing
these antigens in a process known as cytokine storm (39,40).
CD4+ T cells have a critical function in the
pathological process of initiating and amplifying GVHD (34). CD4+ T cells are
categorized into Th1 and Th2 cells according to their active
protein components. In murine models, donor CD4+ Th1
cells preferentially secrete type-1 cytokines (IL-2, IFN-γ and
TNF-α) and induce significant GVHD, whereas donor Th2 cells which
primarily secrete type-2 cytokines (IL-4, IL-5, IL-10 and IL-13),
reduce GVHD and therefore downregulate the GVHD initiated by
Th1/Tc1 cells (3,39,41).
In the present study, pXZ9-imDCs and sTNFR1-imDCs reduced the
secretion of IFN-γ, a Th1 cytokine, and increased the production of
IL-4, a Th2 cytokine, further indicating that pXZ9-imDCs and
sTNFR1-imDCs stimulated immunologic tolerance of donor
CD4+ T cells following activation of recipient antigens.
Th1 cytokines function in immune activation, modulate cellular
immune responses, enhance the activation and recruitment of
macrophages, recruit B cells and promote cytotoxic T-cell
proliferation to promote GVHD. By contrast, Th2 cytokines are
associated with immunologic tolerance and reduce production of
causative agents of GVHD (4,42).
pXZ9-imDCs and sTNFR1-imDCs induce immunologic
tolerance by secreting cytokines that negatively regulate immunity,
which is potentially a mechanism for immunologic tolerance
induction (43). Variation between
pXZ9-imDCs and sTNFR1-imDCs suggests that genetically modified DCs
prevent induction of maturation by exogenous antigens and stabilize
immunologic tolerance. However, half of the mice in the sTNFR1-imDC
group succumbed to acute GVHD, revealing a marked limitation of
sTNFR1-imDCs. This phenomenon may be due to a number of factors;
for example, viral vectors are able to promote DC maturation,
resulting in the gradual maturation of infused imDCs. In addition,
recipient antigen-presenting cells participate in acute GVHD. In
the present study, donor antigen-presenting cells were selectively
manipulated; however, their activation was not completely
inhibited. Therefore, multiple infusions for an extended duration
or the concomitant use of immunosuppressants may improve
therapeutic outcomes. Subsequent experiments are required to
investigate the DC components in blood cells, distribution changes,
homing and chemotaxis.
In conclusion, the EL4 leukemia/lymphoma model was
successfully replicated in a mouse model. Infusion of sTNFR1-imDC
prolonged survival, reduced expression levels of Th1 cytokines,
increased expression levels of Th2 cytokines, significantly
attenuated GVHD and maintained GVL effects in mice. The results of
the present study therefore provided a novel insight into the
clinical discrimination of GVL and GVHD and may therefore aid in
the reduction of mortality following hematopoietic stem cell
transplantation and leukemia relapse.
Acknowledgements
The present study was supported by the Medical
Scientific Research Foundation of Jiangsu Province (grant no.
2014027), the Xuzhou Science and Technology Plan Program (grant no.
XM13B040) and The Ordinary College Postgraduates Practice and
Innovation Projects of Jiangsu Province in 2013–2014 (grant nos.
CXLX13_99 and SJZZ_0200).
References
|
1
|
Mielcarek M, Storer B, Martin PJ, Forman
SJ, Negrin RS, Flowers ME, Inamoto Y, Chauncey TR, Storb R,
Appelbaum FR and Bensinger WI: Long-term outcomes after
transplantation of HLA-identical related G-CSF-mobilized peripheral
blood mononuclear cells versus bone marrow. Blood. 119:2675–2678.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Morecki S, Yacovlev E, Gelfand Y, Shabat Y
and Slavin S: Induction of graft-versus-leukemia (GVL) effect
without graft-versus-host disease (GVHD) by pretransplant donor
treatment with immunomodulators. Biol Blood Marrow Transplant.
15:406–415. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Edinger M, Powrie F and Chakraverty R:
Regulatory mechanisms in graft-versus- host response. Biol Blood
Marrow Transplant. 15(1 Suppl): 2–6. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang Y, Feng S, Tang R, Du B, Xu K and
Pan X: Efficacy of pretreatment of allografts with
methoxypolyethylene glycol-succinimidyl-propionic acid ester in
combination with an anti-OX40L monoclonal antibody in relieving
graft-versus-host disease in mice. Int J Hematol. 92:609–616. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sordi V and Piemonti L: Therapeutic
plasticity of stem cells and allograft tolerance. Cytotherapy.
13:647–660. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou F, Ciric B, Zhang GX and Rostami A:
Immune tolerance induced by intravenous transfer of immature
dendritic cells via up-regulating numbers of suppressive IL-10(+)
IFN-γ(+)-producing CD4(+) T cells. Immunol Res. 56:1–8. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cools N, Van Tendeloo VF, Smits EL, Lenjou
M, Nijs G, Van Bockstaele DR, Berneman ZN and Ponsaerts P:
Immunosuppression induced by immature dendritic cells is mediated
by TGF-beta/IL-10 double-positive CD4+ regulatory T cells. J Cell
Mol Med. 12:690–700. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun X, Jones HP, Dobbs N, Bodhankar S and
Simecka JW: Dendritic cells are the major antigen presenting cells
in inflammatory lesions of murine Mycoplasma respiratory disease.
PLoS One. 8:e559842013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maldonado RA and von Andrian UH: How
tolerogenic dendritic cells induce regulatory T cells. Adv Immunol.
108:111–165. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fukaya T, Takagi H, Taya H and Sato K: DCs
in immune tolerance in steady-state conditions. Methods Mol Biol.
677:113–126. 2011. View Article : Google Scholar
|
|
11
|
Trevejo JM, Marino MW, Philpott N, Josien
R, Richards EC, Elkon KB and Falck-Pedersen E: TNF-α-dependent
maturation of local dendritic cells is critical for activating the
adaptive immune response to virus infection. Proc Natl Acad Sci
USA. 98:12162–12167. 2001. View Article : Google Scholar
|
|
12
|
Kleijwegt FS, Laban S, Duinkerken G,
Joosten AM, Zaldumbide A, Nikolic T and Roep BO: Critical role for
TNF in the induction of human antigen-specific regulatory T cells
by tolerogenic dendritic cells. J Immunol. 185:1412–1418. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jin JO, Park HY, Xu Q, Park JI,
Zvyagintseva T, Stonik VA and Kwak JY: Ligand of scavenger receptor
class A indirectly induces maturation of human blood dendritic
cells via production of tumor necrosis factor-alpha. Blood.
113:5839–5847. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bosè F, Raeli L, Garutti C, et al: Dual
role of anti-TNF therapy: enhancement of TCR-mediated T cell
activation in peripheral blood and inhibition of inflammation in
target tissues. Clin Immunol. 139:164–176. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baldwin HM, Ito-Ihara T, Isaacs JD and
Hilkens CM: Tumour necrosis factor alpha blockade impairs dendritic
cell survival and function in rheumatoid arthritis. Ann Rheum Dis.
69:1200–1207. 2010. View Article : Google Scholar
|
|
16
|
Rossol M, Meusch U, Pierer M, Kaltenhäuser
S, Häntzschel H, Hauschildt S and Wagner U: Interaction between
transmembrane TNF and TNFR1/2 mediates the activation of monocytes
by contact with T cells. J Immunol. 179:4239–4248. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yi HJ and Lu GX: Adherent and non-adherent
dendritic cells are equivalently qualified in GM-CSF, IL-4 and
TNF-α culture system. Cell Immunol. 277:44–48. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xia Y, Dai J, Lu P, Huang Y, Zhu Y and
Zhang X: Distinct effect of CD40 and TNF-signaling on the
chemokine/chemokine receptor expression and function of the human
monocyte-derived dendritic cells. Cell Mol Immunol. 5:121–131.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang YH, Chao YL, Tang RX, Wang SH, Zeng
LY, Chen C, Pan XY and Xu KL: Lentivirus-mediated soluble tumor
necrosis factor receptor 1 expression in mouse bone marrow-derived
immature dendritic cells. J Clin Rehabil Tissue Eng Res.
14:941–946. 2010.
|
|
20
|
Cao J, Chen C, Zeng L, Li L, Li Z and Xu
K: Engineered regulatory T cells prevent graft-versus-host disease
while sparing the graft-versus-leukemia effect after bone marrow
transplantation. Leuk Res. 34:1374–1382. 2010. View Article : Google Scholar
|
|
21
|
Cooke KR, Kobzik L, Martin TR, Brewer J,
Delmonte J Jr, Crawford JM and Ferrara JL: An experimental model of
idiopathic pneumonia syndrome after bone marrow transplantation: I.
The roles of minor H antigens and endotoxin. Blood. 88:3230–3239.
1996.PubMed/NCBI
|
|
22
|
Asai O, Longo DL, Tian ZG, Hornung RL,
Taub DD, Ruscetti FW and Murphy WJ: Suppression of
graft-versus-host disease and amplification of graft-versus-tumor
effects by activated natural killer cells after allogeneic bone
marrow transplantation. J Clin Invest. 101:1835–1842. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun K, Wilkins DE, Anver MR, Sayers TJ,
Panoskaltsis-Mortari A, Blazar BR, Welniak LA and Murphy WJ:
Differential effects of proteasome inhibition by bortezomib on
murine acute graft-versus-host disease (GVHD): delayed
administration of bortezomib results in increased GVHD-dependent
gastrointestinal toxicity. Blood. 106:3293–3299. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Appelbaum FR: Haematopoietic cell
transplantation as immunotherapy. Nature. 411:385–389. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Konuma T, Kato S, Ooi J, Oiwa-Monna M,
Kawamata T, Tojo A and Takahashi S: Comparable long-term outcome of
unrelated cord blood transplantation with related bone marrow or
peripheral blood stem cell transplantation in patients aged 45
years or older with hematologic malignancies after myeloablative
conditioning. Biol Blood Marrow Transplant. 20:1150–1155. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
van Besien K: Allogeneic transplantation
for AML and MDS: GVL versus GVHD and disease recurrence. Hematology
Am Soc Hematol Educ Program. 2013:56–62. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sinkovics JG: Antileukemia and antitumor
effects of the graft-versus-host disease: a new immunovirological
approach. Acta Microbiol Immunol Hung. 57:253–347. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim HJ, Min WS, Cho BS, Eom KS, Kim YJ,
Min CK, Lee S, Cho SG, Jin JY, Lee JW and Kim CC: Successful
prevention of acute graft-versus-host disease using low-dose
antithymocyte globulin after mismatched, unrelated, hematopoietic
stem cell transplantation for acute myelogenous leukemia. Biol
Blood Marrow Transplant. 15:704–717. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Capitini CM, Herby S, Milliron M, Anver
MR, Mackall CL and Fry TJ: Bone marrow deficient in IFN-{gamma}
signaling selectively reverses GVHD-associated immunosuppression
and enhances a tumor-specific GVT effect. Blood. 113:5002–5009.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chorny A, Gonzalez-Rey E, Fernandez-Martin
A, Ganea D and Delgado M: Vasoactive intestinal peptide induces
regulatory dendritic cells that prevent acute graft-versus-host
disease while maintaining the graft-versus-tumor response. Blood.
107:3787–3794. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stenger EO, Turnquist HR, Mapara MY and
Thomson AW: Dendritic cells and regulation of graft-versus-host
disease and graft-versus-leukemia activity. Blood. 119:5088–5103.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Semple K, Yu Y, Wang D, Anasetti C and Yu
XZ: Efficient and selective prevention of GVHD by antigen-specific
induced Tregs via linked-suppression in mice. Biol Blood Marrow
Transplant. 17:309–318. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Toubai T, Mathewson N and Reddy P: The
role of dendritic cells in graft-versus-tumor effect. Front
Immunol. 5:662014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sun W, Wang Q, Zhang L, Pan J, Zhang M, Lu
G, Yao H, Wang J and Cao X: TGF-beta(1) gene modified immature
dendritic cells exhibit enhanced tolerogenicity but induce
allograft fibrosis in vivo. J Mol Med (Berl). 80:514–523. 2002.
View Article : Google Scholar
|
|
35
|
Sela U, Olds P, Park A, Schlesinger SJ and
Steinman RM: Dendritic cells induce antigen specific regulatory T
cells that prevent graft versus host disease and persist in mice. J
Exp Med. 208:2489–2496. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang YG, Sergio JJ, Pearson DA, Szot GL,
Shimizu A and Sykes M: Interleukin-12 preserves the
graft-versus-leukemia effect of allogeneic CD8 T cells while
inhibiting CD4-dependent graft-versus-host disease in mice. Blood.
90:4651–4660. 1997.PubMed/NCBI
|
|
37
|
Mapara MY and Sykes M: Induction of mixed
vs full chimerism to potentiate GVL effects after bone-marrow
transplantation. Methods Mol Med. 109:469–474. 2005.
|
|
38
|
Li YJ, Cao J, Chen C, et al: Establishment
and identification of mouse lymphoma cell line EL4 expressing red
fluorescent protein. J Exp Hematol. 18:107–110. 2010.(In
Chinese).
|
|
39
|
Ball LM and Egeler RM; EBMT Paediatric
Working Party. Acute GvHD: pathogenesis and classification. Bone
Marrow Transplant. 41(Suppl 2): S58–S64. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sun Y, Tawara I, Toubai T and Reddy P:
Pathophysiology of acute graft-versus-host disease: recent
advances. Transl Res. 150:197–214. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Coghill JM, Sarantopoulos S, Moran TP,
Murphy WJ, Blazar BR and Serody JS: Effector CD4+ T cells, the
cytokines they generate, and GVHD: something old and something new.
Blood. 117:3268–3276. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li JM, Giver CR, Lu Y, Hossain MS, Akhtari
M and Waller EK: Separating graft-versus-leukemia from
graft-versus-host disease in allogeneic hematopoietic stem cell
transplantation. Immunotherapy. 1:599–621. 2009.
|
|
43
|
Li X, Yang A, Huang H, et al: Induction of
type 2 T helper cell allergen tolerance by IL-10-differentiated
regulatory dendritic cells. Am J Respir Cell Mol Biol. 42:190–199.
2010. View Article : Google Scholar
|