Introduction
The incidence of acute kidney injury (AKI) following
liver transplantation has been reported to range widely-between 8%
and 78% (1). Several studies have
demonstrated that AKI has an important role in the short-term and
long-term survival rate of patients who have received a liver
transplant (2,3). The causes and mechanism of AKI
induced by liver transplantation are complicated and include
intraoperative renal hypoperfusion and the effects of mediators
produced endogenously following liver transplantation. Several
studies have demonstrated that oxidative stress of the kidney is
activated by the aforementioned factors, in addition to being
involved in the development of AKI (4–7).
Previously, it has been demonstrated that
transcription nuclear factor erythroid 2-related factor 2 (Nrf2),
characterized as ‘an oxidative stress-sensing guarding regulator’,
combines with the activating response element and regulates a
multitude of cytoprotective genes acting in synergy to remove
reactive oxygen species (ROS) through sequential enzymatic
reactions, including heme oxygenase-1 (HO-1) and nicotinamide
adenine dinucleotide phosphate quinine oxidoreductase1 (NQO1)
(7–9). Kelch-like ECH-associated protein 1
(Keap1) is a negative regulator of Nrf2 and sequesters Nrf2 in the
cytoplasm in its homodimeric isoform, promoting Nrf2 degradation
via the ubiquitin-proteasome pathway. An earlier study suggested
that upregulation of Nrf2 and Nrf2-dependent phase 2 enzymes
assisted to ameliorate kidney ischemic-reperfusion (I/R) injury
(10). Nonetheless, the role of
the Nrf2-Keap1 system in AKI associated with liver transplantation
remains to be elucidated.
Propofol, (2,6)-diisopropylphenol, is a common
anesthetic. Propofol has been revealed to ameliorate I/R injury in
several organs through potential anti-inflammatory, antiapoptotic
or antioxidation effects (11–15).
Several studies have demonstrated that propofol may enhance the
expression of antioxidant enzymes, including superoxide dismutase
(SOD) and p47phox (11,12). Nrf2 is the key regulator, which
modulates the expression of numerous antioxidant enzymes. However,
few studies have been conducted on its effects on Nrf2 expression
and its downstream antioxidant and detoxification enzymes,
including HO-1 and NQO1. In the present study, a rat orthotopic
liver autotransplantation (OLAT) model was used to investigate
whether propofol has a protective effect in kidney injury induced
by OLAT via Nrf2 activation.
Materials and methods
Animal and experimental design
A total of 24 male Sprague Dawley (SD) rats (220–280
g) were provided by the Medical Experimental Animal Center of
Guangdong Province (Foshan, China). Ethical approval for the
present study was provided by the Institutional Animal Care and Use
Committee of Sun Yat-Sen University (Guangzhou, China). The animal
studies were performed in accordance with the Guide for the Care
and Use of Laboratory Animals, issued by the National Institutes of
Health (Bethesda, MD, USA).
SD rats were randomly assigned to four groups (n=6
in each group). The sham group (sham), which was subjected to
abdominal incision, vascular dissection and wound closure without
hepatic vascular exclusion and perfusion, and the orthotopic liver
autotransplantation group (OLAT) were administered isotonic sodium
chloride solution (Sigma-Aldrich, St. Louis, MO, USA) through
intraperitoneal (i.p.) injection on each of three consecutive days
prior to the experiment. The OLAT + low dose propofol-treated group
(L-Prop) received propofol at 50 mg/kg i.p. for 3 consecutive days
prior to OLAT and the OLAT + high dose propofol-treated group
(H-Prop) received propofol (Corden Pharma S.P.A, Caponago, Italy)
at 100 mg/kg i.p. for 3 consecutive days prior to OLAT. All rats
were sacrificed 8 h after the sham or OLAT surgeries and kidney
tissue was collected for analysis.
Following administration of an ether inhalation
anesthetic (Sanpin Chemical Technology, Co., Ltd, Shenzen, China),
surgery was performed on the OLAT model, as originally described by
Jin et al (16) and
modified by Chi et al (17). This model simulated the main
surgical steps and pathophysiological course of human liver
transplantation, including blocking and unclamping the hepatic
artery and portal vein, liver I/R injury, intestinal congestion and
hypoxia. The doses of propofol were based on previous experiments
in rats, which demonstrated that 50 mg/kg i.p. of propofol produced
sedation and 100 mg/kg i.p. provided satisfactory anesthesia
(18,19).
Histological examination of kidney
tissue
The harvested kidneys were fixed in a 10%
formaldehyde solution (Sigma-Aldrich) and embedded in paraffin
(Leica Microsystems, Wetzlar, Germany) for histopathological
analysis. The kidney paraffin sections (5 μm) were stained with
hematoxylin and eosin (BeiJingDingGuoChangShengBiotech. Co., Ltd,
Beijing, China). The severity of kidney injury was evaluated, in a
blinded manner, using a semi-quantitative scale evaluating
morphological characteristics of the tubules as suggested by Paller
et al (20). Higher scores
represented more severe damage (the maximum score per tubule was
10), with points administered for tubular dilatation and tubular
epithelial cell flattening (1 point), loss of brush borders (1
point), cell membrane bleb formation (1 or 2 points), interstitial
edema (1 point), cellular vacuolization (1 point), cell necrosis (1
or 2 points) and tubular lumen obstruction (1 or 2 points)
(21).
Biochemical analysis
Blood urea nitrogen (BUN) and Creatinine (Cr), which
were used as renal functional indices, were detected in blood
samples with an automatic biochemistry analyzer (Hitachi
7600-020/7170A; Hitachi, Tokyo, Japan).
Tissue preparation for detection of
protein content
The collected kidney tissue was frozen at −80°C. The
frozen kidney tissue was homogenized on ice in 10 volumes of frozen
saline using a homogenizer (Polytron; Kinematica, Lucerne,
Switzerland). The homogenates were centrifuged for 10 min at 5,000
× g and the supernatant was allocated into 6–8 separate tubes and
preserved at −80°C until use in biochemical assays. The protein
content was determined using a bicinchoninic acid (BCA) protein
assay kit (KeyGen Biotech Co., Ltd, Nanjing, China) according to
the manufacturer’s instructions.
Detection of superoxide anion
(O2•−) and hydroxyl radical (·OH) activity in
kidney tissue
O2•− and ·OH, the main oxygen
free radicals, were quantified using assay kits (KeyGen Biotech
Co., Ltd.) according to the manufacturer’s instructions. The
quantity of O2•− and ·OH in the kidney tissue
was expressed in units per milligram of protein (U/mg).
Measurements of the maleic dialdehyde
(MDA) content in kidney tissue
The content of MDA was determined as an index of
lipid peroxidation, as described by Draper and Hadley (22). The MDA content was assayed using an
assay kit (KeyGen Biotech Co., Ltd.) according to the
manufacturer’s instructions. The kit measures the absorbance at 532
nm and expresses the content as nmol/mg protein.
Western blot analysis
Total protein was extracted from frozen kidney
tissue using total protein extraction kits (KeyGen Biotech Co.,
Ltd.) for NQO1 measurement. Cytosolic and nuclear protein extracts
were prepared using nuclear and cytoplasmic protein extraction kits
(KeyGen Biotech Co., Ltd.) according to the manufacturer’s
instructions. The cytoplasmic protein was collected for Keap1, NQO1
and HO-1 measurements, and the nuclear protein was extracted for
Nrf2 detection. Protein concentrations were determined using a BCA
protein assay reagent kit (KeyGen Biotech Co., Ltd).
Primary antibodies were added to the samples to
detect Nrf2 (1:250 dilution, Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA), Keap1 (1:1,000 dilution, Millipore, Billerica, MA,
USA), HO-1 (1:250 dilution, Santa Cruz Biotechnology, Inc.), NQO1
(1:250 dilution, Santa Cruz Biotechnology, Inc.), β-actin (1:2,000
dilution, Santa Cruz Biotechnology, Inc.) and Lamin B (1:2,000
dilution, Millipore). The relative density of bands was quantified
by computerized scanning of the images using AlphaView software
version 2.2.14407 (ProteinSimple, Santa Clara, CA, USA) and
normalized by β-actin immunoreactivity to correct for any loading
and transfer differences between samples.
Immunohistochemical analysis
For immunohistochemical staining, the kidney
paraffin sections (5 μm) were stained using the
immunohistochemistry technique for Nrf2 as described by Tanaka
et al (23). After the
sections were washed in phosphate-buffered saline three times, the
kidney sections were immersed in 3% hydrogen peroxide
(H2O2; Guangzhou Chemical Reagent Factory,
Guangzhou, China) to quench endogenous peroxidase and then blocked
in bovine serum Boster Biotech Co., Ltd, Wuhan, China) for 1 h. The
kidney sections were incubated with rabbit polyclonal antibody
against Nrf2 (1:50 dilution, Santa Cruz Biotechnology, Inc.)
overnight at 4°C and then incubated with an anti-rabbit IgG
secondary antibody (Santa Cruz Biotechnology, Inc.) and a
horseradish-peroxidase-conjugated streptavidin solution. Brown
granules in the nucleus indicated positive staining for Nrf2.
Statistical analysis
Quantitative data, expressed as the mean ± standard
deviation, were statistically analyzed using a one-way analysis of
variance for inter-group comparisons using SPSS 17.0 (IBM, Armonk,
NY, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
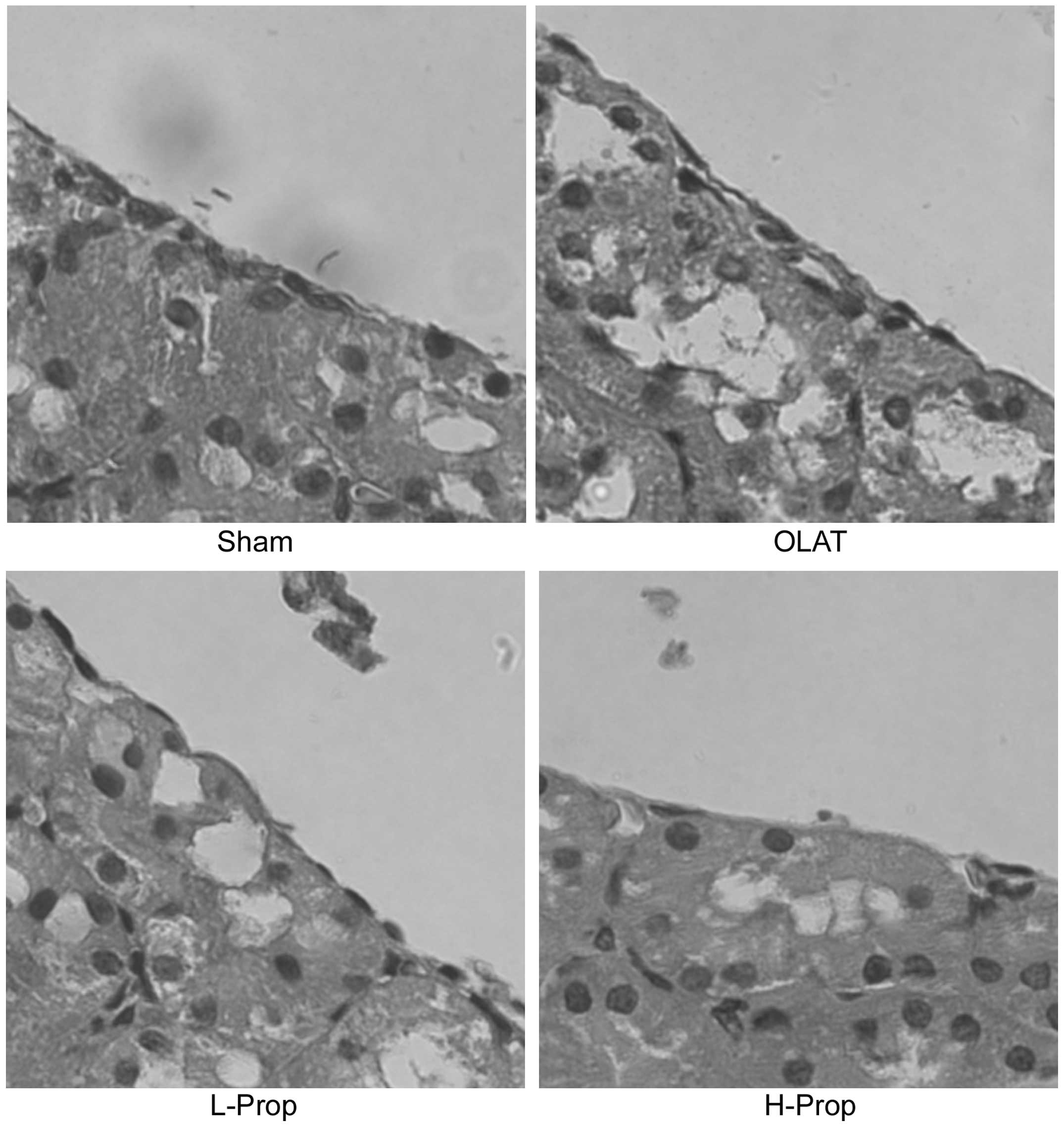
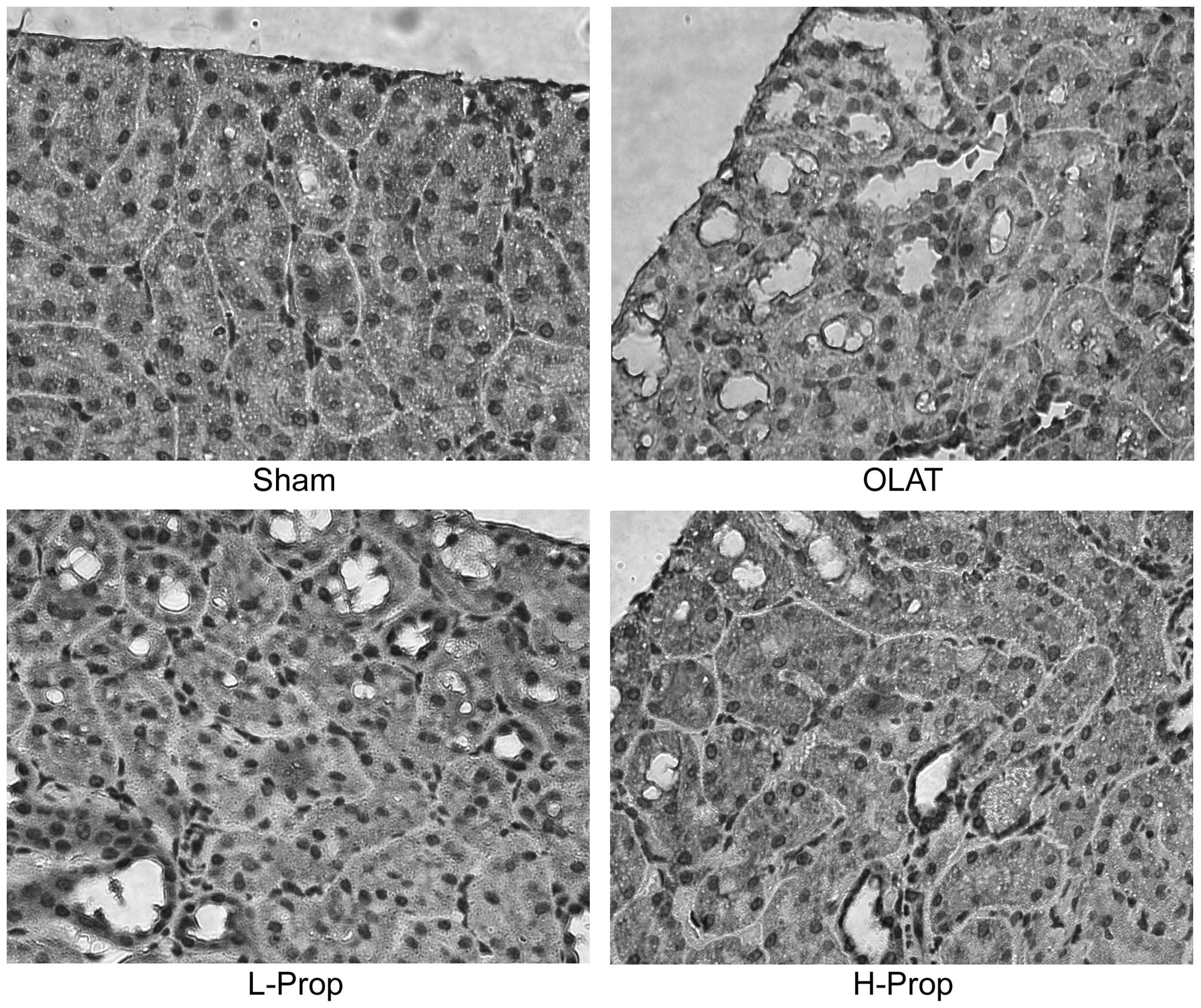
Histopathological analysis of the kidney
using microscopy
As shown in Fig.
1A, there was no detectable change in the sham group, which
exhibited normal nuclei and intact brush borders. Significant
histological injury of the kidney was observed in the OLAT group
(Fig. 1B), including luminal
debris formation, tubular cell flattening, cellular vacuolization
and loss of brush borders. These effects were attenuated by
pretreatment with propofol (Fig. 1C
and D), which exhibited less structural damage than that
observed in the OLAT group. In the L-Prop group, tubular cell
swelling and expansion of renal tubular lumen were observed
(Fig. 1C). Conversely,
pretreatment with a high dose of propofol markedly ameliorated the
renal morphology injury induced by OLAT and resulted in slight
tubular dilatation and flattening of the tubular epithelium cells
(Fig. 1D).
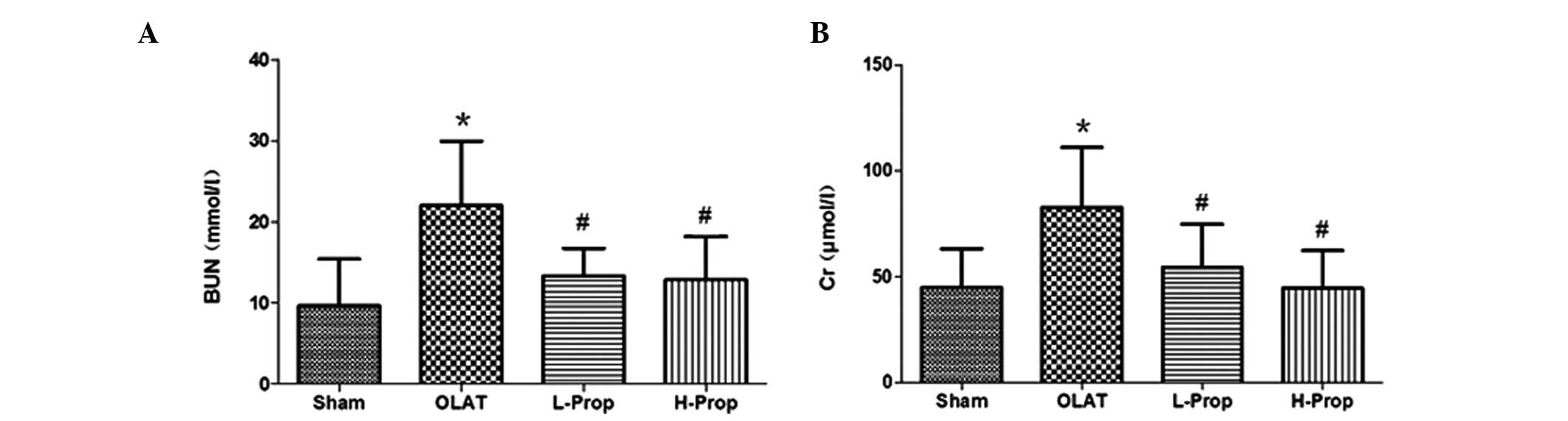
Serum BUN and Cr levels
Plasma Cr and BUN are classical indicators, which
reflect renal function. OLAT, involving 20 min of ischemia followed
by 8 h of reperfusion resulted in significant increases in the
serum concentration of Cr and BUN (Fig. 2; P<0.05, versus the sham group).
Low- and high-dose propofol pretreatment reduced the increases in
serum BUN and Cr levels (P<0.05, versus the OLAT group).
O2•−and ·OH
activity in kidney tissue
O2•− and ·OH are the main
oxygen free radicals. As depicted in Fig. 3A and B, O2•−
and ·OH activity markedly increased in the OLAT and L-Prop groups
as compared with the sham group (P<0.05). When a high dose of
propofol (100 mg/kg i.p.) was administered, the increases in
O2•−and ·OH activity were significantly
suppressed (P<0.05, versus the OLAT group). Low-dose propofol
pretreatment reduced the increases in O2•−and
·OH activity, however no significant difference was observed
(P>0.05, versus the OLAT group; Fig. 3A and B).
MDA content in kidney tissue
MDA is the product of lipid peroxidation damage. The
MDA content in the kidney was significantly higher in the OLAT
group than in the sham group (P<0.05, versus the sham group).
However, this increase was significantly reduced by pretreatment
with propofol (P=0.04, L-Prop group, versus the OLAT group;
P=0.009, H-Prop group, versus the OLAT group; Fig. 3C).
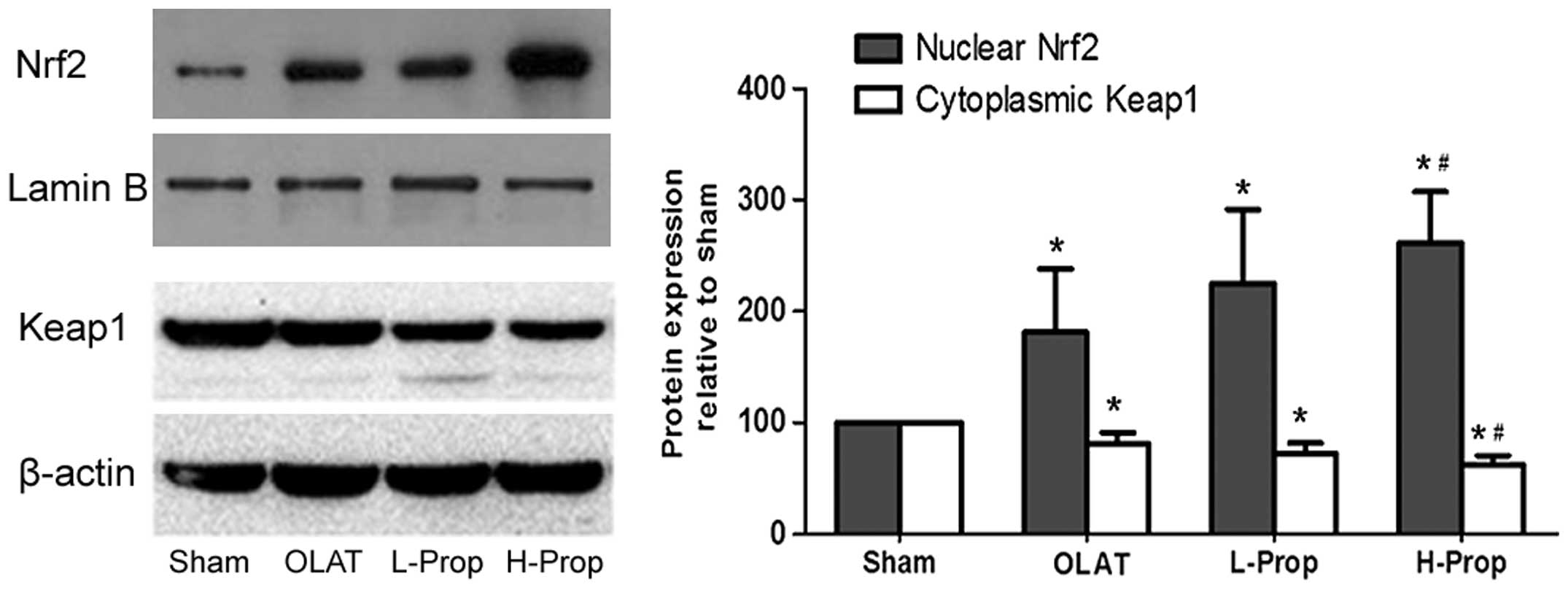
Expression of Nrf2 and Keap1 in kidney
tissue
Nrf2 is an unstable protein with a short half-life
(<20 min) and is dependent on the status of the Keap1/Nrf2
system. Keap1, which sequesters Nrf2 in the cytoplasm and prevents
it from translocation to the nucleus, is crucial in the regulation
of the Nrf2 pathway (7–9). As shown in Fig. 4, there was low expression of Nrf2
in the nucleus in the sham group. By contrast, expression of the
Keap1 protein was very high in the cytoplasm in this group.
Compared with the sham group, the OLAT, L-Prop and H-Prop groups
exhibited a significant increase in expression of Nrf2 in the
nucleus and an evident decrease in expression of Keap1 in the
cytoplasm (P<0.05, versus the sham group). Furthermore, the
increase of nuclear Nrf2 expression and the decrease of cytoplasm
Keap1 expression were more marked in the H-Prop group than those in
the OLAT group (P<0.05, versus the OLAT group), indicating that
the high-dose propofol pretreatment inhibited Keap1 expression in
the cytoplasm and then promoted Nrf2 to translocate into
nucleus.
Consistent with the western blot analysis, the
expression of Nrf2 was minimal, as demonstrated by the lack of
clear visualization of its immunoreactivity in the sham group.
Compared with the sham group, the proportion of Nrf2-positive cells
with brown staining in the nuclei was significantly higher in the
OLAT, L-Prop and H-Prop groups. Furthermore, compared with the OLAT
group, positive expression of Nrf2 was markedly increased in the
H-Prop group (Fig. 5).
Expression of HO-1 and NQO1 in renal
tissue
HO-1 and NQO1 are two typical downstream phase II
antioxidant enzymes mediated by Nrf2 (7–9).
Expression of HO-1 and NQO1 was markedly increased in the OLAT,
L-Prop and H-Prop groups (P<0.05, versus the sham group),
whereas the HO-1 and NQO1 proteins were expressed at low levels in
the sham group. However, pretreatment with high-dose propofol
significantly augmented the expression of HO-1 and NQO1 compared
with the OLAT group (P<0.05, versus the OLAT group; Fig. 6).
Discussion
OLAT is a well-established liver transplantation
model, including a superior vena cava, inferior vena cava and
hepatic portal vein block, hepatic I/R and cold liver preserving
fluid perfusion. The present study provided evidence that liver
transplantation induces remote kidney damage, which contributes to
the progression of oxidative damage. Pretreatment with propofol, a
widely used anesthetic, significantly ameliorated renal dysfunction
and pathology injury induced by OLAT. Furthermore, propofol
inhibited the expression of Keap1 in the cytoplasm, upregulated the
expression of Nrf2 in the nucleus and increased HO1 and NQO1
expression. Propofol also reduced OLAT-induced increases in
O2•− and ·OH activity as well as the MDA
content. These results suggest that propofol’s renal protective
effects against OLAT may be partly due to activation of the
Keap1/Nrf2 pathway.
It has been established that remote kidney injury is
frequently induced by I/R injury during liver transplantation
(3,24). Previous studies revealed that I/R
injury resulted mainly in the production of ROS, which are formed
during reperfusion (4–7). These ROS, including hydroxyl radicals
(·OH) and superoxide anions (O2•−), are key
mediators of renal reperfusion injury (25) and cause lipid peroxidation of the
renal cell membrane, which results in intracellular calcium
overload and subsequent necrotic cell death (26,27).
In the present study, a significant increase in renal
O2•−, ·OH activity and MDA levels was
observed, indicating that liver transplantation induced
considerable ROS generation and lipid peroxidation damage to the
kidney. Kadkhodaee et al (4) reported that 90 min of liver ischemia
and 4 h of reperfusion caused an increase in kidney MDA levels and
a decrease in catalase activities and SOD. The results of the study
of Kadkhodaee et al and the present study provided evidence
of remote kidney injury induced by liver transplantation.
Nrf2 is a critical regulator of the cellular defense
response to protect against oxidative injury (10,28–34).
Under conditions of oxidative stress or in the presence of an
inducer, Nrf2 translocates to the nucleus to activate transcription
of Nrf2-regulated genes and upregulate antioxidant enzymes and
phase II detoxification enzymes, including HO-1 and NQO1. Recent
studies (32) have demonstrated
that hepatic I/R may lead to kidney dysfunction, but renal Nrf2
activation and the subsequent upregulation of HO-1 and NQO1
contributes to the recovery of renal function. Liu et al
(33) observed that
Nrf2-deficiency worsens renal function, histology and survival rate
as well as enhancing susceptibility to ischemic and nephrotoxic
acute kidney injury, indicating a cytoprotective effect of Nrf2,
which functions against oxidative stress. Similar to these studies,
in the present study, it was observed that expression of Nrf2 in
the nucleus was modestly increased, whereas Keap1 expression in the
cytoplasm was decreased at 8 h after OLAT, accompanied by the rise
of expression of HO1 and NQO1, suggesting a defensive mechanism to
protect the kidney from organ dysfunction induced by OLAT. However,
the degree of renal morphological injury and the increase of serum
Cr and BUN concentrations were marked, implying that the increases
in antioxidant enzymes were not sufficient to inhibit oxidative
damage at this time and suggesting that specific drugs are required
to be used to enhance the expression of Nrf2 and its downstream
antioxidant enzymes as well as strengthen the defense response.
Several studies have demonstrated that propofol
ameliorates I/R injury in several organs, including the heart,
liver, kidney and brain, through improvement of antioxidant enzyme
activity (11–15). The association between propofol and
HO-1, a type of Nrf2 downstream regulation antioxidant enzyme, has
been confirmed. Xu et al (35) demonstrated that propofol may
protect cardiomyocytes against H2O2-mediated
cytotoxicity through increased expression of HO-1. Wang et
al (36), suggested that
propofol may improve renal I/R injury in rats partly through the
induction of HO-1 expression and revealed that propofol may induce
antioxidant effects via HO-1 activation. However, whether Nrf2 is
important in propofol-mediated protection remains to be elucidated.
In the current study, sedation and anesthesia doses were selected
to demonstrate that propofol pretreatment prevents OLAT-induced
pathological and functional injury to the kidney. Compared with the
model group, propofol, particularly in the high dose group,
significantly inhibited Keap1 expression, increased Nrf2 nuclear
translocation, promoted the expression levels of HO-1 and NQO1, and
abrogated the increase of O2•− and ·OH
activity, as well as MDA levels, reducing the oxidative damage in
the kidney induced by OLAT.
In conclusion, propofol pretreatment exerted a renal
protective effect against OLAT. The potential mechanism for this
effect is through upregulation of nuclear Nrf2 expression.
Acknowledgements
Financial support and sponsorship: The present study
was in part supported by the National Natural Science Foundation of
China (grant nos. 81170449 and 81372090).
References
|
1
|
Saner FH, Cicinnati VR, Sotiropoulos G, et
al: Strategies to prevent or reduce acute and chronic kidney injury
in liver transplantation. Liver Int. 32:179–188. 2013. View Article : Google Scholar
|
|
2
|
Narayanan Menon KV, Nyberg SL, Harmsen WS,
et al: MELD and other factors associated with survival after liver
transplantation. Am J Transplant. 4:819–825. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gainza FJ, Valdivieso A, Quintanilla N, et
al: Evaluation of acute renal failure in the liver transplantation
perioperative period: incidence and impact. Transplant Proc.
34:250–251. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kadkhodaee M, Mikaeili S, Zahmatkesh M, et
al: Alteration of renal functional, oxidative stress and
inflammatory indices following hepatic ischemia-reperfusion. Gen
Physiol Biophys. 31:195–202. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Heyman SN, Rosen S and Rosenberger C: A
role for oxidative stress. Contrib Nephrol. 174:138–148. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu QQ, Wang Y, Senitko M, et al:
Bardoxolone methyl (BARD) ameliorates ischemic AKI and increases
expression of protective genes Nrf2, PPARγ, and HO-1. Am J Physiol
Renal Physiol. 300:F1180–F1192. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Saito H: Toxico-pharmacological
perspective of the Nrf2-Keap1 defense system against oxidative
stress in kidney diseases. Biochem Pharmacol. 85:865–872. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Copple IM: The Keap1-Nrf2 cell defense
pathway - a promising therapeutic target? Adv Pharmacol. 63:43–79.
2012. View Article : Google Scholar
|
|
9
|
Magesh S, Chen Y and Hu L: Small molecule
modulators of Keap1-Nrf2-ARE pathway as potential preventive and
therapeutic agents. Med Res Rev. 32:687–726. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yoon HY, Kang NI, Lee HK, et al:
Sulforaphane protects kidneys against ischemia-reperfusion injury
through induction of the Nrf2-dependent phase 2 enzyme. Biochem
Pharmacol. 75:2214–2223. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tsai YC, Huang CC, Chu LM and Liu YC:
Differential influence of propofol on different cell types in terms
of the expression of various oxidative stress-related enzymes in an
experimental endotoxemia model. Acta Anaesthesiol Taiwan.
50:159–166. 2012. View Article : Google Scholar
|
|
12
|
Yang S, Chou WP and Pei L: Effects of
propofol on renal ischemia/reperfusion injury in rats. Exp Ther
Med. 6:1177–1183. 2013.PubMed/NCBI
|
|
13
|
Yuzbasioglu MF, Aykas A, Kurutas EB, et
al: Protective effects of propofol against ischemia/reperfusion
injury in rat kidneys. Ren Fail. 32:578–583. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Popic J, Pesic V, Milanovic D, et al:
Propofol-induced changes in neurotrophic signaling in the
developing nervous system in vivo. PLoS One. 7:e343962012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li H, Tan J, Zou Z, Huang CG and Shi XY:
Propofol post-conditioning protects against cardiomyocyte apoptosis
in hypoxia/reoxygenation injury by suppressing nuclear
factor-kappaB translocation via extracellular signal-regulated
kinase mitogen-activated protein kinase pathway. Eur J
Anaesthesiol. 28:525–534. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jin C, Zhang PJ, Wu XM, et al: Impact of
hypoxic preconditioning on apoptosis and its possible mechanism in
orthotopic liver autotransplantation in rats. Hepatobiliary
Pancreat Dis Int. 8:40–45. 2009.PubMed/NCBI
|
|
17
|
Chi X, Zhang A, Luo G, et al: Knockdown of
myeloid differentiation protein-2 reduces acute lung injury
following orthotopic autologous liver transplantation in a rat
model. Pulm Pharmacol Ther. 26:380–387. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu KX, Chen SQ, Huang WQ, et al: Propofol
pretreatment reduces ceramide production and attenuates intestinal
mucosal apoptosis induced by intestinal ischemia/reperfusion in
rats. Anesth Analg. 107:1884–1891. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mu X, Wu A, Wu J, et al: Effects of
anesthetic propofol on release of amino acids from the spinal cord
during visceral pain. Neurosci Lett. 484:206–209. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Paller MS, Hoidal JR and Ferris TF: Oxygen
free radicals in ischemic acute renal failure in the rat. J Clin
Inves. 74:1156–1164. 1984. View Article : Google Scholar
|
|
21
|
Wang HH, Zhou HY, Chen CC, Zhang XL and
Cheng G: Propofol attenuation of renal ischemia/reperfusion injury
involves heme oxygenase-1. Acta Pharmacol Sin. 28:1175–1180. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Draper HH and Hadley M: Malondialdehyde
determination as index of lipid peroxidation. Methods Enzymol.
186:421–431. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tanaka N, Ikeda Y, Ohta Y, et al:
Expression of Keap1-Nrf2 system and antioxidative proteins in mouse
brain after transient middle cerebral artery occlusion. Brain Res.
25:246–253. 2011. View Article : Google Scholar
|
|
24
|
Karapanagiotou A, Kydona C, Dimitriadis C,
et al: Acute kidney injury after orthotopic liver transplantation.
Transplant Proc. 44:2727–2729. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Paller MS and Neumann TV: Reactive oxygen
species and rat renal epithelial cells during hypoxia and
reoxygenation. Kidney Int. 40:1041–1049. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Salahudeen AK: Role of lipid peroxidation
in H2O2 induced renal epithelial (LLCPK1)
cell injury. Am J Physiol. 268:F30–F38. 1995.PubMed/NCBI
|
|
27
|
Sheridan AM, Fitzpatrick S, Wang C,
Wheeler DC and Lieberthal W: Lipid peroxidation contributes to
hydrogen peroxide induced cytotoxicity in renal epithelial cells.
Kidney Int. 49:88–93. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Leonard MO, Kieran NE, Howell K, et al:
Reoxygenation-specific activation of the antioxidant transcription
factor Nrf2 mediates cytoprotective gene expression in
ischemia-reperfusion injury. FASEB J. 20:2624–2626. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun Q, Meng QT, Jiang Y, et al:
Ginsenoside Rb1 attenuates intestinal ischemia reperfusion induced
renal injury by activating Nrf2/ARE pathway. Molecules.
17:7195–7205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang X, Xiao Z, Yao J, et al:
Participation of protein kinase C in the activation of Nrf2
signaling by ischemic preconditioning in the isolated rabbit heart.
Mol Cell Biochem. 372:169–179. 2013. View Article : Google Scholar
|
|
31
|
Li M, Zhang X, Cui L, et al: The
neuroprotection of oxymatrine in cerebral ischemia/reperfusion is
related to nuclear factor erythroid 2-related factor 2
(nrf2)-mediated antioxidant response: role of nrf2 and
hemeoxygenase-1 expression. Biol Pharm Bull. 34:595–601. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tanaka Y, Maher JM, Chen C, et al: Hepatic
ischemia-reperfusion induces renal heme oxygenase-1 via
NF-E2-related factor 2 in rats and mice. Mol Pharmacol. 71:817–825.
2007. View Article : Google Scholar
|
|
33
|
Liu M, Grigoryev DN, Crow MT, et al:
Transcription factor Nrf2 is protective during ischemic and
nephrotoxic acute kidney injury in mice. Kidney Int. 76:277–285.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee YM, Shin JW, Lee EH, et al: Protective
effects of propofol against hydrogen peroxide-induced oxidative
stress in human kidney proximal tubular cells. Korean J
Anesthesiol. 63:441–446. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xu JJ and Wang YL: Propofol attenuation of
hydrogen peroxide-mediated oxidative stress and apoptosis in
cultured cardiomyocytes involves heme oxygenase-1. Eur J Anaesth.
25:395–402. 2008. View Article : Google Scholar
|
|
36
|
Wang HH, Zhou HY, Chen CC, et al: Propofol
attenuation of renal ischemia/reperfusion injury involves heme
oxygenase-1. Acta Pharmacol Sin. 28:1175–1180. 2007. View Article : Google Scholar : PubMed/NCBI
|