Introduction
Hepatocellular carcinoma (HCC) is the fifth and
sixth most prevalent neoplasm in males and females, respectively,
and is the second most frequent cause of cancer-associated
mortality in China, following lung cancer (1). Due to the characteristics of this
type of cancer and limitations in its diagnosis, the majority of
the patients are already in the mid-late stages at the time of
diagnosis and are no longer able to have surgery. Therefore,
dissecting the molecular mechanisms involved in the survival and
growth of HCC cells is essential for the development of targeted
therapies to reduce patient mortality.
It is known that the invasion and metastasis of
tumor cells are commonly associated with proteases, particularly
cysteine protease. There are 11 and 19 members of the cysteine
cathepsin family in humans and mice, respectively (2). Cathepsin B (Cat B) is a cysteine
protease of ~40–45 kD. There is increasing evidence that Cat B is
frequently overexpressed in a variety of tumor tissues (3,4) and
is involved in distinct tumorigenic processes, including
angiogenesis, invasion through extracellular matrices and
metastasis (5–9). However, whether Cat B is involved in
the progression of HCC remains to be elucidated.
In the present study, Cat B promoted the
proliferation and inhibited the apoptosis of HCC cell lines and was
identified as an upstream regulator of the phosphoinositide
3-kinase (PI3K)/Akt signaling pathway. Furthermore, the data
suggested that integrin αvβ3 was essential in the signal
transduction of Cat B into the PI3K/Akt signaling pathway in
HCC.
Materials and methods
Tissue samples and cell culture
A total of eight pairs of HCC tissues and their
adjacent normal tissues were obtained from patients who had
undergone surgery at the Department of General Surgery, Qianfoshan
Hospital, Shandong University (Jinan, China). The present study was
approved by the Hospital Institutional Review Board of Qianfoshan
Hospital, Shandong University. Written informed consent was
obtained from the patient’s family. The HepG2, SMMC-7721 and
BEL-7402 cell lines were obtained from Shandong Province Key
Laboratory of General Surgery Center (Jinan, China). The cells were
cultured in Dulbecco’s modified Eagle’s medium supplemented with
10% fetal calf serum (Invitrogen Life Technologies, Carlsbad, CA,
USA), 100 U/ml penicillin and 100 μg/ml streptomycin (Sigma, St
Louis, MO, USA) at 37°C in 5% CO2.
Transfection
The BEL-7402 cells were transfected with either
pcDNA or pcDNA-Cat B using Lipofectamine 2000 (Invitrogen Life
Technologies) according to the manufacturer’s instructions. The
HepG2 and SMMC-7721 cells were transfected with either 40 nM Cat B
or control siRNA (Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
at 80% confluence using Geneporter 2 transfection reagent
(Genlantis, San Diego, CA, USA) according to the manufacturer’s
instructions.
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol reagent
(Invitrogen Life Technologies) according to the manufacturer’s
instructions. A SYBR RT-PCR kit (Takara Bio, Inc., Shiga, Japan)
was used for RT-qPCR analysis. The following specific primers were
used for RT-qPCR assays: Cat B, forward 5′-CCAGG GAGCA
AGACAGAGAC-3′ and reverse 5′-GAGAC TGGCG TTCTC CAAAG-3′ and
β-actin, forward 5′-GGCCT CCAAG GAGTA AGACC-3′ and reverse 5′-AGGGG
TCTAC ATGGC AACTG-3′. Data from each sample were normalized to the
expression of β-actin.
Tumor growth assay
Male, 4-week-old BALB/c nude mice were purchased
from the Shanghai Laboratory Animal Company (Shanghai, China).
HepG2 cells (1×105) with stable knock down of Cat B and
the negative controls were injected subcutaneously under the front
legs of the nude mice. The mice were observed over 5 weeks for
tumor formation. Subsequently, the mice were sacrificed and,
following tumor removal, the wet weights of each tumor were
determined.
Western blotting
To prepare the proteins, the treated cell monolayers
were rinsed twice using cold phosphate-buffered saline (PBS) and
then scraped and transferred into cell lysis buffer containing 50
mM Tris-HCL (pH 7.5), 150 mM NaCl and 0.5% Nonidet P40 (Sigma) with
Roche complete EDTA-free protease inhibitor cocktail (Sigma). These
suspensions were then centrifuged using a Beckman centrifuge at
10,000 × g for 10 min and the protein concentration was determined
using a BCA protein assay kit (Thermo Fisher Scientific, Waltham,
MA, USA). Equal quantities of the proteins were separated using
SDS-PAGE and transferred onto polyvinylidene difluoride membranes
(Millipore, Billerica, MA, USA) for western blot analysis using
primary antibodies against human Cat B (Abcam, Cambridge,
UK), phosphorylated Akt (Ser473), Akt (Cell Signaling Technology,
Inc., Danvers, MA, USA), β-actin and secondary polyclonal goat
anti-rabbit antibodies conjugated with horseradish peroxidase
(Santa Cruz Biotechnology, Inc.). Following washing with
tris-buffered saline with Tween 20 (Beyotime Institute of
Biotechnology, Haimen, China), bound antibodies were detected using
enhanced chemiluminescence (Thermo Fisher Scientific) according to
the manufacturer’s instructions.
Cell counting kit-8
Cell viability was assessed using a Cell counting
kit-8 (Beyotime Institute of Biotechnology) according to the
manufacturer’s instructions.
Apoptosis
An annexin-V assay was performed using an Annexin
V-fluorescein isothiocyanate (FITC) Apoptosis Detection kit
according to the manufacturer’s instructions (BD Biosciences, San
Diego, CA, USA). Briefly, 2×105 cells were collected,
washed twice with cold PBS, resuspended in 100 μl binding buffer
containing Hepes (10 mM), NaOH (pH 7.4), NaCl (140 mM) and
CaCl2 (2.5 mM) and incubated with Annexin V-FITC at room
temperature for 10 min. This was followed by the addition of 6 μl
propidium iodide (PI; 20 μg/ml) for an additional 5 mins. The
fluorescent intensities were determined using flow cytometry
(Beckman Coulter, Miami, FL, USA).
Statistical analysis
All data are expressed as the mean ± standard
deviation of three or four experiments. Analysis was performed
using Student’s t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Cat B is upregulated in HCC tissues and
cell lines
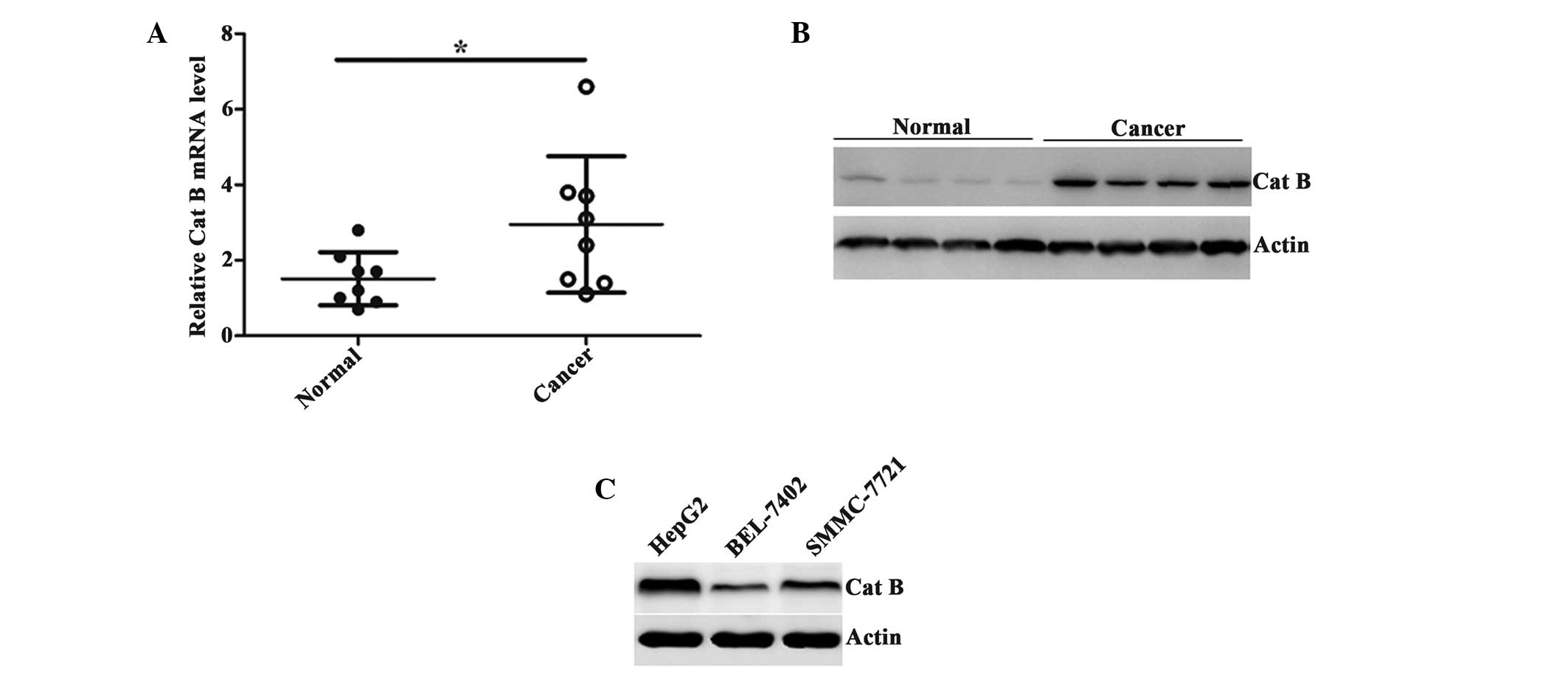
The expression of Cat B was compared between the HCC
tissues and the adjacent normal tissues using RT-qPCR analysis. The
results demonstrated that the expression of Cat B was markedly
increased in cancer tissues (Fig.
1A). The upregulation of Cat B was also confirmed by
representative western blot analysis using the proteins extracted
from the tissues (Fig. 1B).
Furthermore, several HCC cell lines were selected to detect the
expression of Cat B and the results revealed that the expression of
Cat B was highest in the HepG2 cells and lowest in the BEL-7402
cells (Fig. 1C). Taken together,
these data suggested that Cat B was overexpressed in the HCC
tissues and cell lines.
Overexpression of Cat B promotes HCC cell
proliferation
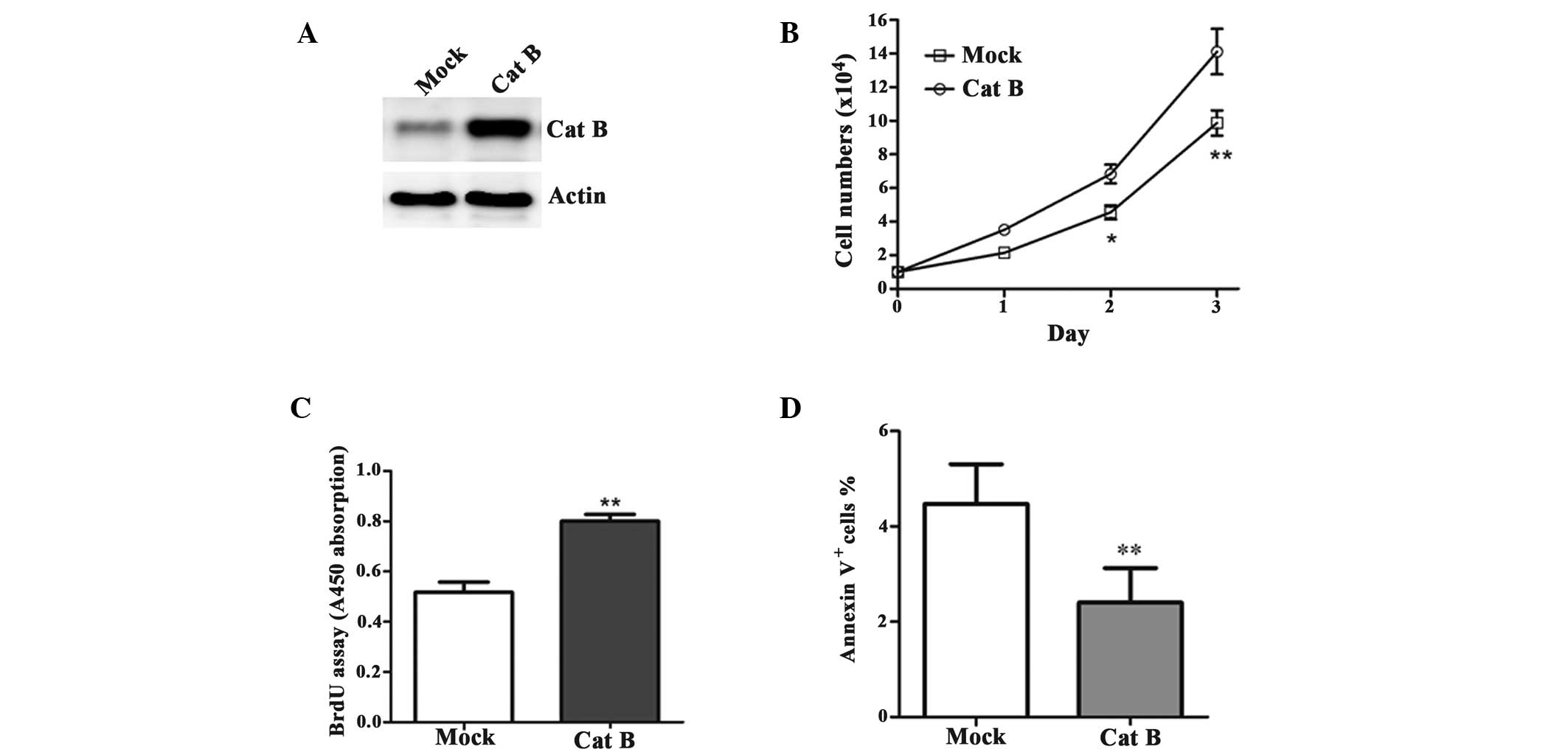
To determine the effect of Cat B on HCC cell growth,
the BEL-7402 cells were transfected with either the pcDNA3-Cat B or
pcDNA3-vector (mock; Fig. 2A).
Cell growth was significantly increased in the Cat B-overexpressed
cells compared with their corresponding controls (Fig. 2B). In addition, the cells
overexpressing Cat B had a high rate of proliferation (Fig. 2C) and the Annexin V/PI analysis of
the Cat B-overexpressed cells revealed a significant decrease in
the rate of apoptosis (Fig.
2D).
Cat B-knockdown inhibits HCC cell
proliferation
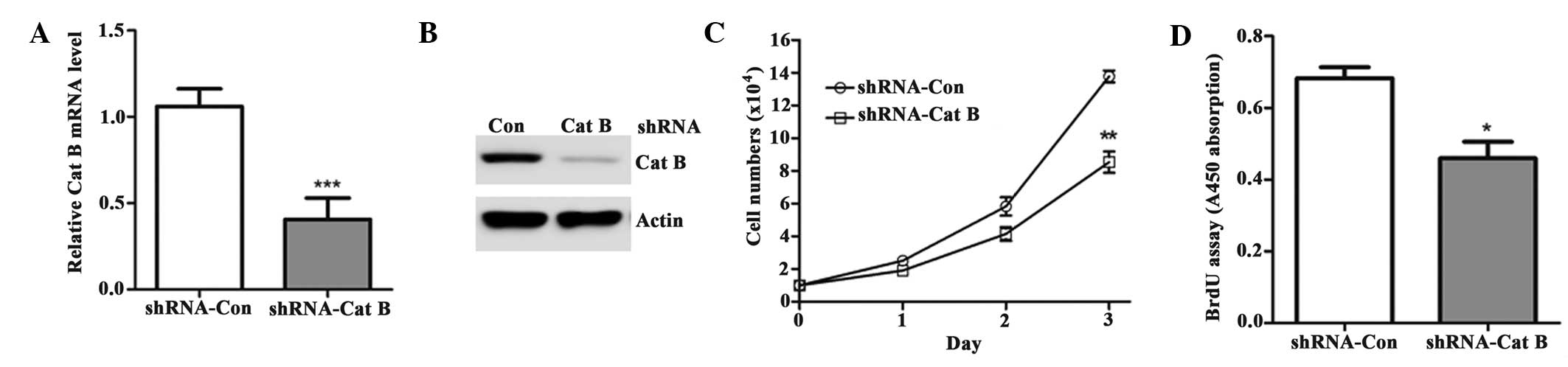
The upregulation of Cat B observed in the present
study prompted further investigation to determine the effect of its
knock down on HCC cell growth. The HepG2 cells were infected with
either a lentivirus targeting Cat B (shRNA-Cat B) or a negative
control (shRNA-Ctrl). As shown in Fig.
3A–B, endogenous Cat B was significantly downregulated by
shRNA-Cat B. Consequently, cell growth was significantly decreased
in the cells transfected with shRNA-Cat B compared with shRNA-Con
(Fig. 3C). These cells also had a
lower rate of proliferation (Fig.
3D).
Cat B-knockdown inhibits HCC growth in
vivo
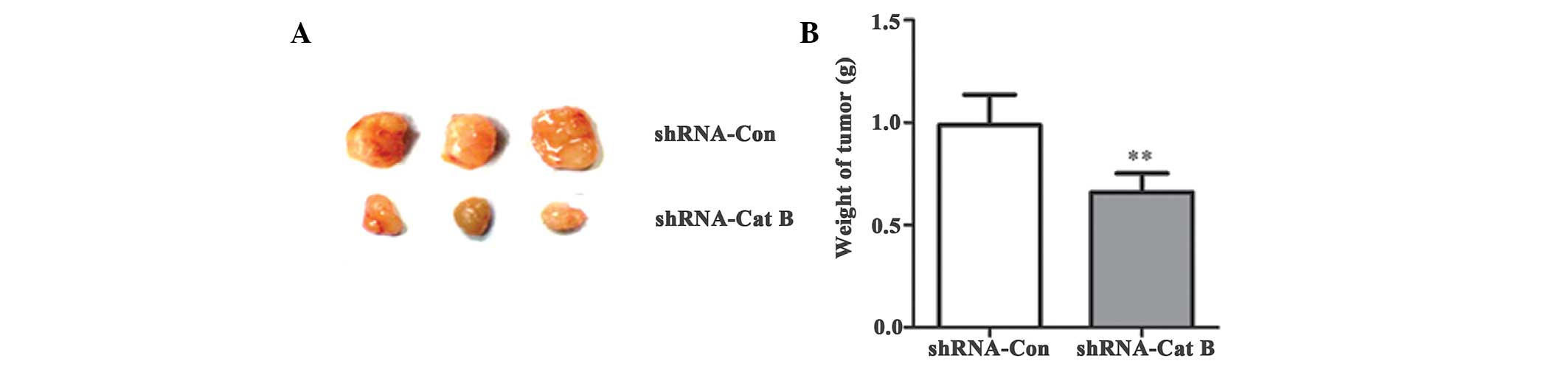
To further determine the role of Cat B knockdown,
HepG2 cells with stable knock down of Cat B or negative controls
were injected subcutaneously under the front legs of the nude mice
and tumor growth was closely monitored for 4 weeks. The results
demonstrated that the tumor size and weight were markedly reduced
following Cat B knockdown compared with the control (Fig. 4A–B), suggesting that Cat B
inhibition suppresses HCC growth in vivo.
Integrin αvβ3 is essential for the
progression of Cat B-induced HCC
It has been demonstrated that Cat B promotes tumor
progression, not only by proteolytic function, but also by a series
of signal transduction pathways (10,11).
Modulation of the PI3K signaling pathway is important in tumor
growth. Therefore, in the present study, this intracellular
signaling pathway was analyzed in HCC cells by manipulating the
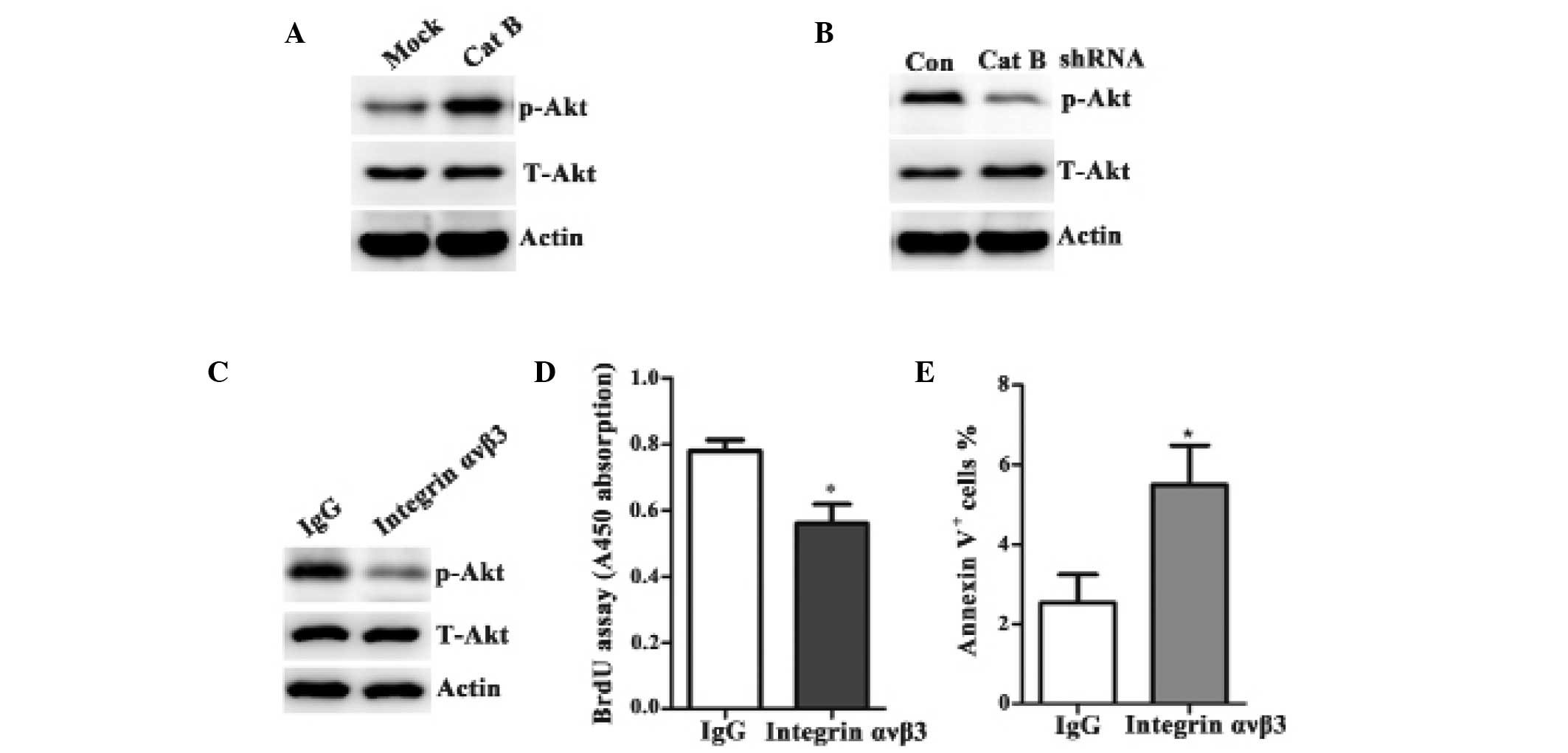
levels of Cat B. As shown in Fig. 5A
and B, the total level of Akt level was unaltered despite the
varying levels of Cat B. However, the phosphorylation of Akt was
markedly increased in the cells overexpressing Cat B compared with
the control vector-transfected cells (Fig. 5A). By contrast, the phosphorylation
of Akt was markedly decreased in the shRNA-Cat B cells compared
with the shRNA-control cells (Fig.
5B).
Our previous study demonstrated that the expression
of integrin αvβ3 was higher than normal in primary liver cancer and
that antisense integrin αvβ3 suppresses the growth of
subcutaneously implanted HCC by inhibiting tumor angiogenesis
(12). Additionally, it has been
suggested that the interaction between integrins and extracellular
components is important in tumor differentiation and progression
(13). Therefore, the present
study hypothesized that Cat B was involved in the progression of
hepatoma through integrin αvβ3 to ultimately affect the
phosphorylation of Akt. To verify this hypothesis, BEL-7402 cells
overexpressing pcDNA3-Cat B were pretreated with either an integrin
αvβ3 inhibitory antibody or with an isotype-matched control,
immunoglobulin G1. The main components of the PI3K signaling
pathway were then detected by western blot analysis. Inhibiting
integrin αvβ3 significantly prevented the Cat B-overexpressing
phosphorylation of Akt (Fig. 5C),
limited Cat B-induced proliferation (Fig. 5D) and led to the apoptosis of Cat
B-overexpressing cells (Fig. 5E).
Taken together, these results demonstrated that Cat B regulated HCC
growth by integrin αvβ3 and subsequently by the PI3K/Akt signaling
pathway.
Discussion
Deregulation of cysteine cathepsins functions,
including Cat B and Cat L, is associated with a number of disease
states, including cancer (5,6,14).
Cat B is a 40–45 kD cysteine protease, composed of a dimer of
disulfide-linked heavy and light chains that are produced from a
single protein precursor. It is initially synthesized on the rough
endoplasmic reticulum, further modified through glycosylation and
sulfation in the Golgi complex and recognized by the
mannose-6-phosphate receptor into lysosomes. Accumulating studies
have demonstrated that Cat B is important in a several types of
tumor. For example, Withana et al observed that Cat B
inhibition limited bone metastasis in breast cancer (15) and Wu et al found that Cat B
may be a potential biomarker in cervical cancer (16). The present study demonstrated that
Cat B is overexpressed in HCC. Notably, Cat B was observed to
promote the progression of HCC, including the enhancement of cell
proliferation and the inhibition of cell apoptosis.
Akt is an important effector kinase, which relays
signaling downstream of the PI3K pathway (17). It regulates a variety of cellular
processes, including cell growth, survival, differentiation,
migration and invasion. Akt activation inhibits cell apoptosis and
promotes cancerous growth and invasion (18). Upon ligand binding to receptor
tyrosine kinases or G protein-coupled receptors, the activation of
Akt is initiated by membrane recruitment, via interaction of its
pleckstrin homology domain with the phospholipid
phosphatidylinositol (3,4,5)-trisphosphate. Subsequent to membrane
recruitment, Akt is sequentially phosphorylated at threonine 308 by
pyruvate dehydrogenase lipoamide kinase isozyme-1 and at serine 473
by mammalian target of rapamycin complex 2 (19,20).
Following this, the phosphorylated Akt translocates from the plasma
membrane to the intracellular compartments, including the cytoplasm
and nucleus where it leads to the phosphorylation of its
substrates. In the present study, phosphorylated Akt notably
increased in the cells overexpressing Cat B compared with the
control vector-transfected cells. By contrast, phosphorylated Akt
decreased markedly in the cells with RNA interference-mediated
knock down of Cat B compared with the control RNAi-transfected
cells. Thus, these data indicated that Cat B regulated the
progression of HCC through the PI3K/Akt signaling pathway.
Cat B is an extracellular molecule and it has been
observed that the interaction of Cat B with urokinase plasminogen
activator (uPA), activates the uPA receptor (uPAR) to promote
cancer cell invasion and migration, as well as angiogenesis
(21,22). It is well established that uPAR has
no intracellular domain, therefore, it may exert its signaling
capacity through interactions with other components of the plasma
membrane, including G protein-coupled receptors, receptor tyrosine
kinases and integrins (13). The
present study confirmed that integrin αvβ3 is essential in the
signal transduction of Cat B into PI3K/Akt. In conclusion, the
results demonstrated that Cat B positively regulated the
progression of HCC, which included accelerating cell growth and
inhibiting cell apoptosis. Furthermore, the present study
demonstrated that Cat B promoted the progression of HCC via the
PI3K/Akt signaling pathway and found that integrin αvβ3 was
essential for the activation of Cat B-mediated PI3K/Akt activation
and the progression of HCC. Therefore, the present study identified
the effect of and the mechanism underlying Cat B in HCC, providing
new potential for HCC therapy in the future.
Acknowledgements
This study was supported by the Natural Science
Foundation of Shandong Province (grant no. ZR2012HL05).
References
|
1
|
Chen JG, Chen WQ, Zhang SW, Zheng RS, Zhu
J and Zhang YH: Incidence and mortality of liver cancer in China:
An analysis on data from the National Registration System between
2003 and 2007. Zhonghua Liu Xing Bing Xue Za Zhi. 33:547–553.
2012.(In Chinese). PubMed/NCBI
|
|
2
|
Turk V, Kos J and Turk B: Cysteine
cathepsins (proteases) - on the main stage of cancer? Cancer Cell.
5:409–410. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Keppler D, Sameni M, Moin K, Mikkelsen T,
Diglio CA and Sloane BF: Tumor progression and angiogenesis:
cathepsin B & Co. Biochem Cell Biol. 74:799–810. 1996.
View Article : Google Scholar
|
|
4
|
Zhang H, Zhong C, Shi L, Guo Y and Fan Z:
Granulysin induces cathepsin B release from lysosomes of target
tumor cells to attack mitochondria through processing of bid
leading to Necroptosis. J Immunol. 182:6993–7000. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gocheva V and Joyce JA: Cysteine
cathepsins and the cutting edge of cancer invasion. Cell Cycle.
6:60–64. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mohamed MM and Sloane BF: Cysteine
cathepsins: multifunctional enzymes in cancer. Nat Rev Cancer.
6:764–775. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vasiljeva O, Reinheckel T, Peters C, Turk
D, Turk V and Turk B: Emerging roles of cysteine cathepsins in
disease and their potential as drug targets. Curr Pharm Des.
13:387–403. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jain M, Bakhshi S, Shukla AA and Chauhan
SS: Cathepsins B and L in peripheral blood mononuclear cells of
pediatric acute myeloid leukemia: potential poor prognostic
markers. Ann Hematol. 89:1223–1232. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nouh MA, Mohamed MM, El-Shinawi M, Shaalan
MA, Cavallo-Medved D, Khaled HM and Sloane BF: Cathepsin B: a
potential prognostic marker for inflammatory breast cancer. J
Transl Med. 9:12011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tummalapalli P, Spomar D, Gondi CS,
Olivero WC, Gujrati M, Dinh DH and Rao JS: RNAi-mediated abrogation
of cathepsin B and MMP-9 gene expression in a malignant meningioma
cell line leads to decreased tumor growth, invasion and
angiogenesis. Int J Oncol. 31:1039–1050. 2007.PubMed/NCBI
|
|
11
|
Malla R, Gopinath S, Alapati K, Gondi CS,
Gujrati M, Dinh DH, Mohanam S and Rao JS: Downregulation of uPAR
and cathepsin B induces apoptosis via regulation of Bcl-2 and Bax
and inhibition of the PI3K/Akt pathway in gliomas. PLoS One.
5:e137312010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li J, Tan H, Dong X, et al: Antisense
integrin alphaV and beta3 gene therapy suppresses subcutaneously
implanted hepatocellular carcinomas. Dig Liver Dis. 39:557–565.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eden G, Archinti M, Furlan F, Murphy R and
Degryse B: The urokinase receptor interactome. Curr Pharm Des.
17:1874–1889. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Premzl A, Zavasnik-Bergant V, Turk V and
Kos J: Intracellular and extracellular cathepsin B facilitate
invasion of MCF-10A neoT cells through reconstituted extracellular
matrix in vitro. Exp Cell Res. 283:206–214. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Withana NP, Blum G, Sameni M, et al:
Cathepsin B inhibition limits bone metastasis in breast cancer.
Cancer Res. 72:1199–1209. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu D, Wang H, Li Z, et al: Cathepsin B may
be a potential biomarker in cervical cancer. Histol Histopathol.
27:79–87. 2012.
|
|
17
|
Franke TF, Yang SI, Chan TO, Datta K,
Kazlauska A, Morrison DK, Kaplan DR and Tsichlis PN: The protein
kinase encoded by the Akt proto-oncogene is a target of the
PDGF-activated phosphatidylinositol 3-kinase. Cell. 81:727–736.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bellacosa A, Kumar CC, Di Cristofano A and
Testa JR: Activation of AKT kinases in cancer: implications for
therapeutic targeting. Adv Cancer Res. 94:29–86. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Manning BD and Cantley LC: AKT/PKB
signaling: navigating downstream. Cell. 129:1261–1274. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sarbassov DD, Guertin DA, Ali SM and
Sabatini DM: Phosphorylation and regulation of Akt/PKB by the
rictor-mTOR complex. Science. 307:1098–1101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Victor BC, Anbalagan A, Mohamed MM, Sloane
BF and Cavallo-Medved D: Inhibition of cathepsin B activity
attenuates extracellular matrix degradation and inflammatory breast
cancer invasion. Breast Cancer Res. 13:R1152011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gupta R, Nalla AK, Gogineni VR, et al:
uPAR/cathepsin B overexpression reverse angiogenesis by rescuing
FAK phosphorylation in uPAR/cathepsin B down regulated meningioma.
PLoS One. 6:e171232011. View Article : Google Scholar : PubMed/NCBI
|