Introduction
Ovarian cancer is one of the most common female
cancers and is associated with a high mortality rate, due to its
high malignancy and the difficulties associated with its treatment.
It remains to be elucidated how ovarian cancer metastasizes and
regulates migration, survival, and growth (1). Previous studies have reported that
cancer stem cells may participate in cancer development (2). Furthermore, it has been identified
that several genes may be indicators of cancer stem cells, and
regulate tumor propagation and invasion (3).
CD44 has been identified as an indicator of cancer
stem cells. It is a widely expressed protein that binds hyaluronic
acid, resulting in cell-cell and cell-matrix adhesion (4,5).
This function of CD44 may provide information regarding its
association with tumor progression. Previous studies have suggested
that numerous tumor types and cancer stem cells express CD44
(6,7). Notably, CD44 transcripts have complex
alternative splice forms that contribute to different isoforms and
are associated with different functions (8,9). In
the intestine, CD44v has been shown to induce gut adenoma
differentiation, whereas CD44s showed no such function (10). The expression of the CD44s isoform
has been widely studied; however, the functions of other isoforms,
including CD44v in tumor progression, remain unknown. Among all of
the CD44v isoforms, it has previously been shown that CD44v6 may
bind hepatocyte growth factor (HGF), osteopontin and cytokines,
which are key factors of the tumor microenvironment (11). In addition, CD44v6 has a central
role in cell migration and invasion. Orian-Rousseau et al
(12,13) showed that CD44v6 mediated the MET
and HGF signaling pathway. CD44v6 has also been identified as a
downstream factor of Wnt signaling, that may stimulate the
β-catenin/Tcf-4 signaling pathway (14,15).
Todaro et al (16) showed
that CD44v6 is a marker of constitutive and reprogrammed cancer
stem cells associated with colon cancer metastasis. These studies
suggest that CD44v6 may be a marker for predicting cancer
metastasis. However, whether the mechanism of CD44v6 is universal
in different tumor types remains to be determined.
Transforming growth factor (TGF)-β is a factor that
is expressed in numerous developmental pathways, and has been shown
to have key roles in apoptosis, the cell cycle and the immune
system (17). Inhibition of TGF-β
expression may be a prospective method for cancer therapy, since
TGF-β stimulation has been shown to induce tumorigenesis and
metastasis (18). Therefore,
understanding the regulation of the TGF-β signaling pathways may
provide novel ways for the treatment of malignant tumors.
Previously, TGF-β has been considered to be a protein which
promotes carcinogenic progression. Simultaneously, Wnt/β-catenin, a
signaling pathway upstream of CD44v6, has also been shown to
participate in tumor progression (19). Inhibition of the Wnt/β-catenin
pathway may stimulate tumorigenesis and angiogenesis. This may be
due to the stabilization of β-catenin by Wnt, which results in the
activation of TCF/LEF family transcription factors (20). Determining the effects of CD44v6 on
TGF-β and Wnt/β-catenin may provide further understanding of the
mechanism, and give indicators of therapeutic targets, in ovarian
cancer.
In the present study, it was hypothesized that an
abnormal CD44v6 expression may result in ovarian cancer aggression.
By transfection of a small hairpin (sh)RNA specifically targeting
CD44v6 expression, it was demonstrated that CD44v6 promoted
β-catenin and TGF-β expression and induced aggression in ovarian
cancer cells. These results may provide a potential therapeutic
target for ovarian cancer.
Materials and methods
Materials
The clinical samples used in the present study were
supplied by the Xiangya School of Medicine, Central South
University (Changsha, China). The samples were obtained during
surgery and the characteristics of the patients are detailed in
Table I. Informed consent was
provided and approval was obtained from the Institutional Review
Board of the Xiangya School of Medicine Research Ethics Committee
(Changsha, China). The CAOV3 ovarian cancer cells were obtained
from American Type Culture Collection (Manassas, VA, USA).
 | Table ICorrelation between CD44v6 expression
levels and clinicopathological factors of the 60 patients with
ovarian cancer. |
Table I
Correlation between CD44v6 expression
levels and clinicopathological factors of the 60 patients with
ovarian cancer.
| CD44v6
expression |
|---|
|
|
|---|
| Characteristics | Low (0,1) | High (2,3) | P-valuea |
|---|
| Age |
| Years, mean ±
SD | 59.23 ± 7.80 | 55.42 ± 10.30 | 0.75 |
| Gender |
| Male | 14 | 14 | 0.53 |
| Female | 16 | 16 | |
| Smoking status |
| Yes | 13 | 18 | 0.48 |
| No | 13 | 16 | |
| Stage |
| I + II | 18 | 10 | 0.01 |
| III + IV | 7 | 25 | |
| Tumor status |
| T1–T2 | 15 | 18 | 0.01 |
| T3–T4 | 15 | 12 | |
| Lymph node
metastasis |
| N0 | 15 | 5 | 0.03 |
| N1–N3 | 9 | 21 | |
| Distal metastasis
status |
| M0 | 23 | 10 | 0.02 |
| M1 | 11 | 16 | |
| Recurrence
status |
| Yes | 8 | 24 | 0.00 |
| No | 23 | 5 | |
Rabbit antibodies against human CD44v6 (HPA005785),
β-Catenin (C2206), TGF-β (SAB4502954) and β-actin (AV40173) were
all purchased from Sigma-Aldrich (St. Louis, MO, USA). The
secondary antibodies, conjugated with horseradish peroxidase,
against rabbit Immunoglobulin G (sc-2030) were purchased from Santa
Cruz Biotechnology Inc., (Dallas, TX, USA).
A CD44v6 interference vector was constructed to
analyze and compare the biophysical properties of knockdown and
over-expression of CD44v6, in ovarian cancer cells. The recombinant
expression CD44v6 shRNA plasmid was purchased from Santa Cruz
Biotechnology Inc. (sc-62576-SH). The recombinant expression
plasmid expressing CD44v6 was then constructed. The CD44v6
fragments were inserted into the plasmid pcDNA3.1(t) (Invitrogen
Life Technologies, Carlsbad, CA, USA) between the XhoI and
BamHI restriction sites, and the recombinant plasmids
pc3.1(t)-CD44v6 were constructed, according to the manufacturer’s
instructions. The cells were transfected with pcDNA3.1(t)-CD44v6
and/or CD44v6 shRNA plasmid using Lipofectamine® 2000
(Invitrogen Life Technologies). The cells were randomly divided
into three groups (five parallel treatments for each group):
Control, non-treated group; pcDNA3.1 control group, no CD44v6
fragment was inserted into the plasmid; and CD44v6 interference
group, 1 μg CD44v6 shRNA plasmid transfection. Following a 24 h
transfection, the cells were harvested and used for the following
experiments.
Quantitative polymerase chain reaction
(qPCR)
Total RNA was extracted from the ovarian cancer and
adjacent normal tissues using TRIzol® reagent
(Invitrogen Life Technologies), according to the manufacturer’s
instructions, and stored at −80°C until further use. A total of 1
μg RNA was reverse transcribed into first-strand cDNA using
SuperScript II Reverse Transcriptase (Invitrogen Life
Technologies). The qPCR was performed using the Applied Biosystems
7500 Real-Time PCR System (Life Technologies, Grand Island, NY,
USA) in a 50 ml reaction volume, containing iQ™ SYBR®
Green Supermix (BioRad Laboratories, Hercules, CA, USA), 100 nM
primers and 20 ng cDNA template. The parameters of the PCR
reactions were as follows: 94°C for 3 min for one cycle, then 94°C
for 30 sec, 60°C for 30 sec, 72°C for 45 sec for 40 cycles, and
72°C for 5 min for one cycle. Following the amplification, the PCR
products were assayed using a dissociation curve, to verify single
product generation. The relative gene expression was calculated
with the SDS 1.3 software on the Applied Biosystems 7500 Real-Time
PCR System, using the comparative cycle threshold (Ct) method
(2−ΔΔCt).
Western blot analysis
Following incubation, the cells of each group were
collected by centrifugation at 15,000 rpm for 15 min and then lysed
in radioimmunoprecipitation assay buffer on ice. The extracted
protein samples were loaded onto 12% SDS-PAGE gels, separated by
electrophoresis and then transferred onto polyvinylidene fluoride
membranes (GE Healthcare Life Sciences, Piscataway, NJ, USA). The
membranes were blocked with 5% skimmed milk for 30 min, and then
incubated with the primary antibodies overnight, at 4°C. The
membranes were washed with phosphate-buffered saline three times
and then incubated with the horseradish peroxidase conjugated
secondary antibody for 1 h, at room temperature. The protein bands
were detected using the chemiluminescence system SuperSignal West
Pico Chemiluminescent Substrate (GE Healthcare Life Sciences,
Piscataway, NJ, USA). Three independent experiments were repeated
to assess the relative protein levels.
Cell invasion assay
A fluorescence Transwell assay was used to determine
cell invasion. The cells were inserted into an 8 μm pore-size PET
membrane (GE Healthcare Life Sciences), and cultured at 4°C
overnight. The cells were then harvested and labeled with the
fluorescent dye Calcein AM (GE Healthcare Life Sciences). The
results were measured using a Beckman DU-8 UV-spectrophotometer
(Eppendorf, Hamburg, Germany), at a wavelength of 494 nm/517
nm.
Migration assay
The number of BrdU-positive cells per 1 mm length
was examined and used to define the cell density. The initial
dissector frame was randomly positioned in the cell and the mean of
10 areas was recorded as the number of migrated cells.
Statistical analysis
The data is expressed as the means ± standard error
of the mean. To determine differences between the groups, the data
were analyzed by a one-way analysis of variance. The normality and
constant variance for experimental data were tested by the Levene’s
test. A Kaplan-Meier survival curves were produced to examine
survival rates. Kaplan-Meiers was defined as the time from
randomization to mortality from any cause. The data underwent
logarithmic transformation to meet the necessary assumptions of
analysis of variance, if the data did not have homogenous variance.
Fisher’s exact test was used to analyze data. The statistical tests
were performed using SPSS version 13.0 software (SPSS Inc.,
Chicago, IL, USA). A P<0.05 was considered to indicate a
statistically significant difference.
Results
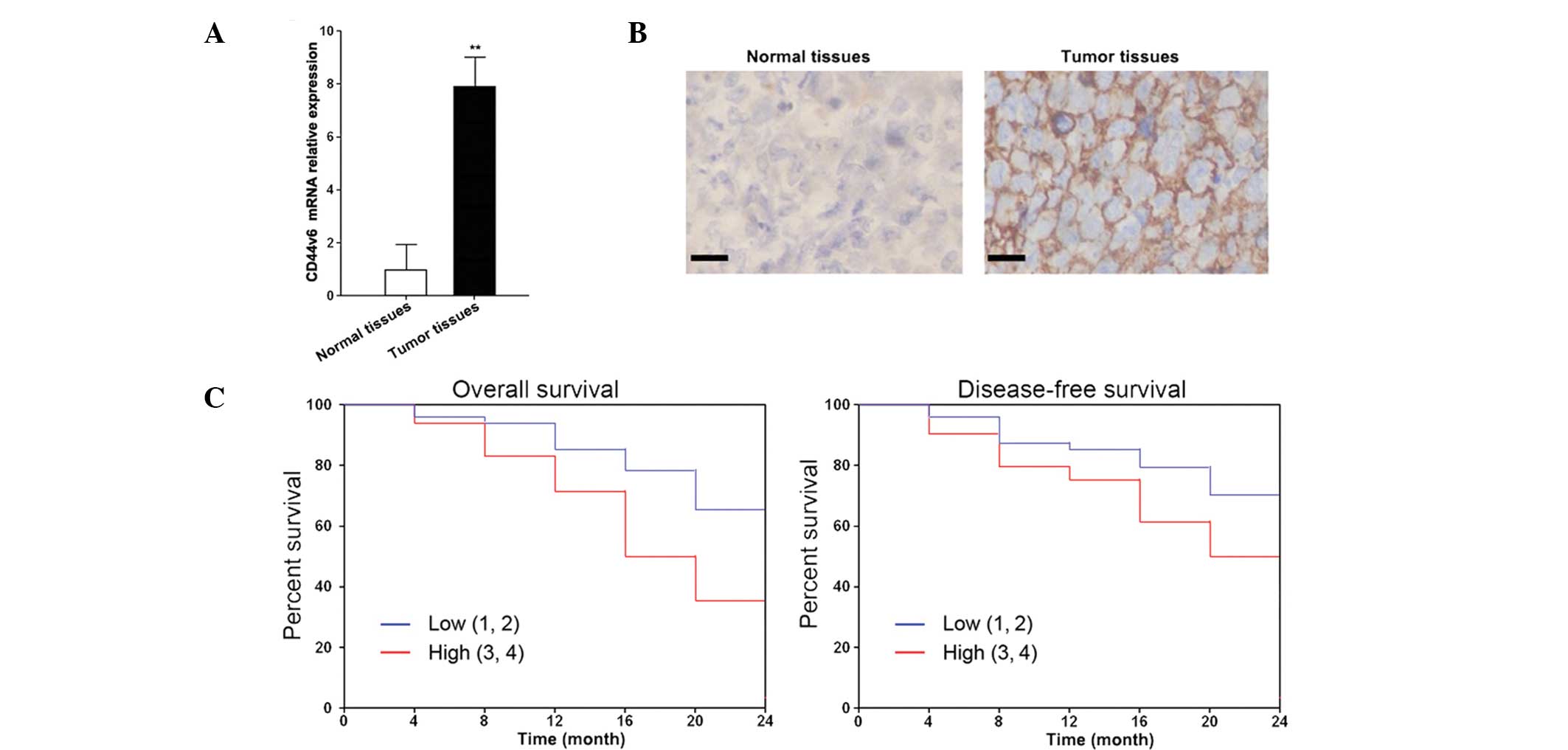
The expression levels of CD44v6 are
higher in ovarian cancer tissues
The relative mRNA expression levels of CD44v6 in
ovarian cancer tissues were determined by qPCR. A total of 60 tumor
samples and their respective adjacent normal tissues were tested.
Out of the 60 tumor samples, 55 had significantly higher expression
levels of CD44v6, as compared with the adjacent normal tissues
(Fig. 1A). Furthermore, the
upregulated expression levels of CD44v6 were correlated with
metastasis and recurrence (Table
I). The protein expression levels of CD44v6 were higher in the
ovarian cancer tissues, as compared with the adjacent normal
tissues (Fig. 1B). The
Kaplan-Meier survival curves showed that CD44v6 expression was
negatively correlated with overall and disease-free survival rates
(Fig. 1C). The high expression
CD44v6 samples, with relative expression levels between 3 and 4,
had higher overall and disease-free survival rates, as compared
with the low expression CD44v6 samples, with relative expression
levels between 1 and 2.
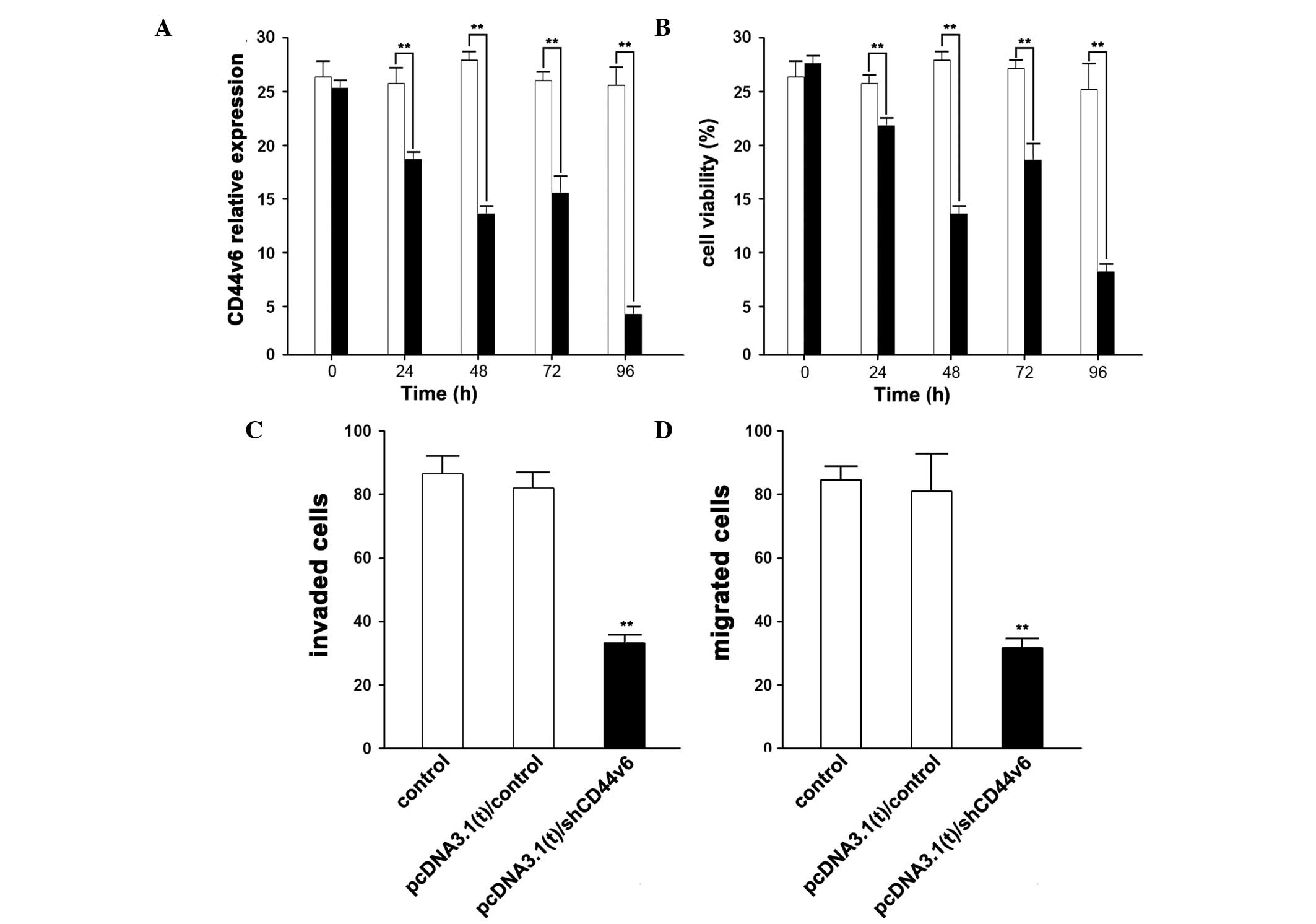
Knockdown of CD44v6 expression in ovarian
cancer cells
The higher expression levels of CD44v6 may induce
ovarian cancer; therefore, the present study aimed to determine
whether knockdown of CD44v6 expression had an effect on ovarian
cancer progression. Following the knockdown of CD44v6 expression by
shCD44v6 vector transfection, the expression levels of CD44v6 were
significantly suppressed, in a time-dependent manner (Fig. 2A).
Simultaneously, following shCD44v6 vector
transfection, the cell-growth and invasive abilities of CAOV3 cells
were reduced(Fig. 2B,C,D). The
cell viability decreased in a time-dependent manner, which is
similar to the reduction in the expression levels of CD44v6. The
cells were assayed 96 h post-transfection with the shCD44v6 vector,
and the invasive and migratory abilities of the CAOV3 cells were
shown to decrease significantly.
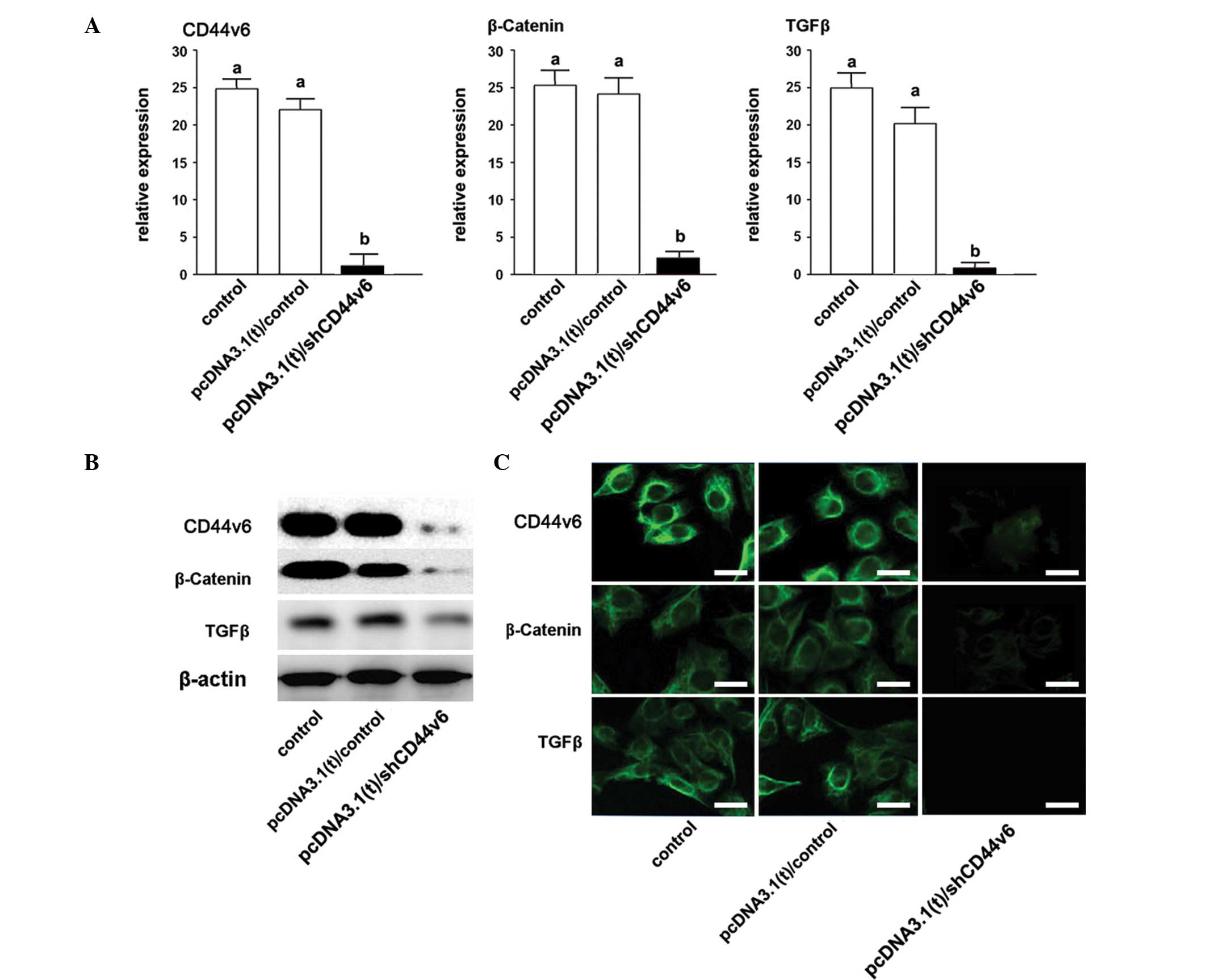
Depression of Wnt/β-catenin and TGF-β by
CD44v6 expression knockdown in ovarian cancer cells
The β-catenin and TGF-β expression levels were also
assayed 96 h post-transfection. The qPCR showed that the mRNA
expression levels of both β-catenin and TGF-β were significantly
decreased, following a knockdown of CD44v6 expression (Fig. 3A). In addition, the protein
expression levels of β-catenin and TGF-β, following a knockdown of
CDv446 expression, were shown to be reduced, as determined by
western blotting and immunofluorescence.
Discussion
The results of the present study suggest that the
expression of CD44v6 is increased in ovarian cancer tissues
obtained from patients, and the knockdown of CD44v6 expression may
result in depression of tumor metastases and cell invasion.
Previously, CD44v6 has been shown to be involved in the progression
of numerous types of cancer, including stomach, prostate, lung and
colon (21–26). In the present study, the CD44v6
expression levels were increased in ovarian cancer tissues, which
indicates that it may have a potential effect on ovarian cancer
development and aggression. In addition, the CD44v6 expression
levels were correlated with overall and disease-free survival
rates, suggesting that CD44v6 may be an indicator and a potential
therapeutic target of ovarian cancer. Notably, other isoforms of
CD44, including CD44s and CD44v3 have converse expression levels,
as compared with CD44v6 (13,27).
The aim of the present study was to determine how the expression of
CD44v6 affects the progression and metastasis of ovarian cancer.
Therefore the viability, invasion and migration of CAOV3 cells were
determined, to understand the effects of CD44v6 on ovarian cancer
cells.
Following a shCD44v6 vector transfection into CAOV3
cells, the CD44v6 expression levels were significantly inhibited.
Furthermore, the metastasis of the CAOV3 cells was depressed. These
results suggest that the knockdown of CD44v6 expression may affect
ovarian cancer cell growth and metastatic ability. The invasive
ability of CAOV3 also decreased significantly, following knockdown
of CD44v6 expression. It has previously been reported that CD44v6
expression may promote the aggression of ovarian cancer cells. Shi
et al (28) demonstrated
that CD44v6, but not CD44s, had a higher expression in ovarian
serous cancer, as compared with primary tumor tissues. Todaro et
al (16) showed that
colorectal cancer stem cells expressed CD44v6 and the expression of
CD44v6 was necessary for the migration and metastasis of cancer
stem cells. It was also indicated that CD44v6 expression levels
were higher in cancer stem cells, as compared with primary tumor
cells. These results suggest that the metastatic process of ovarian
and colorectal cancer cells may be induced by CD44v6, and it may be
required for maintenance of cancer stem cells, which support cancer
progression (29). In the present
study, the expression levels of CD44v6 were shown to promote the
occurrence and development of ovarian cancer. Therefore, it may be
hypothesized that the metastatic process of ovarian cancer is
initiated by cancer stem cells, via CD44v6. CD44v6 may be a
potential indicator of diagnosis and prognosis as well as a
therapeutic target. However, the mechanisms of CD44v6 promotion
remain to be elucidated.
TGF-β has previously been identified as a key factor
in early and late tumor development (30). The results of the present study
suggest that TGF-β signaling may be regulated by CD44v6 in ovarian
cancer cells. In previous studies, TGF-β has been suggested as a
promoter for increasing epithelial-mesenchymal transition and
metastasis in numerous types of cancer, such as colon, ovarian,
stomach, prostate and lung (30,31).
These results indicate that TGF-β may induce tumor progression at
both early and late stages. Besides the TGF-β signaling pathway,
β-catenin is another pathway associated with cancer cell invasion.
β-catenin is located in the plasma membrane and binds E-cadherin;
it also has a crucial role in adherens junctions (32,33).
β-catenin, when located in the nucleus, also participates in the
Wnt signaling pathway. The translocation of β-catenin at the
subcellular level may be activated by the Wnt signaling pathway
(19). The present study reported
that knockdown of CD44v6 expression resulted in the decreased
expression levels of both TGF-β and β-catenin.
In conclusion, the present study indicated that the
upregulated expression levels of CD44v6 in ovarian cancer cells may
contribute to its pathology. Furthermore, knockdown of CD44v6
expression affected the progression of CAOV3 ovarian cancer cells.
The knockdown of CD44v6 expression may downregulate the expression
of β-catenin and TGF-β. These results indicate that CD44v6 may be a
potential therapeutic target in ovarian cancer.
Acknowledgements
The present study was funded by the National Natural
Sciences Foundation of China (no. 21135001).
References
|
1
|
Naora H and Montell DJ: Ovarian cancer
metastasis: integrating insights from disparate model organisms.
Nat Rev Cancer. 5:355–366. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jordan CT, Guzman ML and Noble M: Cancer
stem cells. N Engl J Med. 355:1253–1261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Visvader JE and Lindeman GJ: Cancer stem
cells in solid tumours: accumulating evidence and unresolved
questions. Nat Rev Cancer. 8:755–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lesley J, Hyman R and Kincade PW: CD44 and
its interaction with extracellular matrix. Adv Immunol. 54:271–335.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aruffo A, Stamenkovic I, Melnick M,
Underhill CB and Seed B: CD44 is the principal cell surface
receptor for hyaluronate. Cell. 61:1303–1313. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li C, Heidt DG, Dalerba P, et al:
Identification of pancreatic cancer stem cells. Cancer Res.
67:1030–1037. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takaishi S, Okumura T, Tu S, et al:
Identification of gastric cancer stem cells using the cell surface
marker CD44. Stem Cells. 27:1006–1020. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Günthert U, Stauder R, Mayer B, Terpe HJ,
Finke L and Friedrichs K: Are CD44 variant isoforms involved in
human tumour progression? Cancer Surv. 24:19–42. 1995.PubMed/NCBI
|
|
9
|
Rall CJ and Rustgi AK: CD44 isoform
expression in primary and metastatic pancreatic adenocarcinoma.
Cancer Res. 55:1831–1835. 1995.PubMed/NCBI
|
|
10
|
Chen GY and Wang DR: The expression and
clinical significance of CD44v in human gastric cancers. World J
Gastroenterol. 6:125–127. 2000.
|
|
11
|
Orian-Rousseau V, Chen L, Sleeman JP,
Herrlich P and Ponta H: CD44 is required for two consecutive steps
in HGF/c-Met signaling. Gene Dev. 16:3074–3086. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Orian-Rousseau V, Morrison H, Matzke A, et
al: Hepatocyte growth factor-induced Ras activation requires ERM
proteins linked to both CD44v6 and F-actin. Mol Biol Cell.
18:76–83. 2007. View Article : Google Scholar :
|
|
13
|
Orian-Rousseau V: CD44, a therapeutic
target for metastasising tumours. Eur J Cancer. 46:1271–1277. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cruz MC, Pereira AL, Lopes FF, et al:
Immunohistochemical expression of E-cadherin and CD44v6 in squamous
cell carcinomas of the lower lip and tongue. Braz Dent J. 20:64–69.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cheng XX, Wang ZC, Chen XY, et al:
Frequent loss of membranous E-cadherin in gastric cancers: A
cross-talk with Wnt in determining the fate of beta-catenin. Clin
Exp Metastasis. 22:85–93. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Todaro M, Gaggianesi M, Catalano V, et al:
CD44v6 is a marker of constitutive and reprogrammed cancer stem
cells driving colon cancer metastasis. Cell Stem Cell. 14:342–356.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yingling JM, Blanchard KL and Sawyer JS:
Development of TGF-beta signalling inhibitors for cancer therapy.
Nat Rev Drug Discov. 3:1011–1022. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Siegel PM, Shu W, Cardiff RD, Muller WJ
and Massagué J: Transforming growth factor beta signaling impairs
Neu-induced mammary tumorigenesis while promoting pulmonary
metastasis. Proc Natl Acad Sci USA. 100:8430–8435. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yao H, Ashihara E and Maekawa T: Targeting
the Wnt/β-catenin signaling pathway in human cancers. Expert Opin
Ther Targets. 15:873–887. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Daniels DL and Weis WI: Beta-catenin
directly displaces Groucho/TLE repressors from Tcf/Lef in
Wnt-mediated transcription activation. Nat Struct Mol Biol.
12:364–371. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yamamichi K, Uehara Y, Kitamura N, Nakane
Y and Hioki K: Increased expression of CD44v6 mRNA significantly
correlates with distant metastasis and poor prognosis in gastric
cancer. Int J Cancer. 79:256–262. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Misra S, Ghatak S, Patil N, et al: Novel
dual cyclooxygenase and lipoxygenase inhibitors targeting
hyaluronan-CD44v6 pathway and inducing cytotoxicity in colon cancer
cells. Bioorg Med Chem. 21:2551–2559. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yanamoto S, Yamada S, Takahashi H, et al:
Expression of the cancer stem cell markers CD44v6 and ABCG2 in
tongue cancer: effect of neoadjuvant chemotherapy on local
recurrence. Int J Oncol. 44:1153–1162. 2014.PubMed/NCBI
|
|
24
|
Wang H, Rana S, Giese N, Büchler MW and
Zöller M: Tspan8, CD44v6 and alpha6 beta4 are biomarkers of
migrating pancreatic cancer-initiating cells. Int J Cancer.
133:416–426. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Afify AM, Tate S, Durbin-Johnson B, Rocke
DM and Konia T: Expression of CD44s and CD44v6 in lung cancer and
their correlation with prognostic factors. Int J Biol Markers.
26:50–57. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Klatte T, Seligson DB, Rao JY, et al:
Absent CD44v6 expression is an independent predictor of poor
urothelial bladder cancer outcome. J Urol. 183:2403–2408. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Crispn JC, Keenan BT, Finnell MD, et al:
Expression of CD44 variant isoforms CD44v3 and CD44v6 is increased
on T cells from patients with systemic lupus erythematosus and is
correlated with disease activity. Arthritis Rheum. 62:1431–1437.
2010. View Article : Google Scholar
|
|
28
|
Shi Jm, Zhou Z, Wen Di and Li N:
Correlation of CD44v6 expression with ovarian cancer progression
and recurrence. BMC Cancer. 13:1822013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zöller M: CD44: can a cancer-initiating
cell profit from an abundantly expressed molecule? Nat Rev Cancer.
11:254–267. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Katsuno Y, Lamouille S and Derynck R:
TGF-β signaling and epithelial-mesenchymal transition in cancer
progression. Curr Opin Oncol. 25:76–84. 2013. View Article : Google Scholar
|
|
31
|
Fuxe J and Karlsson MC: TGF-β-induced
epithelial-mesenchymal transition: a link between cancer and
inflammation. Semin Cancer Biol. 22:455–461. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zantek ND, Azimi M, Fedor-Chaiken M, Wang
B, Brackenbury R and Kinch MS: E-cadherin regulates the function of
the EphA2 receptor tyrosine kinase. Cell Growth Differ. 10:629–638.
1999.PubMed/NCBI
|
|
33
|
Nelson WJ and Nusse R: Convergence of Wnt,
beta-catenin, and cadherin pathways. Science. 303:1483–1487. 2004.
View Article : Google Scholar : PubMed/NCBI
|