Introduction
Diabetic retinopathy (DR) is a common complication
of diabetes mellitus (DM) (1,2).
High glucose inflicts oxidative damage on cells of the retinal
pigment epithelium (RPE) and causes pathological changes, which are
responsible for the loss of vision associated with DR (3). Overproduction of reactive oxygen
species (ROS) contributes to oxidative stress-induced cell injury
(4). ROS may mediate RPE cell
dysfunction and lead to hyperglycemic complications in diabetes
(5). It has been reported that ROS
generation is responsible for cell damage in cultured ARPE-19 cells
(6) and that high glucose
increases the generation of ROS (7); therefore, to protect the retina from
oxidative damage, intracellular ROS production in RPE cells must be
inhibited.
In previous years, research into the biological
activities of marine organisms has intensified. The effect of
fucoidan from the seaweed Fucus vesiculosus on the molecular
mechanisms underlying numerous diseases has been investigated. It
exhibits potent antioxidant activities and has effects on processes
involved in cancer and inflammation (8,9). Due
to the potential of fucoidan as an anti-oxidant, anti-cancer and
anti-inflammatory agent, it was the aim of the present study to
determine the protective effect of fucoidan on DR. In the present
study, the hypothesis that fucoidan protects ARPE-19 cells against
high glucose-induced oxidative damage was assessed for the first
time, to the best of our knowledge. The effect of fucoidan on high
glucose-induced ROS generation, apoptosis, Ca2+ influx
and extracellular signal regulated kinase (ERK1/2) phosphorylation
in ARPE-19 cells was investigated. The observations of the present
study indicated that fucoidan may represent novel therapeutic agent
for the treatment of DR.
Materials and methods
ARPE-19 cell culture
The ARPE-19 cell line was purchased by the American
Type Culture Collection (Manassas, VA, USA). ARPE-19 cells were
cultured in Dulbecco’s modified Eagle’s medium/nutrient mixture F12
(DMEM/F12 medium; Life Technologies, Grand Island, NY, USA) with
penicillin (final concentration, 100 U/ml)-streptomycin (final
concentration, 100 μg/ml) (Gibco-BRL, Grand Island, NY, USA) and
10% fetal bovine serum (Moregate Biotech Co., Ltd., Bulimba,
Australia) at 37°C in a 5% CO2 atmosphere. Fresh
conditioned medium was added every third day and subcultures were
digested every seventh to eighth day with 0.25% trypsin
(Gibco-BRL).
Cytotoxicity of fucoidan
Cell Titer 96® AQueous One Solution cell
proliferation assay (Promega, Madison, WI, USA), which is an
effective assay to demonstrate cytotoxicity, was used to determine
the cytotoxicity of fucoidan. According to the manufacturer’s
instructions, ARPE-19 cells at a concentration of 2×104
cells/well were inoculated and incubated in 96-well plates with
conditioned DMEM/F12 medium at 37°C under a 5% CO2
atmosphere for 2 days. The ARPE-19 cells were treated with
different concentrations of fucoidan (0, 1, 10, 100 and 1,000
μg/ml) for another 24 h, followed by addition of 20 μl CellTiter
96® AQueous One Solution cell proliferation assay
solution into all 96 wells. ARPE-19 cells were further incubated
for 1 h and the absorbance at 490 nm was measured by an MTP-800
microplate reader (Corona Electric, Tokyo, Japan).
MTT assay
ARPE-19 cells were cultured at 37°C under a 5%
CO2 atmosphere for 2 days and then exposed to high
D-glucose (30 mM; Melone Biomart, Dailan, China) in the presence or
absence of fucoidan (Sigma-Aldrich, Shanghai, China; 100 μg/ml) for
a further 24 h. The viability of ARPE-19 cells was determined using
a colorimetric MTT assay Sigma-Aldrich) according to a previously
described method (3). Absorbance
at 550 nm was determined using the MTP-800 microplate reader. The
absorbance at 690 nm was also measured to compensate for any
interfering effects of cell debris and the microtiter plate. The
percentage of viable cells was calculated as optical density (OD)
of the treated sample/OD of untreated control × 100%.
Lactate dehydrogenase (LDH) assay
As a result of cytotoxicity, the reduction in the
number of viable cells measured using an MTT assay may have been
the result of inhibition of cell proliferation or cellular damage,
finally leading to cell death. To determine cellular damage, LDH
release into the medium was measured using an LDH assay kit
(Sigma-Aldrich). The rate of LDH release (%) was expressed as the
proportion of LDH released into the medium compared with the total
quantity of LDH present in ARPE-19 cells treated with 2% Triton
X-100 (Sigma-Aldrich). LDH was monitored as the oxidation of
reduced nicotinamide adenine dinucleotide (NADH) at 530 nm. The
cellular damage (%) was determined using the equation
[(OD530 of the treated group - OD530 of the
control group)/(OD530 of the Triton X-100-treated group
- OD530 of the control group)] × 100%.
Microscopy imaging of ARPE-19 cells
ARPE-19 cells were seeded at a density of
5×104 cells/ml in 24-well plates. Following culturing
for 2 days, ARPE-19 cells were exposed to high D-glucose (30 mM) in
the presence or absence of fucoidan (100 μg/ml) for another 24 h.
The microscopy images were prepared using an automatic microscope
(IX70; Olympus, Tokyo, Japan) and a digital CCD camera (Pixera, Los
Gatos, CA, USA). ARPE-19 cells were counted and images were
captured under a phase-contrast microscope (CX22; Olympus, Tokyo,
Japan) in a high-power field (objective lens magnification,
×200).
Apoptosis assay
Apoptosis staining was performed using an Annexin V
(cell apoptosis signaling component)-fluorescein
isothiocyanate-propidium iodide (FITC-PI) apoptosis kit according
to the manufacturer’s instructions (BioVision, Mountain View, CA,
USA). The ARPE-19 cells were grown in a six-well plate at
104 cells/well and were pretreated with high glucose (30
mM) in the presence or absence of fucoidan (100 μg/ml). Stained
cells were analyzed using FACSCalibur™ flow cytometer (BD
Biosciences, San Jose, CA, USA) with Cell-Quest software (version
1.2, Diva 6.1). A total of 10,000 events were collected for each
sample.
Detection of intracellular ROS
Intracellular accumulation of ROS was estimated
using the fluorescent dye H2-DCFDA (Sigma-Aldrich),
which is converted to a membrane-impermeable and highly fluorescent
compound, dichlorofluorescein diacetate (DCF), in the cell in the
presence of ROS (13). ARPE-19
cells were exposed to high glucose (30 mM) in the presence or
absence of fucoidan (150 μM) for 24 h and were then rinsed with
serum-free DMEM/F12 medium and incubated with 5 μM
H2-DCFDA for 60 min at 37°C. The cells were examined
under a fluorescence microscope (C1-T-SM; Nikon, Tokyo, Japan).
Subsequently, the cells were collected with 0.25% trypsin and
analyzed using a fluorescence spectrophotometer (F-2500; Hitachi,
Tokyo, Japan) to detect the fluorescence of DCF inside the cells
(excitation wavelength, 488 nm; emission wavelength, 521 nm).
Measurement of cytoplasmic
Ca2+ influx
Ca2+ imaging was performed as described
previously (10). ARPE-19 cells
(2×106 cells/ml) pre-treated with high glucose (30 mM)
for 24 h in the presence or absence of fucoidan (100 μg/ml) were
loaded with calcium-sensitive Fura 2-AM (1 μM) in
Ca2+-free buffer (Hank’s balanced salt solution
containing 20 mM HEPES and 1% bovine serum albumin, pH 7.4;
Sigma-Aldrich) for 30 min at 37°C according to the manufacturer’s
instructions of the Calcium Kit-Fura 2 (Dojindo Laboratories,
Kumamoto, Japan). Adenosine triphosphate (ATP; 10 μM) was added
directly to the cell suspension after a 3-min baseline recording.
Recordings were made using an F-2500 calcium imaging system from FL
Solutions (Hitachi, Tokyo, Japan) that calculated the ratio of
fluorescent signals obtained at 37°C with excitation wavelengths of
340 and 380 nm and an emission wavelength of 510 nm. The excitation
wavelengths at 380 and 340 nm were used to measure the free Fura-2
and the Ca2+-bound Fura-2, respectively. The fluorescent
activities at 340/500 nm (F1) and of 380/500 nm (F2) as well as the
ratio (R) of F1 to F2 were recorded using the spectrophotometer at
the indicated times. The Ca2+ concentration (C) was then
calculated using the following formula: C = 224 × R, where 224 is
the Kd number.
Western blot analysis
Electrophoresis was performed using a vertical slab
gel with a 12% polyacrylamide content (Sigma-Aldrich) according to
the method described previously (11). Transfer of proteins from the
SDS-PAG to a membrane was performed electrophoretically according
to the method described previously (12) with certain modifications using a
Semi-Dry Electroblotter (Sartorious AG, Göttingen, Germany) for 90
min with an electric current of 15 V. The membrane was treated with
Block Ace™ (4%; Sigma-Aldrich) for 30 min at 22°C. The first
reaction was performed using a rabbit polyclonal immunoglobulin
(Ig) G antibody against unmodified protein or against
phosphorylated protein of ERK1/2 (100 ng/ml; Proteintech Group,
Inc., Chicago, IL, USA) in phosphate-buffered saline containing
0.03% Tween 20 (Haoranbio Biomart, Shanghai, China) for 1 h at
22°C. Following washing in the same buffer, the second reaction was
performed using horseradish peroxidase-conjugated anti-rabbit goat
IgG (20 ng/ml at proper dilution with 1X Tris-buffered saline and
Tween 20; Proteintech Group, Inc.) for 30 min at 22°C. Following
washing, the enhanced chemiluminescence (ECL) reaction was
performed on the membrane using the ECL Plus western blotting
detection system™ (Prime Western Blotting Detection Reagent Kit and
Image Quant 400; GE Healthcare, Tokyo, Japan).
Statistical analysis
Values are expressed as the mean ± standard
deviation. Each experiment was repeated at least three times.
Student’s t-test was used and P<0.05 was considered to indicate
a statistically significant difference. Analyses were performed
using SPSS version 19.0 (IBM SPSS, Armonk, NY, USA).
Results
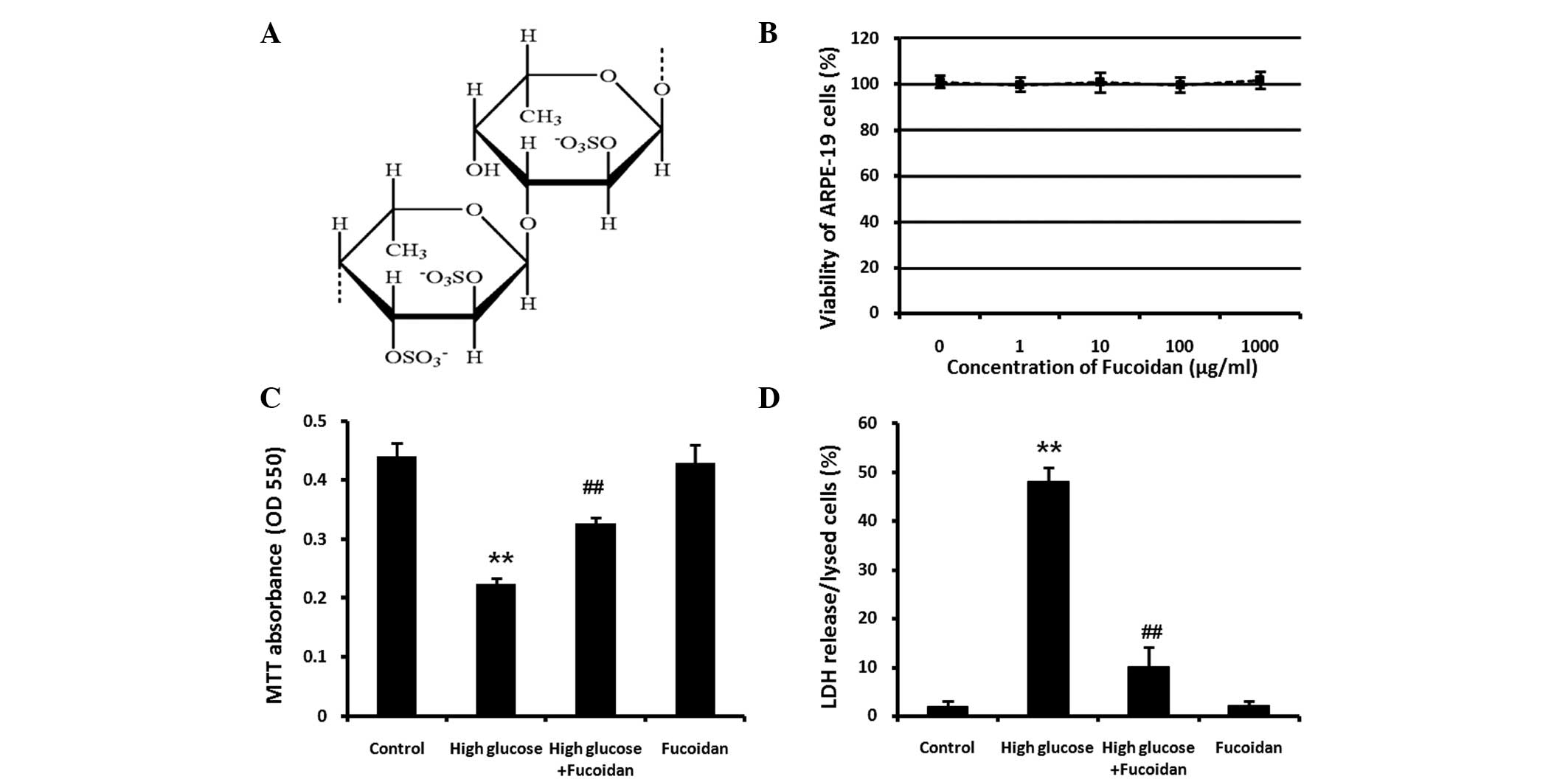
Fucoidan is not cytotoxic to ARPE-19
cells
CellTiter 96® AQueous One Solution Cell
Proliferation Assay was used to determine the cytotoxicity of
fucoidan. At the concentrations used in the present study (0, 1,
10, 100 and 1,000 μg/ml), fucoidan did not have any marked
cytotoxic effect on ARPE-19 cells (Fig. 1B). In subsequent experiments,
fucoidan was used at a concentration of 100 μg/ml study to
investigate its protective effect against high glucose-induced cell
death.
Protective effect of fucoidan against
high glucose-induced cell death
To investigate the protective effect of fucoidan on
ARPE-19 cells against high glucose-induced cell death, ARPE-19
cells exposed to high D-glucose (30 mM) in the presence or absence
of fucoidan (100 μg/ml) for 24 h. The viability of ARPE-19 cells
was determined using a colorimetric MTT assay (Fig. 1C). As the reduction in the number
of viable cells measured using an MTT assay may be due to
inhibition of cell proliferation or cellular damage finally leading
to cell death, cellular damage was also assessed by detecting LDH
release into the medium using an LDH assay kit (Fig. 1D). Furthermore, ARPE-19 cells were
counted and images were captured under a phase-contrast microscope
(Fig. 2A and B). The results of
the abovementioned assays revealed that ARPE-19 cells underwent
apoptosis following treatment with 30 mM glucose (P<0.01), while
the non-toxic fucoidan significantly protected ARPE-19 cells from
high glucose-induced cell death (P<0.01). Fucoidan treatment
alone did not affect the growth of normal ARPE-19 cells.
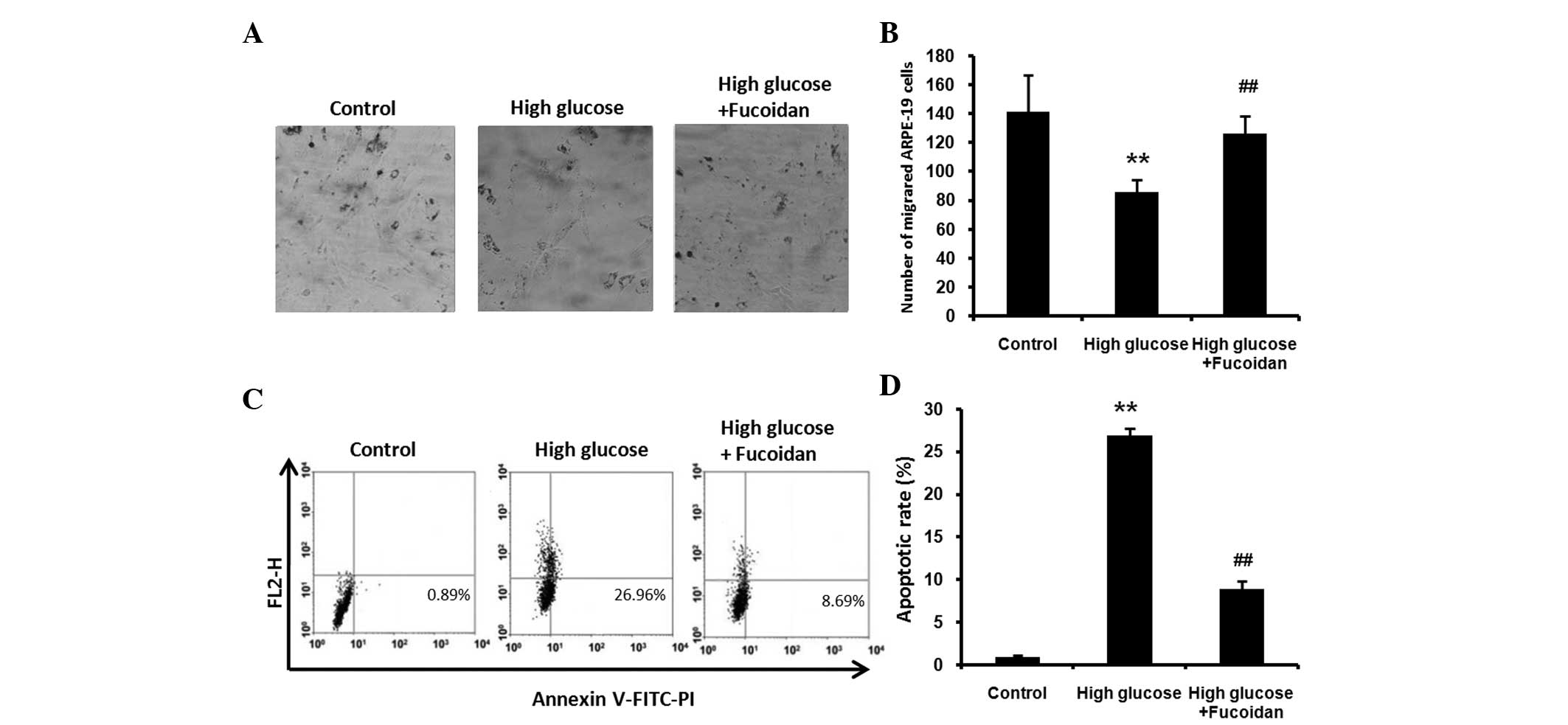
Fucoidan inhibits high glucose-induced
apoptosis in ARPE-19 cells
To examine whether fucoidan protects against high
glucose-induced apoptosis, ARPE-19 cells exposed to high glucose
(30 mM) in the presence or absence of fucoidan (100 μg/ml) for 24
h. Flow cytometric analysis was used to quantify the apoptotic rate
using staining with Annexin V-FITC-PI. The cells exposed to high
glucose exhibited an increase in the apoptotic rate compared with
that of untreated control cells (Fig.
2C and D; P<0.01), while fucoidan significantly reduced the
percentage of apoptotic cells (P<0.01).
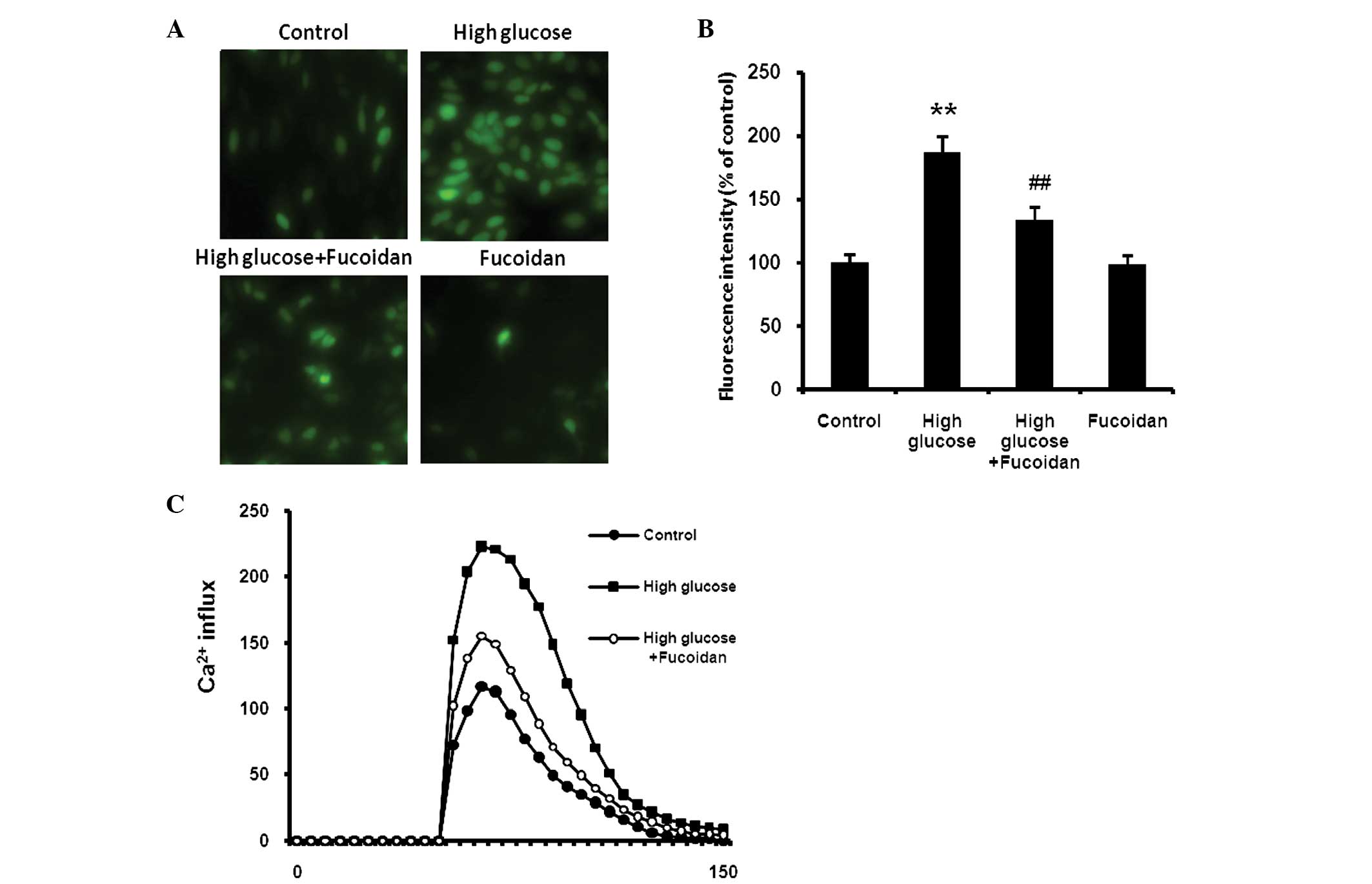
Fucoidan inhibits high glucose-induced
ROS generation
ARPE-19 cells were exposed to high glucose (30 mM)
in the presence or absence of fucoidan (100 μg/ml) for 24 h.
Subsequently, the effect of fucoidan on glucose-induced generation
of ROS in ARPE-19 cells assessed using H2-DCFDA, a
fluorescent ROS indicator. The intracellular ROS generation was
increased in high glucose-exposed cells relative to that in
unexposed control cells. However, this increase in intracellular
ROS was abrogated by treatment with fucoidan (Fig. 3A and B; P<0.01).
Fucoidan inhibits high glucose-mediated
Ca2+ influx in ARPE-19 cells
ARPE-19 cells pretreated with high glucose (30 mM)
in the presence or absence of fucoidan (100 μg/ml) were loaded with
calcium-sensitive Fura 2-AM (1 μM) in Ca2+-free buffer
and ATP (10 μM) was placed directly into the cell suspension after
a 3-min baseline recording. The cells exposed to high glucose
exhibited an increase in Ca2+ influx compared with
untreated control cells. Fucoidan significantly reduced the
increased Ca2+ influx (Fig.
3C).
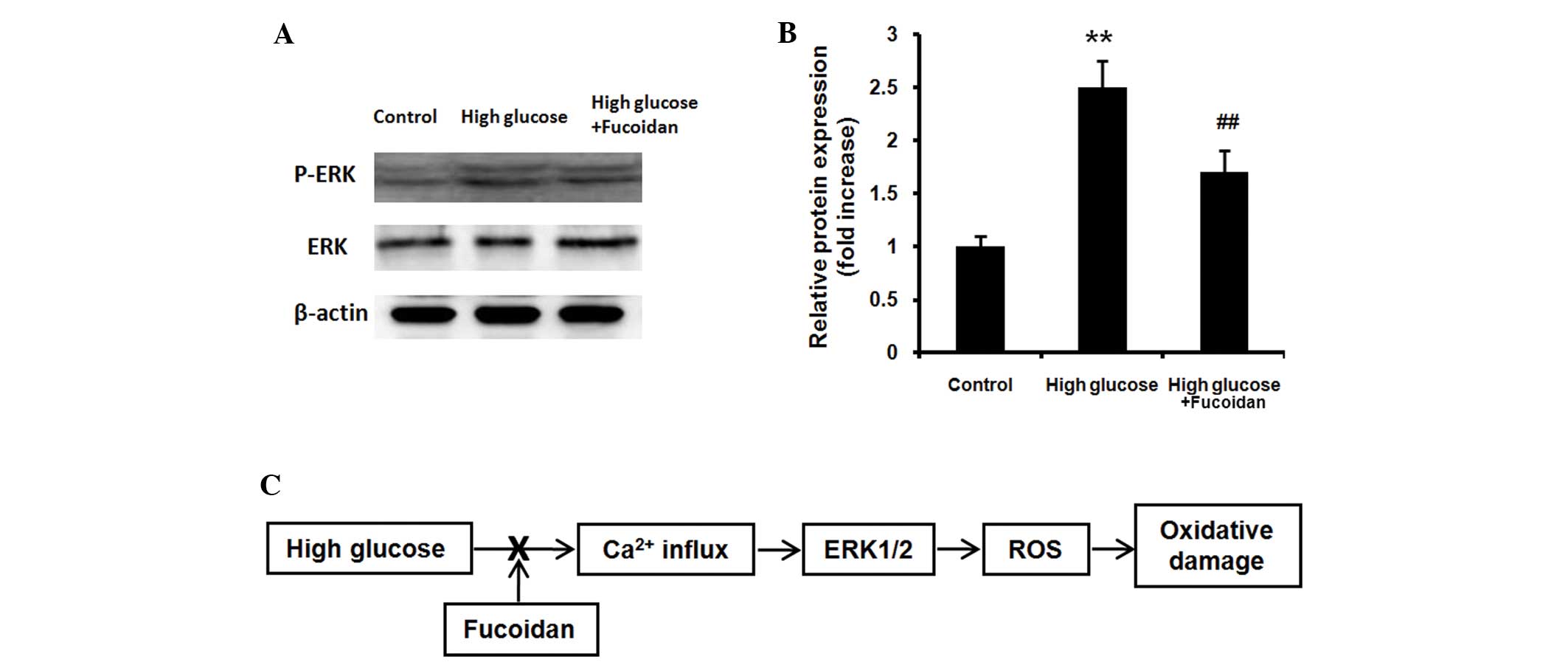
Fucoidan inhibits high glucose-mediated
ERK phosphorylation
ARPE-19 cells exposed to high glucose (30 mM) in the
presence or absence of fucoidan (100 μg/ml) for 24 h. The
phosphorylation of ERK1/2 protein was examined using western blot
analysis. High glucose increased the levels of
activated/phosphorylated ERK1/2 (Fig.
4A and B; P<0.01), while fucoidan significantly reduced the
ERK1/2 phosphorylation induced by high glucose (P<0.01).
Discussion
The present study demonstrated for the first time,
to the best of our knowledge, the beneficial effect of fucoidan on
DR, through protecting ARPE-19 cells against high glucose-induced
oxidative damage via inhibition of ROS generation through a
Ca2+-dependent ERK1/2 signaling pathway. DR is a common
complication of DM and is a main cause of loss of vision (1,2). The
the loss of vision associated with this disease is caused by
oxidative damage inflicted on RPE cells in the presence of high
glucose (3). The RPE is a highly
specialized retinal cell layer, which has important roles in the
regulation and development of photoreceptors in the vertebrate
retina (14). RPE cells function
in transporting and stocking materials, such as retinaldehyde, and
phagocytosing detached photoreceptor outer segments, intercepting
light, removing free radicals, synthesizing cytokines and forming
the blood-retina barrier (10).
The integrity of the RPE is of key importance for maintaining the
integrity and proper functioning of the human retina (15).
In previous years, interest in the biological
activities of marine organisms has intensified. Fucoidan, from the
seaweed Fucus vesiculosus, has been shown to exhibit potent
antioxidant activities and has effects on molecular mechanisms
involved in cancer and inflammation (8). Fucoidan suppresses various
inflammatory cytokines, including interleukin-1β, tumor necrosis
factor-α, interferon-γ and cyclooxygenase-2 (9). In the present study, an MTT assay
indicated a significant decrease in the number of viable ARPE-19
cells following treatment with high glucose. Fucoidan, which was
not cytotoxic to ARPE-19 cells, exerted a significant protective
effect on ARPE-19 cells against high glucose-mediated toxicity. As
the LDH release data were almost inversely identical to the results
of the MTT assay, the glucose-induced toxicity in the present study
was not due to an inhibition of cell proliferation, but a result of
cellular damage leading to cell death.
A high concentration of glucose induced
dysregulation of the electron transport chain activity and caused
increased electron slippage, resulting in overproduction of toxic
ROS (16). The ROS overproduction
contributed to oxidative stress-induced cell injury (4). The vascular and multiorgan
complications in DM are associated with high glucose-induced ROS
overproduction (17). It has been
reported that ROS may mediate RPE cell dysfunction and lead to
hyperglycemic complications in diabetes (5), and ROS generation is responsible for
cell damage in cultured ARPE-19 cells (6). Therefore, to protect the retina from
oxidative damage, it is important to protect RPE cells via
preventing intracellular ROS production.
The RPE provides an ideal environment for the
accumulation of ROS, which in turn leads to mitochondrial
dysfunction and apoptosis (6,7,18).
In normal living cells, phosphatidylserine only exists on the
cytosolic side of the lipid bilayer of the cell membrane, but in
the early phase of cell apoptosis, phosphatidylserine flips to the
extracellular side of the membrane. Annexin V, a type of
Ca2+-dependent phosphatide-conjugated protein, has a
high affinity to and thus binds to phosphatidylserine in early
apoptotic cells (19). Therefore,
Annexin V was used for detecting apoptosis in the present study,
and the results indicated that fucoidan prevented ARPE-19 cells
from entering high glucose-induced apoptosis, which was consistent
with the results of the MTT and LDH assays.
Consistent with the theories and results presented
in the present study, a previous study on ROS generation
demonstrated that high glucose led to an increase in ROS generation
in ARPE-19 cells (20). The
present study reported that fucoidan inhibited the generation of
ROS, indicating that fucoidan offers distinct protection against
high glucose-induced oxidative damage. To better understand the
protective mechanism of fucoidan on ARPE-19 cells, it was
investigated whether fucoidan may inhibit high glucose-induced
increase of Ca2+ influx in ARPE-19 cells. It was
observed that, as an early signaling factor, Ca2+ influx
was increased by high glucose in ARPE-19 cells, while fucoidan
effectively inhibited the increased Ca2+ influx.
Furthermore, it has been demonstrated that the
ERK1/2 pathway includes cytoplasmic Ca2+ influx
(21) and the activation of ERK1/2
may be involved in defense signaling against oxidative damage in
cells (22). ERK1/2 is the
signaling cascade involved in protection from oxidative damage and
its activation is generally hypothesized to mediate cell survival
(23). High glucose accelerates
the rate of oxidative phosphorylation and eventually causes a
change in redox signaling to activate the mitogen-activated protein
kinase pathway (24). In the
present study, western blot analysis indicated an increase in the
expression of phosphorylated ERK1/2 in high glucose-treated ARPE-19
cells, which was inhibited by fucoidan. Changes in the expression
of activated ERK1/2 closely reflect cell damage, suggesting that
high glucose-induced oxidative damage of ARPE-19 cells was mediated
by ERK1/2 activation. This was in agreement with the results on
Ca2+ influx, indicating that the Ca2+ influx
and ERK1/2 signaling are the upstream components activating ROS
generation.
As presented in the schematic in Fig. 4C, fucoidan inhibits high
glucose-induced oxidative damage via normalization of ROS
generation through the Ca2+-dependent ERK1/2 signaling
pathway in ARPE-19 cells. The Ca2+-dependent cascade is
a complex process and it has been reported that ROS themselves may
lead to transient and rapid activation of ERK (25). Therefore, although the protective
effect of fucoidan on ARPE-19 cells against high glucose-induced
oxidative damage was demonstrated, the complex underlying mechanism
requires further study.
In conclusion, the present study was the first, to
the best of our knowledge, to demonstrate that treatment with
fucoidan significantly protected ARPE-19 cells from high
glucose-induced oxidative damage via inhibition of ROS generation
through the Ca2+-dependent ERK1/2 signaling pathway. The
activity and mechanism of action of fucoidan are expected to
provide important information for the use of biological agents and
the development of novel clinical treatments of DR.
References
|
1
|
Wang J, Yang MM, Rong SS, Ng TK, Li YB and
Liu XM: Association of paraoxonase gene polymorphisms with diabetic
nephropathy and retinopathy. Mol Med Rep. 8:1845–1851.
2013.PubMed/NCBI
|
|
2
|
Lorenzi M and Gerhardinger C: Early
cellular and molecular changes induced by diabetes in the retina.
Diabetologia. 44:791–804. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yuan Z, Feng W, Hong J, Zheng Q, Shuai J
and Ge Y: p38MAPK and ERK promote nitric oxide production in
cultured human retinal pigmented epithelial cells induced by high
concentration glucose. Nitric Oxide. 20:9–15. 2009. View Article : Google Scholar
|
|
4
|
Yu T, Robotham JL and Yoon Y: Increased
production of reactive oxygen species in hyperglycemic conditions
requires dynamic change of mitochondrial morphology. Proc Natl Acad
Sci USA. 103:2653–2658. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu T, Sheu SS, Robotham JL and Yoon Y:
Mitochondrial fission mediates high glucose-induced cell death
through elevated production of reactive oxygen species. Cardiovasc
Res. 79:341–351. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Winkler BS, Boulton ME, Gottsch JD and
Sternberg P: Oxidative damage and age-related macular degeneration.
Mol Vis. 5:321999.PubMed/NCBI
|
|
7
|
Cai J, Nelson KC, Wu M, Sternberg P Jr,
Jones DP, et al: Oxidative stress and protection of the RPE. Prog
Retin Eye Res. 19:205–221. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Myers SP, O’Connor J, Fitton JH, Brooks L,
Rolfe M, Connellan P, Wohlmuth H, Cheras PA and Morris C: A
combined Phase I and II open-label study on the immunomodulatory
effects of seaweed extract nutrient complex. Biologics. 5:45–60.
2011.PubMed/NCBI
|
|
9
|
Li C, Gao Y, Xing Y, Zhu H, Shen J and
Tian J: Fucoidan, a sulfated polysaccharide from brown algae,
against myocardial ischemia-reperfusion injury in rats via
regulating the inflammation response. Food Chem Toxicol.
49:2090–2095. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nishiura H, Tokita K, Li Y, Harada K,
Woodruff TM, Taylor SM, Nsiama TK, Nishino N and Yamamoto T: The
role of the ribosomal protein S19 C-terminus in Gi
protein-dependent alternative activation of p38 MAP kinase via the
C5a receptor in HMC-1 cells. Apoptosis. 15:966–981. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Laemmli UK: Cleavage of structural
proteins during the assembly of the head of bacteriophage T4.
Nature. 227:680–685. 1970. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kyhse-Andersen J: Electroblotting of
multiple gels: a simple apparatus without buffer tank for rapid
transfer of proteins from polyacrylamide to nitrocellulose. J
Biochem Biophys Methods. 10:203–209. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rastogi RP, Singh SP, Häder DP and Sinha
RP: Detection of reactive oxygen species (ROS) by the
oxidant-sensing probe 2′,7′-dichlorodihydrofluorescein diacetate in
the cyanobacterium Anabaena variabilis PCC 793. Biochem Biophys Res
Commun. 397:603–607. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jackson GR, Owsley C and Curcio CA:
Photoreceptor degeneration and dysfunction in aging and age-related
maculopathy. Ageing Res Rev. 1:381–396. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zarbin MA: Age-related macular
degeneration: review of pathogenesis. Eur J Ophthalmol. 8:199–206.
1998.
|
|
16
|
Allen DA, Yaqoob MM and Harwood SM:
Mechanisms of high glucose-induced apoptosis and its relationship
to diabetic complications. J Nutr Biochem. 16:705–713. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brownlee M: Biochemistry and molecular
cell biology of diabetic complications. Nature. 414:813–820. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Giddabasappa A, Bauler M, Yepuru M, Chaum
E, Dalton JT and Eswaraka J: 17-β estradiol protects ARPE-19 cells
from oxidative stress through estrogen receptor-β, Invest.
Ophthalmol Vis Sci. 51:5278–5287. 2010. View Article : Google Scholar
|
|
19
|
Lee JE, Lee RA, Kim KH and Lee JH:
Induction of apoptosis with diallyl disulfide in AGS gastric cancer
cell line. J Korean Surg Soc. 81:85–95. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dong X, Li Z, Wang W, Zhang W, Liu S and
Zhang X: Protective effect of canolol from oxidative stress-induced
cell damage in ARPE-19 cells via an ERK mediated antioxidative
pathway. Mol Vis. 17:2040–2048. 2011.PubMed/NCBI
|
|
21
|
Castro R, Sun XH, Liu XB, Martinez JR and
Zhang GH: Inhibition of Ca2+ influx by surfactant in
NR8383 alveolar macrophages. Inflamm Res. 57:489–496. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Glotin AL, Calipel A, Brossas JY, Faussat
AM, Tréton J and Mascarelli F: Sustained versus transient ERK1/2
signaling underlies the antiand proapoptotic effects of oxidative
stress in human RPE cells, Invest. Ophthalmol Vis Sci.
47:4614–4623. 2006. View Article : Google Scholar
|
|
23
|
Guillonneau X, Régnier-Ricard F, Dupuis C,
Courtois Y and Mascarelli F: Paracrine effects of phosphorylated
and excreted FGF1 by retinal pigmented epithelial cells. Growth
Factors. 15:95–112. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cai L: Suppression of nitrative damage by
metallothionein in diabetic heart contributes to the prevention of
cardiomyopathy. Free Radic Biol Med. 41:851–861. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qin S, McLaughlin AP and De Vries GW:
Protection of RPE cells from oxidative injury by
15-deoxy-delta12,14-prostaglandin J2 by augmenting GSH and
activating MAPK. Invest Ophthalmol Vis Sci. 47:5098–5105. 2006.
View Article : Google Scholar : PubMed/NCBI
|