Introduction
Atherosclerosis and cardiovascular disease remain
one of the major causes of mortality worldwide. Ox-LDL has a
central proatherogenic role in the arterial wall (1–3). It
has a multitude of actions on vascular smooth muscle cells,
including inducing their migration and proliferation as well as
altering their phenotype to foam cells (4–6).
Ox-LDL also results in the generation of reactive oxygen species
(ROS) from vascular smooth muscle cells (7). Ox-LDL, through increasing ROS
production, leads to an increase in the generation of a variety of
growth factors, including fibroblast growth factor (8,9),
insulin-like growth factor-1 (10)
and epidermal growth factor (11)
as well as expression of their receptors, therefore inducing
vascular smooth muscle cell proliferation and hypertrophy. Ox-LDL
is also able to induce apoptosis in smooth muscle cells (12).
The (pro)renin receptor (PRR) constitutes a novel
component of the renin-angiotensin system (RAS) and has attracted
significant attention in previous years due to its versatile
functions (13). Numerous studies
have verified that when the renin precursor binds to its receptor,
it directly triggers angiotensin II independent reactions, which
may be potentially active in the development of atherosclerotic
plaques (14,15). The binding to PRR and the ability
to induce a signal transduction cascade independent of the
generation of angiotensin II (13,16),
including the activation of mitogen-activated protein kinase (MAPK)
(17) and enhancement of the
phosphorylation of extracellular signal-regulated kinase (ERK1/2)
(18), promotes fibrosis gene
expression, including transforming growth factor-β, plasminogen
activator inhibitor-1, fibronectin and collagen proteins (19,20).
However, to the best of our knowledge, there is little data
demonstrating the direct effect of the atherogenic condition on the
PRR.
A previous study by our group demonstrated that the
conditions of atherogenesis, including high glucose and high blood
lipids were able to upregulate the expression of PRR in cultured
human umbilical vein endothelial cells (21). Therefore, cultured human aortic
smooth muscle cells (HASMCs) were utilized to investigate the
effect of ox-LDL on the expression of PRR.
Materials and methods
Cell culture
HASMCs were obtained from Lifeline Cell Technology
(Beijing, China) and cultured according to the manufacturer’s
instructions. The cells were cultured in Vasculife SMC cell culture
medium, containing VascuLife basal medium and LifeFactors SMC (cat
no. LS-1040; Lifeline Cell Technology) at 37°C in an atmosphere of
95% air and 5% CO2 according to the manufacturer’s
instructions. Cells were seeded in six-well plates and the culture
medium was changed daily. Prior to each experimental treatment,
cells were serum starved for 24 h under serum-free conditions. The
concentration of human ox-LDL (24.5 nmoles of MDA/mg protein;
Qingdao Haicon Biotechnology Co., Ltd., Qingdao, China) was
primarily based on a previously published study demonstrating their
effectiveness in vascular smooth muscle cells (21). According to these results, 100
μg/ml ox-LDL was used to stimulate HASMCs for different time
periods, which were divided into 1, 2, 4, 6, 12 and 24 h subgroups.
Subsequently, the strongest expression time point of PRR was
selected and cells were treated with different concentrations of
ox-LDL, which were divided into 25, 50, 100, 150, 200 and 300
μg/ml subgroups. At the end of each experiment, cells were
harvested for the preparation of whole-cell lysates and total RNA
extraction.
Immunofluorescence
The expression of PRR in HASMCs was detected using
immunofluorescence staining. HASMCs were seeded at a density of
5×104 cells/ml into 24-well plates, cultivated and
divided into groups. At the end of culture, the HASMCs were fixed
with paraformaldehyde for 30 min. Cells were incubated with the
primary antibody (polyclonal rabbit anti-human ATP6IP2 antibody;
1:700 dilution; ab64975; Abcam, Cambridge, MA, USA) at 4°C
overnight. Following incubation with the fluorescein-labeled
secondary antibody (fluorescein isothiocyanate-conjugated goat
anti-rabbit immunoglobulin G; 1:200; sc-2012, Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) at 37°C for 40 min, HASMCs
were observed using fluorescence microscopy (DM4000 B LED; Leica,
Mannheim, Germany) and images were immediately captured. The same
process without the primary antibody and secondary antibody was
used as a negative control. Green fluorescence of the cell membrane
indicated positive expression of PRR.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted immediately from cultured
cells using TRIzol reagent (Gibco-BRL, Dalian, China) according to
the manufacturer’s instructions. Total RNA was reverse transcribed
using the Prime Script RT reagent kit with gDNA Eraser (Perfect
Real Time; Takara Bio Inc., Dalian, China). RT-qPCR was performed
using SYBR Premix Ex Taq™ II (Takara Bio Inc.) with the Roche
LightCycler® 480 Sequence Detection System (Roche
Diagnostics GmbH, Mannheim, Germany). Samples were run in
triplicate in separate tubes to permit quantification of the target
gene normalized to GAPDH, which was used for equal loading. Primer
sequences are shown in Table I.
The PCR amplification program was as follows: 95°C for 30 sec, 95°C
for 5 sec and 60°C for 20 sec for 40 cycles.
 | Table IPrimers used for reverse
transcription-quantitative polymerase chain reaction. |
Table I
Primers used for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Sequence (5′–3′) |
|---|
ATP6AP2
(NM_005765) |
| Forward |
TGGAAATTGGCCTATACCAGGAG |
| Reverse |
GTAGCCCGAGGACGATGAAAC |
GAPDH
(NM_002046) |
| Forward |
GCACCGTCAAGGCTGAGAAC |
| Reverse |
TGGTGAAGACGCCAGTGGA |
Western blot analysis
Cells were lysed in 100 μl of lysis buffer
(Nanjing KeyGen Biotech. Co., Ltd., Nanjing, China). Protein
concentrations were measured using a protein assay kit (Nanjing
KeyGen Biotech. Co., Ltd.). The protein (25 μg) obtained
from each lysate was electrophoresed on a 15% SDS-polyacrylamide
gel (90 mV, 30 min; 130 mV, 90 min) and transferred onto a
nitrocellulose membrane (Merck Millipore, Darmstadt, Germany) using
a Mini-PROTEAN 3 system (120 mV, 1.5 h; Bio-Rad, Hercules, CA,
USA). The membrane was incubated overnight with polyclonal rabbit
anti-human ATP6AP2 antibody (1:700 dilution; Abcam) and polyclonal
rabbit anti-human β-actin (1:500 dilution; bs-0061R; Bioss,
Beijing, China). The specific binding was detected using
horseradish peroxidase-labeled goat anti-rabbit IgG (1:7,500;
ZSGB-BIO, Beijing, China) and an enhanced chemiluminescence
detection kit (Perkin-Elmer, Waltham, MA, USA). The bands were
quantified using Image Pro-Plus 5.0 software (Media Cybernetics,
Rockville, MD, USA).
Statistical analysis
Standard statistical methods from the SPSS
statistical analysis system 16.0 (SPSS, Inc., Chicago, IL, USA)
were used. Statistical comparisons were made using a two-way
analysis of variance. Data are presented as the mean ± standard
error of the mean. P<0.05 was considered to indicate a
statistically significant difference. Duplicate wells were analyzed
for each experiment and each experiment was performed independently
at least three times.
Results
PRR expression in HASMCs
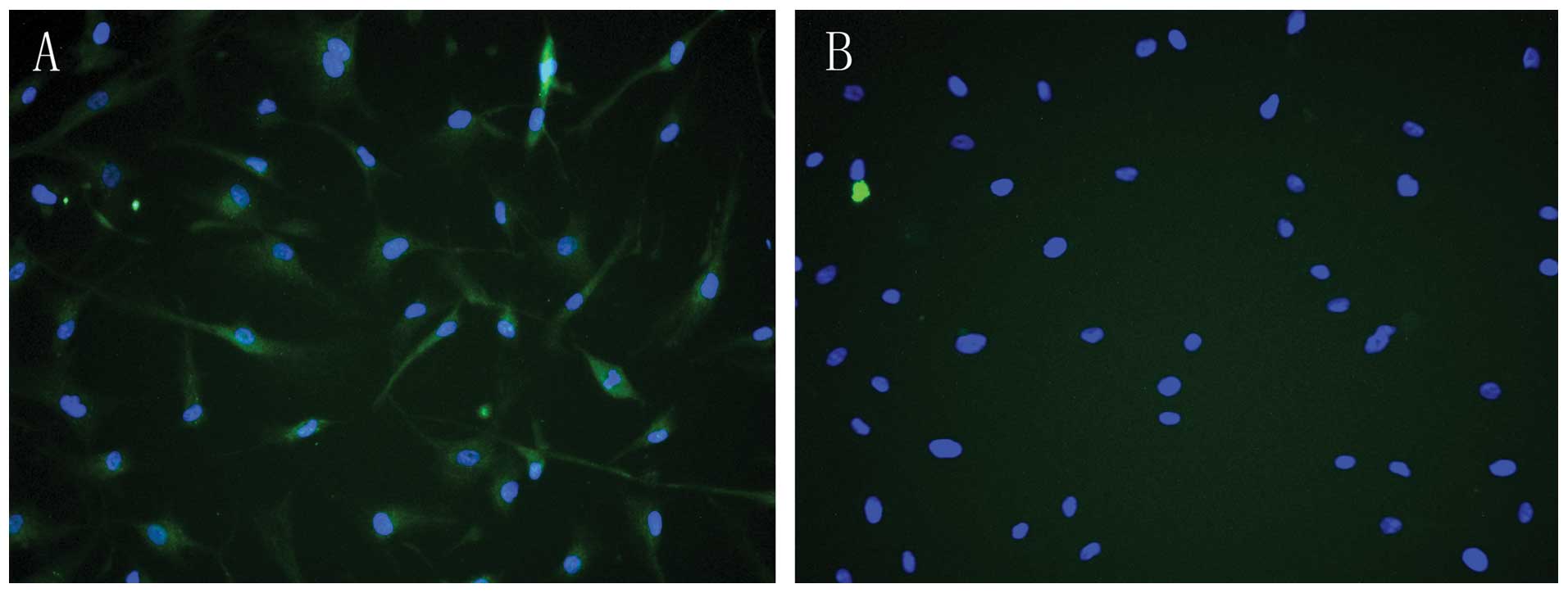
Immunofluorescence techniques verified that PRR was
expressed in HASMCs (Fig. 1),
indicating that PRR was abundant in HASMCs and mainly present in
the cell membrane and cytoplasm.
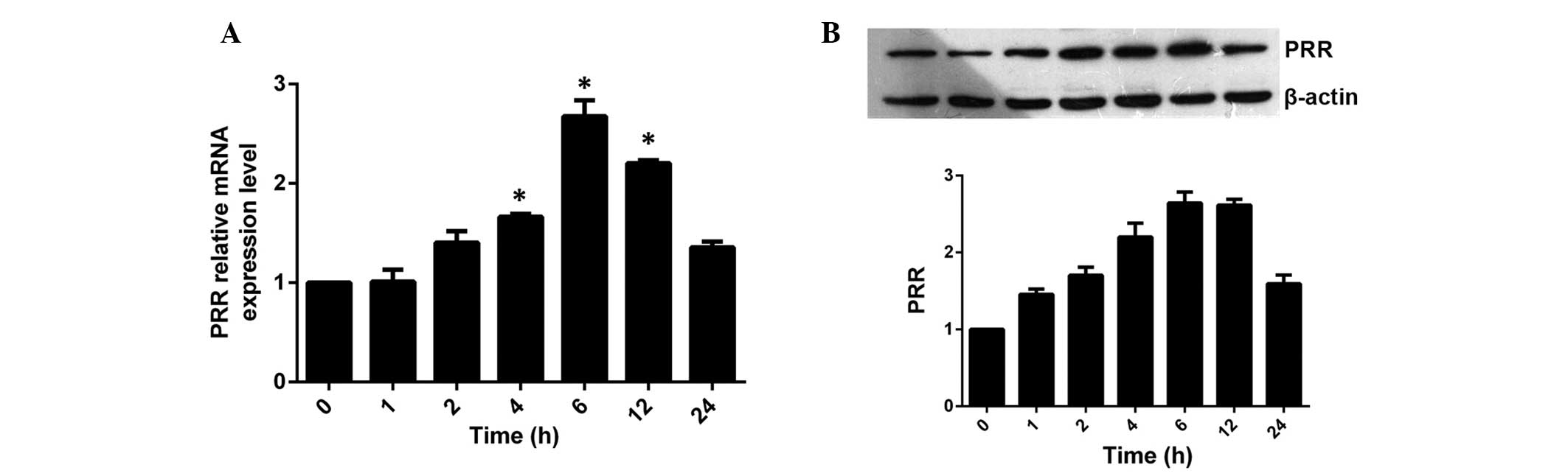
Effect of ox-LDL on the expression of PRR
at different time periods
HASMCs were incubated with a final concentration of
100 μg/ml ox-LDL for 0, 1, 2, 4, 6, 12 and 24 h. It was
found that the expression of PRR was significantly upregulated by
ox-LDL in a time-dependent manner (Fig. 2). The mRNA and protein expression
of PRR began to increase 2 h following incubation and reached a
peak level at 6 h and then decreased moderately, however,
maintaining a higher level of expression than the control group at
24 h.
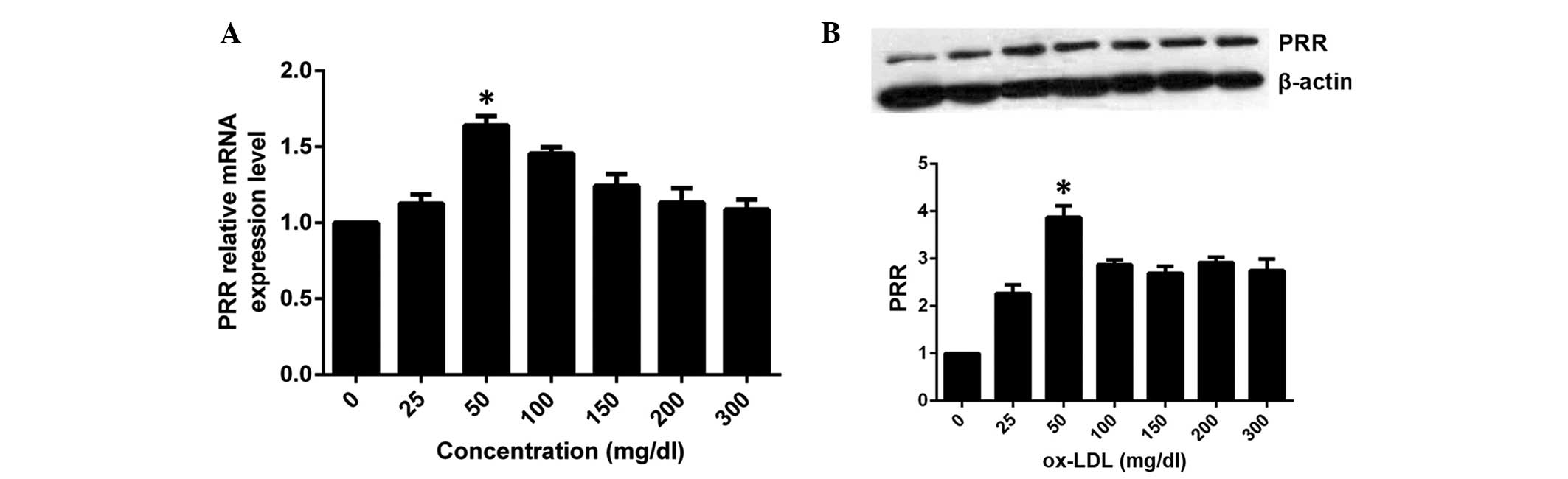
Effect of ox-LDL on the expression of PRR
at different concentrations
Based on the above results, HASMCs were incubated
with 0, 25, 50, 100, 150, 200 and 300 μg/ml ox-LDL for 6 h,
respectively. Compared with the control group, the expression of
PRR mRNA increased in the group treated with 25 μg/ml,
reached a peak in the 50 μg/ml group, then decreased
moderately in the remaining groups (Fig. 3A), revealing a
concentration-dependent effect. The expression of PRR protein was
similar to that of the mRNA, however, maintained significantly
higher levels than the control group following decrease (Fig. 3B).
Discussion
In the present study, immunofluorescence
demonstrated that PRR was expressed on the cell membrane and
cytoplasm in HASMCs (Fig. 1A),
which is consistent with a previous study (22) and the PRR was abundant in
HASMCs.
Binding of (pro)renin to the PRR increases the
catalytic activity of prorenin and renin, resulting in increased
RAS activation (13).
Additionally, intracellular signaling cascades, including the MAPK
and ERK1/2 pathways as well as the phosphorylation of heat shock
protein 27 (HSP 27) (18,23,24)
are triggered, resulting in the expression of profibrotic and
inflammatory molecules. These effects promote the occurrence and
development of atherosclerosis.
The present study demonstrated that the expression
of PRR was upregulated in a time- and concentration-dependent
manner stimulated by ox-LDL, indicating that PRR is involved in
ox-LDL-induced atherosclerosis. This demonstrated the effect of
ox-LDL on the expression of PRR for the first time, to the best of
our knowledge.
However, the mechanisms underlying atherosclerosis
formation induced by ox-LDL are varied, including via its own LOX-1
receptor, promoting the generation of ROS, promoting the formation
of foam cells and promoting the phenotypic transformation of
SMC.
Therefore, whether the upregulation of PRR
expression was induced by ox-LDL directly remains to be elucidated.
Further studies are required to determine the association between
PRR and LOX-1, the receptor of ox-LDL.
References
|
1
|
Ananyeva NM, Tjurmin AV, Berliner JA, et
al: Oxidized LDL mediates the release of fibroblast growth
factor-1. Arterioscler Thromb Vasc Biol. 17:445–453. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li D, Liu L, Chen H, Sawamura T,
Ranganathan S and Mehta JL: LOX-1 mediates oxidized low-density
lipoprotein-induced expression of matrix metalloproteinases in
human coronary artery endothelial cells. Circulation. 107:612–617.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cai H and Harrison DG: Endothelial
dysfunction in cardiovascular diseases: The role of oxidant stress.
Circ Res. 87:840–844. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chisolm GM 3rd and Chai Y: Regulation of
cell growth by oxidized LDL. Free Radic Biol Med. 28:1697–1707.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chai YC, Binion DG, Macklis R and Chisolm
GM 3rd: Smooth muscle cell proliferation induced by oxidized
LDL-borne lysophosphatidylcholine. Evidence for FGF-2 release from
cells not extracellular matrix. Vascul Pharmacol. 38:229–237. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shen CM, Mao SJ, Huang GS, Yang PC and Chu
RM: Stimulation of smooth muscle cell proliferation by ox-LDL- and
acetyl LDL-induced macrophage-derived foam cells. Life Sci.
70:443–452. 2001. View Article : Google Scholar
|
|
7
|
Hsieh CC, Yen MH, Yen CH and Lau YT:
Oxidized low density lipoprotein induces apoptosis via generation
of reactive oxygen species in vascular smooth muscle cells.
Cardiovasc Res. 49:135–145. 2001. View Article : Google Scholar
|
|
8
|
Chang PY, Luo S, Jiang T, et al: Oxidized
low-density lipoprotein downregulates endothelial basic fibroblast
growth factor through a pertussis toxin-sensitive G-protein
pathway: Mediator role of platelet-activating factor-like
phospholipids. Circulation. 104:588–593. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen CH, Jiang W, Via DP, et al: Oxidized
low-density lipoproteins inhibit endothelial cell proliferation by
suppressing basic fibroblast growth factor expression. Circulation.
101:171–177. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Higashi Y, Peng T, Du J, et al: A
redox-sensitive pathway mediates oxidized LDL-induced
downregulation of insulin-like growth factor-1 receptor. J Lipid
Res. 46:1266–1277. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gao P, Wang XM, Qian DH, et al: Induction
of oxidative stress by oxidized LDL via meprinα-activated epidermal
growth factor receptor in macrophages. Cardiovasc Res. 97:533–543.
2013. View Article : Google Scholar
|
|
12
|
Nishio E, Arimura S and Watanabe Y:
Oxidized LDL induces apoptosis in cultured smooth muscle cells: a
possible role for 7-ketocholesterol. Biochem Biophys Res Commun.
223:413–418. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nguyen G, Delarue F, Burcklé C, Bouzhir L,
Giller T and Sraer JD: Pivotal role of the renin/prorenin receptor
in angiotensin II production and cellular responses to renin. J
Clin Invest. 109:1417–1427. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nguyen G: Renin/prorenin receptors. Kidney
Int. 69:1503–1506. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oliver JA: Receptor-mediated actions of
renin and prorenin. Kidney Int. 69:13–15. 2006. View Article : Google Scholar
|
|
16
|
Funke-Kaiser H, Zollmann FS, Schefe JH and
Unger T: Signal transduction of the (pro)renin receptor as a novel
therapeutic target for preventing end-organ damage. Hypertens Res.
33:98–104. 2010. View Article : Google Scholar
|
|
17
|
Sakoda M, Ichihara A, Kaneshiro Y, et al:
(Pro)renin receptor-mediated activation of mitogen-activated
protein kinases in human vascular smooth muscle cells. Hypertens
Res. 30:1139–1146. 2007. View Article : Google Scholar
|
|
18
|
Feldt S, Batenburg WW, Mazak I, et al:
Prorenin and renin-induced extracellular signal-regulated kinase
1/2 activation in monocytes is not blocked by aliskiren or the
handle-region peptide. Hypertension. 51:682–688. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang Y, Wongamorntham S, Kasting J, et
al: Renin increases mesangial cell transforming growth factor-beta1
and matrix proteins through receptor-mediated, angiotensin
II-independent mechanisms. Kidney Int. 69:105–113. 2006. View Article : Google Scholar
|
|
20
|
Huang Y, Noble NA, Zhang J, Xu C and
Border WA: Renin-stimulated TGF-beta1 expression is regulated by a
mitogen-activated protein kinase in mesangial cells. Kidney Int.
72:45–52. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu X: Oxidized low density lipoprotein
activates ERK 1/2 pathway via (pro)renin receptor in human
umbilical vein endothelial cells. Chinese Journal of Integrative
Medicine on Cardio/Cerebrovascular Disease. 10:1235–1237. 2013.In
Chinese.
|
|
22
|
Greco CM, Camera M, Facchinetti L, et al:
Chemotactic effect of prorenin on human aortic smooth muscle cells:
a novel function of the (pro)renin receptor. Cardiovasc Res.
95:366–374. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kaneshiro Y, Ichihara A, Sakoda M, et al:
Slowly progressive, angiotensin II-independent glomerulosclerosis
in human (pro)renin receptor-transgenic rats. J Am Soc Nephrol.
18:1789–1795. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Abassi Z, Winaver J and Feuerstein GZ: The
biochemical pharmacology of renin inhibitors: implications for
translational medicine in hypertension, diabetic nephropathy and
heart failure: expectations and reality. Biochem Pharmacol.
78:933–940. 2009. View Article : Google Scholar : PubMed/NCBI
|