Introduction
Prostate cancer is the second most common type of
cancer and the sixth leading cause of cancer-related mortality
among males worldwide (1).
Currently, prostate-specific antigen testing, digital rectal
examination and histopathological evaluation of prostate needle
biopsies, are performed in order to detect and monitor the
progression of prostate cancer (2,3).
However, there is increasing evidence to suggest that the
resistance of prostate cancer cells to conventional drugs is a
significant problem. The investigation of potential novel and
effective drugs for the treatment of prostate cancer is therefore
required (4).
Traditional Chinese herbs are sources of compounds
that may serve as potential therapeutic drugs for cancer (5). Ku Shen, the dried root of Sophora
flavescens Aiton, is a commonly used, traditional Chinese
herbal medicine. Oxymatrine, an alkaloid present in Ku Shen,
exhibits anti-inflammatory, anti-allergic, antiviral, antifibrotic
and cardiovascular-protective properties (6–8).
Furthermore, oxymatrine has been reported to exhibit anticancer
properties, such as the inhibition of cancer cell proliferation,
the cell cycle and angiogenesis, the promotion of cell apoptosis
and reversal of multi-drug resistance in patients with cancer
(9–11). A previous study suggested that
oxymatrine may suppress angiogenesis by modulating the expression
of the NF-κB-mediated vascular endothelial growth factor signaling
pathway (12). Furthermore,
oxymatrine may induce mitochondria-dependent apoptosis in human
osteosarcoma cells by inhibiting the phosphatidylinositol-3
kinase/protein kinase B pathway (13). A number of studies have
demonstrated that oxymatrine may inhibit cell growth and the cell
cycle, and promote apoptosis in human gastric and breast cancers
(9,14,15).
However, to the best of our knowledge, the effects of oxymatrine on
prostate cancer cells have yet to be investigated. Therefore, the
present study aimed to investigate the anticancer effects of
oxymatrine on human prostate cancer cells.
Materials and methods
Reagents
Dulbecco’s modified Eagle’s medium (DMEM), fetal
bovine serum (FBS) and antibiotics (penicillin and streptomycin)
were purchased from Invitrogen Life Technologies (Carlsbad, CA,
USA). Oxymatrine, obtained from Sigma-Aldrich (St. Louis, MO, USA),
was dissolved in dimethyl sulfoxide (Sigma-Aldrich) with the stock
concentration of 10 mg/ml, and further diluted in the culture
medium. Each experiment was repeated at least three times and new
dilutions were prepared for each experiment.
Cell culture
DU145 and PC-3 human prostate cancer cell lines and
the PNT1B healthy human prostate cell line were obtained from the
Chinese Academy of Sciences (Shanghai, China). Cell lines were
cultured in DMEM supplemented with 10% FBS, 100 IU/ml penicillin
and 100 mg/ml streptomycin, and incubated at 37°C in 5%
CO2 for 48 h.
Cell proliferation assay
DU145, PC-3 and PNT1B cell lines were seeded into
96-well plates, incubated overnight and treated with oxymatrine (0,
2, 4, 6 and 8 mg/ml). Cell viability was determined using an MTT
assay (Sigma-Aldrich). Cells (3×104 cells/well) were
seeded into 96-well plates and incubated overnight at 37°C in 5%
CO2. Subsequently, the cells were incubated with
different concentrations of oxymatrine (0, 2, 4, 6 and 8 mg/ml).
MTT (10 ml; 5 mg/ml) was added and the mixture was incubated in
darkness at 37°C for 2 h. Absorbance was measured at a wavelength
of 490 nm using a microplate reader (FluoDia T70; Photon Technology
International, Lawrenceville, NJ, USA).
Flow cytometric analysis
Human prostate cancer cell lines were treated with
different concentrations of oxymatrine (0, 4 and 8 mg/ml).
Following treatment with oxymatrine for 48 h, cells were
trypsinized (Sigma-Aldrich) and centrifuged at 1,000 x g and the
pellet was washed twice using PBS. Cells were resuspended and
washed with PBS three times. Apoptotic cells were detected using an
annexin V-fluorescein isothiocyanate/propidium iodide (annexin
V-FITC/IP) cell apoptosis detection kit, according to the
manufacturer’s instructions (BD Biosciences, Franklin Lakes, NJ,
USA).
Western blot analysis
Following oxymatrine treatment, proteins were
extracted and separated using a sodium dodecyl sulfate
polyacrylamide electrophoresis gel (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Proteins were then transferred to
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA). Membranes were blocked and incubated with the following
primary antibodies: Mouse anti-human p53 monoclonal antibody
(1:1,000 dilution; cat. no. sc-126), mouse anti-human bcl-2
monoclonal antibody (1:1,000 dilution; cat. no. sc-7382), mouse
anti-human bax monoclonal antibody (1:1,000 dilution; cat. no.
sc-20067) and mouse anti-human GAPDH monoclonal antibody (1:5,000
dilution; cat. no. sc-365062) (Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) overnight at 4°C. Following washing with
Tris-buffered saline and Tween, membranes were incubated with a
goat anti-mouse secondary antibody conjugated with horseradish
peroxidase (1:10,000 dilution; cat. no. sc-2072; Santa Cruz
Biotechnology, Inc.) and visualized using an enhanced
chemiluminescent detection reagent from Pierce Biotechnology, Inc.
(Rockford, IL, USA).
In vivo xenografts
Approval was obtained from the ethics committee of
the First Affiliated Hospital of Wenzhou Medical University
(Wenzhou, China). BALB/c homozygous (nu/nu) nude mice (aged 6–8,
weeks; weight, 18–20 g), bred in-house, were maintained in a
specific pathogen-free environment. PC-3 cells (3×106)
were suspended in 100 μl PBS and subcutaneously injected into the
left axilla of recipient mice. On day five, 24 tumor-bearing mice
were randomly divided into three groups: The control group was
treated with PBS, and two groups were treated with different
concentrations of oxymatrine (50 mg/kg and 100 mg/kg body weight).
Oxymatrine was administered to the mice, using daily
intraperitoneal injections. Tumor volume was calculated using the
formula A × B2 × π/6, where A was the length of the
longest aspect of the tumor, and B was the length of the tumor
perpendicular to A. Following five weeks of treatment the mice were
sacrificed by cervical dislocation and tumor weight was
measured.
A terminal deoxynucleotidyl
transferase-mediated dUTP-biotin nick end-labeling (TUNEL)
assay
Cell apoptosis in mouse tumor samples from six
BALB/c mice, was measured in vivo, using a TUNEL assay kit
(Roche diagnostics, Indianapolis, IN, USA). Brown nuclei were
considered apoptotic. The number of apoptotic cells/1,000 cells was
recorded in each field of view, using a microscope (LZ12; Leica
Microsystems GmbH, Wetzlar, Germany) at magnification ×200.
Statistical analysis
Data are expressed as the mean ± standard deviation
and statistical analysis was carried out using SPSS version 10.0
(SPSS, Inc., Chicago, IL, USA). Comparisons between groups were
made using analysis of variance. P<0.05 was considered to
indicate a statistically significant difference.
Results
Oxymatrine inhibits the proliferation of
prostate cancer cells
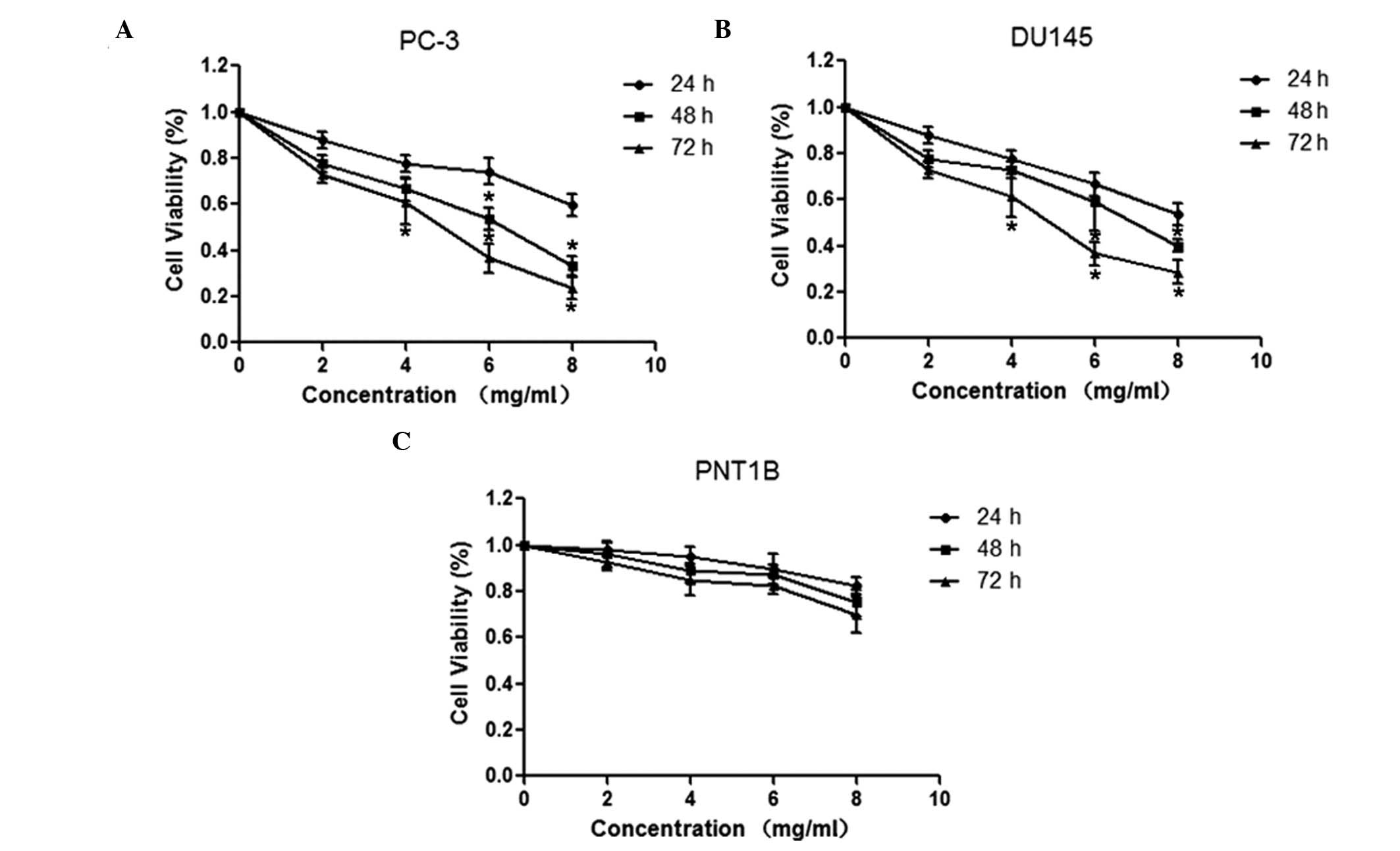
In order to investigate the antiproliferative
effects of oxymatrine on prostate cancer cells, DU145 and PNT1B
cell lines were treated with different concentrations of oxymatrine
(0, 2, 4, 6 and 8 mg/ml) for 24, 48 and 72 h. An MTT assay
suggested that oxymatrine significantly inhibited the proliferation
of DU145 and PC-3 cell lines in a time- and dose-dependent manner
(Fig. 1A and B). By contrast,
following treatment with oxymatrine, PNT1B healthy human prostate
cell proliferation was not inhibited (Fig. 1C).
Oxymatrine promotes prostate cancer cell
apoptosis
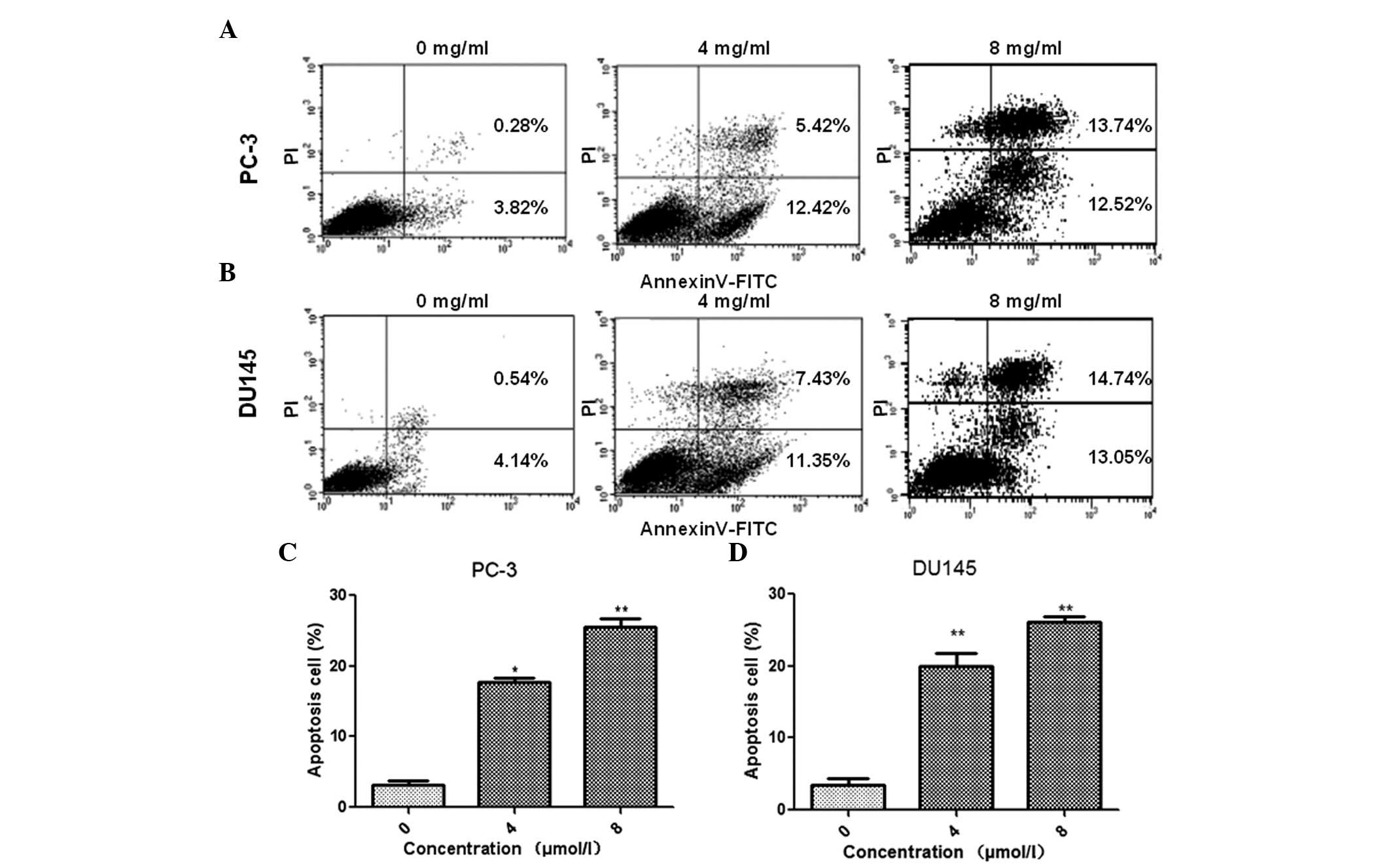
Oxymatrine-induced apoptosis in prostate cancer
cells was measured using annexin V-FITC/PI double staining. Flow
cytometry analysis demonstrated that treatment with oxymatrine
resulted in a significant increase in cell apoptosis of PC-3
(Fig. 2A and C) and DU145
(Fig. 2B and D) cell lines, in a
dose-dependent manner. These data suggested that oxymatrine
treatment may promote prostate cancer cell apoptosis.
Effect of oxymatrine on the expression of
apoptosis-related proteins
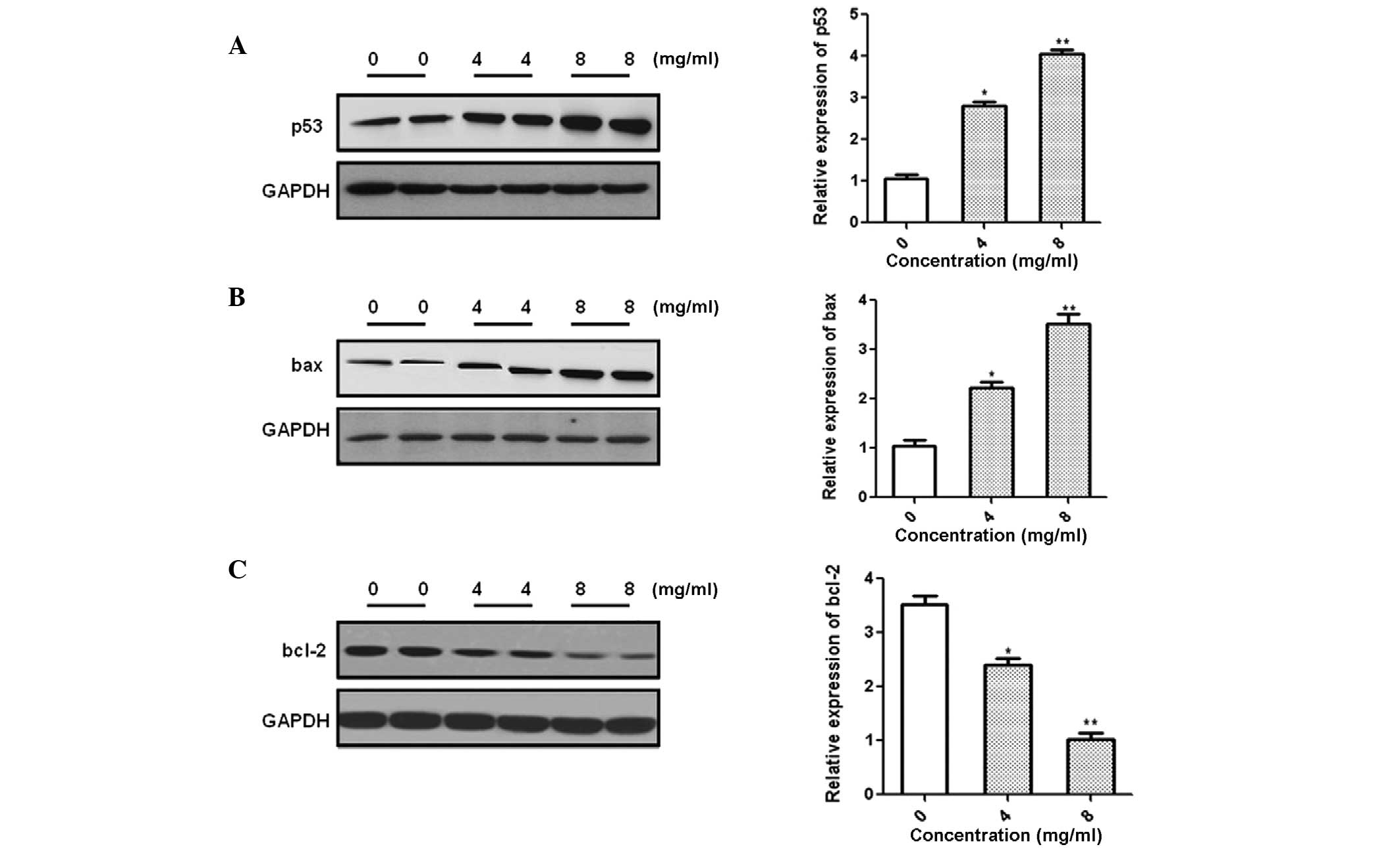
In order to investigate the possible molecular
mechanisms underlying oxymatrine-induced apoptosis of prostate
cancer cells, the expression of p53, bax and bcl-2 was analyzed
following treatment with different concentrations of oxymatrine.
Western blotting suggested that the expression of p53 and bcl-2
decreased, whereas that of bax increased, in a dose-dependent
manner (Fig. 3).
Oxymatrine reduces prostate cancer cell
proliferation in vivo
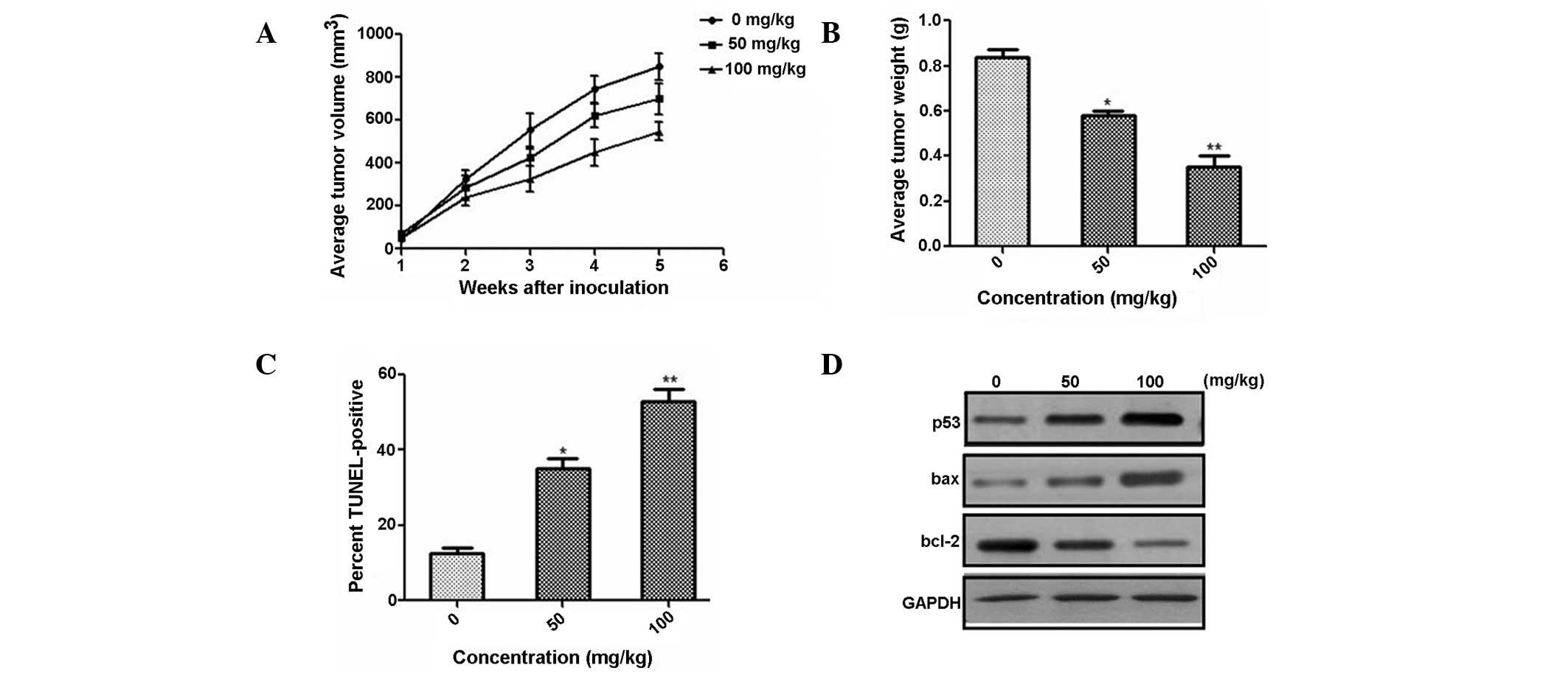
In order to investigate the effect of oxymatrine on
tumor growth in vivo, three concentration levels of
oxymatrine were intraperitoneally injected into nude mice, using
PC-3 subcutaneous xenografts. The results suggested that the volume
(Fig. 4A) and weight (Fig. 4B) of tumors in mice significantly
decreased in a dose-dependent manner. A TUNEL assay suggested that
the number of apoptotic cells increased significantly in a
dose-dependent manner (Fig. 4C).
In accordance with the in vitro analyses, the expression of
apoptosis-associated proteins, p53 and bcl-2 decreased and that of
bax increased, in a dose-dependent manner (Fig. 4D). Oxymatrine may therefore reduce
prostate cancer cell growth by promoting cell apoptosis in
vivo.
Discussion
Oxymatrine is an alkaloid, which is derived from Ku
Shen and has been shown to be a potential treatment for a number of
types of cancers, such as pancreatic (11), gastric (14) and breast cancer (15). However, to the best of our
knowledge, the effects of oxymatrine on prostate cancer and the
underlying molecular mechanisms of these effects have yet to be
investigated. In the present study, oxymatrine treatment was found
to promote prostate cancer cell apoptosis and inhibit prostate
cancer cell proliferation in vitro and in vivo.
In vitro, an MTT assay demonstrated that
oxymatrine treatment significantly inhibited cell proliferation in
DU145 and PC-3 prostrate cancer cell lines in a time- and
dose-dependent manner. Xenograft tumorigenesis analysis in
vivo, demonstrated that following oxymatrine treatment, the
weight and size of tumors in PC-3 subcutaneous xenografts were
significantly reduced, in a dose-dependent manner. The results of
the present study, therefore, indicated that oxymatrine treatment
inhibited the proliferation of prostate cancer cells, in
vitro and in vivo.
Apoptosis is the process of cell death,
characterized by cellular and molecular processes, such as
phosphatidylserine externalization, cell shrinkage and chromatin
condensation (16,17). Uncontrolled cell proliferation is
involved in tumor initiation and progression. Therefore, apoptosis
induction provides a potential mechanism for the development of
antitumor therapies (18,19). In the present study, oxymatrine
treatment induced prostate cancer cell apoptosis in vitro,
in a dose-dependent manner, which was demonstrated using flow
cytometry and TUNEL analysis.
A number of signalling pathways are involved in the
regulation of apoptosis, and numerous molecular markers involved in
these pathways have been identified (20–22).
For example, p53, encoded by the tumor protein 53 gene is
associated with cell apoptosis and cell cycle regulation, in
multi-cellular organisms (21,22).
Upon internal and external stimuli, such as oxidative stress and
viral infection, p53 may activate or suppress a number of
downstream target genes involved in apoptosis, such as bax, p53
upregulated modulator of apoptosis and bcl-2 (23,24).
Bax is a p53 primary-response gene, involved in a p53-regulated
pathway. p53 accumulates in the cytosol and promotes the expression
of bax, which permeabilizes mitochondria and promotes cell
apoptosis (25,26). The antiapoptotic protein, bcl-2 has
been shown to prevent mitochondrial disruption and block cytochrome
c release from the mitochondria (27,28).
The results of the present study demonstrated that oxymatrine
treatment of prostate cancer cells may result in an increase in p53
and bax expression and a decrease in bcl-2 expression, in a
dose-dependent manner. Overall, the results suggested that
oxymatrine is capable of regulating the expression of
apoptosis-associated proteins in prostate cancer cells, in
vitro and in vivo.
In conclusion, the results of the present study
demonstrated that oxymatrine exhibits antitumor properties in
prostate cancer cells, in vitro and in vivo.
Furthermore, the results suggested that the antitumor properties of
oxymatrine may be attributed to the inhibition of proliferation and
the induction of apoptosis via regulation of the expression of
apoptosis-associated proteins. Therefore, these findings may
provide a novel approach for the development of prostate cancer
therapy, using oxymatrine, which is derived from the traditional
Chinese herb, Sophora flavescens.
Acknowledgments
This study was sponsored by Zhejiang Provincial
Natural Science Foundation of China (grant no. LY12H10004).
References
|
1
|
Dunn MW and Kazer MW: Prostate cancer
overview. Semin Oncol Nurs. 27:241–250. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Katahira K, Takahara T, Kwee TC, Oda S,
Suzuki Y, Morishita S, Kitani K, Hamada Y, Kitaoka M and Yamashita
Y: Ultra-high-b-value diffusion-weighted MR imaging for the
detection of prostate cancer: evaluation in 201 cases with
histopathological correlation. Eur Radiol. 21:188–196. 2011.
View Article : Google Scholar
|
|
3
|
Gosselaar C, Roobol MJ, Roemeling S, van
der Kwast TH and Schröder FH: Screening for prostate cancer at low
PSA range: the impact of digital rectal examination on tumor
incidence and tumor characteristics. Prostate. 67:154–161. 2007.
View Article : Google Scholar
|
|
4
|
Shi GH, Ye DW, Yao XD, Zhang SL, Dai B,
Zhang HL, Shen YJ, Zhu Y, Zhu YP, Xiao WJ and Ma CG: Involvement of
microRNA-21 in mediating chemo-resistance to docetaxel in
androgen-independent prostate cancer PC3 cells. Acta Pharmacol Sin.
31:867–873. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu YH, Li ML, Hsu MY, Pang YY, Chen IL,
Chen CK, Tang SW, Lin HY and Lin JY: Effects of a Chinese herbal
medicine, Guan-Jen-Huang (Aeginetia indica Linn.), on renal cancer
cell growth and metastasis. Evid Based Complement Alternat Med.
2012:9358602012. View Article : Google Scholar
|
|
6
|
Huang M, Hu YY, Dong XQ, Xu QP, Yu WH and
Zhang ZY: The protective role of oxymatrine on neuronal cell
apoptosis in the hemorrhagic rat brain. J Ethnopharmacol.
143:228–235. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chai NL, Fu Q, Shi H, Cai CH, Wan J, Xu SP
and Wu BY: Oxymatrine liposome attenuates hepatic fibrosis via
targeting hepatic stellate cells. World J Gastroenterol.
18:4199–4206. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hong-Li S, Lei L, Lei S, Dan Z, De-Li D,
Guo-Fen Q, Yan L, Wen-Feng C and Bao-Feng Y: Cardioprotective
effects and underlying mechanisms of oxymatrine against ischemic
myocardial injuries of rats. Phytother Res. 22:985–989. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu Y, Xu Y, Ji W, Li X, Sun B, Gao Q and
Su C: Antitumor activities of matrine and oxymatrine: literature
review. Tumour Biol. 35:5111–5119. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Song G, Luo Q, Qin J, Wang L, Shi Y and
Sun C: Effects of oxymatrine on proliferation and apoptosis in
human hepatoma cells. Colloids Surf B Biointerfaces. 48:1–5. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ling Q, Xu X, Wei X, Wang W, Zhou B, Wang
B and Zheng S: Oxymatrine induces human pancreatic cancer PANC-1
cells apoptosis via regulating expression of Bcl-2 and IAP
families, and releasing of cytochrome c. J Exp Clin Cancer Res.
30:662011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen H, Zhang J, Luo J, Lai F, Wang Z,
Tong H, Lu D, Bu H, Zhang R and Lin S: Antiangiogenic effects of
oxymatrine on pancreatic cancer by inhibition of the NF-κB-mediated
VEGF signaling pathway. Oncol Rep. 30:589–595. 2013.PubMed/NCBI
|
|
13
|
Zhang Y, Sun S, Chen J, Ren P, Hu Y, Cao
Z, Sun H and Ding Y: Oxymatrine induces mitochondria dependent
apoptosis in human osteosarcoma MNNG/HOS cells through inhibition
of PI3K/Akt pathway. Tumour Biol. 35:1619–1625. 2014. View Article : Google Scholar
|
|
14
|
Song MQ, Zhu JS, Chen JL, Wang L, Da W,
Zhu L and Zhang WP: Synergistic effect of oxymatrine and
angiogenesis inhibitor NM-3 on modulating apoptosis in human
gastric cancer cells. World J Gastroenterol. 13:1788–1793. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Y, Piao B, Zhang Y, Hua B, Hou W, Xu
W, Qi X, Zhu X, Pei Y and Lin H: Oxymatrine diminishes the side
population and inhibits the expression of β-catenin in MCF-7 breast
cancer cells. Med Oncol. 28(Suppl 1): S99–S107. 2011. View Article : Google Scholar
|
|
16
|
Vogler M, Weber K, Dinsdale D, Schmitz I,
Schulze-Osthoff K, Dyer MJ and Cohen GM: Different forms of cell
death induced by putative BCL2 inhibitors. Cell Death Differ.
16:1030–1039. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Silva MT: Secondary necrosis: the natural
outcome of the complete apoptotic program. FEBS Lett.
584:4491–4499. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Frew AJ, Johnstone RW and Bolden JE:
Enhancing the apoptotic and therapeutic effects of HDAC inhibitors.
Cancer Lett. 280:125–133. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hou J, Wang D, Zhang R and Wang H:
Experimental therapy of hepatoma with artemisinin and its
derivatives: in vitro and in vivo activity, chemosensitization, and
mechanisms of action. Clin Cancer Res. 14:5519–5530. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vazquez A, Bond EE, Levine AJ and Bond GL:
The genetics of the p53 pathway, apoptosis and cancer therapy. Nat
Rev Drug Discov. 7:979–987. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu W, Ge Y, Ojcius DM, Sun D, Dong H, Yang
XF and Yan J: p53 signalling controls cell cycle arrest and
caspase-independent apoptosis in macrophages infected with
pathogenic Leptospira species. Cell Microbiol. 15:1642–1659.
2013.PubMed/NCBI
|
|
22
|
Ben Sahra I, Laurent K, Giuliano S,
Larbret F, Ponzio G, Gounon P, Le Marchand-Brustel Y,
Giorgetti-Peraldi S, Cormont M, Bertolotto C, et al: Targeting
cancer cell metabolism: the combination of metformin and
2-deoxyglucose induces p53-dependent apoptosis in prostate cancer
cells. Cancer Res. 70:2465–2475. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gomez-Lazaro M, Galindo MF, Concannon CG,
Segura MF, Fernandez-Gomez FJ, Llecha N, Comella JX, Prehn JH and
Jordan J: 6-Hydroxydopamine activates the mitochondrial apoptosis
pathway through p38 MAPK-mediated, p53-independent activation of
Bax and PUMA. J Neurochem. 104:1599–1612. 2008. View Article : Google Scholar
|
|
24
|
Ramaiah MJ, Pushpavalli SN, Lavanya A,
Bhadra K, Haritha V, Patel N, Tamboli JR, Kamal A, Bhadra U and
Pal-Bhadra M: Novel anthranilamide-pyrazolo[1,5-a]pyrimidine
conjugates modulate the expression of p53-MYCN associated micro
RNAs in neuroblastoma cells and cause cell cycle arrest and
apoptosis. Bioorg Med Chem Lett. 23:5699–5706. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Deng Y and Wu X: Peg3/Pw1 promotes
p53-mediated apoptosis by inducing Bax translocation from cytosol
to mitochondria. Proc Natl Acad Sci USA. 97:12050–12055. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gogada R, Prabhu V, Amadori M, Scott R,
Hashmi S and Chandra D: Resveratrol induces p53-independent,
X-linked inhibitor of apoptosis protein (XIAP)-mediated Bax protein
oligomerization on mitochondria to initiate cytochrome C release
and caspase activation. J Biol Chem. 286:28749–28760. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bishayee K, Chakraborty D, Ghosh S,
Boujedaini N and Khuda-Bukhsh AR: Lycopodine triggers apoptosis by
modulating 5-lipoxygenase, and depolarizing mitochondrial membrane
potential in androgen sensitive and refractory prostate cancer
cells without modulating p53 activity: signaling cascade and
drug-DNA interaction. Eur J Pharmacol. 698:110–121. 2013.
View Article : Google Scholar
|
|
28
|
Liu Y, Yang Y, Ye YC, Shi QF, Chai K,
Tashiro S, Onodera S and Ikejima T: Activation of ERK-p53 and
ERK-mediated phosphorylation of Bcl-2 are involved in autophagic
cell death induced by the c-Met inhibitor SU11274 in human lung
cancer A549 cells. J Pharmacol Sci. 118:423–432. 2012. View Article : Google Scholar : PubMed/NCBI
|