Introduction
Pancreatic cancer constitutes ~3% of all diagnosed
cancers; however, it is the third and fourth most common cause of
cancer-associated mortality in females and males, respectively.
Pancreatic cancer ranks 10th in the number of new cases, and 4th in
cancer-associated mortality in the US (1). There is no specific treatment for
pancreatic cancer; therefore, patients suffering from pancreatic
cancer have a very poor prognosis, with a 5-year relative survival
rate of <5% (2). Currently,
there are no successful treatments for this cancer; therefore, it
is crucial for doctors and scientists to identify methods to
improve the diagnosis and treatment of pancreatic cancer.
Endocrine gland-derived vascular endothelial growth
factor (EG-VEGF), was first cloned by LeCourter et al
(3) in 2001. EG-VEGF selectively
acts on the endothelium of endocrine gland cells. The coding region
of EG-VEGF encodes 305 amino acids, and has a molecular weight of
8.6 kDa. The mature protein has been predicted to consist of 86
amino acids, containing 10 cysteine residues. EG-VEGF has high
homology (80%) with a nontoxic protein purified from the venom of
the black mamba snake, but has low homology with VEGF (4). Human EG-VEGF has been shown to exert
its functions through two G protein-coupled receptors (GPCRs). The
expression of EG-VEGF is induced by hypoxia, and the protein is
expressed in various endocrine tissues, including the testis,
adrenal gland, ovary and placenta. The effects of EG-VEGF appear to
be restricted to endothelial cells derived from these endocrine
tissues (3). In pancreatic cancer
cells, EG-VEGF was previously shown to protect the cells from
apoptosis, through the upregulation of the myeloid cell leukemia-1
protein (5), but no disruption was
observed in the function of EG-VEGF in pancreatic cancer cells.
To investigate the effects on EG-VEGF, at the
molecular level in pancreatic cancer, the expression of EG-VEGF was
determined in human pancreatic cancer tissue. Furthermore, the
association between EG-VEGF expression and clinico-pathological
characteristics was assessed, and the in vitro activity of
EG-VEGF was observed in pancreatic cancer cells.
Materials and methods
Cell lines
The present study used the Mia PaCa-2 human
pancreatic cancer cell line, which were provided by Dr Friess
(University of Heidelberg, Heidelberg, Germany) and originally
purchased from the American Type Culture Collection (ATTC CRL-1420;
Manassas, VA, USA). The cells were cultured in Dulbecco's modified
Eagle's medium (DMEM), supplemented with 10% fetal calf serum, 100
U/ml penicillin and 100 mg/ml streptomycin, and maintained at 37°C
in a humidified atmosphere containing 5% CO2.
Tissue chip
A tissue chip was constructed by Cybrdi (Potomac,
MD, USA), which included 60 pancreatic cancer tissue samples and 10
normal pancreatic tissue samples. The majority of the pancreatic
cancer patients were male (65%), with 39 samples from males and 21
from females. The age of the patients with pancreatic carcinoma
ranged between 34 and 86 years, with a median age of 65 years. A
histopathological diagnosis was made by two experienced
pathologists. The tumors were staged according to the International
Union against Cancer's Tumor-Node-Metastasis classification, and
then histologically subtyped and graded according to the World
Health Organization guidelines (6).
Immunohistochemistry
Immunostaining for EG-VEGF was performed manually at
room temperature, using the Ultra Sensitive Solid Phase
Immunohistochemistry kit (Maixin Biotech, Fuzhou, China).
Phosphate-buffered saline (PBS) was used as a negative control. A
tumor epithelial cell proportion score was determined and a
staining intensity score was calculated for each slide. Staining
was assessed in numerous areas on the slide, and given a + or −
score and the average score was then determined for each slide. The
slides were microwaved in EDTA buffer (pH 8.0) for 60 min, for
antigen retrieval. The slides were then rinsed with water, and 3%
hydrogen peroxide was applied to the slides for 4 min at room
temperature. A further rinse with Tris buffer was performed, prior
to incubation of the slides with EG-VEGF antibody (1:100 dilution),
or PBS (control) overnight, at 4°C. The slides were rinsed twice
with Tris buffer, and then incubated with Biotin (Ventana, Tucson,
AZ, USA) for 10 min, rinsed again as before, and incubated with
streptavidin for 8 min. Following a final rinse with Tris buffer,
chromogen (dimethylaminoazobenzene; Ventana) was applied for 8 min,
followed by a copper solution for 4 min. Counterstaining was
performed using a commercially prepared hematoxylin for 4 min.
Post-counterstaining was performed with a bluing solution (Beijing
Biosynthesis Biotechnology Co., Ltd, Beijing, China), and the
slides were dehydrated and cover-slipped with Permount (Beijing
Biosynthesis Biotechnology Co., Ltd). A BenchMark XT (Ventana) was
used to measure the staining. Two independent pathologists viewed
and interpreted the stained slides without knowledge of the patient
outcome. The intensity of immunostaining for EG-VEGF was visually
scored, and stratified into four groups: No tumor cells (−),
<10% tumor cells (±), 10–50% tumor cells (+), or >50% tumor
cells (++). Any cytoplasmic staining with EG-VEGF was considered to
indicate positive expression. Staining in the basal epithelial
cells of the normal pancreatic cancer epithelium served as an
internal control.
Cell proliferation assay
The anti-proliferative effects of EG-VEGF on the Mia
PaCa-2 cells were determined using the MTT dye uptake method.
Briefly, the cells (2.5×103/ml), with or without EG-VEGF
(100 ng/ml), were incubated in triplicate in a 96-well plate. The
plates were then cultured for 0, 24, 48, 72 or 96 h. At each time
point, 5 mg/ml MTT dye was added to each well. Following a 4 h
incubation, the formed crystals were dissolved in 0.04 m HCl, in
isopropanol. Absorbance was measured at 490 nm using a microplate
reader (Bio-Rad Laboratories, Hercules, CA, USA). The cell
proliferation rates of the EG-VEGF-treated and untreated cells were
compared.
Apoptosis assay
Apoptosis was measured using an
Annexin-V/Fluorescein isothiocyanate (FITC) Apoptosis Detection kit
(BD Pharmingen, San Diego, CA, USA) and a
4,6-diamidino-2-phenylindole staining kit. A total of
~0.3×106 cells were seeded in each well of a six-well
plate. The cells were grown for 24 h in DMEM, without fetal bovine
serum (FBS), with or without EG-VEGF (100 ng/ml). Both adherent and
floating cells were collected, washed twice with cold PBS, and
resuspended in cold annexin-V binding buffer, at a concentration of
1×107 cells/ml. Propidium iodide (PI) staining was
performed in order to identify the dead cells. The cells were
incubated at 37°C for 24 h. Following further washing, the pellets
were resuspended in 100 μl binding buffer, containing
Annexin V-FITC. The cells were incubated in the dark at room
temperature for 15 min, washed once with Annexin binding buffer,
and stained with 10 μl PI, on ice, for 30 min. The cell
pellet was analyzed using a FACSCalibur flow cytometer (BD
Biosciences, Franklin Lakes, NJ, USA). The untreated cells were
stained using the same protocol, and served as the controls.
Assessment of EG-VEGF migration
Wound healing assay
A wound healing assay was performed to determine the
effects of EG-VEGF on the motility of the Mia PaCa-2 cells (n=3).
The cells were seeded in complete medium (DMEM, supplemented with
5% FBS), at a density of 4×105 cells/well in six-well
plates. Once the cells had reached confluency, the complete medium
was replaced with serum-free medium, and the cells were scratched
using a sterile tip, in order to create an artificial wound. The
cells were allowed to heal for the next 24 h, with or without
EG-VEGF treatment (100 ng/ml). Images were captured at regular time
intervals (0 and 24 h) using an IX70-CoolSNAP microscope-camera
combination (Olympus Corp., Tokyo, Japan). The size of the wound
was measured from three separate experiments. The results are
presented as a percentage of the wound closure, 24 h following the
treatment.
Cell migration assay
The rates of migration were evaluated using a
Matrigel®-based assay, as previously described. Briefly,
the cells were plated in triplicate at 2.5×104
cells/well in serum-free medium on 8 μm pore polycarbonate
membranes of Transwell chambers, precoated with
Matrigel® (BD Biosciences). The lower Transwell chambers
contained EG-VEGF (100 ng/ml), without serum, as a chemoattractant.
Matrigel-coated filters were placed between the upper and lower
compartments. The cells were suspended in DMEM at a concentration
of 2.5×104 cells/ml and then loaded onto the upper
chambers. The cells that had migrated through the Matrigel-coated
filters were recovered from the lower compartments following a 24 h
incubation, and counted. To determine the number of cells migrating
(uncoated membrane), or invading (extracellular matrix-coated
membrane), through the membrane, the membranes were fixed and
stained with 0.5% crystal violet solution. Following a wash with
water, the non-migrating or non-invading cells were removed by
wiping the top of the membrane with a cotton-wool tip. The cells
which had migrated or invaded through the membrane were manually
counted, using magnified (×200) digital pictures of the
insert/membranes (five fields for each membrane). The experiment
was performed at least twice, with each sample being run in
triplicate. An invasion index was calculated as the number of cells
invading through the membrane in the treated cell group, divided by
the number of cells migrating through the membrane in the control
group. The migration of the cells was calculated from the ratio of
the number of cells recovered from the lower compartment : the
total number of cells initially loaded into the upper
compartment.
Western blot analysis
In order to investigate whether the MAPK pathway was
activated in response to EG-VEGF, Mia PaCa 2 cells were: 1)
Incubated with various concentrations of EG VEGF (0, 50, 100 and
200 ng/ml) for 30 min; or 2) pretreated with 200 ng/ml Pertussin
toxin (PTX) and 50 ng/ml PD98059, an MEK1
(MAPK2)-specific-inhibitor, for 1 h followed by exposure to 100
ng/ml EG-VEGF for 30 min at 37°C with 5% CO2. The cells
were then centrifuged, washed with cold PBS, and lysed on ice for
30 min, using a lysis buffer containing protease and phosphatase
inhibitors. The concentration of the protein samples were
determined using a Bio-Rad protein assay (Bio-Rad Laboratories).
Total protein was separated by electrophoresis on a 12%
SDS-polyacrylamide gel, and transferred to a polyvinylidene
fluoride membrane (Thermo Fisher Scientific, Inc., Rockford, IL,
USA). The membranes were then blocked with 5% non-fat milk and
incubated with the following primary antibodies: Anti-EG-VEGF
(1:200 dilution; BD Pharmingen), anti-MAPK (1:1,000 dilution; Dako,
Glostrup, Denmark) or anti-β-actin (Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA). Following an overnight incubation at 4°C, the
membranes were washed and incubated with horseradish
peroxidase-conjugated secondary antibodies for 1 h. The bands were
visualized using an Enhanced Chemiluminescence substrate (Thermo
Fisher Scientific, Inc.). The bands were quantified using Image
Lab™ 4.1 software (Bio-Rad Laboratories, Inc.) for densitometric
analysis.
Statistics
Statistical comparisons of significance between the
expression and clinicopathological features of the pancreatic
cancer patients were evaluated using Cochran-Mantel-Haenszel
Statistics or Fisher's Exact. Statistical analysis was performed
using SAS version 9.0 software (SAS Institute Inc., Cary, NC, USA).
The results are expressed as the means of the percentage ± the
standard deviation of the mean. Two-tailed student's t-tests were
used when appropriate for statistical analysis. A P<0.05 was
considered to indicate a statistically significant difference.
Results
EG-VEGF expression in pancreatic cancer
and normal pancreatic tissue
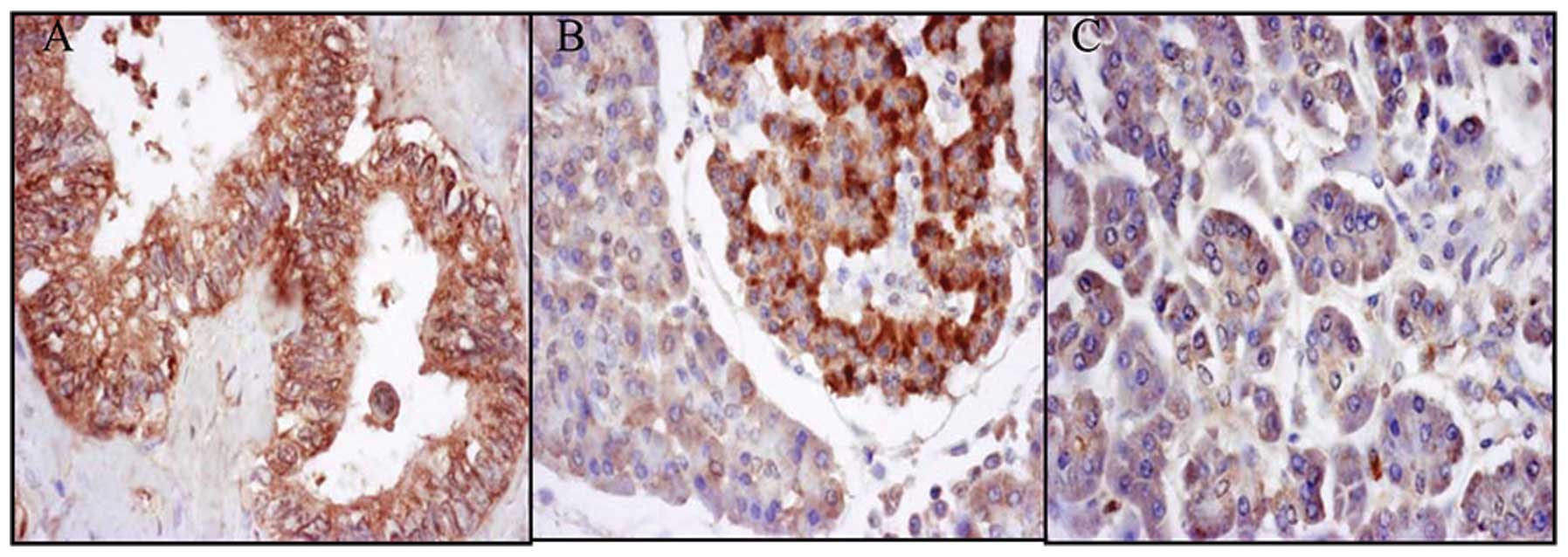
Positive staining for EG-VEGF expression was
observed in the pancreatic islets of 8/10 normal patients. However,
only 1/10 normal pancreatic tissue samples stained positively for
EG-VEGF, whereas 57/60 of the tissue samples from the pancreatic
cancer patients stained positively (Fig. 1).
The expression of EG-VEGF in pancreatic carcinomas
had a linear correlation with the clinical stage of pancreatic
cancer (0.0299, P<0.05; Table
I). The expression of EG-VEGF was much higher in the pancreatic
cancer tissue from patients in the later stages of cancer. The
differences in the expression of EG-VEGF between the pancreatic
tumor and normal pancreas tissue were also statistically
significant (4.167E-08, P<0.05). Furthermore, the expression of
EG-VEGF was detected in the islet cells of the normal pancreatic
tissue.
 | Table IAssociation between
clinicopathological characteristics of the pancreatic cancer
patients and endocrine gland-derived vascular endothelial growth
factor (EG-VEGF) expression. |
Table I
Association between
clinicopathological characteristics of the pancreatic cancer
patients and endocrine gland-derived vascular endothelial growth
factor (EG-VEGF) expression.
| Characteristic | Patients (n) | EG-VEGF Positive
(%) | P-value |
|---|
| Age (years) | | | |
| <50 | 16 | 100.0 | |
| ≥50 | 44 | 93.1 | >0.05 |
| Gender | | | |
| Male | 39 | 94.9 | |
| Female | 21 | 95.2 | >0.05 |
| Stage | | | |
| I | 25 | 92.0 | |
| II | 26 | 96.2 | |
| III | 3 | 100.0 | |
| IV | 6 | 100.0 | <0.05 |
EG-VEGF promotes Mia PaCa-2 cell
proliferation and protects the cells from apoptosis
As compared with the untreated cells, the cells
exposed to EG-VEGF had an increased rate of proliferation 48, 72
and 96 h, following treatment (P<0.05; Fig. 2A). The Mia PaCa-2 cells were
starved for 24 h, and stained with Annexin-V/PI, the apoptotic
cells were then identified using a fluorescence microscope. In the
serum-deprived, untreated cells, 27.74% were positive for
annexin-V/FITC staining; whereas among the cells cultured in the
presence of 100 ng/ml EG-VEGF, only 13.21% cells were positive for
Annexin-V/FITC staining (Fig.
2B).
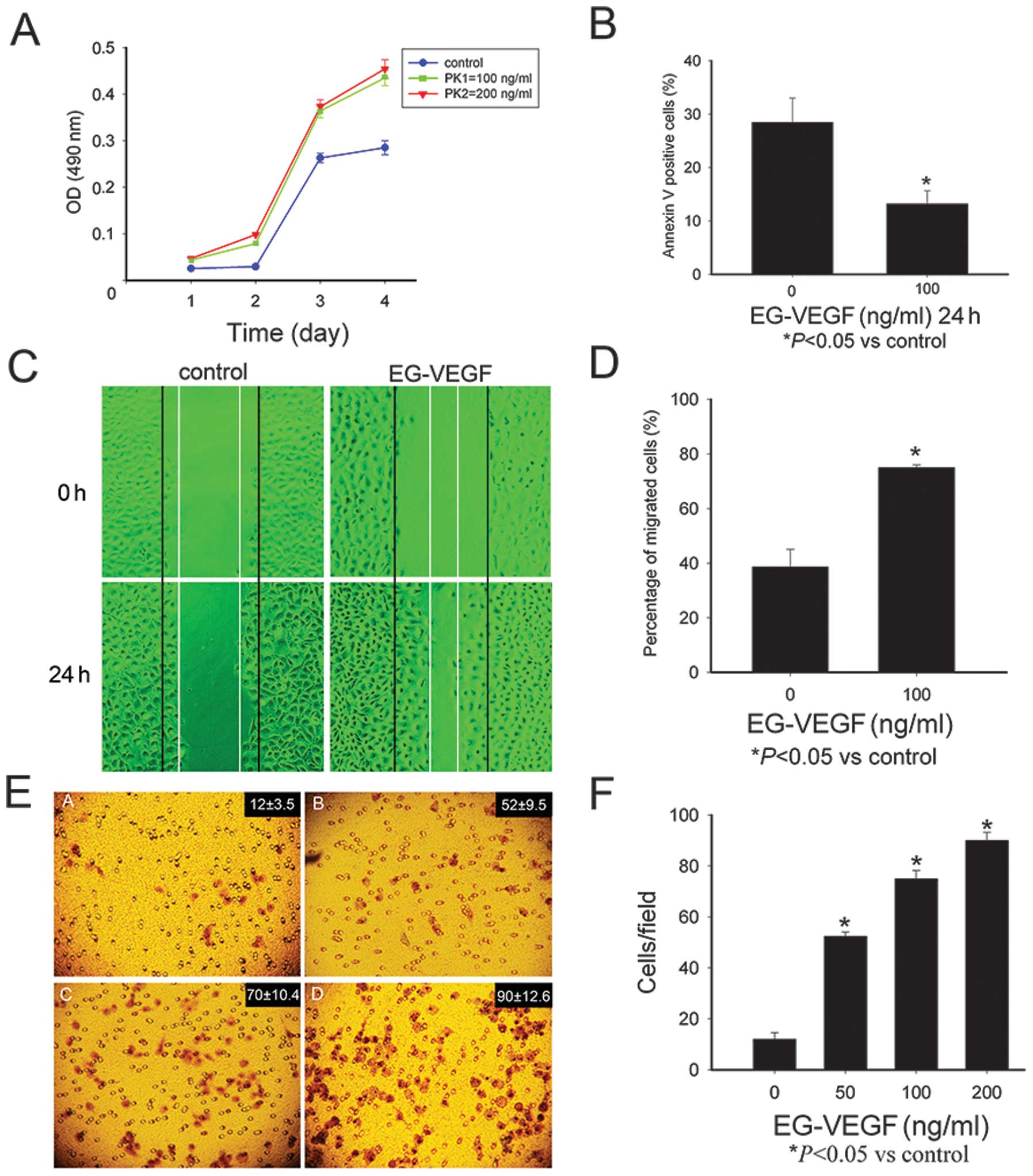 | Figure 2Endocrine gland-derived vascular
endothelial growth factor (EG-VEGF) can modulate the malignant
phenotype of the Mia PaCa-2 pancreatic cancer cells. (A) The Mia
PaCa-2 cells were treated with EG-VEGF (100 or 200 ng/ml), and
cultured for 24, 48, 72, and 96 h. EG-VEGF treatment significantly
promoted growth of the cells (P<0.05, as compared with the
control cells), but there was no marked difference between the 100
and 200 ng/ml EG-VEGF groups. (B) Protective effects of EG-VEGF on
the apoptosis of Mia PaCa-2 cells. The apoptotic rate of cells
cultured in the absence of fetal bovine serum was 27.74%, whereas
the percentage of apoptosis of the cells cultured in the presence
of EG-VEGF was significantly lower (13.21%, P<0.05). These data
represent the average values derived from three independent
experiments. (C and D) Effects of EG-VEGF on the migration of the
Mia PaCa-2 cells. (C) Images of the Mia PaCa-2 cells 0 h and 24 h
following the wounding, treated with (100 ng/ml) or without
EG-VEGF. (D)The percentage of wound closure 24 h following
treatment with EG-VEGF (100 ng/ml). (E) Images of the Mia PaCa-2
cells treated with EG-VEGF (0, 25, 50, 100 ng/ml), following a
migration assay. (F) The number of invasive cells was counted under
a microscope. The data is expressed as the means ± standard
deviation of three independent experiments. *P<0.05,
as compared with the control cells. OD, optical density; nm,
nanometers. |
The effects of EG-VEGF on Mia PaCa-2
migration
The effects of EG-VEGF on the migration of the Mia
PaCa-2 cells were then determined. Images of the Mia PaCa-2 cells
were captured 0 and 24 h following wound simulation by scraping the
cells with a pipette tip, and incubation with or without 100 ng/ml
EG-VEGF (Fig. 2C). Following a 24
h incubation, the wound was almost completely healed in the EG-VEGF
treated cells. The closure of the wound reached 79±5.43% in the
EG-VEGF treated cells, as compared with 39±2.65% in the untreated
controls (Fig. 2D).
The effects of EG-VEGF on Mia PaCa-2
invasion
The role of EG-VEGF in the invasiveness of human
pancreatic cancer cells was determined using a Matrigel®
migration assay. The cells treated with EG-VEGF, had a
significantly higher number of cells migrating through the
Matrigel, as compared with the control cells (P<0.05). In the
presence of 0, 50, 100, or 200 ng/ml EG-VEGF, the number of
invading cells was 12±3.5, 52±9.5, 70±10.4, and 90±12.6
respectively (Fig. 2E and F).
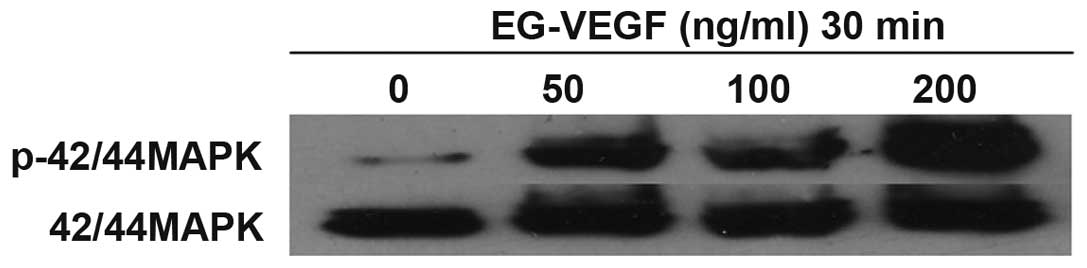
EG-VEGF activates the MAPK pathway in Mia
PaCa-2 cells
EG-VEGF induced the phosphorylation of p44/42 MAPK
in the cultured Mia PaCa-2 pancreatic cancer cells. Numerous
downstream signaling pathways were investigated that may
potentially be associated with EG-VEGF signaling in Mia PaCa-2
cells. Following exposure of the cells to various concentrations of
EG-VEGF for 30 min, the cell lysates were separated by
electrophoresis on an SDS-polyacrylamide gel, and transferred onto
a polyvinylidene difluoride membrane. The membranes were then
blotted with a phospho-p44/42 MAPK antibody (Fig. 3). A dose-dependent phosphorylation
of p44/42 MAPK was observed, in response to EG-VEGF treatment. The
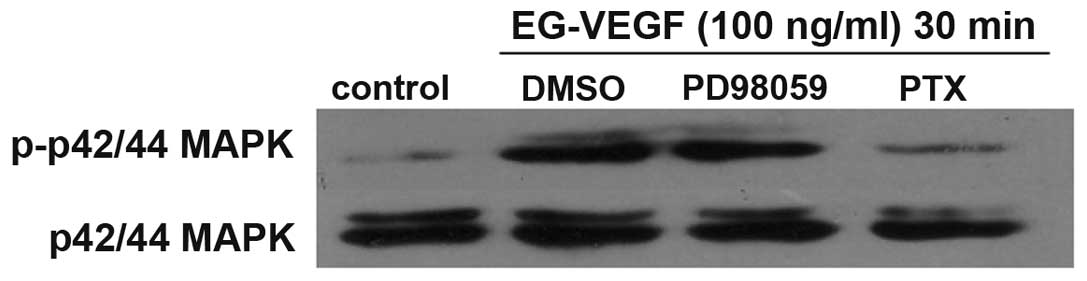
effects of PTX were determined on EG-VEGF-induced MAPK activation.
The MAPK activation was shown to be PTX-sensitive (Fig. 3). Pretreatment of the Mia PaCa-2
cells with 200 ng/ml PTX abolished EG-VEGF-induced p42/44 MAPK
phosphorylation. Since PTX near completely abolished the
EG-VEGF-induced phosphorylation of MAPK, it is likely that the
EG-VEGF receptor is G protein coupled. To determine the specificity
of the MAPK pathway being activated by EG-VEGF, the effects of the
MEK1 (MAPK2)-specific-inhibitor PD98059 were examined on
EG-VEGF-induced phosphorylation of p44/42 MAPK. Pretreatment of the
Mia PaCa-2 cells with PD98059 blocked EG-VEGF-induced
phosphorylation of p44/42 MAPK (Fig.
4).
Discussion
Patients suffering from pancreatic cancer have a
very poor prognosis, with only 1–4% of patients surviving for five
years. Pancreatic ductal adenocarcinoma (PDAC), composing 70% of
all cases of pancreatic cancer, is not only the most common form,
but also has the worst prognosis, of all of the pancreatic cancer
subtypes (7). Currently,
improvements in the treatment of pancreatic cancer patients are
limited. Therefore, the identification of molecular markers that
may aid the development of new therapeutic agents, is required.
There have been numerous reports on the role of
EG-VEGF as a novel angiogenic factor expressed in malignant tumors
(8–12), including colorectal cancer and
metastatic renal cell carcinoma. Therefore, evaluation of the
expression and action of EG-VEGF in pancreatic cancer may have
important clinical applications. It was previously shown that
EG-VEGF has a critical role in mediating anti-apoptotic effects in
a pancreatic cancer cell line (5).
The present study extended these findings, comparing the expression
of EG-VEGF in pancreatic tumor and normal pancreatic tissues. The
expression of EG-VEGF was significantly increased in the pancreatic
cancer tissues, as compared with the normal pancreatic tissues. The
expression of EG-VEGF was also shown to be much higher in the later
stages of pancreatic cancer, and there was a linear correlation to
this association. Therefore, it may be concluded that EG-VEGF has
an important role in the development and progression of pancreatic
neoplasms. Notably, the expression of EG-VEGF was also detected in
the normal pancreatic islets. This phenomenon has also been
observed in other studies (13,14).
The positive expression of the EG-VEGF in the normal pancreatic
islets suggests a function for EG-VEGF in the mediation of
endocrine function.
The malignant phenotype is an important biological
phenomenon in cancer (15–18). Proliferation, migration, invasion
and apoptosis are significant functions of cancer cells (19). It was previously demonstrated that
EG-VEGF protects pancreatic cancer cells from apoptosis (4). In the present study EG-VEGF was shown
to promote proliferation, apoptosis, migration and invasion in the
Mia PaCa-2 pancreatic cell line. This function has also been
reported in human neuroblastoma (20). The function of EG-VEGF in mediating
the malignant phenotype may indicate that this cytokine is a
crucial regulatory peptide in pancreatic cancer cells.
Further investigation is required to fully
understand the regulatory mechanisms of EG-VEGF. The MAPK pathway
is critical for cellular proliferation, migration and apoptosis
(21), therefore the potential for
EG-VEGF to stimulate p44/42 MAPK phosphorylation was also assessed
in the present study. The results demonstrated that EG-VEGF was
capable of inducing the activation of MAPK in pancreatic cancer
cells, leading to proliferation, migration, and survival (22,23).
The dose-dependent phosphorylation of MAPK p44/p42
is a sensitive indicator of EG-VEGF activity. The near complete
inhibition of MAPK phosphorylation by pretreatment with the MEK-1
inhibitor PD-98059 indicated that the activation of MEK1/2 is
necessary for EG-VEGF-induced MAPK p44/42 phosphorylation. These
data suggest that the EG-VEGF receptor may be a GPCR mediated by
MAPK activation. PTX has been shown to specifically modify the
heterotrimeric G-protein Gαi, blocking the signaling pathways of
GPCRs which involve Gαi. EG-VEGF-induced MAPK phosphorylation was
also shown to be PTX-sensitive (24).
The results of the present study show a strong
linear correlation between the expression of EG-VEGF in pancreatic
tumors and the stage of pancreatic cancer. Furthermore, EG-VEGF
modulated cellular proliferation, migration, and survival. In
conclusion, the results suggest that EG-VEGF may be a potential
candidate for gene therapy, or a target for other pancreatic cancer
therapies.
Acknowledgments
The present study was supported in part by the
Chinese National Natural Science Foundation (no. 81071982) and the
Doctoral Research Found of Liaoning Province (no. 20111094).
References
|
1
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics, 2006. CA Cancer J Clin. 56:106–130. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wolfgang CL, Herman JM, Laheru DA, et al:
Recent progress in pancreatic cancer. CA Cancer J Clin. 63:318–348.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
LeCouter J, Kowalski J, Foster J, et al:
Identification of an angiogenic mitogen selective for endocrine
gland endothelium. Nature. 412:877–884. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Monnier J and Samson M: Cytokine
properties of prokineticins. FEBS J. 275:4014–4021. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ren LN, Li QF, Xiao FJ, et al: Endocrine
glands-derived vascular endothelial growth factor protects
pancreatic cancer cells from apoptosis via upregulation of the
myeloid cell leukemia-1 protein. Biochem Biophys Res Commun.
386:35–39. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bosman FT: WHO Classification of Tumours
of the Digestive System. 4th. IARC Press; Lyon: 2010
|
|
7
|
Song JW and Lee JH: New morphological
features for grading pancreatic ductal adenocarcinomas. Biomed Res
Int. 2013:1752712013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nagano H, Goi T, Koneri K, et al:
Endocrine gland-derived vascular endothelial growth factor
(EG-VEGF) expression in colorectal cancer. J Surg Oncol.
96:605–610. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
LeCouter J, Lin R, Tejada M, et al: The
endocrine-gland-derived VEGF homologue Bv8 promotes angiogenesis in
the testis: Localization of Bv8 receptors to endothelial cells.
Proc Natl Acad Sci USA. 100:2685–2690. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kisliouk T, Levy N, Hurwitz A and Meidan
R: Presence and regulation of endocrine gland vascular endothelial
growth factor/prokineticin-1 and its receptors in ovarian cells. J
Clin Endocrinol Metab. 88:3700–3707. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Morales A, Vilchis F, Chávez B, et al:
Expression and localization of endocrine gland-derived vascular
endothelial growth factor (EG-VEGF) in human pancreas and
pancreatic adenocarcinoma. J Steroid Biochem Mol Biol. 107:37–41.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rini BI: Vascular endothelial growth
factor-targeted therapy in metastatic renal cell carcinoma. Cancer.
115:2306–2312. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang X, Abiatari I, Kong B, et al:
Pancreatic islet and stellate cells are the main sources of
endocrine gland-derived vascular endothelial growth
factor/prokineticin-1 in pancreatic cancer. Pancreatology.
9:165–172. 2009. View Article : Google Scholar
|
|
14
|
Morales A, Morimoto S, Díaz L, Robles G
and Díaz-Sánchez V: Endocrine gland-derived vascular endothelial
growth factor in rat pancreas: genetic expression and testosterone
regulation. J Endocrinol. 197:309–314. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dingli D, Chalub FA, Santos FC, Van
Segbroeck S and Pacheco JM: Cancer phenotype as the outcome of an
evolutionary game between normal and malignant cells. Br J Cancer.
101:1130–1136. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stasinopoulos I, Mori N and Bhujwalla ZM:
The malignant phenotype of breast cancer cells is reduced by COX-2
silencing. Neoplasia. 10:1163–1169. 2008.PubMed/NCBI
|
|
17
|
Nielsen JD, Moeslund M, Wandall HH and
Dabelsteen S: Influences of tumor stroma on the malignant
phenotype. J Oral Pathol Med. 37:412–416. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Melnikova VO and Bar-Eli M:
Transcriptional control of the melanoma malignant phenotype. Cancer
Biol Ther. 7:997–1003. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Grzesiak JJ and Bouvet M: Divalent cations
modulate the integrin-mediated malignant phenotype in pancreatic
cancer cells. Cancer Sci. 99:1553–1563. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ngan ES, Sit FY, Lee K, et al:
Implications of endocrine gland-derived vascular endothelial growth
factor/prokineticin-1 signaling in human neuroblastoma progression.
Clin Cancer Res. 13:868–875. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Koul HK, Pal M and Koul S: Role of p38 MAP
kinase signal transduction in solid tumors. Genes Cancer.
4:342–359. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Arlt A, Müerköster SS and Schäfer H:
Targeting apoptosis pathways in pancreatic cancer. Cancer Lett.
332:346–358. 2013. View Article : Google Scholar
|
|
23
|
Bayraktar S and Rocha-Lima CM: Advanced or
metastatic pancreatic cancer: molecular targeted therapies. Mt
Sinai J Med. 77:606–619. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Labasque M, Reiter E, Becamel C, et al:
Physical interaction of calmodulin with the 5-hydroxytryptamine2C
receptor C-terminus is essential for G protein-independent,
arrestin-dependent receptor signaling. Mol Biol Cell. 19:4640–4650.
2008. View Article : Google Scholar : PubMed/NCBI
|