Introduction
Epithelial-mesenchymal transition (EMT) was
initially described by developmental biologists, who indicated
specific morphological and phenotypic alterations in epithelial
cells during embryonic development (1). During this process, epithelial cells
lose their polarity and become similar in morphology to
fibroblasts, with reduced adhesion to the surrounding cells and
matrix, thus acquiring an enhanced migratory ability (2).
It is currently hypothesized that EMT is accompanied
by alterations in the expression of specific epithelial and
mesenchymal markers. Epithelial cell markers such as E-cadherin,
desmoplakin, tight junction protein, keratin, α-catenin, β-catenin
and γ-catenin are downregulated in EMT, whereas the expression of
mesenchymal tissue markers such as N-cadherin, α-smooth muscle
actin, vimentin and fibronectin protein is increased (3). Furthermore, other EMT-related
proteases, cytokines and transcription factors, including matrix
metalloproteinase-2/9, transforming cell growth factor, fibroblast
growth factor, Snail, Slug and Twist, are also upregulated
(4). In addition to its role in
embryonic development, previous studies have indicated that EMT is
closely associated with tumor progression and resistance to
chemotherapy (5). This has
resulted in an increased focus on EMT by academics, clinicians and
pharmaceutical researchers.
A large body of evidence indicates that the
transcription factor Brachyury, which is a member of the T-box
family, serves a key function during the process of EMT (5–7).
T-box family members contain the highly-conserved DNA-binding
domain, known as the T-box domain (8). As is the case with the other family
members, Brachyury is among the proteins that are conserved during
the differentiation process of the mesoderm (9–12).
Previous studies have suggested that Brachyury is vital in the
formation of the notochord (9).
In vitro experiments have also indicated that Brachyury may
induce mesenchymal differentiation in the embryonic stem cells of
the rhesus monkey (9,11,12).
A previous study demonstrated high expression levels of Brachyury
in a number of types of human cancer (13), which suggests that Brachyury is
important in the process of tumorigenesis, and may therefore be a
novel therapeutic target in human cancer.
In the current study, the expression levels of
Brachyury in normal human lung tissues and in tumor samples from
patients with non-small cell lung cancer (NSCLC) were examined. The
associations between Brachyury and various clinicopathological
factors were analyzed in 115 NSCLC samples. The impact of Brachyury
on the proliferative and invasive capacities of lung cancer cells,
in addition to NSCLC cell chemosensitivity, was also
investigated.
It was hypothesized that Brachyury is involved in
the induction of EMT, and that via this induction, upregulation of
Brachyury in NSCLC is able to exacerbate tumor malignancy.
Materials and methods
Patients and specimens
Ethical approval for the current study was obtained
from the Ethics committee of the Liaoning Cancer Hospital and
Institute (Shenyang, China). Primary tumor specimens were obtained
from 115 patients diagnosed with NSCLC, who underwent complete
tumor resection in the Liaoning Cancer Hospital and Institute
between January and December 2007. Control samples were taken from
adjacent non-cancerous normal lung tissues of the same patients.
Written informed consent was obtained from each patient or their
family. All 115 patients had complete follow-up records and
received no radiotherapy or chemotherapy prior to surgery. The 115
patients with NSCLC comprised 80 males and 35 females, with a
median age of 67.3 years (range, 47–86 years). The pathological TNM
(pTNM) staging system of the Union for International Cancer Control
(seventh edition) (14) was used
to classify specimens as: Stage I (n=40), stage II (n=33) and stage
III-IV (n=42). According to the classification of lung cancer by
the World Health Organization classification guidelines, 70 cases
were categorized as squamous cell carcinoma and 45 cases as
adenocarcinoma.
Immunohistochemistry
Surgically excised specimens were fixed with 10%
neutral formalin (Fuzhou Maixin Biotechnology Development Co.,
Fuzhou, China), embedded in paraffin (Leica, Wetzlar, Germany), and
cut into 4-μm sections. Immunostaining was conducted using
the avidin-biotin-peroxidase complex method (Ultrasensitive™;
Fuzhou Maixin Biotechnology Development Co.). The expression levels
of Brachyury, E-cadherin and N-cadherin were measured in 115 NSCLC
specimens, using the corresponding antibodies (goat anti-human
polyclonal anti-Brachyury, 1:100, sc-17745; mouse anti-human
monoclonal anti-E-cadherin, 1:100, sc-8426; and goat anti-human
polyclonal anti-N-cadherin, 1:100, sc-31030; all from Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), and the correlation
between the expression of these markers and clinicopathological
factors was analyzed. A total of 400 tumor cells were counted and
the percentage of cells with positive staining was calculated. The
proportion of cells exhibiting Brachyury expression was categorized
as follows: 0= absent; 1=1–25%; 2=26–50%; 3=51–75%; 4=≥76%. The
staining intensity was categorized as follows: 0= negative; 1=
weak; 2= moderate; 3= strong. The proportion and intensity scores
were then multiplied in order to obtain a total score. To obtain
final statistical results, scores <2 were considered to be
negative, while scores ≥2 were considered to be positive.
Cell culture and transfection
The A549 human lung adenocarcinoma cell line was
purchased from the American Type Culture Collection (Manassas, VA,
USA) cultured in RPMI 1640 medium (Invitrogen Life Technologies,
Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS;
Invitrogen Life Technologies). Small interfering RNAs (siRNAs) for
Brachyury, in addition to non-targeting siRNA, were purchased from
Santa Cruz Biotechnology, Inc., and consisted of pools of three to
five siRNAs to avoid off-target effects. Brachyury siRNA was
transfected into the A549 cell line using Lipofectamine®
2000 (Invitrogen Life Technologies).
MTT assay
Cells were plated in four 96-well plates in RPMI
1640 medium containing 10% FBS at a density of ~1×105/ml
cells per well. Each plate was divided into three groups: Blank,
control siRNA and Brachyury siRNA. For quantification of cell
viability, 20 μl 5 mg/ml MTT (Sigma-Aldrich, St. Louis, MO,
USA) was added to each well at 24, 48, 72 and 96 h
post-transfection. Following 4 h incubation, the media was removed
from each well and the resultant MTT formazan was solubilized in
150 μl dimethyl sulfoxide (Sigma-Aldrich). The results were
quantitated spectrophotometrically (Bio-Rad 550; Bio-Rad
Laboratories, Inc., Hercules, CA, USA) at a wavelength of 490 nm.
The experiments were repeated three times and growth curves of the
cells were plotted accordingly.
Western blot analysis
Total proteins from cells were extracted in lysis
buffer (Pierce, Rockford, IL, USA) and quantified using the
Bradford method. Fifty micrograms of protein was separated by 10%
sodium dodecyl sulfate polyacrylamide gel electrophoresis.
Separated proteins were transferred to polyvinylidene fluoride
membranes (Millipore, Billerica, MA, USA), and the membranes were
incubated overnight at 4°C with goat anti-human polyclonal
anti-Brachyury (1:100; sc-17745; Santa Cruz Biotechnology). After
incubation with peroxidase-coupled anti-goat immunoglobulins (Santa
Cruz Biotechnology) at 37°C for 2 h, bound proteins were
visual-ized by electrochemiluminescence (Pierce) and detected by
BioImaging System (UVP, Upland, CA, USA).
Matrigel invasion assay
The bottom of the Transwell® chambers
(Costar, Cambridge, MA, USA) was covered with Matrigel™ (BD
Biosciences, San Jose, CA, USA), 1:8, diluted in RPMI 1640 medium
(~50 μg/chamber). For sterilization, the prepared chambers
were irradiated with ultraviolet rays for 2 h. Prior to use, a
small amount of serum-free medium (Invitrogen Life Technologies)
was added, in order to hydrate the chambers. A total of 0.5 ml RPMI
1640 medium containing 1% FBS was added to the lower chamber,
1.5×10/ml cells were added to the upper chamber and the chambers
were subsequently incubated at 37°C for 48 h. The small chamber was
removed and rinsed with PBS and the cells from the upper chamber
were then removed from the microporous membrane using a cotton
swab. Cells were then fixed in 95% ethanol and stained with 0.5%
methylene blue (Sigma-Aldrich). The microporous membranes of the
lower chamber of cells was counted under an inverted microscope
(×200; TE2000; Nikon, Tokyo, Japan).
DDP toxicity analysis
A549 cells (3×105 cells/well) were
cultured in a 96-well plate for 18 h. Each plate was divided into
three groups: Blank, control siRNA and Brachyury siRNA, according
to transfection treatment. After 48 h, 1, 3 or 5 μg/ml
cisplatin (DDP) was added to the culture medium of each group and
incubated for 24 h. The control siRNA group with DDP treatments
formed the DDP treatment groups; the Brachyury siRNA group with DDP
treatments formed the combination treatment groups. The endpoint
viability of the A549 cells was quantified at 24 h following DDP
treatment using the MTT assay, as described above. The effect of
DDP alone or in combination with Brachyury-specific siRNA on the
cell proliferation inhibition rate after 24 h was calculated as
follows: Cell proliferation inhibition rate (%) = (1 - the
OD570 value of the experimental group/the
OD570 value of the control group) × 100. The experiment
was repeated three times.
Statistical analysis
SPSS version 13.0 for Windows was used for all
statistical analyses (SPSS, Inc., Chicago, IL, USA). The Pearson
χ2 test was used to examine possible correlations
between Brachyury and clinicopathological factors of NSCLC. The
Kaplan-Meier method was used to evaluate the prognosis of patients.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Clinical significance of Brachyury
expression in NSCLC
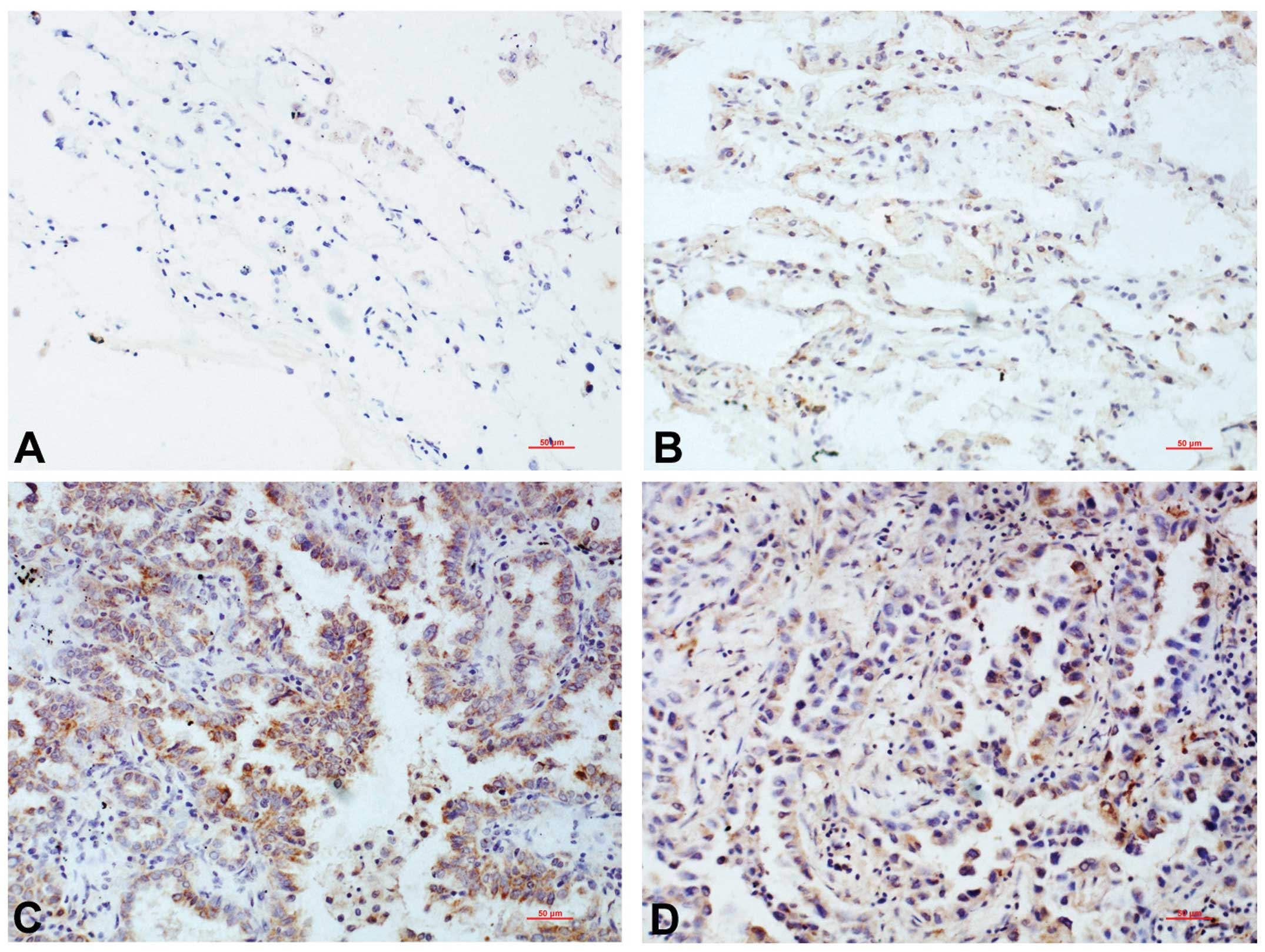
Brachyury was observed to be negatively or weakly
expressed in the cytoplasm of the matched normal lung tissues,
adjacent to the cancer tissues, and was defined as negative
expression according to the rating criteria (Fig. 1A and B). In the lung cancer
tissues, Brachyury was predominantly located in the nucleus, with a
low level of cytoplasmic expression. The proportion of cells that
was positive for Brachyury expression was 40.87% (47/115), which
was significantly higher than that in the normal lung tissues
(0/115; P<0.001) (Fig. 1C and
D).
Associations between Brachyury expression and
clinicopathological factors are presented in Table I. The positive expression of
Brachyury in lung adenocarcinoma was 55.56% (25/45), which was
significantly higher than that in squamous cell carcinoma samples
(31.43%, 22/70; P=0.01). The positive expression of Brachyury was
also correlated with higher tumor stages (III+IV vs. I+II; P=0.007)
and lymph node metastases (P<0.001). However, no correlation was
observed between Brachyury expression and patient age, gender or
tumor differentiation (P>0.05, Table I).
 | Table ISummary of the correlation between
Brachyury expression and clinicopathological characteristics of
NSCLC. |
Table I
Summary of the correlation between
Brachyury expression and clinicopathological characteristics of
NSCLC.
| Characteristic | Brachyury expression
|
|---|
| n | Negative | Positive | χ2 | P-value |
|---|
| Age (years) |
| <63 | 46 | 28 | 18 | 0.096 | 0.757 |
| ≥63 | 69 | 40 | 29 | | |
| Gender |
| Male | 80 | 52 | 28 | 3.747 | 0.053 |
| Female | 35 | 16 | 19 | | |
| Histology |
| Squamous cell
carcinoma | 70 | 48 | 22 | 6.6 | 0.01* |
| Adenocarcinoma | 45 | 20 | 25 | | |
| Tumor
differentiation |
| Well to
moderate | 93 | 54 | 39 | 0.229 | 0.633 |
| Low | 22 | 14 | 8 | | |
| TNM stage |
| I+II | 73 | 50 | 23 | 7.25 | 0.007a |
| III+IV | 42 | 18 | 24 | | |
| Lymph node
metastasis |
| Yes | 61 | 46 | 15 | 14.25 | <0.001a |
| No | 54 | 22 | 32 | | |
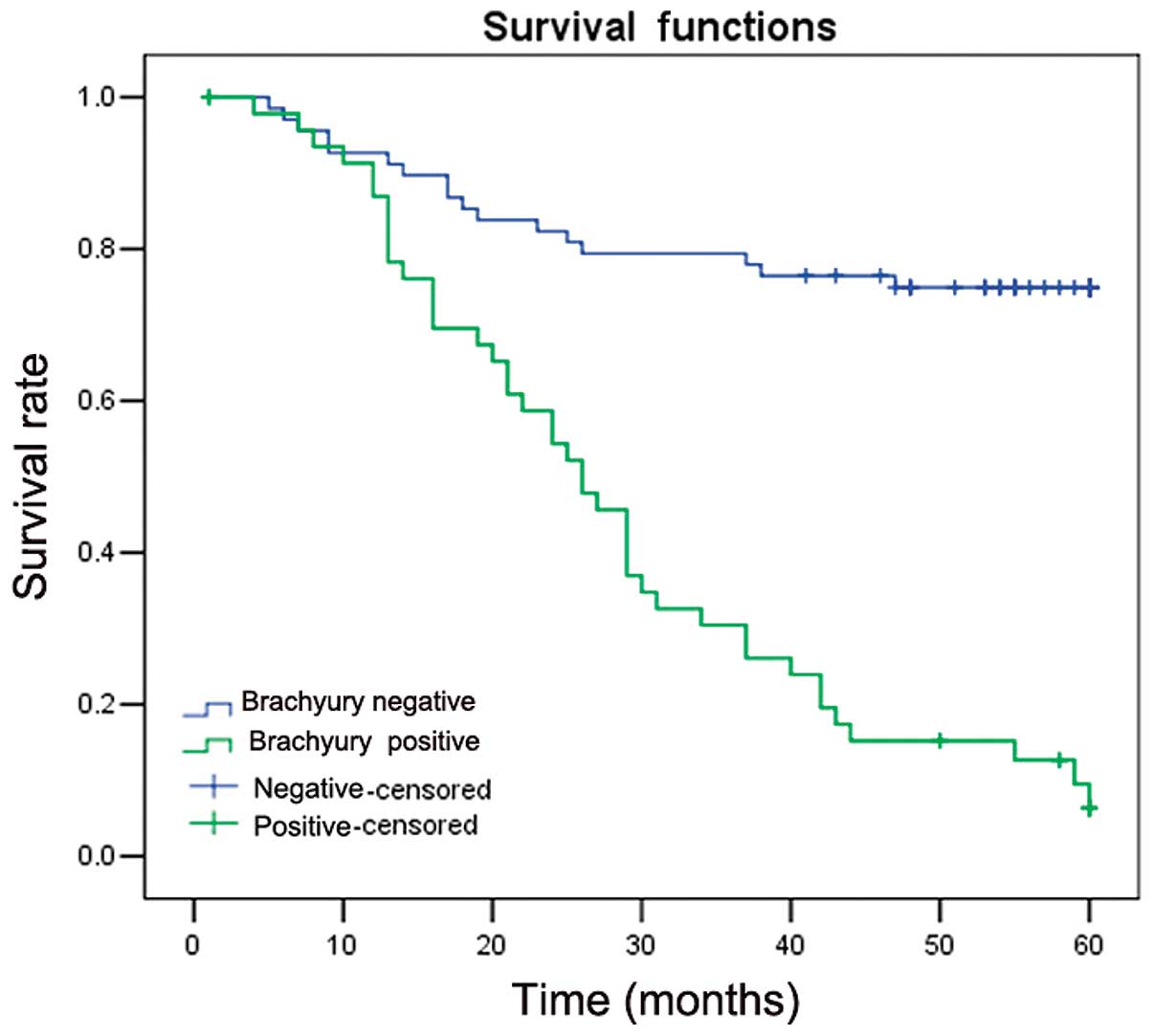
Kaplan-Meier survival analysis showed that patients
with Brachyury expression exhibited shorter average survival times
compared with patients with negative expression of Brachyury
(34.21±3.01 vs. 45.81±2.37 months; P=0.001). This suggested that
Brachyury expression is associated with a poorer prognosis
(Fig. 2).
The impact of Brachyury on the expression
of N-cadherin and E-cadherin in lung cancer
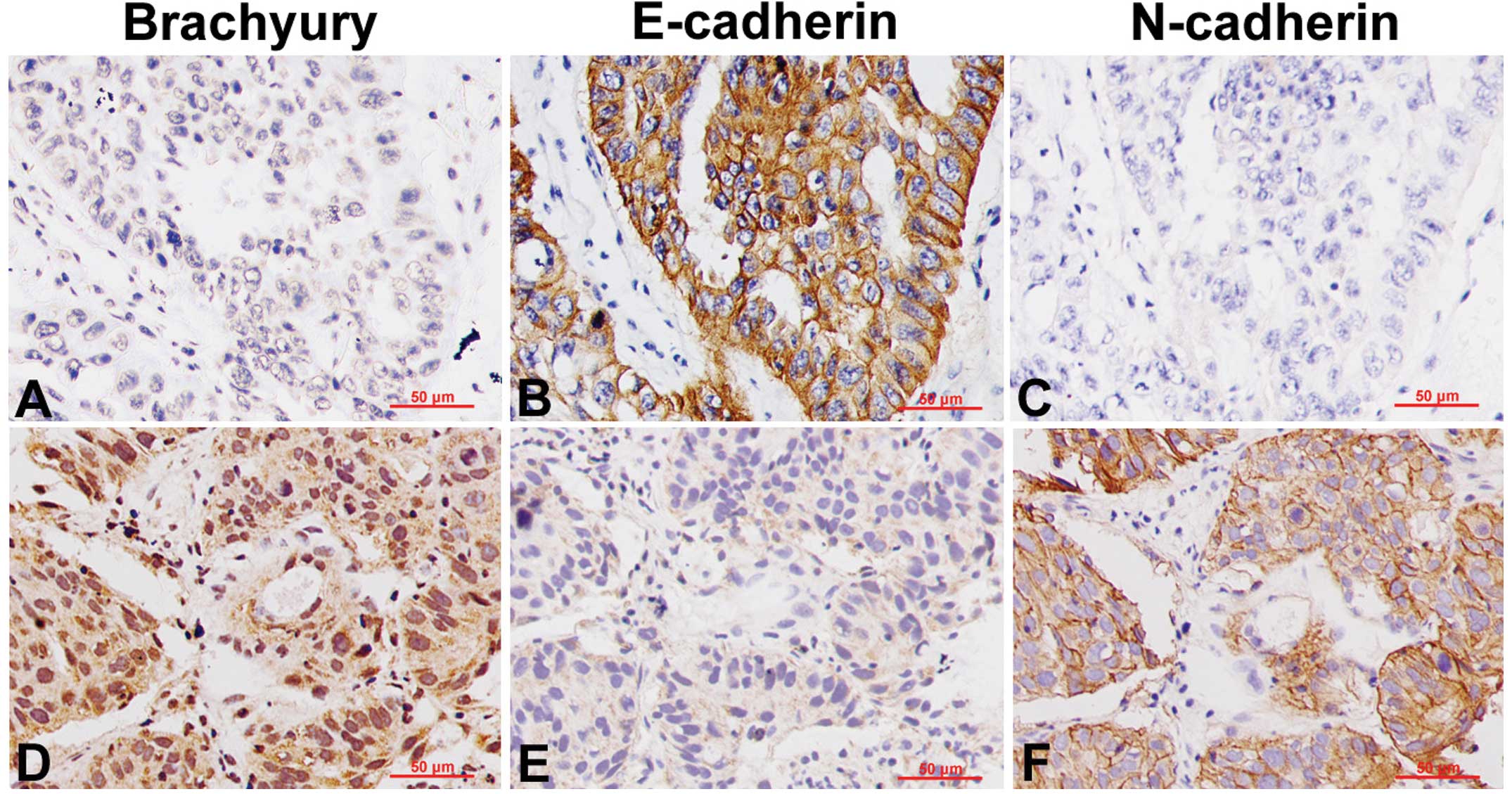
Immunohistochemical analysis demonstrated a high
expression of E-cadherin in the Brachyury-negative lung cancer
tissues, with obvious staining of the cell membrane (Fig. 3A and B), whereas N-cadherin
expression was either negative or low (Fig. 3C). By contrast, in the
Brachyury-positive lung cancer tissues, E-cadherin expression was
significantly reduced (Fig. 3D and
E), while N-cadherin expression was increased, with marked
membrane staining (Fig. 3F).
Brachyury promotes lung cancer cell
proliferation and invasion
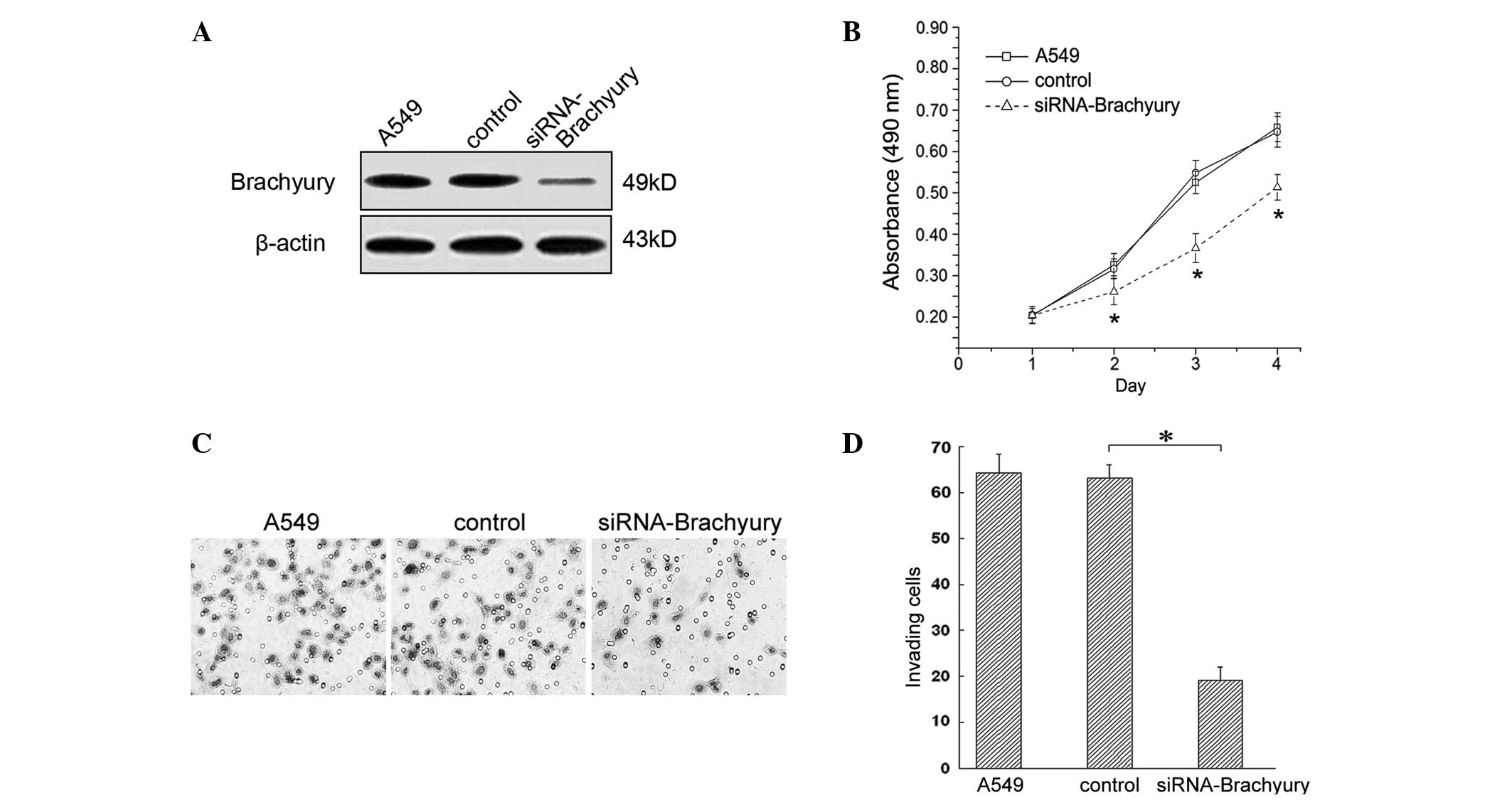
In order to study the impact of Brachyury expression
on cell proliferation, an MTT assay was used. In the A549 human
lung adenocarcinoma cell line, 24 h following treatment with
Brachyury-specific siRNA, no significant difference in the
proliferative capacity of each group was observed (P>0.05;
Fig. 4A and B). However, from the
second day, the lung cancer cells treated with Brachyury-specific
siRNA, demonstrated a lower proliferative capacity compared with
the control group (Fig. 4B,
P<0.05).
A Matrigel assay was performed to study the impact
of Brachyury on the invasive capacity of A549 cells. The results
indicated that 48 h after Brachyury knockdown, the average number
of invasive cells was 19.17±2.89, which was significantly lower
than that in the control group (63.47±2.93; P<0.05; Fig. 4C and D).
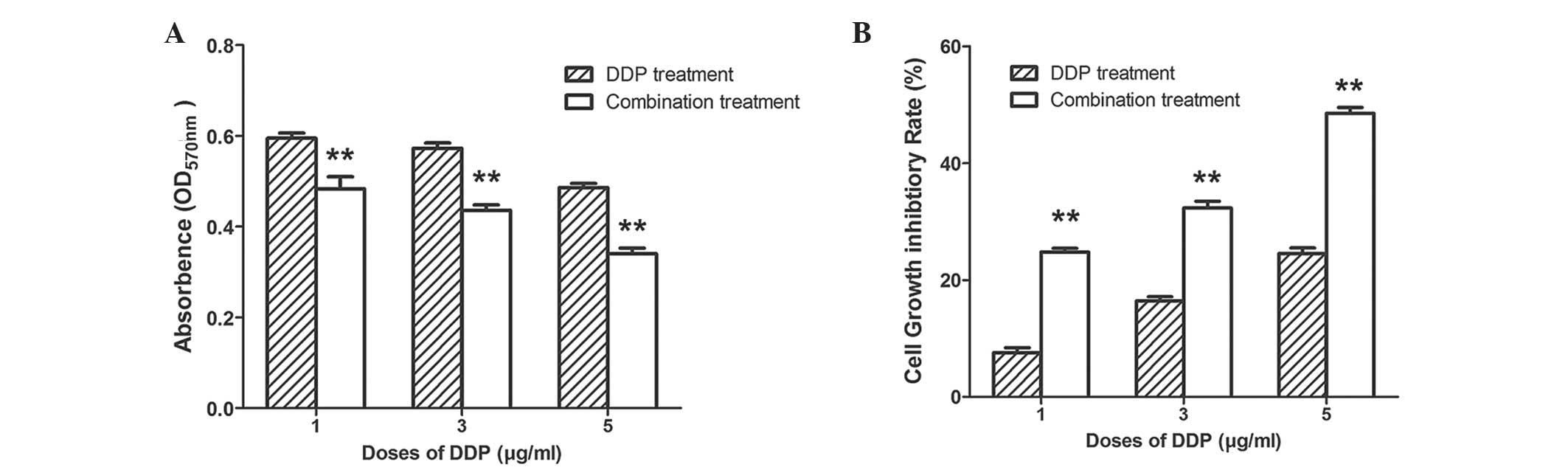
Knockdown of Brachyury increases cell
sensitivity to DDP
DDP is a widely used chemotherapeutic drug and is
frequently administered as combination chemotherapy (15). Therefore, the cell toxicity of DDP
was tested in combination with silencing of Brachyury in A549
cells. In the MTT assay, the OD570 of cells in the
control group without DDP treatment was 0.703±0.006. The
OD570 of cells treated with 1, 3 or 5 μg/ml DDP
was observed to be 0.598±0.014, 0.573±0.012 and 0.490±0.013,
respectively. However, when combining DDP (1, 3 and 5 μg/ml)
with Brachyury-specific siRNA, the OD570 of cells was
0.483±0.027, 0.436±0.012 and 0.334±0.018, respectively (Fig. 5A; P<0.01 between the DDP and
combination groups at each corresponding concentration). This
indicated that the combination of these treatment may result in a
higher cytotoxicity in A549 cells than DDP treatment alone.
For cells treated with 1, 3 or 5 μg/ml DDP,
the cell growth inhibition rates were 7.53±0.88%, 16.40±0.73% and
24.54±0.95%, respectively. When combining DDP with
Brachyury-specific siRNA, the cell growth inhibitory rates were
24.79±0.64, 32.33±1.16 and 48.54±1.00%, respectively (Fig. 5B; P<0.01 between the DDP and
combination groups at each corresponding concentration). Thus, with
the same concentration of DDP, there was a higher cytotoxic effect
for the combined treatment group than the DDP alone group.
Discussion
Brachyury is a T-box transcription factor,
containing the highly conserved T-domain, which has the capacity to
bind to DNA (5–7). Overexpression of Brachyury in human
carcinoma cells is reported to induce alterations in the
characteristics of EMT, including an increase in mesenchymal
markers, a reduction of epithelial markers, and the upregulation of
cell migration and invasion (16).
However, the mechanisms underlying the involvement of Brachyury in
EMT remain to be fully elucidated in human lung cancer. Therefore,
the current study aimed to investigate the role of Brachyury in EMT
and chemosensitivity in 115 NSCLC samples.
A previous study observed that Brachyury is highly
expressed in various human tumor cell lines, but not in the
majority of healthy human adult tissues (13). In accordance with this finding, the
current study identified that Brachyury was negatively or weakly
expressed in the cytoplasm of normal lung tissue samples, whereas
it was predominantly positively expressed in the NSCLC tissues. The
associations between Brachyury expression and clinicopathological
factors were also investigated. In accordance with the previously
proposed hypothesis that the expression of Brachyury is positively
associated with tumorigenesis and malignancy (16–19),
the expression of Brachyury in samples of NSCLC from patients with
lymph node metastases was observed to be higher than in tumors from
patients without metastases. The positive correlation between
Brachyury expression and lung tumor malignancy suggests that
Brachyury may be associated with lung tumor progression and makes
Brachyury a potential target for lung cancer therapy.
Brachyury has widely been reported to function in
the initiation and promotion of EMT (16,20).
In the current study, the data obtained was consistent with that
from previous studies, and suggested that in NSCLC, EMT was
associated with the expression of Brachyury. E-cadherin, a
cell-adhesion molecule, is a well-known suppressor of cell invasion
(3,21). In lung cancer tissues with positive
expression of Brachyury, the downregulation of E-cadherin may
result in the initiation of EMT.
A clinical trial of a vaccine for Brachyury-positive
tumors is currently underway in patients with solid tumours, as a
result of further evidence supporting the hypothesis that Brachyury
may be an important potential target for tumor therapy (22). In the current study, the data
demonstrated that knockdown of Brachyury by specific siRNAs
significantly attenuates the resistance of A549 cells to DDP
treatment. Kobayashi et al (17) demonstrated that knockdown of
Brachyury increases the sensitivity of adenoid cystic carcinoma
cells to chemotherapy and radiation in vivo; therefore,
Brachyury may be involved in the regulation of cell cycle
progression, which alters the therapeutic effects of conventional
cancer treatments, including chemotherapy, radiotherapy and immune
therapy. Further investigation of Brachyury is required to provide
more evidence for its function in cancer therapy.
In conclusion, the present study demonstrated that
Brachyury expression is associated with TNM staging, lymph node
metastasis and the prognosis of NSCLC. Brachyury expression is also
accompanied by the downregulation of E-cadherin and the
upregulation of N-cadherin. Brachyury may promote lung cancer via
the induction of EMT, which leads to invasion and metastasis of
NSCLC, and a consequent poor prognosis. These results, in
combination with those of previous studies, support the hypothesis
that Brachyury may be a novel target for the prevention and
treatment of lung cancer.
Acknowledgments
This study was supported by the Liaoning BaiQianWan
Talents Program (grant no. 2012921017).
References
|
1
|
Thiery JP: Epithelial-mesenchymal
transitions in development and pathologies. Curr Opin Cell Biol.
15:740–746. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Radisky DC: Epithelial-mesenchymal
transition. J Cell Sci. 118:4325–4326. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guarino M, Rubino B and Ballabio G: The
role of epithelial-mesenchymal transition in cancer pathology.
Pathology. 39:305–318. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang J, Mani SA, Donaher JL, et al: Twist,
a master regulator of morphogenesis, plays an essential role in
tumor metastasis. Cell. 117:927–939. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Herrmann BG, Labeit S, Poustka A, King TR
and Lehrach H: Cloning of the T gene required in mesoderm formation
in the mouse. Nature. 343:617–622. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kispert A and Hermann BG: The Brachyury
gene encodes a novel DNA binding protein. EMBO J. 12:4898–4899.
1993.PubMed/NCBI
|
|
7
|
Edwards YH, Putt W, Lekoape KM, Stott D,
Fox M, Hopkinson DA and Sowden J: The human homolog T of the mouse
T(Brachyury) gene; gene structure, cDNA sequence, and assignment to
chromosome 6q27. Genome Res. 6:226–233. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Papaioannou VE and Silver LM: The T-box
gene family. Bioessays. 20:9–19. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Technau U and Scholz CB: Origin and
evolution of endoderm and mesoderm. Int J Dev Biol. 47:531–539.
2003.
|
|
10
|
Kispert A, Herrmann BG, Leptin M and
Reuter R: Homologs of the mouse Brachyury gene are involved in the
specification of posterior terminal structures in Drosophila,
Tribolium, and Locusta. Genes Dev. 8:2137–2150. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Behr R, Heneweer C, Viebahn C, Denker HW
and Thie M: Epithelial-mesenchymal transition in colonies of rhesus
monkey embryonic stem cells: A model for processes involved in
gastrulation. Stem cells. 23:805–816. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vidricaire G, Jardine K and McBurney MW:
Expression of the Brachyury gene during mesoderm development in
differentiating embryonal carcinoma cell cultures. Development.
120:115–122. 1994.PubMed/NCBI
|
|
13
|
Palena C, Polev DE, Tsang KY, Fernando RI,
Litzinger M, Krukovskaya LL, Baranova AV, Kozlov AP and Schlom J:
The human T-box mesodermal transcription factor Brachyury is a
candidate target for T-cell-mediated cancer immunotherapy. Clin
Cancer Res. 13:2471–2478. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Goldstraw P: Updated staging system for
lung cancer. Surg Oncol Clin N Am. 20:655–666. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
D’Antonio C, Milano A, Righini R, et al:
Pharmacogenomics in lung cancer chemotherapy: a review of what the
oncologist should know. Anticancer Res. 34:5241–5250. 2014.
|
|
16
|
Fernando RI, Litzinger M, Trono P,
Hamilton DH, Schlom J and Palena C: The T-box transcription factor
Brachyury promotes epithelial-mesenchymal transition in human tumor
cells. J Clin Invest. 120:533–544. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kobayashi Y, Sugiura T, Imajyo I, Shimoda
M, Ishii K, Akimoto N, Yoshihama N and Mori Y: Knockdown of the
T-box transcription factor Brachyury increases sensitivity of
adenoid cystic carcinoma cells to chemotherapy and radiation in
vitro: Implications for a new therapeutic principle. Int J Oncol.
44:1107–1117. 2014.PubMed/NCBI
|
|
18
|
Haro A, Yano T, Kohno M, Yoshida T, Koga
T, Okamoto T, Takenoyama M and Maehara Y: Expression of Brachyury
gene is a significant prognostic factor for primary lung carcinoma.
Ann Surg Oncol. 20(Suppl 3): S509–S516. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kilic N, Feldhaus S, Kilic E, et al:
Brachyury expression predicts poor prognosis at early stages of
colorectal cancer. Eur J Cancer. 47:1080–1085. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Imajyo I, Sugiura T, Kobayashi Y, et al:
T-box transcription factor Brachyury expression is correlated with
epithelial-mesenchymal transition and lymph node metastasis in oral
squamous cell carcinoma. Int J Oncol. 41:1985–1995. 2012.PubMed/NCBI
|
|
21
|
Onder TT, Gupta PB, Mani SA, Yang J,
Lander ES and Weinberg RA: Loss of E-cadherin promotes metastasis
via multiple downstream transcriptional pathways. Cancer Res.
68:3645–3654. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hamilton DH, Litzinger MT, Jales A, et al:
Immunological targeting of tumor cells undergoing an
epithelial-mesenchymal transition via a recombinant brachyury-yeast
vaccine. Oncotarget. 4:1777–1790. 2013.PubMed/NCBI
|