Introduction
Nicotine is a major component of cigarette smoke.
The adverse effects of nicotine on the cardiovascular system have
been well documented (1,2). A growing body of evidence has
revealed that offspring exposed to an adverse intrauterine
environment develop a series of cardiovascular disorders, including
atherosclerosis (3), obesity
(4), cardiac arrhythmias (5), coronary artery disease (6) and hypertension (7,8). In
addition, compelling evidence has demonstrated that acute and
chronic cigarette smoking exacerbates arterial stiffness (9), as well as myocardial fibrosis
(10). In canine models exposed to
nicotine, increased left ventricle (LV) chamber stiffness is
identified due to increased collagen deposition and collagen
cross-links in the myocardium (11). However, insufficient data are
available on the effects of prenatal nicotine exposure (PNE) on
combined ventricular-arterial stiffening, designated
‘ventricular-arterial uncoupling’.
Ventricular-arterial coupling, meaning the
interaction of the heart with the systemic vasculature, is a key
determinant of cardiovascular performance. Ventricular-arterial
integration can be quantified via examination of the ratio of
effective arterial elastance (Ea) and LV end-systolic elastance
(Ees). Ea, the ratio of end systolic pressure (PES)/stroke volume,
is representative of arterial loading, while Ees is an indicator of
ventricular end-systolic elastance. The ratio is used to index
relative coupling between the heart and vascular system (12).
Boychuk et al (13) reported that PNE gender-dependently
compromised cardiorespiratory integration in vivo during
early postnatal development and primarily affected male offspring.
Clinically, convincing evidence has indicated that smoking disrupts
complex hemodynamic mechanisms even in young smokers with a
resultant increase in myocardial workload, a decreased capacity for
coronary perfusion and blunted ventricular-vascular dynamics
(14). However, to date, few
animal studies have been designed to investigate the effects of PNE
on ventricular-vascular integration in adult offspring in
vivo. Thus, the aim of the present study was to examine whether
PNE causes a similar alteration in ventricular-arterial coupling
and subsequently to investigate whether this is associated with
myocardial fibrosis, aortic elasticity properties and the
morphology of resistance vessels.
Materials and methods
Ethics statement
All the procedures and protocols were approved by
the Fujian Medical University Institutional Animal Care and Use
Committee (Fuzhou, China) and followed the guidelines outlined by
the National Institutes of Health Guide for the Care and Use of
Laboratory Animals (National Institutes of Health, Bethesda, MD,
USA).
Animals and experimental protocol
Female Sprague-Dawley rats (n=10) weighing 300±35 g
were purchased from the Shanghai Laboratory Animal Center of the
Chinese Academy of Sciences (Shanghai, China). The rats were
exposed to nicotine (n=8, 8 mg/kg/day) or saline (n=4) via
subcutaneous osmotic mini-pumps (Alzct Model 2ML4; Alza Corp., Palo
Alto, CA, USA) throughout gestation as described previously
(8,15). Natal pups were kept with their
mothers until weaning. At weaning, male and female pups were
separated and housed in temperature and humidity-controlled rooms
with a 12 h light-dark cycle. They were administered standard chow
and mineral water ad libitum. Caudal artery systolic blood
pressure (SBP), diastolic blood pressure (DBP) and pulse pressure
(PP) was monitored in nonanesthetized pups every 2 weeks with a
tail cuff system (BP-98A; Softron, Tokyo, Japan). Male and female
offspring were sacrificed by intraperitoneal injection of 200 mg/kg
pentobarbital (Sigma-Aldrich, St. Louis, MO, USA) at 12 months-old
to determine the effects of PNE on the coupling conditions of the
LV and artery system.
Quantification of cardiac geometry and
function using echocardiography
Cardiac geometry and function of anesthetized
offspring (ketamine 50 mg/kg and diazepam 2.5 mg/kg) were evaluated
by transthoracic echocardiography (Sonos 7500; Philips Healthcare,
Eindhoven, Netherlands) with a 15 MHz-transducer. The dosage
regimen was previously demonstrated to have minimal
cardiorespiratory effects when compared with other suitable
anesthetics (16). For calculation
of intraobserver variability, examinations were repeated by the
same examiner and for interobserver variability, examinations were
performed independently by two investigators. The probe was placed
to obtain short and long-axis and four-chamber views. From the
long-axis view, an M mode trace of the LV was obtained, and left
ventricular end systolic diameter, LV end diastolic diameter and LV
wall thickness were measured. Left ventricular end diastolic volume
(EDV) and end systolic volume were determined using the biplane
Simpson method (17). Stroke
volume was the difference between end-diastolic volume and
end-systolic volume. LV end-systolic meridional wall stress was
calculated using the following formula (18,19):
0.34PD/[(1+h/D)h], where P is LV pressure (mmHg), D is LV cavity
diameter, h is wall thickness and 0.34 is the conversion factor
from mmHg to gram-force/cm2.
Assessment of left ventricular and
arterial hemodynamics with a conductance catheter
Following the echocardiography study, a 1.5 F
high-fidelity manometer-tipped catheter (SPR-407; Millar
Instruments, Houston, TX, USA) was introduced through the right
carotid artery into the left ventricle. PES and the maximal rates
of increases and decreases in LV pressure (dP/dtmax and
dP/dtmin, respectively) were recorded and analyzed using
PowerLab Chart 4.1.2 software (ADInstruments, Inc., Bella Vista,
New South Wales, Australia). Successively, central aortic SBP, DBP
and PP were recorded when the conductance catheter was withdrawn
from the LV into the ascending aorta. To quantitate the
ventricular-arterial interaction, the LV and the arterial system
are considered elastic chambers with known LV Ees and Ea,
respectively. Effective pulmonary arterial elastance, as a measure
of right ventricular (RV) afterload, was calculated as end-systolic
pressure/stroke volume. An indicator of ventricular end-systolic
elastance can be determined according to the following formula
(20):
Ees=0.10e0.15/EDVxdP/dtmax. Subsequently, the coupling
parameter Ea/Ees was examined. The augmentation index (AI) was
calculated as the ratio of ΔP to PP, where ΔP was defined as the
height from the shoulder of the reflected wave to the systolic peak
(P2-P1) (21). For each rat, all
the above-mentioned parameters were determined in 100–150
consecutive cardiac cycles and the results were averaged.
Elastic properties of the aorta
Following in vivo measurement of mechanical
parameters, a 5 mm sample of the descending aorta and left
ventricle was rapidly harvested. Fragmentation of the medial
elastic fiber network (excluding the external and internal laminae)
was evaluated on 6 μm thick sections stained with van
Gieson’s solution by measuring the increase in the number of
elastic lamellae (22). The number
of elastic lamellae in each of four quadrants were counted using
light microscopy (BX51; Olympus Corp., Tokyo, Japan).
Medial thickness to internal diameter
ratio in the descending aorta and mesenteric artery
The aorta and mesenteric artery were stained with
hematoxylin & eosin (H&E) and the aortic medium thickness
(MT), internal diameter (ID) and the ratio of MT/ID were
determined.
Myocardial cell cross-sectional area and
collagen volume fraction
Quantitative histomorphometry of the left
ventricular myocardium was performed following H&E staining.
The mean of the cardiomyocyte cross-sectional area and the diameter
were calculated using Image-Pro Plus 6.0 software (Media
Cybernetics, Rockville, MD, USA). Collagen volume fraction was
assessed using Sirius-red staining.
Collagen composition
The LV collagen content was estimated from the
hydroxyproline content determined by the colorimetric method
described by Leipner et al (23). The quantity of hydroxyproline was
multiplied by the conversion factor 7.46 to calculate total
collagen (24). To determine
soluble collagen content, myocardium was extracted and digested
with cyanogen bromide (CNBr) according to the modified procedure of
Yamamoto et al (25). The
quantity of non-cross-linked (soluble) and cross-linked (insoluble)
collagen in the myocardium was determined from the product of the
percentage of collagen soluble to CNBr digestion, the total
myocardial collagen concentration and the difference between the
total collagen concentration and soluble collagen concentration,
respectively. The association between insoluble and total collagen
was used as an index of the degree of collagen cross-linking.
Statistical analysis
All values are expressed as the mean ± standard
deviation unless otherwise indicated. Differences were determined
by unpaired Student’s t-test. Intraobserver reproducibility was
assessed by calculating the coefficient of variation and
interobserver reproducibility by two-way analysis of variance. All
reported probability values are two-tailed and P<0.05 was
considered to indicate a statistically significant difference.
Statistical analyses were performed using SPSS 17.0 (SPSS, Inc.,
Chicago, IL, USA).
Results
Basic hemodynamic parameters
Maternal treatment with nicotine modified neither
body weight (BW) nor LV/BW in either gender group (Table I). Similarly, maternal nicotine
administration failed to affect SBP and DBP in the two groups,
although the SBP in the male offspring tended to increase. However,
caudal artery PP markedly increased in 12 month-old males, while it
remained comparable in females compared with their gender-matched
controls.
 | Table IBody weight and basic caudal artery
hemodynamic parameters. |
Table I
Body weight and basic caudal artery
hemodynamic parameters.
| Parameters | Female offspring
| Male offspring
|
|---|
| Control (n=9) | PNE (n=12) | Control (n=10) | PNE (n=8) |
|---|
| BW (g) | 348.17±21.42 | 362.87±17.31 | 628.58±26.01 | 595.43±20.36 |
| LV/BW (mg/g) | 3.10±0.43 | 2.91±0.53 | 2.54±0.34 | 2.42±0.44 |
| Heart rate
(beats/min) | 394±17 | 425±10 | 410±15 | 430±12 |
| SBPc (mmHg) | 141.95±5.16 | 151.49±9.48 | 148.41±7.14 | 155.31±10.27 |
| DBPc (mmHg) | 89.37±4.26 | 92.33±6.28 | 98.42±9.84 | 97.31±7.06 |
| PPc (mmHg) | 47.77±3.47 | 53.28±6.36 | 50.16±4.94 | 56.36±7.41a |
Echocardiographic properties
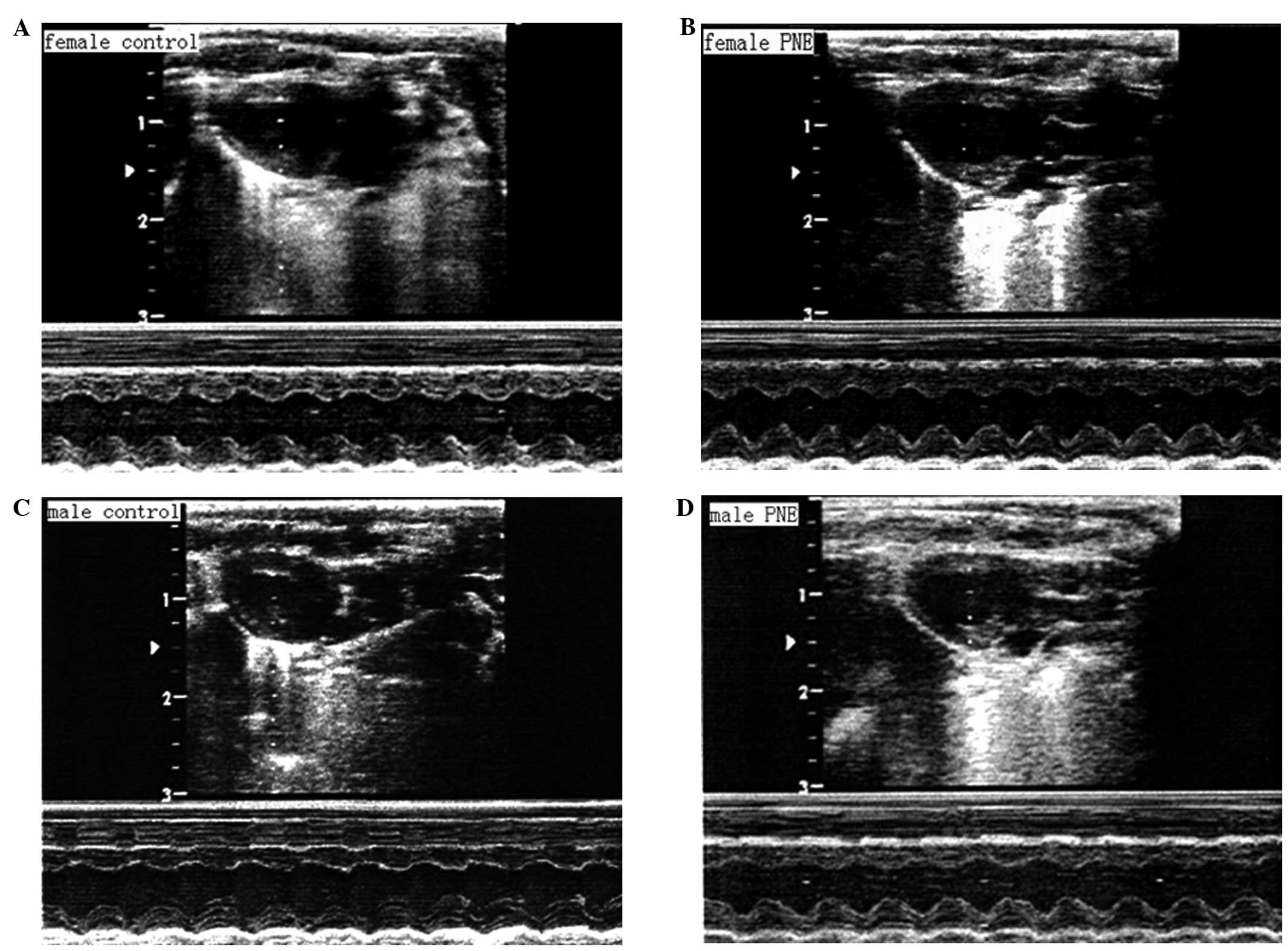
Echocardiographic examination revealed comparable LV
end-systolic diameter, end-diastolic diameter and LV wall thickness
(posterior and septal wall diastolic thickness) among the groups
(Table II; Fig. 1). Prenatal nicotine exposure
markedly reduced stroke volume by 25.71% in 12 month-old male
offspring, but had no significant effect on stroke volume in female
pups. Estimated left ventricular meridional wall stress was
significantly enhanced in males whereas it remained unaltered in
females following maternal nicotine administration. The
intraobserver (3.05%) and interobserver (6.13%) variabilities were
acceptable.
 | Table IIEchocardiographic properties. |
Table II
Echocardiographic properties.
| Properties | Female offspring
| Male offspring
|
|---|
| Control (n=9) | PNE (n=12) | Control (n=10) | PNE (n=8) |
|---|
| SWTd (cm) | 0.16±0.03 | 0.15±0.05 | 0.23±0.04 | 0.24±0.03 |
| PWTd (cm) | 0.19±0.04 | 0.20±0.03 | 0.26±0.06 | 0.25±0.05 |
| EDD (cm) | 0.85±0.11 | 0.80±0.09 | 0.82±0.13 | 0.72±0.14 |
| ESD (cm) | 0.59±0.10 | 0.61±0.07 | 0.63±0.10 | 0.52±0.08 |
| EDV (ml) | 0.65±0.13 | 0.53±0.14 | 0.59±0.09 | 0.37±0.11 |
| SV (ml) | 0.33±0.02 | 0.32±0.03 | 0.35±0.03 | 0.26±0.03a |
| LVMWS
(kdyne/cm2) | 62.62±7.34 | 64.92±9.64 | 69.64±7.58 | 78.25±9.12a |
Ventricular and arterial
hemodynamics
As shown in Table
III, SBP, DBP and PP in males and females tended to be higher
compared with age and gender-matched controls, however, no
statistical significance was noted. Conversely, increased AI and
dP/dtmax and decreased dP/dtmin were found in
males and females following maternal nicotine treatment compared
with their respective control. These effects were more pronounced
in males than in females. Ea/Ees did not differ significantly
between PNE and control females due to tandem increases in Ea and
Ees, suggesting that ventricular-arterial coupling was matched. By
contrast, Ea/Ees was evidently lower in PNE males than control
males (0.77±0.04 vs. 0.30±0.09; P<0.05), owing to a
disproportionate increase in Ees (by 2.19-fold) vs. Ea (by
1.40-fold).
 | Table IIIHemodynamic parameters. |
Table III
Hemodynamic parameters.
| Hemodynamic
parameters | Female offspring
| Male offspring
|
|---|
| Control (n=9) | PNE (n=12) | Control (n=10) | PNE (n=8) |
|---|
| Central SBP
(mmHg) | 132.05±12.36 | 145.37±14.91 | 147.87±12.15 | 155.47±17.06 |
| Central DBP
(mmHg) | 92.07±8.15 | 100.38±7.30 | 105.26±9.13 | 112.04±10.25 |
| PP (mmHg) | 32.47±3.11 | 40.38±6.07 | 41.42±4.06 |
47.29±6.03* |
| Augmentation index
(%) | 27.64±3.05 | 34.46±4.25a | 29.12±5.21 | 37.34±5.72a |
| Ea
(mmHg/μl) | 0.35±0.09 | 0.42±0.07a | 0.40±0.08 | 0.56±0.10a |
| dP/dtmax
(mmHg/ms) | 5694.03±312.47 |
6624.38±483.15a | 6490.25±547.86 |
7295.36±634.03a |
| dP/dtmin
(mmHg/ms) | 4384.82±596.36 |
3852.27±302.89a | 4918.75±368.25 |
3156.89±248.26b |
| Ees
(mmHg/μl) | 0.37±0.15 | 0.65±0.13a | 0.53±0.09 | 1.92±0.15b |
| Ea/Ees | 0.94±0.07 | 0.65±0.05 | 0.77±0.04 | 0.30±0.09b |
Left ventricular cardiomyocyte
cross-sectional area and total and insoluble collagen content
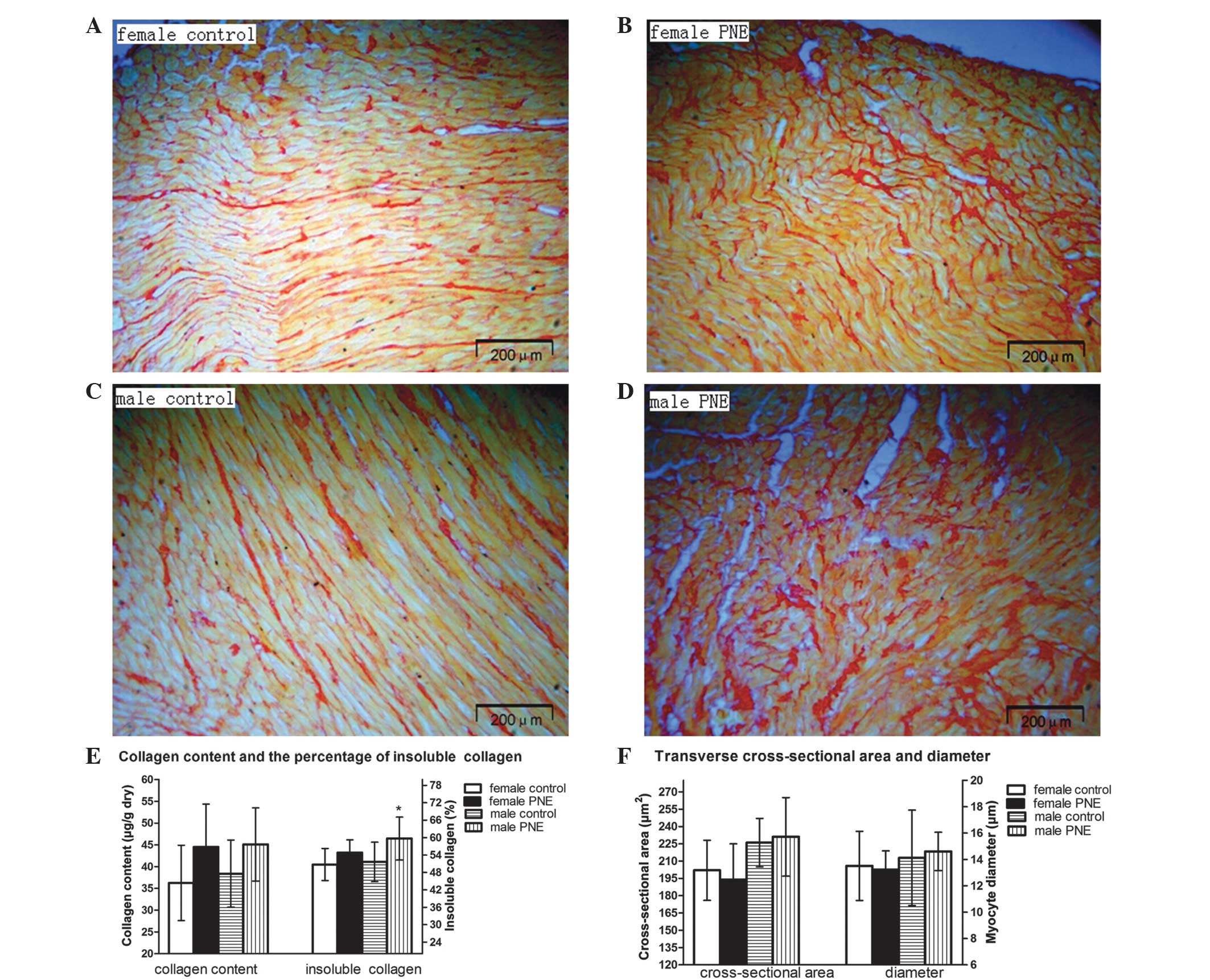
Maternal nicotine administration showed no
significant affect of LV collagen content, as evidenced by similar
collagen volume fractions and hydroxyproline quantification in the
two groups (Fig. 2A–D). However,
insoluble collagen (collagen cross-linking) content was markedly
higher in male offspring (51.64±6.83 vs. 59.68±7.41 μg/g;
P<0.05; Fig. 2E), whereas it
exhibited only a mild but not significant increment in females
compared with their controls (from an average of 50.74–54.81%;
P>0.05). In addition, no significant morphological alterations
in cardiomyocyte transverse cross-sectional area and diameter were
identified among the groups (Fig.
2F).
Elastic properties of the aorta
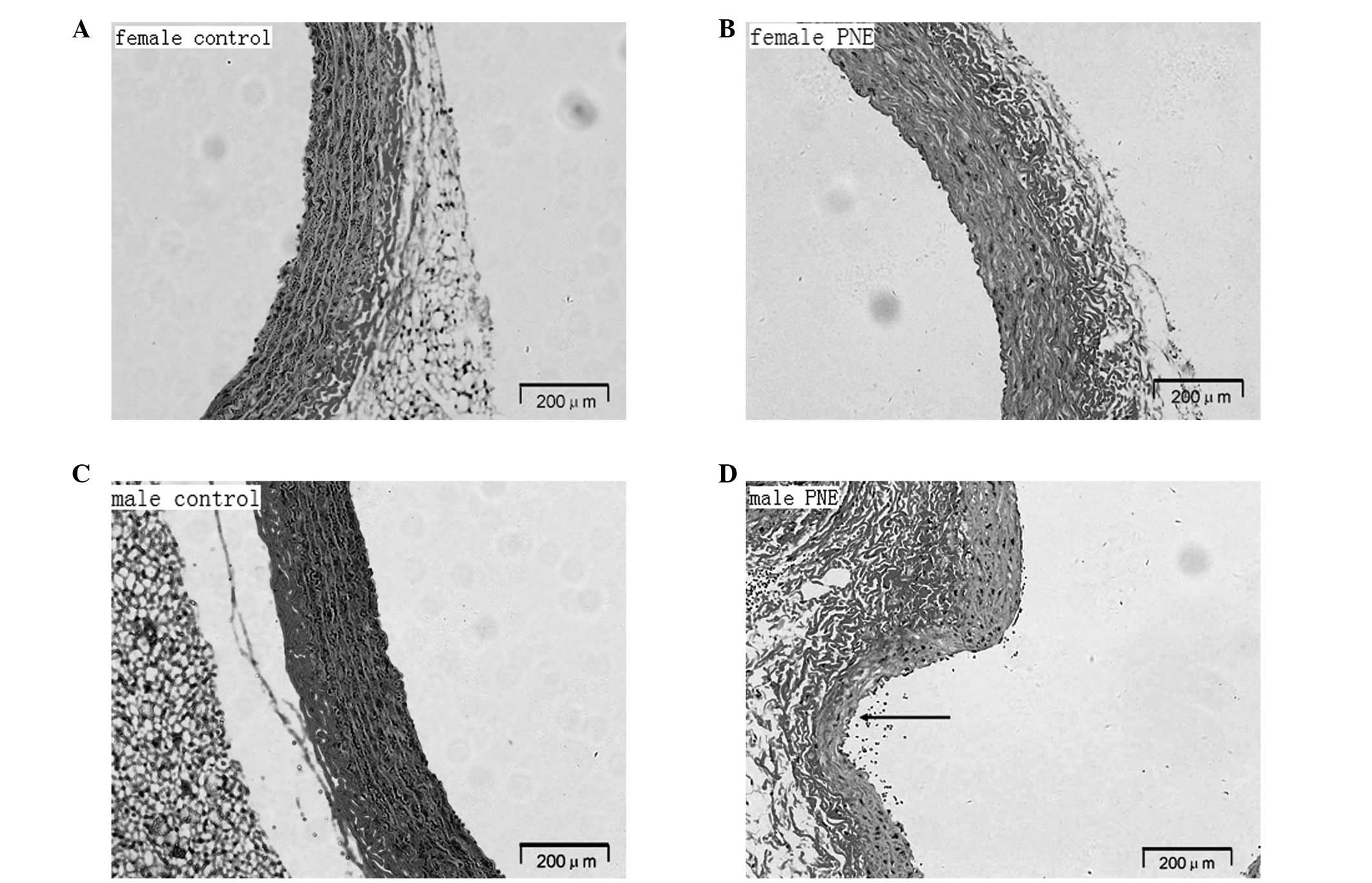
PNE offspring presented with a significantly lower
elastin density as manifested by elastic lamellae (Fig. 3). The arterial media revealed
degeneration and fragmentation of the elastic network and
disorganization of collagen fibers. Compared with female pups,
aortic elastic fibers appeared to be more fractured and disrupted
in male offspring. In addition, aortic aneurysm occurred due to a
localized weakness of the artery wall.
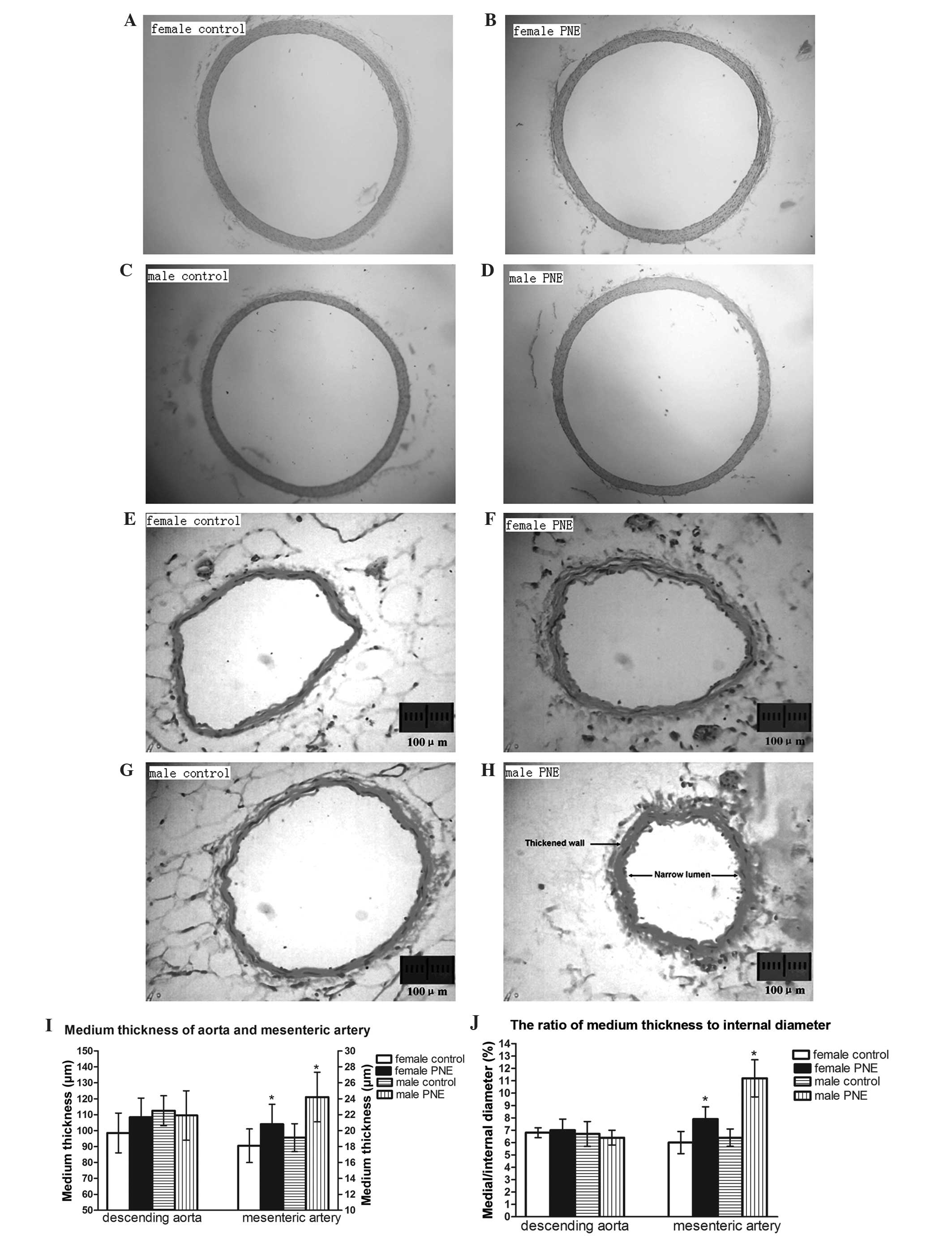
Medial thickness to internal diameter
ratio in the descending aorta and mesenteric artery
In the descending aorta, medial thickness, internal
diameter and ratio of medial thickness to internal diameter were
comparable between PNE offspring and their gender-matched control
rats. Conversely, in resistance-sized mesenteric arteries, the
ratio of medial thickness to lumen diameter increased by 35 and 75%
in males and females, respectively (Fig. 4).
Discussion
The present study revealed the following novel
observations, namely, PNE induced ventricular-arterial stiffness
characterized with a lower Ea/Ees in males, whereas Ea/Ees was
preserved in females. Furthermore, enhanced collagen cross-linking
in myocardium, underdeveloped elastic fibers in the aorta and
remodeled resistance vessels were noted in male and female pups,
with a lesser extent in female offspring. The finding that PNE
affected predominantly male offspring is in line with a previous
study indicating that male offspring were more sensitive to the
manifestation of hypertension caused by adverse prenatal stimuli
(26). These results also
supported a study demonstrating that PNE altered vascular function,
which predisposed male offspring to hypertension during adulthood
(27). However, the present data
revealed that prenatal nicotine insult modified mesenteric artery
medial thickness to internal diameter ratio in male and female
offspring, which was somewhat at odds with the observations that
mesenteric artery media thickness did not alter significantly in
female offspring. A plausible explanation may be that different
ages were investigated, 12 months in the present study compared
with 5 months in the previous study (15).
Another important finding of the present study was
that central systolic pressure and PP demonstrated no significant
differences in the male or female pups as compared with their age
and gender-matched control. While central diastolic pressure was
modestly elevated in PNE offspring, it may represent a compensatory
mechanism whereby an increase in pressure in the ascending aorta
during diastole may increase coronary blood flow to compensate for
shorter diastole.
The present study revealed that Ea as well as AI was
elevated following maternal nicotine insult. Ea and AI are
primarily an assessment of peripheral resistance and the elastic
properties of large conduit arteries (28). The present data revealed increased
fragmentation of the elastic network. There was also an increase in
the ratio of medial thickness to internal diameter in mesenteric
arteries. These results were in line with a previous study
demonstrating that smoking had acute and chronic detrimental
effects on ascending aortic elastic properties in healthy male
subjects (29). Elevated AI in
male PNE offspring implies that peripheral waves arrive earlier. It
occurs early in the cardiac cycle during systole and thus results
in increased myocardial load and reduced coronary perfusion,
eventually compromising cardiac function and structure.
The present observations implied LV myocardium CSA
and LV weight/BW were altered insignificantly; however, collagen
solubility (an index of collagen cross-linking) was decreased in
parallel with increased left ventricular meridional wall stress.
These results were substantiated by other studies demonstrating
that enhanced collagen cross-linking exacerbated myocardium
distensibility without LV hypertrophy (30,31).
Ees principally mirrors LV systolic stiffening. Elevated
ventricular elastance may augment systolic pressure sensitivity to
cardiac loading, increase cardiac energy cost and myocardial oxygen
consumption to deliver stroke volume and further impair cardiac
structure and function.
Under normal conditions, the Ea/Ees ratio is close
to 1 (0.3–1.3) resulting in maximal stroke work and cardiac
metabolic efficiency (32). The
present results suggested that although cardiovascular stiffness
was evident in males and females by 12 months of age, only males
exhibited suboptimal arterial-ventricular coupling. These findings
may assist in elucidating the reasons that intrauterine insults
predisposed male offspring to cardiovascular function
disequilibrium (33).
Decreased aortic elasticity concomitant with
enhanced peripheral resistance led to increased arterial loading,
wave reflection velocity and AI, which in turn resulted in LV
augmented end-systolic stress, modified collagen composition and
diminished cardiac performance.
The mechanisms through which PNE affects
ventricular-arterial coupling are various. It has been demonstrated
that nicotine downregulates miR-133 and miR-590 by the nicotinic
acetylcholine receptor, α7-nAChR, subsequently activates the
transforming growth factor (TGF)-β1 receptor and increases the
synthesis of TGF-β1 protein, resulting in structural alterations of
increased collagen and reduced elastin content in vascular media
(34). Additionally, maternal
nicotine administration caused programming of protein kinase Cε
gene repression through promoter methylation, simultaneously
activating the sympathetic nervous system in the fetal heart, which
compromised cardiovascular homeostasis (35).
The exact mechanisms underlying the gender
dimorphism in nicotine-mediated cardiovascular homeostasis remain
to be elucidated. These gender-specific alterations may be directly
associated with differences in sensitivity and adaptation to the
specific insult while in utero, or females may have greater
vascular compensatory mechanisms following birth.
There are, however, several limitations regarding
the present study. With reference to the study design, Ea/Ees was
not invasively quantified from catheterization-derived
pressure-volume loops. However, the validations of noninvasive
single-beat determination of left ventricular end-systolic
elastance with echocardiography have been published previously
(36). Additionally, studies were
performed under anesthesia. Future studies that investigate
hemodynamics in a conscious animal may be useful to confirm this
data. Finally, several studies have suggested collagen phenotype
affects myocardial stiffness (30,37).
Further studies are required in order to clarify its independent
role in determining myocardial stiffness in the present model.
In conclusion, the present data indicated that PNE
caused combined ventricular-arterial stiffening in male and female
offspring, with lower Ea/Ees in males but preserved Ea/Ees in
females. Enhanced collagen cross-linking in myocardium,
underdeveloped elastic fibers in the aorta and remodeled resistance
vessels were associated with the pathological ventricular arterial
mismatching. Higher ventricular and arterial stiffness has
important implications for blood pressure instability and loading
sensitivity. It is essential for early intervention and the
development of treatment strategies to understand how the fetus
adapts to an adverse intrauterine environment and how this
permanently affects cardiovascular function, particularly prior to
the onset of detectable cardiovascular complications. Further
studies at a molecular level are encouraged to elucidate the
effects of PNE on ventricular and arterial coupling.
Acknowledgments
This study was supported in part by the National
Natural Science Foundation of China (grant no. 81000129), the
Natural Science Foundation of Fujian Province (grant nos. 13131037
and 2014J06018), the Scientific Research Foundation for the
Returned Overseas Chinese Scholars, State Education Ministry (grant
no. 2009-1549), the Foundation from Medical Innovation Project of
Fujian Province (grant no. 2011-CX-25) and by the Major Program
Foundation of Fujian Medical University (grant no. 09ZD015). The
authors would like to thank Mr. Changsheng Xu and Mr. Liangming
Zhang (Fujian Institute of Hypertension) for their technical
assistance with histomorphometric analysis. The abstract for the
present study has previously been published (http://content.onlinejacc.org/article.aspx?articleID=1913711).
References
|
1
|
Hanna ST: Nicotine effect on
cardiovascular system and ion channels. J Cardiovasc Pharmacol.
47:348–358. 2006.PubMed/NCBI
|
|
2
|
Benowitz NL and Gourlay SG: Cardiovascular
toxicity of nicotine: implications for nicotine replacement
therapy. J Am Coll Cardiol. 29:1422–1431. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang Z, Huang Z, Lu G, et al: Hypoxia
during pregnancy in rats leads to early morphological changes of
atherosclerosis in adult offspring. Am J Physiol Heart Circ
Physiol. 296:H1321–H1328. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gao YJ, Holloway AC, Zeng ZH, et al:
Prenatal exposure to nicotine causes postnatal obesity and altered
perivascular adipose tissue function. Obes Res. 13:687–692. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rueda-Clausen CF, Morton JS and Davidge
ST: Effects of hypoxia-induced intrauterine growth restriction on
cardiopulmonary structure and function during adulthood. Cardiovasc
Res. 81:713–722. 2009. View Article : Google Scholar
|
|
6
|
Lawrence J, Xiao D, Xue Q, et al: Prenatal
nicotine exposure increases heart susceptibility to
ischemia/reperfusion injury in adult offspring. J Pharmacol Exp
Ther. 324:331–341. 2008. View Article : Google Scholar
|
|
7
|
Gao YJ, Holloway AC, Su LY, et al: Effects
of fetal and neonatal exposure to nicotine on blood pressure and
perivascular adipose tissue function in adult life. Eur J
Pharmacol. 590:264–268. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xiao D, Huang X, Lawrence J, et al: Fetal
and neonatal nicotine exposure differentially regulates vascular
contractility in adult male and female offspring. J Pharmacol Exp
Ther. 320:654–661. 2007. View Article : Google Scholar
|
|
9
|
Kim JW, Park CG, Hong SJ, et al: Acute and
chronic effects of cigarette smoking on arterial stiffness. Blood
Press. 14:80–85. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goette A, Lendeckel U, Kuchenbecker A, et
al: Cigarette smoking induces atrial fibrosis in humans via
nicotine. Heart. 3:1056–1063. 2007. View Article : Google Scholar
|
|
11
|
Rajiyah G, Agarwal R, Avendano G, et al:
Influence of nicotine on myocardial stiffness and fibrosis during
chronic ethanol use. Alcohol Clin Exp Res. 20:985–989. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fourie PR, Coetzee AR and Bolliger CT:
Pulmonary artery compliance: its role in right ventricular-arterial
coupling. Cardiovasc Res. 26:839–844. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Boychuk CR and Hayward LF: Prenatal
nicotine exposure alters postnatal cardiorespiratory integration in
young male but not female rats. Exp Neurol. 232:212–221. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fennessy F, Casey RG and Bouchier-Hayes D:
Peripheral and central arterial haemodynamic interactions are early
abnormalities in young male cigarette smokers. Eur J Vasc Endovasc
Surg. 25:152–158. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xiao D, Xu Z, Huang X, et al: Prenatal
gender-related nicotine exposure increases blood pressure response
to angiotensin II in adult offspring. Hypertension. 51:1239–1247.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sumitra M, Manikandan P, Rao KV, et al:
Cardiorespiratory effects of diazepam-ketamine, xylazine-ketamine
and thiopentone anesthesia in male Wistar rats - a comparative
analysis. Life Sci. 75:1887–1896. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jegger D, da Silva R, Jeanrenaud X, et al:
Ventricular-arterial coupling in a rat model of reduced arterial
compliance provoked by hypervitaminosis D and nicotine. Am J
Physiol Heart Circ Physiol. 291:H1942–H1951. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Weinberg EO, Thienelt CD, Katz SE, et al:
Gender differences in molecular remodeling in pressure overload
hypertrophy. J Am Coll Cardiol. 34:264–273. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sahlén A, Shahgaldi K, Aagaard P, et al:
Altered ventriculo-arterial coupling during exercise in athletes
releasing biomarkers after endurance running. Eur J Appl Physiol.
112:4069–4079. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Blaudszun G and Morel DR: Relevance of the
volume-axis intercept, V0, compared with the slope of end-systolic
pressure-volume relationship in response to large variations in
inotropy and afterload in rats. Exp Physiol. 96:1179–1195. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
London GM, Blacher J, Pannier B, et al:
Arterial wave reflections and survival in end-stage renal failure.
Hypertension. 38:434–438. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lartaud I, Gaillard V, Dauca M, et al:
Pioglitazone protects against elastocalcinosis and improves aortic
wall elasticity. Ann Pharm Fr. 65:189–194. 2007.In French.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Leipner C, Grün K, Müller A, et al:
Imatinib mesylate attenuates fibrosis in coxsackievirus b3-induced
chronic myocarditis. Cardiovasc Res. 79:118–126. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Marque V, Kieffer P, Gayraud B, et al:
Aortic wall mechanics and composition in a transgenic mouse model
of Marfan syndrome. Arterioscler Thromb Vasc Biol. 21:1184–1189.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yamamoto K, Masuyama T, Sakata Y, et al:
Myocardial stiffness is determined by ventricular fibrosis, but not
by compensatory or excessive hypertrophy in hypertensive heart.
Cardiovasc Res. 55:76–82. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
do Carmo Pinho Franco M, Nigro D, Fortes
ZB, et al: Intrauterine undernutrition - renal and vascular origin
of hypertension. Cardiovasc Res. 60:228–234. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xiao D, Huang X, Yang S and Zhang L:
Estrogen normalizes perinatal nicotine-induced hypertensive
responses in adult female rat offspring. Hypertension.
61:1246–1254. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Heffernan KS, Patvardhan EA, Hession M, et
al: Elevated augmentation index derived from peripheral arterial
tonometry is associated with abnormal ventricular-vascular
coupling. Clin Physiol Funct Imaging. 30:313–317. 2010.PubMed/NCBI
|
|
29
|
Sassalos K, Vlachopoulos C, Alexopoulos N,
et al: The acute and chronic effect of cigarette smoking on the
elastic properties of the ascending aorta in healthy male subjects.
Hellenic J Cardiol. 47:263–268. 2006.PubMed/NCBI
|
|
30
|
Norton GR, Tsotetsi J, Trifunovic B, et
al: Myocardial stiffness is attributed to alterations in
cross-linked collagen rather than total collagen or phenotypes in
spontaneously hypertensive rats. Circulation. 96:1991–1998. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Antonini-Canterin F, Carerj S, Di Bello V,
et al: Arterial stiffness and ventricular stiffness: a couple of
diseases or a coupling disease? A review from the cardiologist’s
point of view. Eur J Echocardiogr. 10:36–43. 2009. View Article : Google Scholar
|
|
32
|
Frenneaux M and Williams L:
Ventricular-arterial and ventricular-ventricular interactions and
their relevance to diastolic filling. Prog Cardiovasc Dis.
49:252–262. 2007. View Article : Google Scholar
|
|
33
|
Xue Q and Zhang L: Prenatal hypoxia causes
a sex-dependent increase in heart susceptibility to ischemia and
reperfusion injury in adult male offspring: role of protein kinase
C epsilon. J Pharmacol Exp Ther. 330:624–632. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Goette A: Nicotine, atrial fibrosis and
atrial fibrillation: do microRNAs help to clear the smoke?
Cardiovasc Res. 83:421–422. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lawrence J, Chen M, Xiong F, et al: Foetal
nicotine exposure causes PKCε gene repression by promoter
methylation in rat hearts. Cardiovasc Res. 89:89–97. 2011.
View Article : Google Scholar
|
|
36
|
Chen CH, Fetics B, Nevo E, et al:
Noninvasive single-beat determination of left ventricular
end-systolic elastance in humans. J Am Coll Cardiol. 38:2028–2034.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kato S, Spinale FG, Tanaka R, et al:
Inhibition of collagen cross-linking: effects on fibrillar collagen
and ventricular diastolic function. Am J Physiol. 269(3 Pt 2):
H863–H868. 1995.PubMed/NCBI
|