Introduction
Recent evidence has indicated that certain solid
tumors, including brain gliomas (1,2) and
breast (3), prostate (4), colon (5) liver (6) and lung cancer (7) contain a small population of cancer
stem cells (CSCs), which have a high capacity for self-renewal,
multilineage differentiation, inducing malignancy, drug-resistance
and radiotherapy resistance, as well as recurrence and metastasis
(8,9). These cells are responsible for tumor
maintenance and metastasis. It is postulated that therapies for
cancer that specifically target stem cell signaling pathways
utilized by CSCs may be beneficial (10,11).
The phosphatidylinositol 3-kinase (PI3K)/Akt
signaling pathway has been demonstrated to be involved in the
regulation of cell proliferation and apoptosis, and is pivotal in
the initiation and progression of malignancies, enhancing cell
survival by stimulating cell proliferation and inhibiting apoptosis
(12,13). More recent studies have identified
that the PI3K/Akt signaling pathway is overactivated in several
types of human cancer, including brain glioma (14), pancreatic cancer (15), lung cancer (16), and high expression of PI3K and
p-Akt is often associated with a poor prognosis.
The overactivation of the PI3K/Akt signaling pathway
has also been observed in several CSCs (17–19).
However, there are no reports with regard to the correlation
between the PI3K/Akt signaling pathway, and proliferation and
self-renewal of lung cancer stem cells (LCSCs) in the
English-language literature. In the present study, a cell
population with a CD133+ phenotype was isolated from the
single cell suspension of lung adenocarcinoma tissue using a
magnetic-activated cell sorting (MACS) technique, and enriched by
serum free cultures. AKT1 and PI3K/p85 were suppressed by RNA
interference. The expression of proliferating cell nuclear antigen
(PCNA), cyclin Dl and p53 were also detected by western blot
analysis. The effects of AKT1 and PI3K/p85 on the self-renewal and
proliferation of LCSCs were investigated using an
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay, sphere forming assay and xenograft formation assay. In
addition, a cell cycle assay was also conducted using flow
cytometry following AKT1 and PI3K/p85 silencing in LCSCs. The
present study demonstrated that the PI3K/Akt signaling pathway is
consistently overactivated in LCSCs. Additionally, it was revealed
that the downregulation of AKT1 and PI3K/p85 suppressed the
self-renewal and proliferation of LCSCs and decreased the rate of
tumor formation in vivo.
Materials and methods
LCSC isolation and cell culture
In our previous study (7), CD133+ cells were
successfully isolated from the single cell suspension of lung
adenocarcinoma tissue using a MACS technique, and enriched by serum
free culture. The sorting was verified with properties of LCSCs
through experiments of self-renewal, multipotential differentiation
capacity, drug resistance and tumorigenic capacity in vivo.
LCSCs were harvested and cultured in serum-free Dulbecco’s modified
Eagle’s medium (DMEM)-F12 (Beijing Beiruo Biothechnology Co., Ltd.,
Beijing, China) containing 50 μg/ml insulin (Sigma-Aldrich,
St. Louis, MO, USA), 100 μg/ml apotransferrin
(Sigma-Aldrich), 10 μg/ml putrescine (Sigma-Aldrich), 0.03
mM sodium selenite (Sigma-Aldrich), 2 μM progesterone (Pure
Chemistry Scientific, Inc., Sugarland, TX, USA), 0.6% glucose (LGM
Pharma, Nashville, TN, USA), 5 mM HEPES (Nanjing Search Biotech
Co., Ltd., Nanjing, China), 0.1% sodium bicarbonate, 0.4% bovine
serum albumin (BSA; Wuhan Boster Biological Engineering Co., Ltd.,
Wuhan, China), glutamine (Ameresco, Inc., Solon, OH, USA) and 1%
penicillin and 1% streptomycin (Beijing Beiruo Biothechnology Co.,
Ltd.), as well as 20 ng/ml epidermal growth factor (EGF; PeproTech,
Rocky Hill, NJ, USA) and 10 ng/ml basic fibroblast growth factor
(bFGF; PeproTech) at 37°C and 5% CO2.
Generation of rAd5-small interfering
(si)AKT1-siPI3K/p85 RNAi lentiviruses (Wuhan Boster Biological
Engineering Co., Ltd.)
After testing knock down efficiencies of several
shRNA constructs, the following shRNA oligonucleotides
(rAd5-siAKT1-siPI3K-shRNA) were utilized: 5′-GGAGATCATGCAGCATCGC-3′
[to target the 19 bp interference sequence of AKT1 gene
(intervention sites: 1540–1558)] and 5′-GAAAGGAGGAAATAACAAA-3′ [to
target the 19 bp interference sequence of PI3K/p85 gene
(intervention sites: 371–389]. A non-specific shRNA
(rAd5-siCtrl-shRNA) was also synthesized as a control, and each
shRNA was cloned into a pGCL-GFP plasmid containing the U6 promoter
and green fluorescent protein (GFP). The plasmids pDONR221 and
pAD/CMV/V5-DEST were also included to provide the necessary
packaging elements for lentivirus production. For viral
transduction, shRNA lentiviral vectors at a multiplicity of
infection (MOI) of 25 were added to dispersed LCSCs rapidly
following plating. GFP fluorescence was measured 72 h
post-transduction.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The primer sequences used included: Forward:
5′-GGCCCAGATGATCACCATCAC-3′ and reverse: 5′-CTATCGTCCAGCGCAGTCCA-3′
for AKT1; forward: 5′-AGCATTGGGACCTCACATTACACA-3′ and reverse:
5′-ACTGGAAACACAGTCCATGCACATA-3′ for PI3K/p85; and forward:
5′-CCTGGCACCCAGCACAAT-3′ and reverse: 5′-GCCGATCCACACGGAGTACT-3′
for β-actin. The total RNA was isolated using TRIzol reagent
(Invitrogen Life Technologies) according to the manufacturer’s
instructions. cDNA was synthesized using a reverse transcription
kit (Toyobo Co., Ltd., Osaka, Japan), according to the
manufacturer’s instructions. cDNA (2.5 μl) was subjected to
qPCR using SYBR-Green as a fluo rescent reporter and 2.5X Real
Master mix (Toyobo, Osaka, Japan). Specific gene primers (Wuhan
Boster Biological Engineering Co., Ltd.) for AKT1, PI3K/p85 and
β-actin, were amplified in separate reac tion tubes. Threshold
cycle numbers of triplicate reactions were determined using
ABI-7500 software (v2.0; Invitrogen Life Technologies, Carlsbad,
CA, USA) and aver aged. Relative fold changes were calculated using
the 2−ΔΔCt method and standard curves were produced.
Western blot analysis
Cell extracts from the control group (untreated
cells), Ad5-Control-shRNA group (cells infected with
Ad5-Control-shRNA) and Ad5-siAKT1-siPI3K-shRNA group (cells
infected with Ad5-siAKT1-siPI3K-shRNA), as well as primary lung
cancer cells were collected, and protein samples (50 μg)
were then electrophoresed on 12% SDS-polyacrylamide gels and
transferred onto polyvinylidene fluoride (PVDF) membranes (Wuhan
Boster Biological Engineering Co., Ltd.). Membranes were blocked in
5% non-fat dry milk in Tris-buffered saline (TBS) for 1 h at room
temperature and incubated with anti-AKT1 (sc-514032), anti-PI3K/p85
(sc-131324), anti-PCNA (sc-71858), anti-cyclin D1 (sc-70899) and
anti-P53 (sc-377567) antibodies (diluted 1:1,000, Santa Cruz
Biotechnology Inc., Santa Cruz, CA, USA) overnight at 4°C.
Membranes were incubated with 50 μl IgG/horseradish
peroxidase secondary antibody (diluted 1:2,000, Wuhan Boster
Biological Engineering Co., Ltd. Wuhan, China) for 2 h at room
temperature after three washes with TBS-Tween-20 (TBST). Each
membrane was also incubated with an anti-β-actin antibody (Santa
Cruz Biotechnology Inc.) as a loading control. Membranes were
washed three times with TBST and bound antibodies were detected
using enhanced chemiluminescence (Beyotime, Jiangsu, China).
Protein levels were quantitated by densitometry using Quantity One
4.62 software (Bio-Rad Laboratories, Munich, Germany).
Sphere-forming assay
Cell extracts from the control group,
Ad5-Control-shRNA group and Ad5-siAKT1-siPI3K-shRNA group were
dissociated and cultured in 96-well plates in serum-free DMEM-F12
medium containing 50 μg/ml insulin, 100 μg/ml
apotransferrin, 10 μg/ml putrescine, 0.03 mM sodium
selenite, 2 μM progesterone, 0.6% glucose, 5 mM HEPES, 0.1%
sodium bicarbonate, 0.4% BSA, glutamine and 1% penicillin and 1%
streptomycin, as well as 20 ng/ml EGF and 10 ng/ml bFGF. Wells
containing more than one cell or no cells were marked and dismissed
from the statistical data. The cells were cultured under conditions
of 5% CO2 at 37°C for 10 days. The medium was replaced
or supplemented with fresh growth factors twice a week. Wells that
contained spheres were counted using inverted phase contrast
microscopy (DMIL-PH1; Leica, Mannheim, Germany) and the percentage
of cells with sphere-forming capacity was calculated.
Proliferation assay
The proliferation of the cells was detected using an
MTT assay on days 1, 3, 5 and 7. Three groups were plated onto
96-well plates (2,000 cells in 0.2 ml cell culture medium/well).
The cells were then incubated with 20 μl MTT (5 mg/ml;
Sigma-Aldrich, St. Louis, MO, USA) for 4 h prior to collection. The
culture medium was finally removed, and 150 μl
dimethylsulfoxide was added to the well. After shaking thoroughly
for 10 min, the plates were read for absorbance in an enzyme immuno
assay at 490 nm using an Automatic enzyme-linked immunity analyzer
(Diasorin S.p.A, Italy). Six wells were analyzed for each
group.
Cell cycle phase distribution
A total of 1×106 cells from the control
group, Ad5-Control-shRNA group and Ad5-siAKT1-siPI3K-shRNA group
were centrifuged at 375 × g for 5 min, resuspended in 0.2 ml
phosphate-buffered saline and then fixed in l ml of 70% ethanol at
4°C for 16 h. Subsequent to washing with PBS, the cells were
incubated with 300 μl Green-DNA Dye (Nanjing Search Biotech
Co., Ltd., Nanjing, China) at room temperature for 30 min. Cell
cycle status was assessed by flow cytometry (Beckman Coulter, Brea,
CA, USA). The relative proportions of cells in the
G0/G1, S and G2/M phases were
analyzed, and the percentages of cells in each phase were
calculated.
Tumorigenicity in NOD/SCID mice
Cells from the control group, Ad5-Control-shRNA
group and Ad5-siAKT1-siPI3K-shRNA group were diluted in growth
factor-containing medium alone prior subcutaneous injection. Serial
dilutions of cells (102, 103, 104
and 105 cells) were injected subcutaneously into the
abdominal wall of 4-week-old NOD/SCID mice (5 mice/group; Beijing
Vitalriver Experimental Animal Technical Co., Ltd,. Beijing,
China). Tumor size was measured using calipers and tumor volume was
calculated using the equation V=π/6 (length × width × height). The
mice were sacrificed 8 weeks following the subcutaneous injection.
The present animal study was approved by the Ethics Committee of
Taizhou People’s Hospital (Jiangsu, China) and was performed
according to the Declaration of Helsinki.
Statistical analysis
Statistical analysis was performed using SPSS 13.0
software (SPSS, Inc., Chicago, IL, USA). All experiments were
performed at least three times and representative results are
presented as the mean ± standard deviation. Statistical analysis
was performed by one-way analysis of variance and comparisons among
groups were achieved using independent sample t-tests. P<0.05
was considered to indicate a statistically significant
difference.
Results
AKT1 and PI3K/p85 expression in primary
lung cancer cells and LCSCs
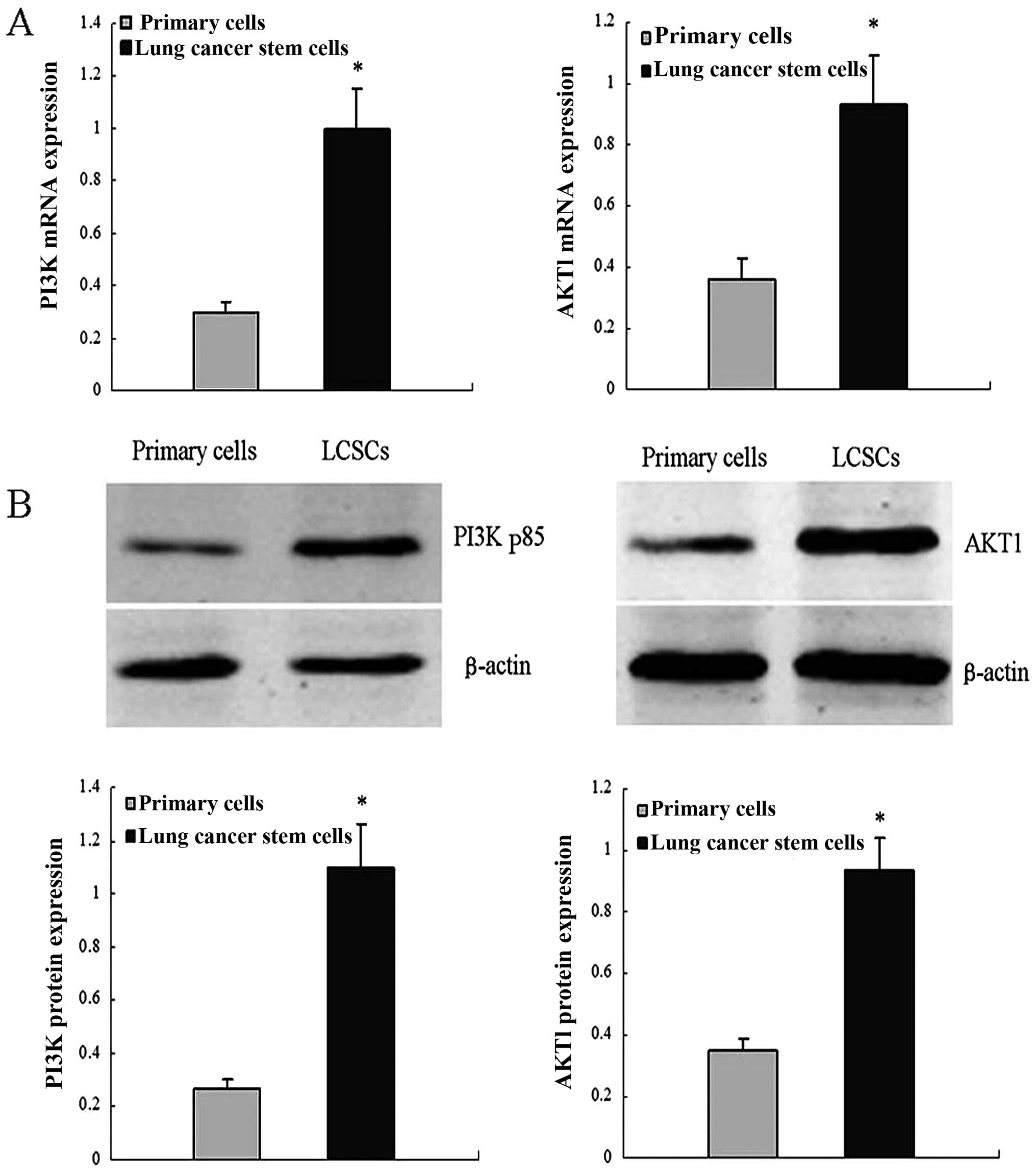
RT-qPCR assays were performed to detect AKT1 and
PI3K/p85 mRNA levels. In these assays, AKT1 and PI3K/p85 mRNA abun
dance was higher in LCSCs compared with the primary lung cancer
cells. Similar results from western blot assays using the same cell
types confirmed the results. These results indicated that AKT1 and
PI3K/p85 expression is upregulated in LCSCs (Fig. 1).
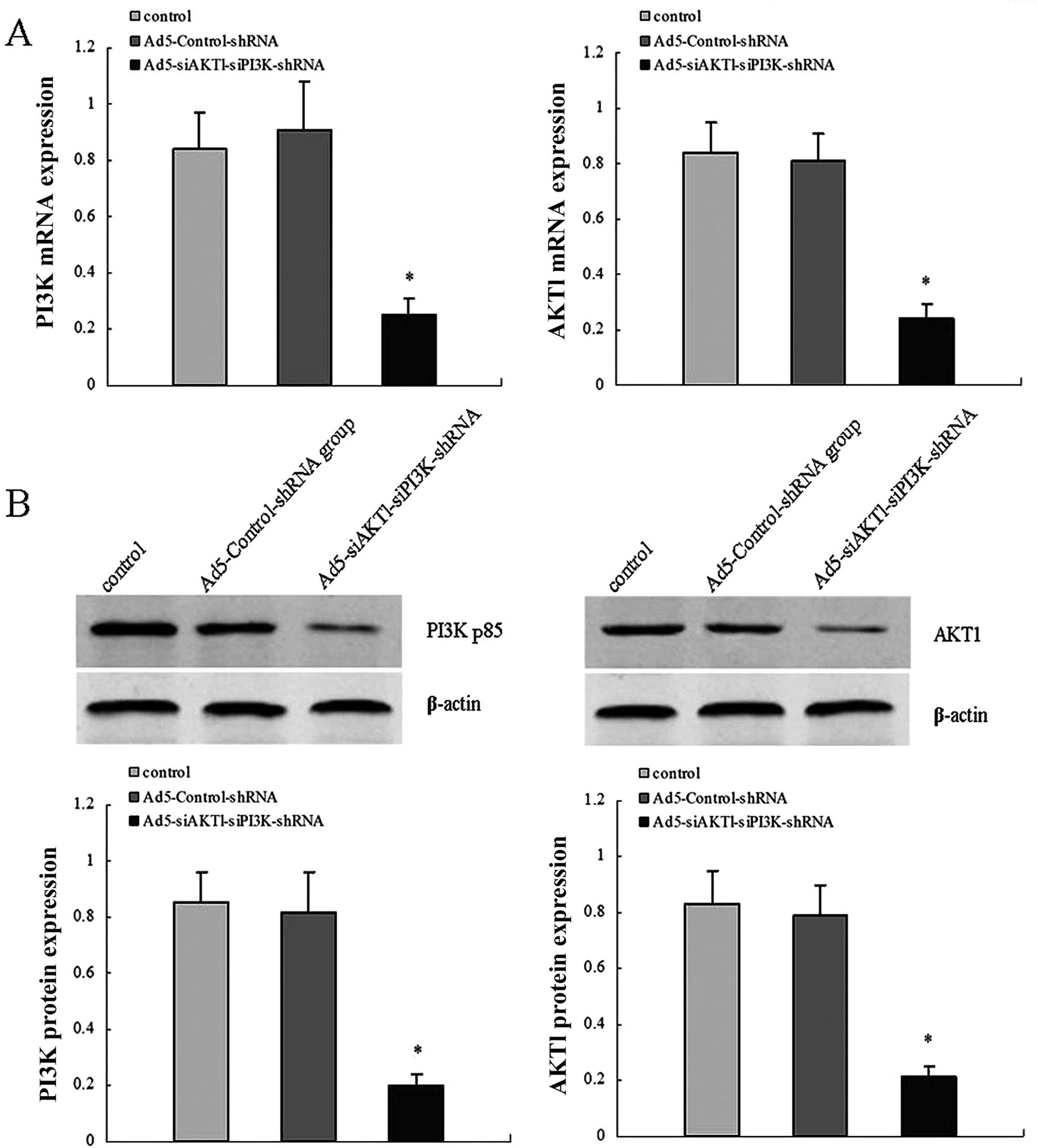
Efficiency of adenovirus
transfection
For viral transduction, shRNA lentiviral vectors at
an MOI of 25 were added to dispersed LCSCs shortly following
plating. After three days, the GFP expression of the LCSCs
confirmed that the lentiviral infection was achieved, compared with
the corresponding white field image of the same cell population.
RT-qPCR and western blot assays were performed to detect AKT1 and
PI3K/p85 mRNA and protein levels in control, Ad5-Control-shRNA and
Ad5-siAKT1-siPI3K-shRNA groups. The results showed that the
expression levels of AKT1 and PI3K/p85 mRNA and protein were
significantly decreased in the Ad5-siAKT1-siPI3K-shRNA group,
compared with the control or Ad5-Control-shRNA groups. The results
demonstrated the high knockdown efficiency of
Ad5-siAKT1-siPI3K-shRNA in vitro (Fig. 2).
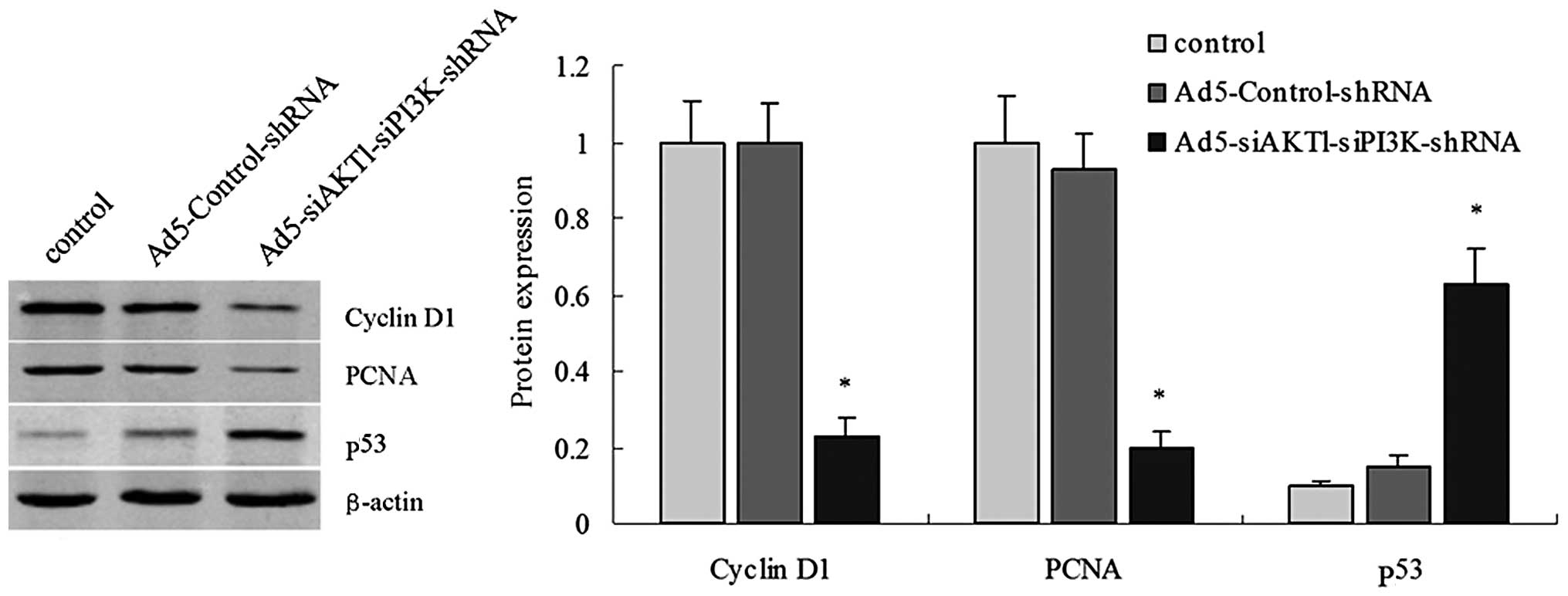
PCNA, cyclin D1 and p53 protein
expression in control, Ad5-Control-shRNA and
Ad5-siAKT1-siPI3K-shRNA groups
Western blot assays were performed to detect PCNA,
cyclin D1 and p53 protein levels. In these assays, expression
levels of PCNA and cyclin D1 were significantly decreased in the
Ad5-siAKT1-siPI3K-shRNA group, compared with the control or
Ad5-Control-shRNA groups. While expression of p53 was significantly
increased in the Ad5-siAKT1-siPI3K-shRNA group, compared with
control or Ad5-Control-shRNA groups (Fig. 3).
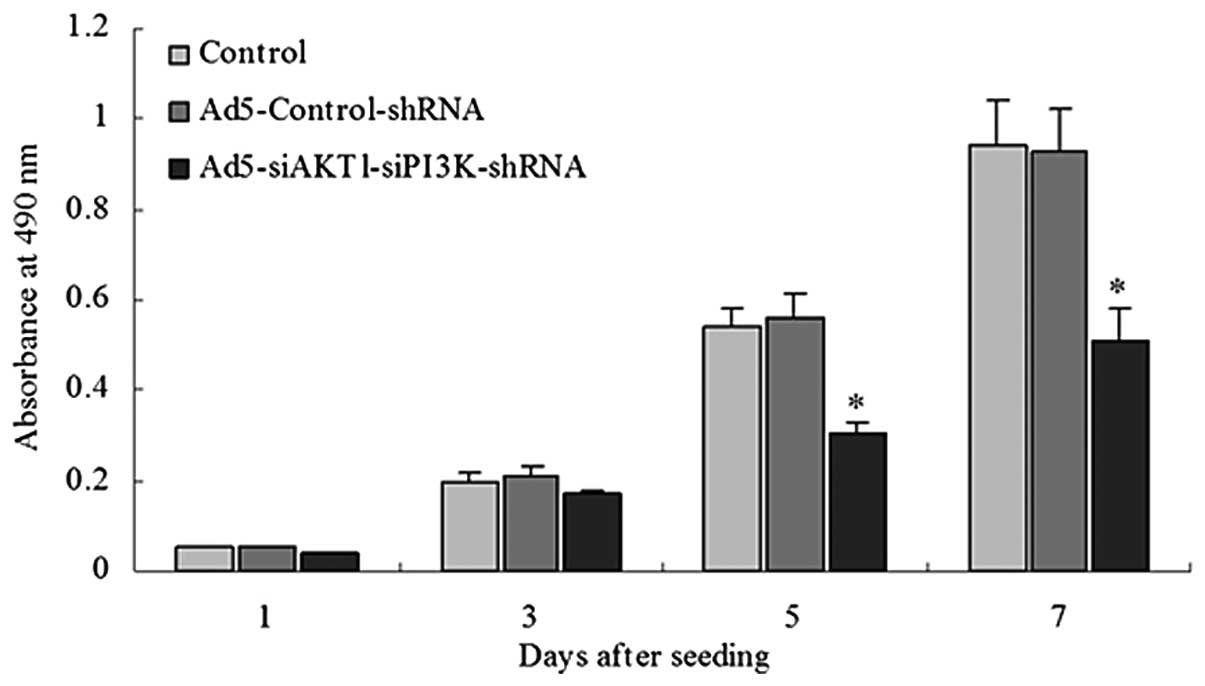
AKT1 and PI3K/p85 knockdown decreases the
proliferation rate of LCSCs
The Ad5-siAKT1-siPI3K-shRNA group exhibited a low
proliferation rate when compared with the rate in the control group
or Ad5-Control-shRNA groups beginning the fifth day after seeding
(Fig. 4).
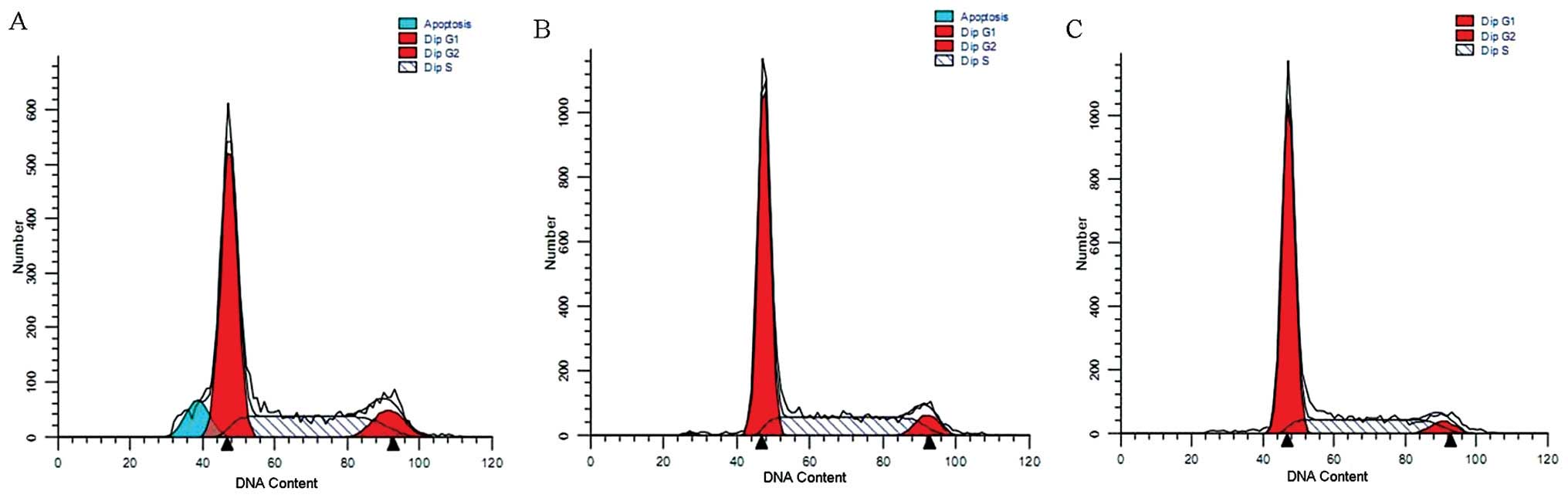
AKT1 and PI3K/p85 knockdown induces the
arrest of the cell cycle of LCSCs
The cell cycle distribution was assessed using flow
cytometry. The results revealed a marked arrest in the
G0/G1 phase in the Ad5-siAKT1-siPI3K-shRNA
group relative to the control group or Ad5-Control-shRNA group
(67.15±3.31% vs. 57.89±2.86% and 60.22±2.29%; P<0.01,
respectively) (Fig. 5).
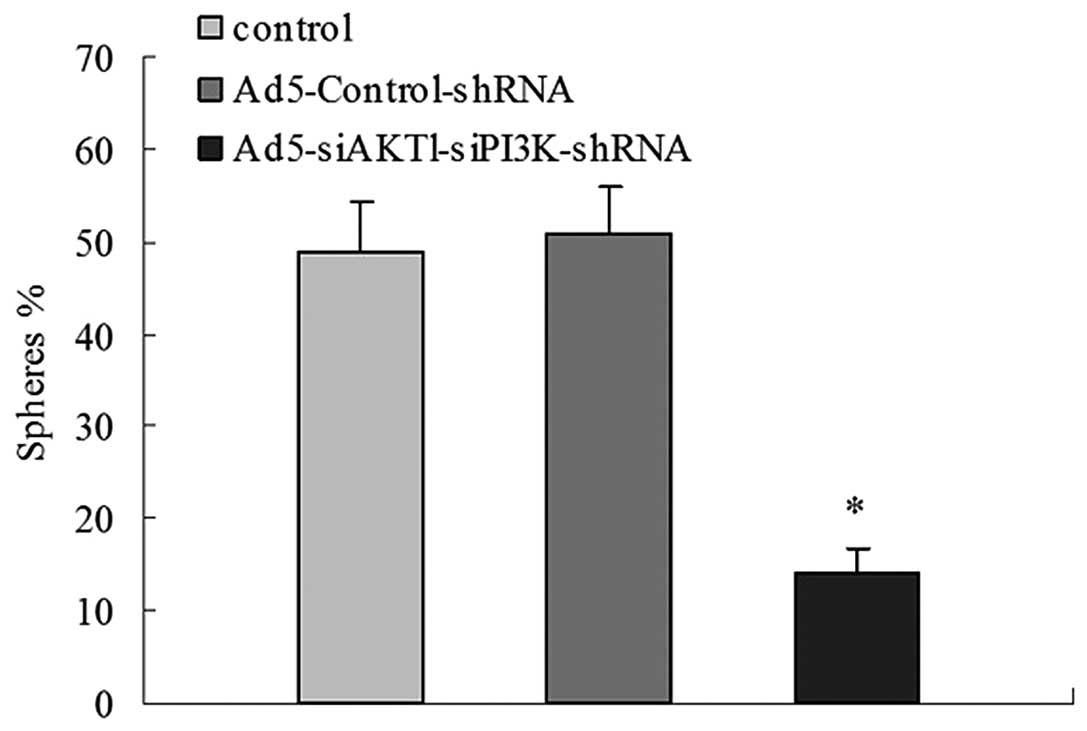
AKT1 and PI3K/p85 knockdown slows the
sphere-forming ability of LCSCs
The control, Ad5-Control-shRNA and
Ad5-siAKT1-siPI3K-shRNA groups were examined for their ability to
form new spheres after being initially cultured as a single cell.
After 10 days, 49±5.4 and 51±4.9% of wells with a single cell
derived from the control group and the Ad5-Control-shRNA group
formed a novel set of spheres, while only 14±2.7% of wells with a
single cell derived from the Ad5-siAKT1-siPI3K-shRNA group was able
to form spheres. These results indicate that the downregulation of
AKT1 and PI3K/p85 leads to the loss of self-renewal in LCSCs
(Fig. 6).
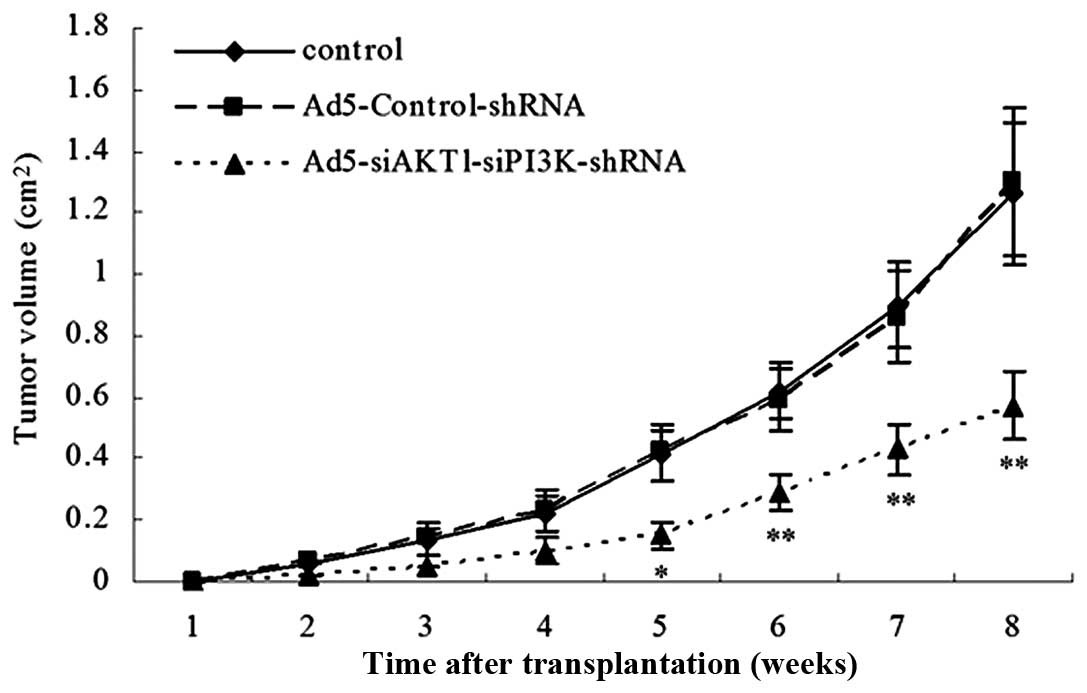
AKT1 and PI3K/p85 knockdown decreases
tumorigenic potential in LCSCs in NOD/SCID mice
The control, Ad5-Control-shRNA and
Ad5-siAKT1-siPI3K-shRNA cells were injected subcutaneously into
NOD/SCID mice in a limiting dilution experiment (i.e.,
102, 103, 104 and 105
cells). The results showed that rAd5-siAKT1-siPI3K cells were
associated with a decreased level of tumorigenicity relative to
that of the control or Ad5-Control-shRNA groups. In order to
further compare the size of the xenograft tumor of the three groups
in vivo, the xenograft tumor induced by 105 cells
in the three groups was observed and it was found that the tumor
volume induced by the Ad5-siAKT1-siPI3K-shRNA group was also
significantly lower than that induced by the control or
Ad5-Control-shRNA group (Table I,
Fig. 7).
 | Table IIncidence of tumors of control group,
Ad5-Control-shRNA group and Ad5-siAKT1-siPI3K-shRNA group cells
serially transplanted into NOD/SCID mice. |
Table I
Incidence of tumors of control group,
Ad5-Control-shRNA group and Ad5-siAKT1-siPI3K-shRNA group cells
serially transplanted into NOD/SCID mice.
| Group | Cell number
|
|---|
| 102 | 103 | 104 | 105 |
|---|
| Control | 2/5 | 4/5 | 5/5 | |
|
Ad5-Control-shRNA | 2/5 | 4/5 | 5/5 | |
|
Ad5-siAKT1-siPI3K-shRNA | 0/5 | 0/5 | 1/5 | 3/5 |
Discussion
Current studies suggest that drug resistance
mechanisms in tumor cells have a close association with CSCs, which
may lead to resistance to radiation and chemotherapy through the
following mechanisms: i) The majority of CSCs (such as leukemia
stem cells and liver stem cells) are in the G0 phase.
Due to their slow growth and primarily dormant state, CSCs are
resistant to cell cycle-specific agents (20–22).
ii) Various types of CSCs overexpress membrane ABC transporter and
drug resistance genes, including P-glycoprotein, encoded by the
ABCB1 gene, multidrug resistance-related protein 1 and breast drug
resistance protein 2, encoded by the ABCG2/MXR gene. These proteins
can pump chemotherapeutic drugs out of the cell, decreasing the
intracellular concentration and weakening their cytotoxic effects
on cancer cells; thus, resulting in drug resistance (23–25).
iii) CSCs express DNA repair protein and thymidylate synthase
excision repair cross-complementation group 1 at a high level,
increasing the capacity of cancer cells for repair following
antitumor treatment, and thereby increasing resistance to
anticancer drugs (26). iv) In
comparison to normal stem cells, signaling pathways associated with
self-renewal and proliferation in CSCs are often over-activated,
becoming sources for tumor recurrence and metastasis (27,28).
Although there have been few studies of the PI3K/AKT
signaling pathway in stem cells, a previous study has confirmed
that this pathway is important in the proliferation of breast
cancer stem cells (18). Gong
et al (29) found that the
proliferation of osteosarcoma cancer stem cells was significantly
decreased by the PI3K inhibitor LY294002 in a dose- and
time-dependent manner, suggesting that the PI3K/AKT signaling
pathway is an important mediator of osteosarcoma cancer stem cell
proliferation. Targeted inhibition of this pathway can effectively
inhibit the proliferation of cancer stem cells (29). Sunayama et al (30) used rapamycin in combination with
LY294002 to treat glioma stem cells and showed that proliferation
and the self-renewal capacity of these cells were diminished, as
was tumorigenicity in vivo. Yang et al (19) successfully extracted
bronchioloalveolar stem cells (BASCs) from a mouse model of
K-ras-driven cancer. Following treatment with a PI3K/AKT pathway
inhibitor, migration and proliferation of BASCs were reduced and
the PTEN gene was inactivated.
Currently, to the best of our knowledge, there are
no reports demonstrating the involvement of the PI3K/AKT signaling
pathway in the regulation of self-renewal, proliferation and
differentiation of human LCSCs. In the present study, shRNA
technology was used to downregulate the expression of AKT1 and the
PI3K/p85 subunit in LCSCs and changes were detected in cell
proliferation in three groups. LCSC proliferation slowed and the
cell doubling time increased when AKT1 and PI3K/p85 were silenced,
suggesting that the PI3K/AKT signaling pathway promoted LCSC
proliferation. In addition, the self-renewal capacity of LCSCs was
analyzed through LCSC sphere formation. The results showed that the
self-renewal capacity of LCSCs in the rAd5-siAKT1-siPI3K-shRNA
group was lower than in the control group or Ad5-Control-shRNA
group, indicating an important role for the PI3K/AKT signaling
pathway in LCSC self-renewal.
To further investigate the mechanism by which the
PI3K/AKT pathway controls LCSC proliferation, gene expression of
PCNA, cyclin D1 and p53 was examined in three groups of LCSCs. PCNA
is a nuclear protein closely associated with cell proliferation,
while p53 was the first identified tumor suppressor gene.
Diminished or absent expression of p53 is an important mechanism
for promoting tumorigenesis. PCNA and p53 are closely related to
each other. p53 protein can induce the synthesis of p21 and GAPD45
protein, which binds to, inactivates and degrades PCNA (31). Cyclin D1 is highly expressed in a
number of different cancer cells, promoting the G0/S
transition and stimulating tumor growth (32). In the present study, downregulation
of AKT1 and PI3K/p85 expression was accompanied by decreased
expression of cyclin D1 and PCNA, and increased expression of p53.
These results show that silencing of AKT1 and PI3K/p85 can result
in cell cycle arrest at the G0/G1 phase,
while S phase cells are significantly decreased, resulting in
inhibition of cell proliferation.
The present study also showed that LCSC
tumorigenicity in the rAd5-siAKT1-siPI3K-shRNA group was
significantly reduced, compared with the control groups or
Ad5-Control-shRNA group, but was still present in the treated
cells, implying that LCSCs retained part of their self-renewal
capacity when AKT1 and PI3K/p85 had been silenced. It was
speculated that silencing of AKT1 and PI3K/p85 blocks LCSCs from
entering the cell cycle, inhibits the self-renewal rate of LCSCs
and reduces the number of stem cells with tumorigenic properties by
causing asymmetric division.
In conclusion, AKT1 and PI3K were overexpressed in
LCSCs. Silencing of AKT1 and PI3K/p85 gene transcription reduced
cell proliferation, tumor sphere formation and tumor development in
NOD/SCID mice. This study provides a potential novel therapeutic
strategy for the treatment of drug resistance and recurrence of
lung cancer.
References
|
1
|
Zhou XD, Wang XY, Qu FJ, et al: Detection
of cancer stem cells from the C6 glioma cell line. J Int Med Res.
37:503–510. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Qiu B, Zhang D, Tao J, Wu A and Wang Y: A
simplified and modified procedure to culture brain glioma stem
cells from clinical specimens. Oncol Lett. 3:50–54. 2012.PubMed/NCBI
|
|
3
|
Hwang-Verslues WW, Kuo WH, Chang PH, et
al: Multiple lineages of human breast cancer stem/progenitor cells
identified by profiling with stem cell markers. PLoS ONE.
4:e83772009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Collins AT, Berry PA, Hyde C, Stower MJ
and Maitland NJ: Prospective identification of tumorigenic prostate
cancer stem cells. Cancer Res. 65:10946–10951. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
O’Brien CA, Pollett A, Gallinger S and
Dick JE: A human colon cancer cell capable of initiating tumour
growth in immunodeficient mice. Nature. 445:106–110. 2007.
View Article : Google Scholar
|
|
6
|
Suetsugu A, Nagaki M, Aoki H, Motohashi T,
Kunisada T and Moriwaki H: Characterization of CD133+
hepatocellular carcinoma cells as cancer stem/progenitor cells.
Biochem Biophys Res Commun. 351:820–824. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang DG, Jiang AG, Lu HY, Zhang DG, Zhang
LX and Gao XY: Isolation, cultivation and identification of human
lung adenocarcinoma stem cells. Oncol Lett. 9:47–54. 2015.
|
|
8
|
Varnat F, Duquet A, Malerba M, et al:
Human colon cancer epithelial cells harbour active HEDGEHOG-GLI
signalling that is essential for tumour growth,
recurrence,metastasis and stem cell survival and expansion. EMBO
Mol Med. 1:338–351. 2009. View Article : Google Scholar
|
|
9
|
Sullivan JP, Minna JD and Shay JW:
Evidence for self-renewing lung cancer stem cells and their
implications in tumour initiation, progression and targeted
therapy. Cancer Metastasis Rev. 29:61–72. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Meng M, Zhao XH, Ning Q, Hou L, Xin GH and
Liu LF: Tumor stem cells: A new approach for tumor therapy
(Review). Oncol Lett. 4:187–193. 2012.PubMed/NCBI
|
|
11
|
Xu Y, Hu YD, Zhou J, Zhang MH, Yuan WW and
Luo Y: shRNA targeting Bmi1 impedes the self-renewal of
cisplatin-enriched stem-like cells in human A549 cells. Oncol Rep.
28:629–639. 2012.PubMed/NCBI
|
|
12
|
Cantrell DA: Phosphoinositide 3-kinase
signaling pathways. J Cell Sci. 114:1439–1445. 2001.PubMed/NCBI
|
|
13
|
Hennessy BT, Smith DL, Ram PT, Lu Y and
Mills GB: Exploiting the PI3K/AKT pathway for cancer drug
discovery. Nat Rev Drug Discov. 4:988–1004. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun XY, Ding LS, Jin XD, et al:
Correlation of PI3K/Akt/mTOR signal transduction pathway with both
malignancy progression and prognosis of human gliomas. Chin J
Neuromed. 10:24–28. 2011.In Chinese.
|
|
15
|
Missiaglia E, Dalai I, Barbi S, et al:
Pancreatic endocrine tumors:expression profiling evidences a role
for AKT-mTOR pathway. J Clin Oncol. 28:245–255. 2010. View Article : Google Scholar
|
|
16
|
Jiang AG, Yu H and Huang JA: Expression
and clinical significance of the PI3K/Akt signal transduction
pathway in non-small cell lung carcinoma. Oncol Lett. 8:601–607.
2014.PubMed/NCBI
|
|
17
|
Sunayama J, Sato A, Matsuda K, et al: Dual
blocking of mTor and PI3K elicits a prodifferentiation effect on
glioblastoma stem-like cells. Neuro Oncol. 12:1205–1219.
2010.PubMed/NCBI
|
|
18
|
Zhou J, Wulfkuhle J, Zhang H, et al:
Activation of the PTEN/mTOR/STAT3 pathway in breast cancer
stem-like cells is required for viability and maintenance. Proc
Natl Acad Sci USA. 104:16158–16163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang Y, Iwanaga K, Raso MG, et al:
Phosphatidylinositol 3-kinase mediates bronchioalveolar stem cell
expansion in mouse models of oncogenic k-ras-induced lung cancer.
PLoS One. 3:e22202008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Loebinger MR, Eddaoudi A, Davies D and
Janes SM: Mesenchymal stem cell delivery of TRAIL can eliminate
metastatic cancer. Cancer Res. 69:4134–4142. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Levi E, Misra S, Du J, Patel BB and
Majumdar AP: Combination of aging and dimethylhydrazine treatment
causes an increase in cancer-stem cell population of rat colonic
crypts. Biochem Biophys Res Commun. 385:430–433. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ghotra VP, Puigvert JC and Danen EH: The
cancer stem cell microenvironment and anti-cancer therapy. Int J
Radiat Biol. 85:955–962. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ho MM, Ng AV, Lam S and Hung JY: Side
population in human lung cancer cell lines and tumors is enriched
with stem-like cancer cells. Cancer Res. 67:4827–4833. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sung JM, Cho HJ, Yi H, et al:
Characterization of s stem cell population in lung cancer A549
cells. Biochem Biophys Res Commun. 371:163–167. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Levina V, Marrangoni AM, DeMarco R,
Gorelik E and Lokshin AE: Drug-selected human lung cancer stem
cells: cytokine network, tumorigenic and metastatic properties.
PLoS One. 3:e30772008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ota S, Ishii G, Goto K, et al:
Immunohistochemical expression of BCRP1 and ERCC1 in biopsy
specimen predicts survival in advanced non-small-cell lung cancer
treated with cisplatin-based chemotherapy. Lung Cancer. 64:98–104.
2009. View Article : Google Scholar
|
|
27
|
Smith KS, Chanda SK, Lingbeek M, et al:
Bmi-1 regulation of INK4A-ARF is adownstream requirement for
transformation of hematopoietic progenitors by E2a-Pbx1. Mol Cell.
12:393–400. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lessard J and Sauvageau G: Bmi-1
determines the proliferative capacity of normal and leukaemic stem
cells. Nature. 423:255–260. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gong C, Guo FJ, Qin L, et al: LY294002
inhibits proliferation of cancer stem-like cells from human
osteosarcoma via down-regulation of PI3K/AKT signaling pathwa. Chin
J Exp Surg. 28:2215–2217. 2011.In Chinese.
|
|
30
|
Sunayama J, Sato A, Matsuda K, et al: Dual
blocking of mTor and PI3K elicits a prodifferentiation effect on
glioblastoma stem-like cells. Neuro Oncol. 12:1205–1219.
2010.PubMed/NCBI
|
|
31
|
Cayrol C, Knibiehler M and Ducommun B: p21
binding to PCNA causes G1 and G2 cell cycle arrest in p53-deficient
cells. Oncogene. 16:311–320. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lebeau A, Unholzer A, Amann G, et al:
EGFR, HER-2/neu, cyclin D1, p21 and p53 in correlation to cell
proliferation and steroid hormone receptor status in ductal
carcinoma in situ of the breast. Breast Cancer Res Treat.
79:187–198. 2003. View Article : Google Scholar : PubMed/NCBI
|