Introduction
Orostachys japonicus is a perennial herb that
is primarily ubiquitous in Korea, China and Japan. Dried whole
plants of this species have previously been used as a Chinese
medicinal therapy for the treatment of fever, hemostasis,
hepatitis, arthritis, eczema and intoxication; in addition, O.
japonicus has traditionally been used in folk medicine as an
anticancer agent (1). However, the
extract nature of its physiological activity and the signaling
pathway involved remain to be elucidated.
Matrix metalloproteinases (MMPs) have an important
involvement in numerous physiological processes, including wound
healing, angiogenesis and tissue remodeling. An alteration in their
expression has been associated with the development of severe
pathological conditions (2). MMP
expression is mediated pre- and post-transcription. Numerous
extracellular factors, including cytokines, growth factors, cell
contact with the extracellular matrix as well as inducers and
inhibitors, have been suggested to be involved in regulating MMP
expression in various types of tumor cells (3,4). The
gelatinases, MMP-2 and MMP-9, digest components of the connective
tissue matrix and type IV collagen within the basement membrane
(5). MMP expression is known to be
mediated by numerous stimulatory factors, including cytokines,
growth factors and chemical agents (6). Various types of human tumors were
reported to be associated with upregulated MMP-2 and MMP-9
expression (7–9). In addition, several experimental and
clinical studies have reported a significant correlation between
the extent of tumor aggression and increased levels of MMP-2 and
MMP-9 (10–13).
Cyclooxygenase-2 (COX-2) is rapidly induced by
cytokines and tumor promoters (14). COX-2 is upregulated in the majority
of human tumor cells (15) and may
be downregulated by the antitumoral effects of nonsteroidal
anti-inflammatory drugs. Nitric oxide (NO) was reported to promote
cancer progression by regulating tumor angiogenesis (16); in vivo studies have
demonstrated an interaction between NO synthase (NOS) and the COX-2
pathway in inflammation (17). The
inducible NOS (iNOS) and COX-2 genes were found to be upregulated
in human colorectal cancer (18,19)
and tumor-associated macrophages reportedly express iNOS and COX-2
in certain malignant, borderline and benign tumors (20). In addition, nuclear factor-κB
(NF-κB) is involved in the regulation of iNOS and COX-2 expression
at the gene transcriptional or translational levels (21). Numerous studies have indicated that
MMP gene expression was regulated specifically by mitogen-activated
protein kinases (MAPKs), a family of serine/threonine kinases
including ERKs, JNK and p38 MAPK (22–24).
The present study aimed to investigate the mechanism by which O.
japonicus extract affects the expression of MMP-2 and MMP-9,
its association with the expression of iNOS and COX-2 in phorbol
myristate acetate (PMA)-differentiated THP-1 human monocytic
leukemia cells and how it mediates the regulation of the NF-κB and
MAPK pathways.
Materials and methods
Extraction of O. japonicas
A total of 20 g O. japonicus was extracted by
overnight incubation at 60°C in 500 ml 80% methanol. The solution
was filtered through Whatman No. 1 filter paper (Whatman
International Ltd., Maidstone, UK) and concentrated using a rotary
evaporator (Rotavapor R-220; BÜCHI Labortechnik AG, Flawil,
Switzerland). The concentrated extract was freeze-dried (FD-1;
EYELA, Tokyo, Japan) and stored at 4°C in a vacuum container until
further use.
Cell culture
THP-1 human monocytic leukemia cells were supplied
by the Korean Cell Line Bank (Seoul, Korea). Cells were cultured in
RPMI 1640 medium containing 10% fetal bovine serum and antibiotics
(all from Gibco-BRL, Grand Island, NY, USA). Cells were incubated
at 37°C in a humidified atmosphere of 5% CO2. THP-1
cells were treated with 100 nM PMA (Sigma-Aldrich, St. Louis, MO,
USA) for 72 h to induce differentiation of the cells into
macrophages. Following differentiation, non-attached cells were
removed by aspiration. The adherent macrophages were then washed
three times with RPMI 1640 medium and incubated in cell culture
medium at 37°C.
Cell viability
Cell proliferation was measured with Cell Titer 96
Aqueous One solution (Promega, Madison, WI, USA). Cells were seeded
at a density of 1×104 cells/well in 96-well plates and
incubated with various concentrations (0, 5, 10 and 25
μg/ml) of O. japonicus at 37°C for 24, 48 and 72 h,
respectively. Cell viability was determined using a colorimetric
assay with phenazine methosulfate/MTS solution. Absorbance was
determined at 490 nm, with background subtraction at 650 nm (Emax,
Molecular Devices, Sunnyvale, CA, USA).
Treatment with O. japonicas
THP-1 cells were incubated for 24 h in serum-free
medium with O. japonicus (0, 5, 10, and 25 μg/ml). At
each time point, the cell culture supernatant, total RNA and total
protein were isolated from the cultured THP-1 cells.
RNA extraction and reverse transcription
quantitative polymerase chain reaction (RT-qPCR) procedures
Total RNA was purified from cultured cells using
TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA)
according to the manufacturer’s instructions. First-strand cDNA
synthesis was performed with 1 μg total RNA and transcribed
to cDNA using a reverse-transcription system with random hexamers
(A3500; Promega) according to the manufacturer’s instructions. The
sequences for gene-specific primers (Bioneer, Daejeon, Korea) were
as follows: MMP-2 forward, 5′-CAACTACAACTTCTTCCCTCGCA-3′ and
reverse, 5′-GGTCACATCGCTCCAGACTTG-3′ (141 bp); MMP-9 forward,
5′-GCATAAGGACGACGTGAATGGC-3′ and reverse,
5′-CGGTGTGGTGGTGGTTGGAG-3′ (83 bp); iNOS forward,
5′-TGGATGCAACCCCATTGTC-3′ and reverse, 5′-CCCGCTGCCCCAGTTT-3′ (59
bp); COX-2 forward, 5′-CAAATCCTTGCTGTTCCCACCCAT-3′ and reverse,
5′-GTGCACTGTGTTTGGAGTGGGTTT-3′ (173 bp); and β-actin forward,
5′-GCGAGAAGATGACCCAGATC-3′ and reverse, 5′-GGATAGCACAGCCTGGATAG-3′
(77 bp). qPCR was performed on a StepOnePlus real-time PCR system
with Power SYBR Green PCR Master Mix (Applied Biosystems, Foster
City, CA, USA). PCR was performed with 1 μl cDNA in
20-μl reaction mixtures that were comprised of 10 μl
Power SYBR Green PCR Master Mix, 2 μl primers and 7
μl PCR-grade water (in A3500 reverse transcription system).
The reactions were performed with a denaturation step at 95°C for
10 min, 40 cycles of 95°C for 15 sec and 60°C for 1 min. The
crossing point of the target genes with β-actin was calculated
using the formula 2−(target gene-β-actin) and the
relative amounts were quantified.
Western blot analysis
The cells were collected and washed with cold
phosphate-buffered saline (Welgene, Daegu, Korea) and lysed using
lysis buffer [20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM
Na2 EDTA, 1 mM ethylene glycol tetraacetic acid, 1%
Triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM
Na3VO4 and 1 μg/ml leupeptin]
containing 1 mM phenylmethylsulfonyl fluoride (Cell Signaling
Technology, Boston, MA, USA). The protein concentration was
determined using a bicinchoninic acid protein assay (#23227; Thermo
Fisher Scientific, Rockford, IL, USA) according to the
manufacturer’s instructions. A total of 30 μg protein (per
group) was fractionated by 12% SDS-PAGE and transferred by
electrophoresis onto nitrocellulose membranes. The membranes were
blocked with 5% nonfat dry milk for 1 h at room temperature then
incubated overnight at 4°C with antibodies against iNOS (AB5382;
Chemicon, Billerica, MA, USA), COX-2 (SC-19999; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), NF-κB p65 (#8242),
phospho-NF-κB p65 (#3031), p38 MAPK (#9228), phospho-p38 MAPK
(#9215), MAPK kinase (MEK; #4694), phospho-MEK (#9154), ERK
(#4696), phospho-ERK (#4376) (Cell Signaling Technology) and
β-actin (A5441; Sigma-Aldrich); diluted 1:1,000 with Tris-buffered
saline containing 0.05% Tween 20 (TBS-T). Following washing with
TBS-T for 1 h, the membranes were incubated for 1 h at room
temperature with anti-rabbit (#7074) and anti-mouse (#7076)
horseradish peroxidase-conjugated secondary antibodies diluted
1:2,500 in TBS-T. The membranes were subsequently washed with TBS-T
for 1 h and proteins were detected using an enhanced
chemiluminescence kit (Santa Cruz Biotechnology, Inc.). The protein
expression was analyzed using a Davinch-Chemi™ Chemiluminescence
Imaging System (CAS-400 Chemi-DOC Image Analyzer; Davinch-K Co.
Ltd., Seoul, Korea).
Statistical analysis
Values are expressed as the mean ± standard
deviation. Student’s t-test was used to evaluate differences
between the control and O. japonicus-treated samples.
*P<0.05 and **P<0.01 were considered to
indicate a statistically significant difference between values.
Results
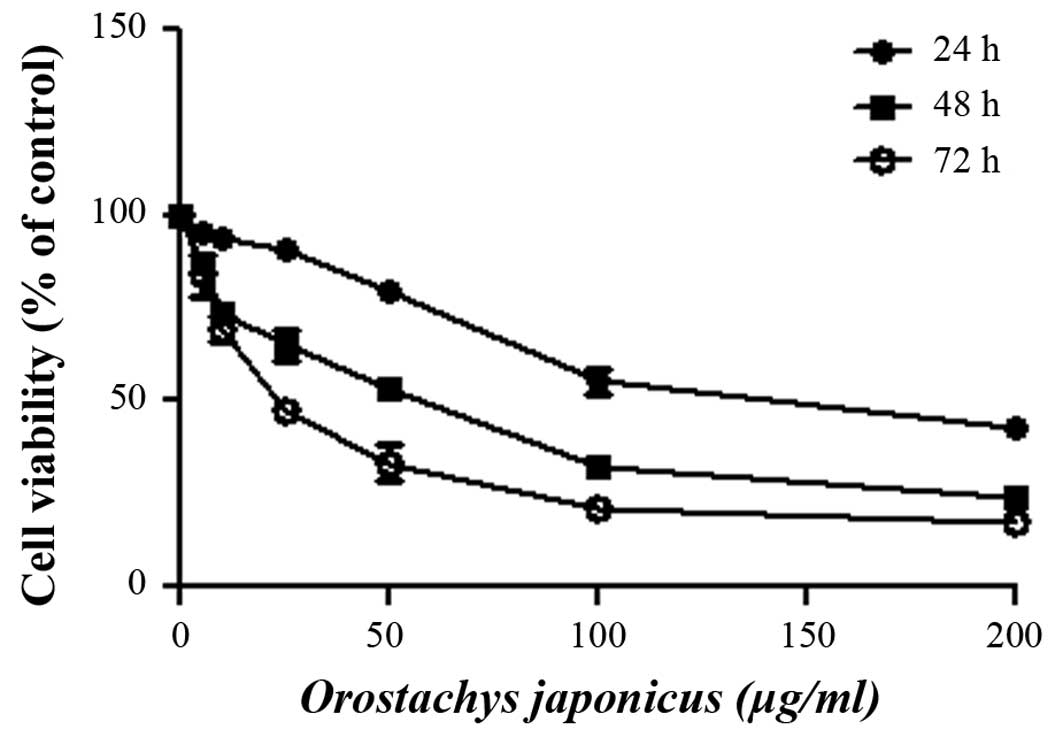
Inhibition of cell viability by O.
japonicas
The cytotoxic effect of O. japonicus on THP-1
cells was determined by exposing the cells to various
concentrations of O. japonicus for 24, 48, and 72 h. Cell
viability was measured using the MTT assay. O. japonicus
inhibited cell viability in THP-1 cells in a dose- and
time-dependent manner (Fig. 1).
However, O. japonicus had no effect on cell viability at a
low concentrations; therefore, concentrations of 5, 10 and 25
μg/ml were considered to be appropriate for the subsequent
experiments.
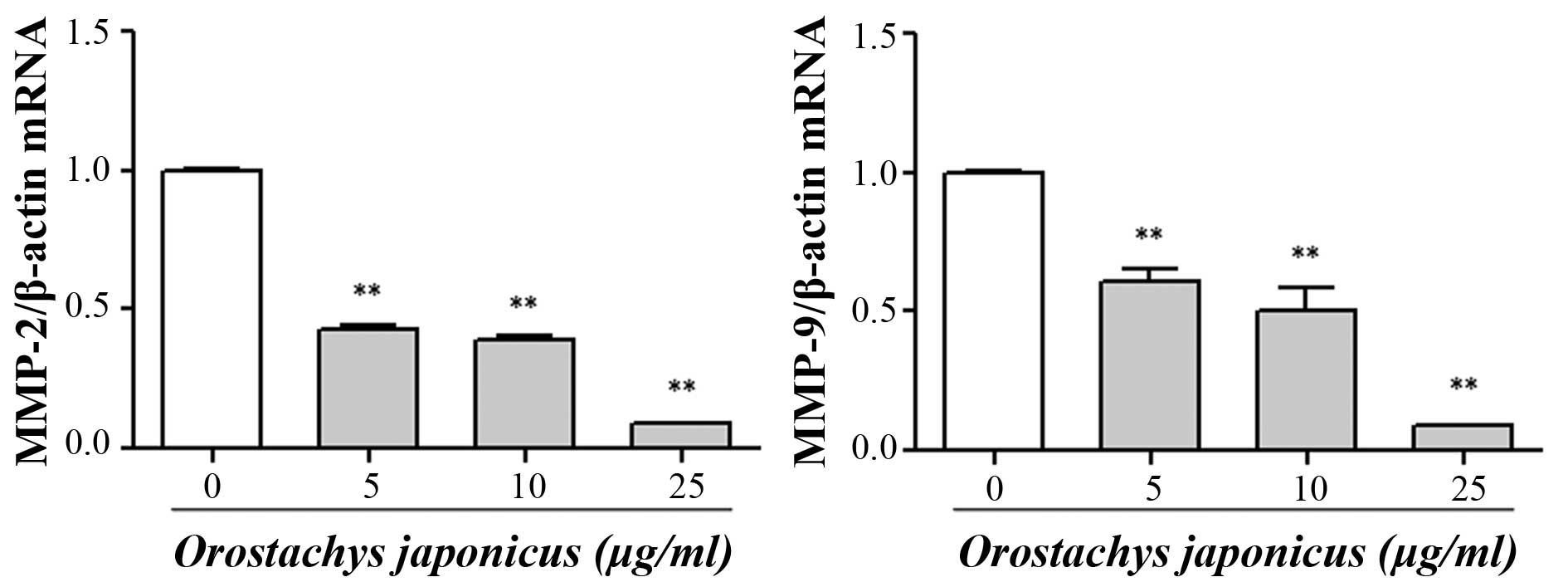
Inhibition of MMP-2 and MMP-9 mRNA
expression by O. japonicas
In order to investigate the effect of O.
japonicus on the expression of MMP-2 and MMP-9, THP-1 cells
were treated with various concentrations of O. japonicus (0,
5, 10, and 25 μg/ml) for 24 h. The expression of MMP mRNA
was then determined using RT-qPCR. The results showed that
treatment with O. japonicus led to a significant decrease in
the MMP-2 and MMP-9 mRNA expression at each tested concentration
compared with that of the control group (P<0.01) (Fig. 2).
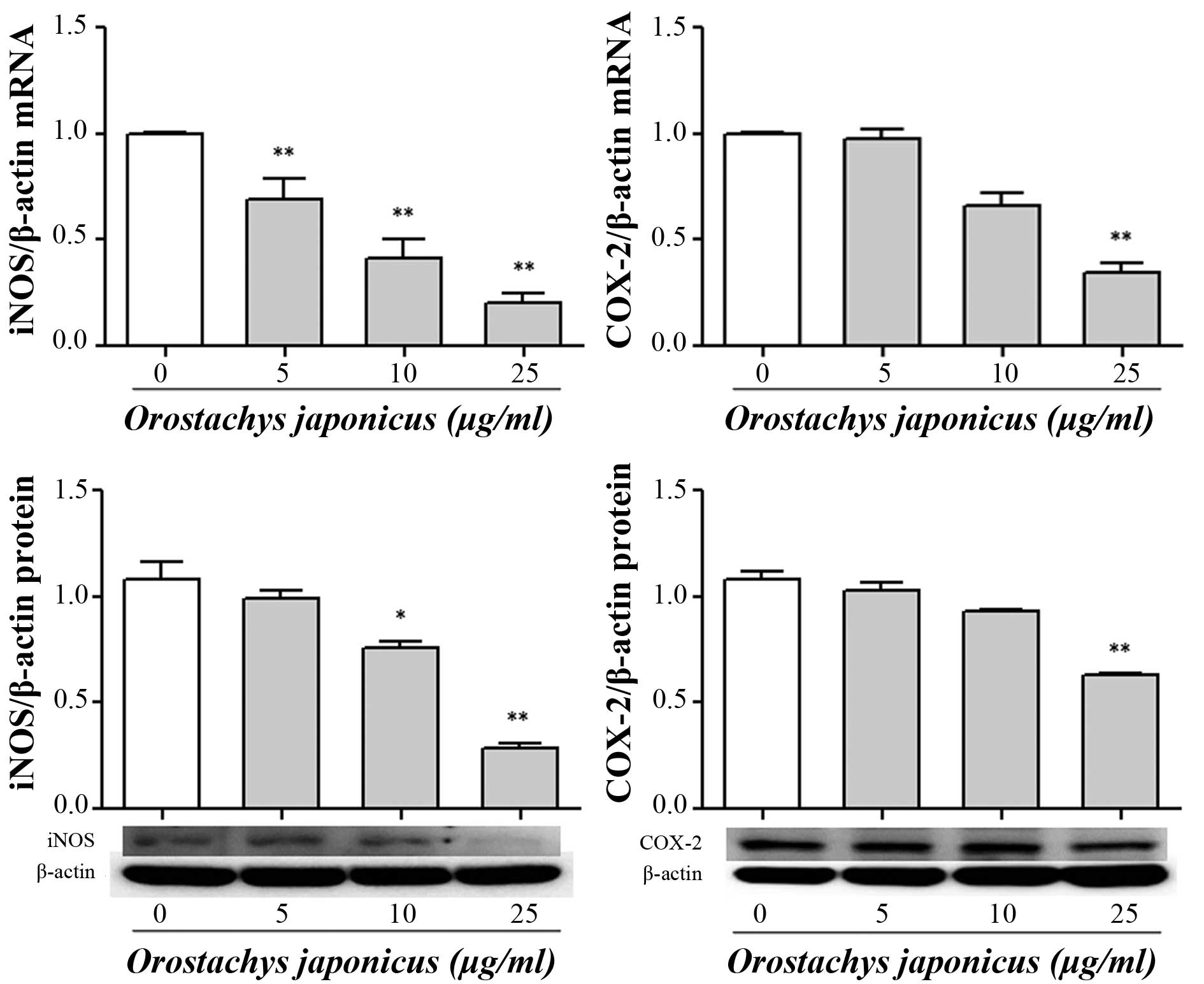
Inhibition iNOS and COX-2 expression by
O. japonicas
To examine the effects of O. japonicus on the
expression of iNOS and COX-2 mRNA and protein, THP-1 cells were
treated with various concentrations of O. japonicus (5, 10
and 25 μg/ml) for 24 h. The expression of mRNA and protein
were measured using RT-qPCR and western blot analysis. Treatment
with O. japonicus significantly suppressed iNOS mRNA
expression at all tested concentrations (P<0.01) and iNOS
protein expression at 10 and 25 μg/ml O. japnicus
(P<0.05 and 0.01, respectively) compared with that of the
control. In addition, COX-2 transcription and translocation were
significantly reduced at 25 μg/ml O. japonicus
compared with the control (P<0.01) (Fig. 3).
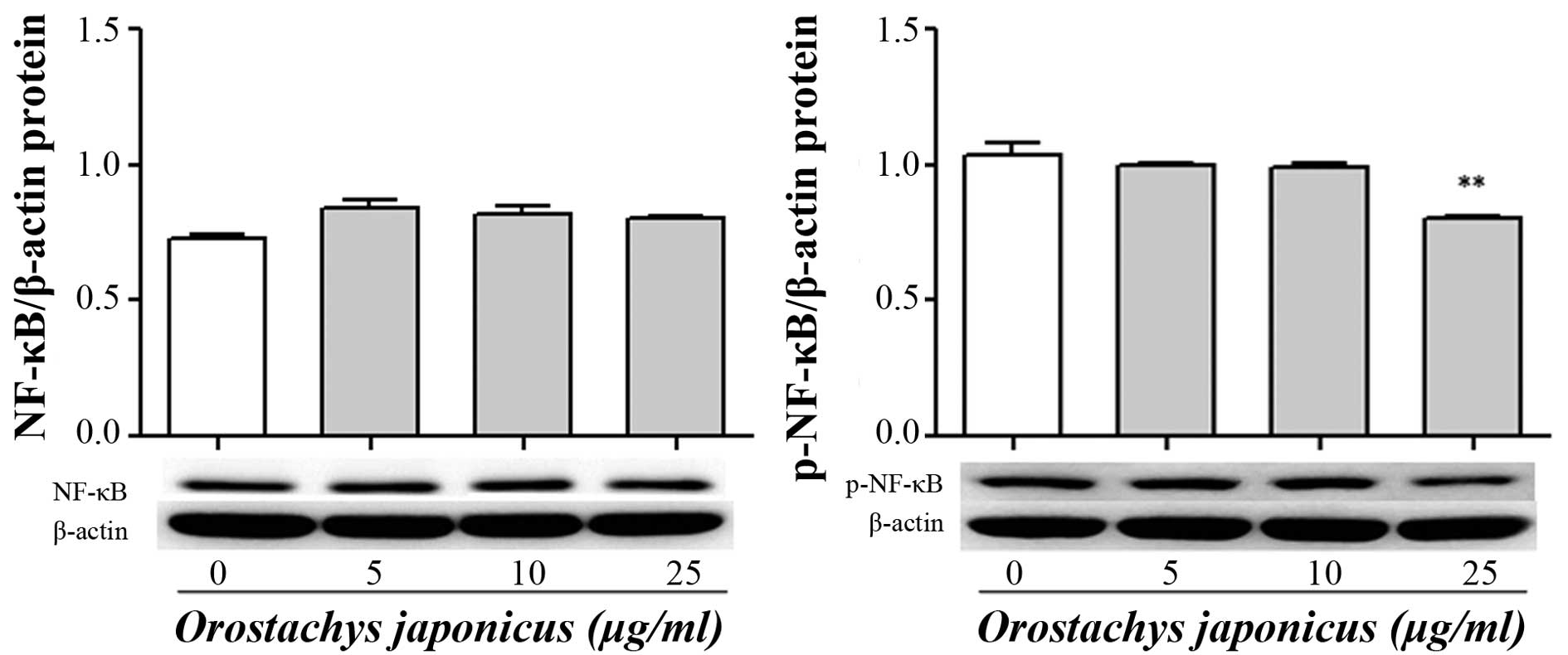
Inhibition of NF-κB p65 nuclear
translocation by O. japonicas
In order to investigate the mechanisms involved in
the inhibition of the NF-κB activity, THP-1 cells were treated with
various concentrations of O. japonicus (5, 10 and 25
μg/ml) for 24 h. The expression of NF-κB p65 protein was
determined by western blot analysis. The results showed that
phosphorylation of NF-κB p65 was significantly inhibited by O.
japonicus at 25 μg/ml compared with the control
(Fig. 4).
Inhibition of p38 MAPK, MEK and ERK
phosphorylation by O. japonicas
The activation of signal transduction by O.
japonicus was evaluated in THP-1 cells. The cells were treated
with various concentrations of O. japonicus (5, 10, and 25
μg/ml) for 24 h. The expression of p38 MAPK, MEK and ERK
protein was then determined by western blot analysis. O.
japonicus had no effects on total p38 MAPK and ERK expression
compared with the control, whereas O. japonicus markedly
suppressed their phosphorylation at concentrations of 10 and 25
μg/ml (P<0.01) (Fig. 5).
However, O. japonicus showed no significant affect on MEK
phosphorylation.
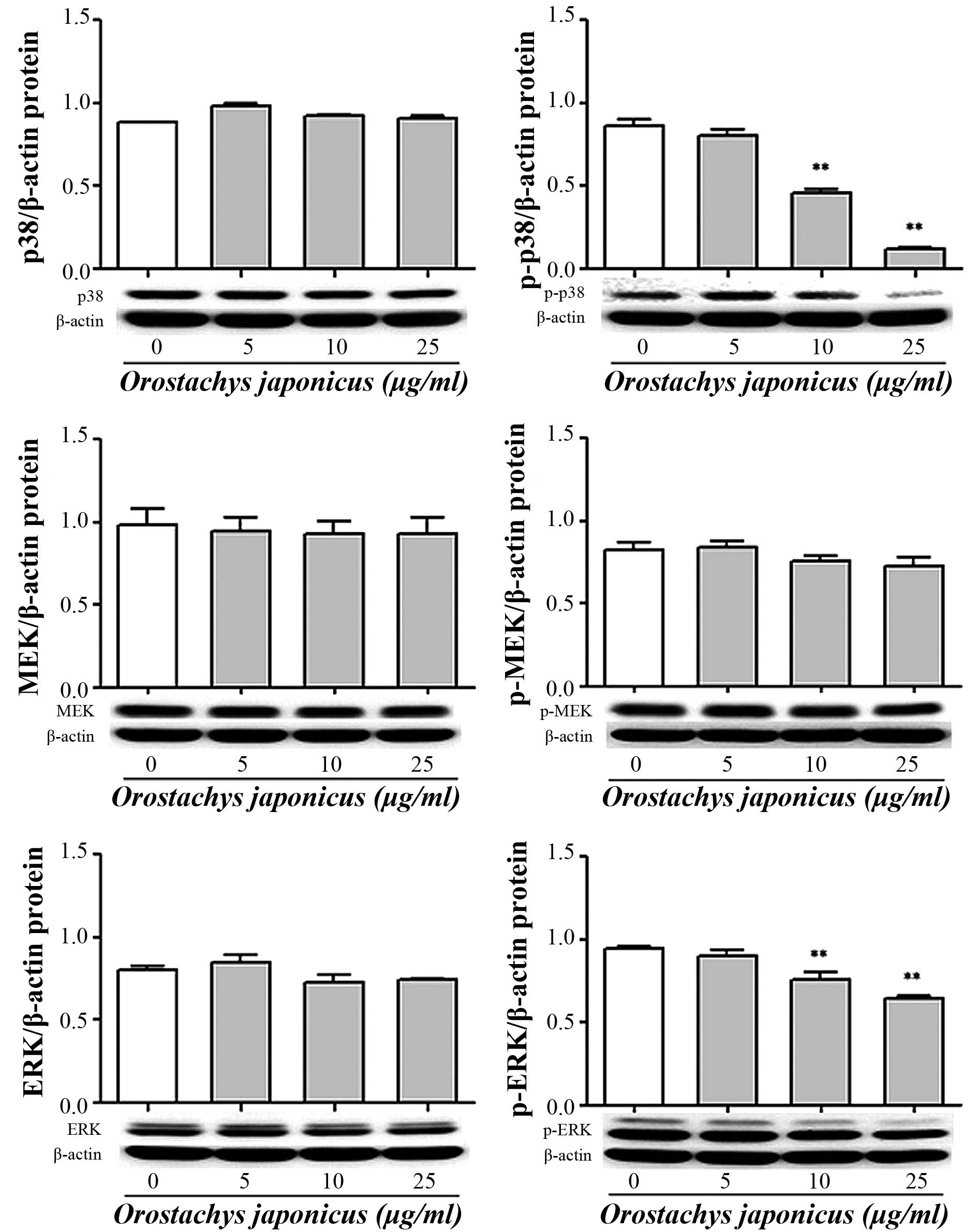 | Figure 5Orostachys japonicus inhibits
p38 MAPK, MEK and ERK phosphorylation in THP-1 cells. Cells were
cultured with various concentrations of O. japonicus (0, 5,
10 and 25 μg/ml) for 24 h and the expression of p38 MAPK,
MEK and ERK protein was examined by immunoblotting. Densitometric
analyses are presented as the relative ratios of p38 MAPK, p-p38
MAPK, MEK, p-MEK, ERK and p-ERK against β-actin. Values are
expressed as the mean ± standard deviation of three independent
samples. **P<0.01 vs. 0 μg/ml O.
japonicus (control). MAPK, mitogen activated protein kinase;
MEK, MAPK kinase; ERK, extracellular related kinase; p-,
phosphorylated. |
Discussion
In the present study, THP-1 human monocytic leukemia
cells were induced to differentiate into macrophages using PMA and
the cells were then treated with O. japonicus at various
concentrations. It was identified that O. japonicus was able
to inhibit cell proliferation in THP-1 cells in a time- and
dose-dependent manner at a high concentration, whereas lower
concentrations had no marked effect. Therefore, concentrations of
5, 10 and 25 μg/ml were considered to be appropriate for the
subsequent experiments.
In a previous study, the invasion and migration
abilities of THP-1 human monocytic leukemia cells were
significantly inhibited by Sinnomenine in a dose-dependent manner;
in addition, the levels of MMP-2 and MMP-9 were downregulated
(25). Another previous study
demonstrated that the mRNA and protein expression of MMP-2 and
MMP-9 in LnCaP prostate carcinoma cells were decreased following
treatment with flavonoids extracted from O. japonicus (FEOJ)
in a dose-dependent manner. These findings supported the idea that
the suppression of MMPs by FEOJ is associated with the
anti-invasive activity of FEOJ (26). It is possible that MMPs are not
directly produced by cancer or stromal cells, but that other sites
may be responsible for the increased levels of MMP-9, which were
reported to correlate with the existence of tumor tissues (27). In the present study, it was
demonstrated that O. japonicus inhibited MMP-2 and MMP-9
gene transcription. The present results suggested that O.
japonicus inhibited migration and invasion of cancer cells by
inhibiting the expression of MMP-2 and MMP-9 in THP-1 cells.
The NOS and COX systems are important in similar
pathophysiological conditions, such as inflammation and cancer
(28). In the present study, the
effects of O. japonicus on NO were investigated and it was
identified that NO was not detected in the culture supernatants of
THP-1 cells. The present results revealed that O. japonicus
inhibited iNOS and COX-2 mRNA and protein expression. Therefore,
these findings suggested that O. japonicus mediated iNOS and
COX-2 at the gene transcription and translocation levels in THP-1
cells. NF-κB has been reported to mediate the expression of
specific genes, the products of which were found to be involved in
tumorigenesis. In addition, NF-κB is able to induce the activation
of MMP-9 and COX-2 (29,30). Activation of NF-κB promotes
proinflammatory cytokines and enzymes, including TNF-α,
interleukins, NO, prostaglandin E2, iNOS and COX-2,
which may ultimately induce neuronal damage (31). In one study, treatment with
shikonin was demonstrated to down-regulate levels of MMP-2 and
MMP-9 through the suppression of AKT activation as well as the
inhibition of the NF-κB signaling pathway (32).
The MAPK cascade is an important signal transduction
pathway. Members of the MAPK gene family, including JNK, p38 MAPK
and ERK1/2, enhance MMP production via activation of the
transcription factor activation protein (AP-1) (33,34).
Silibinin reportedly reduces ERK1/2 phosphorylation, but has no
observable effects on the phosphorylation of JNK 1/2, p38 MAPK or
Akt (23). Expression of iNOS and
COX-2 is upregulated by activated MAPKs with induction of the
transcription factor AP-1 (35).
ERK and p38 MAPK pathway suppression was reported to result in
MMP-2 and MMP-9 downregulation in U937 leukemia cells (36). The inhibitory mechanism of
andrographolide may proceed via the inhibition of NF-κB activation
and subsequent attenuation of MMP-9 expression (37). In the present study, the
phosphorylation levels of p38 MAPK and ERK1/2 were inhibited by
O. japonicus. This notable finding suggested that modulation
of the expression of the MMPs and inflammatory genes (COX-2 and
iNOS) in THP-1 cells treated with O. japonicus was likely to
be mediated by MAPKs, including p38 MAPK, MEK and ERK1/2.
In conclusion, O. japonicus treatment of
PMA-differentiated THP-1 cells inhibited not only MMP-2 and MMP-9,
but also iNOS and COX-2 transcription and translocation. The
activation of NF-κB and the phosphorylation of p38 MAPK, MEK and
ERK1/2 were also found to be reduced. However, O. japonicus
may also inhibit the expression of MMP-2 and MMP-9 mRNA and the
transcription and translation of iNOS and COX-2 by inhibiting the
activation of NF-κB and phosphorylation of the MAPK pathway in
THP-1 cells.
References
|
1
|
Kim JK: Illustrated Natural Drugs
Encyclopedia. 1. 1st. Namsandang Press; Korea: pp. 14–167. 1984
|
|
2
|
Visse R and Nagase H: Matrix
metalloproteinases and tissue inhibitors of metalloproteinases:
structure, function and biochemistry. Circ Res. 92:827–839. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Apodaca G, Rutka JT, Bouhana K, et al:
Expression of metal-loproteinases and metalloproteinase inhibitors
by fetal astrocytes and glioma cells. Cancer Res. 50:2322–2329.
1990.PubMed/NCBI
|
|
4
|
Ray JM and Stetler-Stevenson WG: The role
of matrix metal-loproteases and their inhibitors in tumour
invasion, metastasis and angiogenesis. Eur Respir J. 7:2062–2072.
1994.PubMed/NCBI
|
|
5
|
Stetler-Stevenson WG: Type IV collagenases
in tumor invasion and metastasis. Cancer Metastasis Rev. 9:289–303.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mauviel A: Cytokine regulation of
metalloproteinase gene expression. J Cell Biochem. 53:288–295.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Di Nezza LA, Misajon A, Zhang J, et al:
Presence of active gelatinases in endometrial carcinoma and
correlation of matrix metalloproteinase expression with increasing
tumor grade and invasion. Cancer. 94:1466–1475. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sato T, Sakai T, Noguchi Y, Takita M,
Hirakawa S and Ito A: Tumor-stromal cell contact promotes invasion
of human uterine cervical carcinoma cells by augmenting the
expression and activation of stromal matrix metalloproteinases.
Gynecol Oncol. 92:47–56. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Berube M, Deschambeault A, Boucher M,
Germain L, Petitclerc E and Guerin SL: MMP-2 expression in uveal
melanoma: differential activation status dictated by the cellular
environment. Mol Vis. 11:1101–1111. 2005.PubMed/NCBI
|
|
10
|
Cottam DW, Rennie IG, Woods K, Parsons MA,
Bunning RA and Rees RC: Gelatinolytic metalloproteinase secretion
patterns in ocular melanoma. Invest Ophthalmol Vis Sci.
33:1923–1927. 1992.PubMed/NCBI
|
|
11
|
Garzetti GG, Ciavattini A, Lucarini G, et
al: Tissue and serum metalloproteinase (MMP-2) expression in
advanced ovarian serous cystoadenocarcinomas: clinical and
prognostic implications. Anticancer Res. 15(6B): 2799–2804.
1995.PubMed/NCBI
|
|
12
|
Fishman DA, Bafetti LM, Banionis S, Kearns
AS, Chilukuri K and Stack MS: Production of extracellular
matrix-degrading proteinases by primary cultures of human
epithelial ovarian carcinoma cells. Cancer. 80:1457–1463. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stetler-Stevenson WG: The role of matrix
metalloproteinases in tumor invasion, metastasis and angiogenesis.
Surg Oncol Clin N Am. 10:383–392. 2001.
|
|
14
|
Smith WL and Langenbach R: Why there are
two cyclooxygenase isozymes. J Clin Invest. 107:1491–1495. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Koki AT, Leahy KM, Harmon JM and Masferrer
JL: Cyclooxygenase-2 and cancer. COX-2 blockade in cancer
prevention and therapy. Harris RE: Humana Press; Totowa, NJ: pp.
185–203. 2003
|
|
16
|
Gallo O, Fabbroni V, Sardi I, Magnelli L,
Boddi V and Franchi A: Correlation between nitric oxide and
cyclooxygenase-2 pathways in head and neck squamous cell
carcinomas. Biochem Biophys Res Commun. 299:517–524. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Han M, Wen JK, Zheng B and Zhang DQ:
Acetylbritannilatone suppresses NO and PGE2 synthesis in
RAW 264.7 macrophages through the inhibition of iNOS and COX-2 gene
expression. Life Sci. 75:675–684. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cianchi F, Cortesini C, Bechi P, et al:
Up-regulation of cyclooxygenase 2 gene expression correlates with
tumor angiogenesis in human colorectal cancer. Gastroenterology.
121:1339–1347. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cianchi F, Cortesini C, Fantappie O, et
al: Inducible nitric oxide synthase expression in human colorectal
cancer: correlation with tumor angiogenesis. Am J Pathol.
162:793–801. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Klimp AH, Hollema H, Kempinga C, van der
Zee AG, de Vries EG and Daemen T: Expression of cyclooxygenase-2
and inducible nitric oxide synthase in human ovarian tumors and
tumor-associated macrophages. Cancer Res. 61:7305–7309.
2001.PubMed/NCBI
|
|
21
|
Chiang YM, Lo CP, Chen YP, et al: Ethyl
caffeate suppresses NF-κB activation and its downstream
inflammatory mediators, iNOS, COX-2 and PGE2 in vitro or
in mouse skin. Br J Pharmacol. 146:352–363. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen PN, Hsieh YS, Chiang CL, Chiou HL,
Yang SF and Chu SC: Silibinin inhibits invasion of oral cancer
cells by suppressing the MAPK pathway. J Dent Res. 85:220–225.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hsieh YS, Chu SC, Yang SF, Chen PN, Liu YC
and Lu KH: Silibinin suppresses human osteosarcoma MG-63 cell
invasion by inhibiting the ERK-dependent c-Jun/AP-1 induction of
MMP-2. Carcinogenesis. 28:977–987. 2007. View Article : Google Scholar
|
|
24
|
Weng CJ, Chau CF, Hsieh YS, Yang SF and
Yen GC: Lucidenic acid inhibits PMA-induced invasion of human
hepatoma cells through inactivating MAPK/ERK signal transduction
pathway and reducing binding activities of NF-κB and AP-1.
Carcinogenesis. 29:147–156. 2008. View Article : Google Scholar
|
|
25
|
Ou YQ, Chen LH, Li XJ, Lin ZB and Li WD:
Sinomenine influences capacity for invasion and migration in
activated human monocytic THP-1 cells by inhibiting the expression
of MMP-2, MMP-9 and CD147. Acta Pharmacol Sin. 30:435–441. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shin DY, Lee WS, Jung JH, et al:
Flavonoids from Orostachys japonicus A. Berger inhibit the invasion
of LnCaP prostate carcinoma cells by inactivating Akt and
modulating tight junctions. Int J Mol Sci. 14:18407–18420. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Quaranta M, Daniele A, Coviello M, et al:
MMP-2, MMP-9, VEGF and CA 15.3 in breast cancer. Anticancer Res.
27(5B): 3593–3600. 2007.PubMed/NCBI
|
|
28
|
Ohshima H, Tazawa H, Sylla BS and Sawa T:
Prevention of human cancer by modulation of chronic inflammatory
processes. Mutat Res. 591:110–122. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pahl HL: Activators and target genes of
Rel/NF-κB transcription factors. Oncogene. 18:6853–6866. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Garg A and Aggarwal BB: Nuclear
transcription factor-κB as a target for cancer drug development.
Leukemia. 16:1053–1068. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bi X, Yan B, Fang S, et al: Quetiapine
regulates neurogenesis in ischemic mice by inhibiting NF-κB p65/p50
expression. Neurol Res. 31:159–166. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wei PL, Tu CC, Chen CH, et al: Shikonin
suppresses the migratory ability of hepatocellular carcinoma cells.
J Agric Food Chem. 61:8191–8197. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kanangat S, Postlethwaite A, Hasty K, et
al: Induction of multiple matrix metalloproteinases in human dermal
and synovial fibroblasts by Staphylococcus aureus: implications in
the pathogenesis of septic arthritis and other soft tissue
infections. Arthritis Res Ther. 8:R1762006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Menke NB, Ward KR, Witten TM, Bonchev DG
and Diegelmann RF: Impaired wound healing. Clin Dermatol. 25:19–25.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim JM, Jung HY, Lee JY, Youn J, Lee CH
and Kim KH: Mitogen-activated protein kinase and activator
protein-1 dependent signals are essential for Bacteroides fragilis
enterotoxin-induced enteritis. Eur J Immunol. 35:2648–2657. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu WH and Chang LS: Caffeine induces
matrix metallopro-teinase-2 (MMP-2) and MMP-9 down-regulation in
human leukemia U937 cells via Ca2+/ROS-mediated
suppression of ERK/c-fos pathway and activation of p38 MAPK/c-jun
pathway. J Cell Physiol. 224:775–785. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee WR, Chung CL, Hsiao CJ, et al:
Suppression of matrix metalloproteinase-9 expression by
andrographolide in human monocytic THP-1 cells via inhibition of
NF-κB activation. Phytomedicine. 19:270–277. 2012. View Article : Google Scholar : PubMed/NCBI
|