Introduction
Postoperative pain is a common clinical symptom,
predominantly caused by peripheral and central sensitization from
the persistent excitement of nociceptors (1). The mechanisms involved in the
occurrence and development of postoperative pain remains
unclear.
Previous studies have demonstrated that vascular
dysfunction and vascular endothelial cells have a role in
mechanical pain (2,3); however, few studies have investigated
the effects of microvessel density (MVD) on postoperative pain. MVD
is generally considered to be a quantitative parameter for vascular
endothelial cell function and angiogenesis (4). It has been previously observed that
MVD is positively correlated with the expression of glucose
transporter protein-1 (GLUT1), which functions in basal metabolism
and can enhance glucose utilization (5,6).
Furthermore, GLUT1 has been demonstrated as having a crucial role
in osteoarthritic pain (7). Nerve
growth factor (NGF), a multi-functional nutritional factor, is
produced by innervated target organs, and undergoes transportation
into nerve terminals (8–10). An overproduction of NGF has been
shown to be capable of inducing central sensitization (11,12).
In addition, previous studies have shown that the persistent
activation of C-jun N-terminal kinases (JNK) in spinal astrocytes,
following nerve injury and inflammation, can induce central
sensitization (13,14). Furthermore, the activation of
ATP-sensitive potassium (KATP) channels has been implicated in
mediating anti-nociceptive effects following ventricular,
intrathecal or epidural injection of KATP activators in numerous
animal models (15,16); however, the direct effects of KATP
activators on sensory neurons remains unclear (17).
Previous studies have investigated the
anti-nociceptive effects of KATP inhibitors or activators
administered peripherally (18,19),
yet studies regarding the histopathological changes around the
incision site, in response to KATP stimulation, are scarce
(16,17). Thus, there remains uncertainty
regarding the direct effects of KATP activators on nociceptors.
The present study established a rat model of
postoperative pain evoked by skin/muscle incision and retraction
(SMIR) surgery (20). The KATP
activator pinacidil was intraperitoneally injected prior to
surgery. The direct effects of pinacidil on nociceptors around the
incision site, were assessed by detecting the mechanical withdrawal
thresholds (MWT). The changes to the levels of MVD, as well as the
changes to the relative protein expression levels of GLUT1, NGF and
spinal JNK were observed both prior to and following the surgery.
In addition the effects of the microenvironment surrounding the
incision site on mechanical allodynia, following SMIR surgery, and
the effects of pinacidil on mechanical allodynia and its mechanisms
of action, were determined.
Materials and methods
Animal grouping
The present study was approved by the Experimental
Animal Protection and Care Committee of Nantong University
(Jiangsu, China). A total of 24 male Sprague Dawley rats (weighing
200–250 g) were obtained from the Laboratory Animal Center of
Nantong University (Jiangsu, China), and maintained in conventional
housing. The rats were randomly assigned to four groups
(n=6/group): Control, sham (incision operation), SMIR (incision
plus retraction), and pinacidil (SMIR plus pinacidil) groups. The
rats in the control group did not receive any treatment. The rats
in the sham operation group had an incision made through the skin
and muscle. The rats in the SMIR group underwent 1 h retraction
after the skin/muscle incision. The rats in the pinacidil group
were further divided into three subgroups, and were
intraperitoneally injected with either low (10 μg/kg),
middle (25 μg/kg), or high-dose pinacidil (50 μg/kg)
(Sigma-Aldrich, St Louis, MO, USA; lot number: D9035-250MG) prior
to the SMIR procedure.
Behavioral assessments
All of the rats were adapted to the testing
conditions for three days prior to experimentation. To quantify
mechanical allodynia, the MWT was determined using von Frey
filaments (range 1.4-2.6 g; North Coast Medical Inc., Morgan Hill,
CA, USA), as described by previous methods (21). Briefly, each rat was placed in a
Plexiglass® box (Nantong Jingxin Optical Glass, Co.,
Ltd., Nantong, China) with a wire mesh floor. Following habituation
for 30 min to the environment, the von Frey filament was pressed
perpendicular to the plantar surface of both hind paws and held for
≤4 sec. A positive response was noted if the rats showed paw
withdrawal, flinches or licking. If there was no response
(negative), the next heavier filament was tested. Each trial was
repeated five times. At each 30 sec interval, the 50% threshold was
determined using the “up and down” method, as previously described
(22); in which if no two
consecutive positive responses appeared, a further heavier
stimulation was administered; if two consecutive positive responses
appeared, a lighter stimulation was administered until the
alternation of one positive and one negative response was reached.
The alternations with five repetitions were recorded.
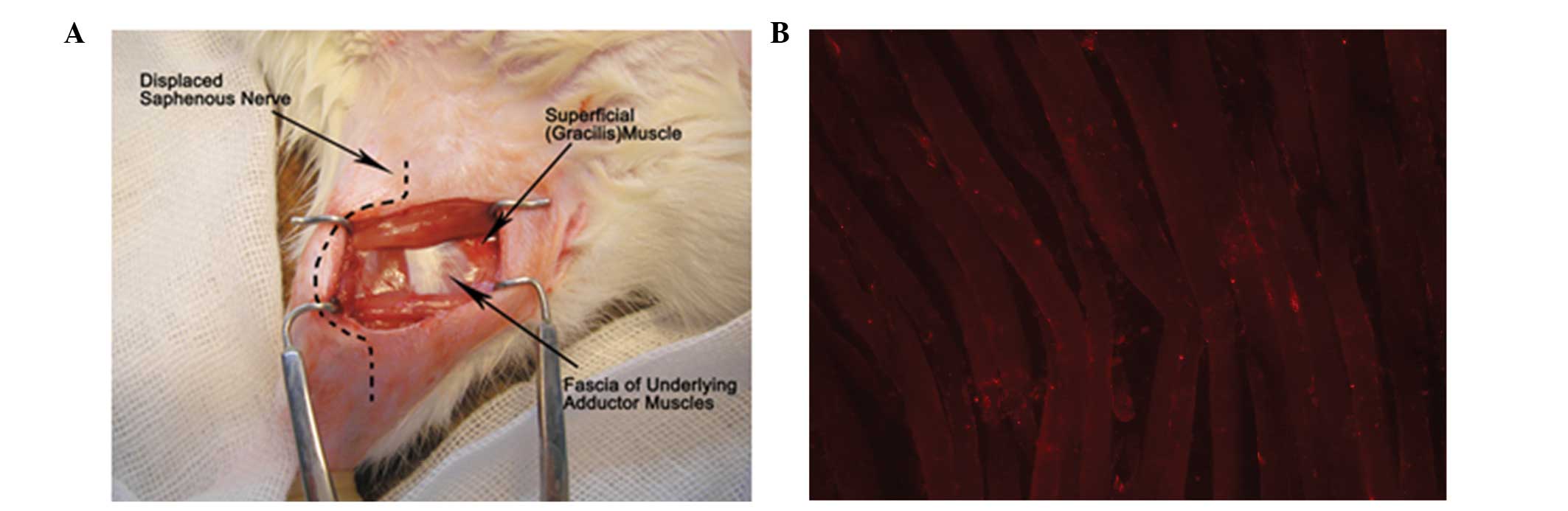
Establishment of the SMIR model
The rats were anesthetized with an intraperitoneal
injection of nembutal (40 mg/kg; Beijing Propbs Biotechnology Co.,
Ltd., Beijing, China), and laid in a supine position under sterile
conditions. A 1.5–2 cm incision was made in the medial side of the
right hind limb ~4 mm medial to the saphenous vein, in order to
reveal the thigh muscle. An incision (7–10 mm long) was made in the
superficial muscle layer of the thigh. The superficial muscle was
then retracted 2 cm by spreading blunt scissors within the muscle
incision site. This retraction was maintained for 1 h. During the
retraction period, the incision site was covered with gauze and
moistened with sterile saline, in order to prevent dehydration of
the surgical site. Following the SMIR procedure, the incision was
covered with gauze coated with gentamycin (Yantai Justaware
Pharmaceutical Co., Ltd., Yantai, China) to prevent infection. The
establishment of the injury site during the 1 h retraction period
of the SMIR surgery is shown in Fig.
1.
Western blot analysis
Three days after the SMIR surgery, the rats in each
group were anesthetized as described previously. The peripheral
muscle and lumbar regions 3–5 of the spinal cord were harvested and
homogenized on ice in SDS sample buffer (10 ml/mg tissue),
containing a cocktail of proteinase and phosphatase inhibitors
(Sigma-Aldrich), using a hand-held pestle. The protease inhibitor
cocktail (P2714) contains AEBSF, E-64, bestatin, leupeptin,
aprotinin, and sodium EDTA, and the phosphatase cocktail (P5726)
contains sodium orthovanadate, sodium molybdate, sodium tartrate,
and imidazole. The cell lysates were collected and transferred to a
1.5 ml centrifuge tube. After centrifugation at 10,000 × g for 18
min at 4°C, the protein was extracted, denatured and stored at 4°C,
until further use. To determine the protein concentrations, the
protein samples were diluted with double- distilled H2O
five times, and the diluted samples were plated into a 96-well
plate. The protein concentration was measured using a bicinchoninic
acid kit (Pierce Biotechnology, Inc., Rockford, IL, USA). A Synergy
2 Multi-Mode reader (Biotek Instruments, Inc., Winooski, VT, USA)
was used to measure the optical density of each sample, at a
wavelength of 562 nm (OD>0.995). The protein concentration of
each sample was calculated by referring to a standard curve of the
standard reference.
Subsequently, equal amounts (40 μg/lane) of
total protein from each sample were separated by 5 and 10% SDS-PAGE
(Beyotime Biotech Inc., Nanjing, China) sequentially, and
transferred to polyvinylidene difluoride membranes (EMD Millipore,
Shanghai, China). The required protein volume per lane was
calculated by dividing the total protein amount loaded per lane, by
the protein concentration. The membranes were incubated overnight
at 4°C with one of the following primary antibodies: Rabbit
anti-NGF (1:200 dilution), goat anti-GLUT1 (1:200 dilution) and
goat anti-JNK (1:200 dilution), followed by an incubation with the
corresponding horseradish peroxidase-conjugated secondary
antibodies (1:3,000 dilution; GE Healthcare Life Sciences,
Chalfont, UK), at room temperature for 2 h. All of the primary
antibodies used for western blotting were purchased from Santa Cruz
Biotechnology Inc. (Santa Cruz, CA, USA). Following several washes,
the intensity of the visualization signal was detected using an
Enhanced Chemiluminescence Substrate kit (Thermo Fisher Scientific
Inc., Shanghai, China), and the relative protein levels were
quantified using the Image J software system (National Institutes
of Health, Bethesda, MD, USA). GAPDH served as an endogenous
internal reference. The relative expression of each target protein
was calculated as the ratio of the intensity of the target protein
band as compared with GAPDH.
Measurement of MVD
Any endothelial cell or endothelial cell cluster was
considered a single countable microvessel, as described by previous
methods (23). The tissues from
the different groups were cut into 5-μm serial sections, and
immunohistochemical staining was used to detect factor VIII, in
order to evaluate the MVD. Briefly, the rats were terminally
anesthetized with isoflurane and the ascending aorta was perfused
with saline, followed by 4% paraformaldehyde (Sigma-Aldrich) with
1.5% picric acid (Sigma-Aldrich) in 0.16 M phosphate buffer (pH
7.2–7.4). Following the perfusion, the muscle tissue around the
incision site was harvested and post-fixed in the same fixative for
3–6 h, then replaced with 15% sucrose (Sigma-Aldrich) overnight.
Muscle tissue sections (15 mm) were cut in a cryostat and processed
for immunofluorescence. All of the sections were blocked with 5%
donkey serum (Gibco-BRL, Carlsbad, CA, USA) in 0.3% Triton
(Gibco-BRL) for 2 h at room temperature and incubated overnight at
4°C with factor VIII antibody (Santa Cruz Biotechnology Inc.). The
sections were then incubated for 2 h at room temperature with
Cy3-conjugated secondary antibody (1:300, Jackson ImmunoResearch
Laboratories, Inc., West Grove, PA, USA). The stained sections were
examined using a Leica fluorescence microscope (Leica Microsystems
GmbH, Wetzlar, Germany), and the images were captured with a
charge-coupled device Spot camera (Leica Microsystems GmbH). For
the negative controls the primary antibodies were omitted. The
factor VIII-positive sections were stained orange and were
initially scanned at ×100 magnification. The three areas with the
highest number of microvessels were selected, and were subsequently
scanned at ×200 magnification. The mean number of microvessels was
defined as the MVD (15).
Statistical analyses
All statistical analyses were performed using SPSS
version 17.0 (SPSS Inc., Chicago, IL, USA), and all data are
expressed as the means ± standard deviation. Differences between
the two groups were compared using a Student’s t-test and a one-way
analysis of variance was used to compare the differences among ≥3
groups. Statistical diagrams were drawn using Excel (Microsoft
Corporation, Redmond, WA, USA). A P<0.05 was considered to
indicate a statistically significant difference.
Results
Changes in MWT values in response to SMIR
and pinacidil administration
It has been previously reported that SMIR may induce
mechanical allodynia in rats, but that it has no effects on thermal
or cold hyperalgesia (14). As
shown in Table I, the MWT was
significantly decreased at each time point in the SMIR group, as
compared with the control group, in a time-dependent manner.
However, there were no significant differences in the MWT of rats
from the sham operation group, comparing between before and after
the sham surgery. Furthermore, pinacidil administration was shown
to significantly attenuate the SMIR-induced reduction in MWT, in a
dose-dependent manner. As compared with the control group, the MWT
was significantly reduced in the low-dose pinacidil (10
μg/kg) group 3, 7 and 12 days after the SMIR surgery. A
significantly decreased MWT was also observed in the rats in the
middle-dose pinacidil (25 μg/kg) group 12 days after the
SMIR surgery, as compared with the rats in the control group
(P<0.05). Administration of high-dose pinacidil (50
μg/kg) completely inhibited the SMIR-induced decrease in the
MWT at all time points, 1,3, 7 and 12 days after the SMIR
surgery.
 | Table IComparison of the mechanical
withdrawal threshold in the control, sham, SMIR, and pinacidil
groups of rats. |
Table I
Comparison of the mechanical
withdrawal threshold in the control, sham, SMIR, and pinacidil
groups of rats.
| Control (g) | Sham (g) | SMIR (g) | SMIR + pinacidil (g)
|
|---|
| 10 μg/kg | 25 μg/kg | 50 μg/kg |
|---|
| Before SMIR
surgery | 23.1±1.3 | 23.4±1.3 | 23.0±1.4 | 24.3±1.2 | 23.1±1.3 | 23.34±1.34 |
| After SMIR
surgery |
| 1 day | 23.7±1.3 | 22.0±0.3 | 18.4±1.7a | 24.1±1.3 | 24.1±1.3 | 24.59±1.26 |
| 3 days | 23.8±1.6 | 20.3±1.3c | 10.9±1.6b | 16.7±1.3a | 24.5±1.3 | 23.54±1.15 |
| 7 days | 23.4±1.2 | 22.1±1.2c | 6.2±0.7b | 19.0±1.0a | 22.9±1.2 | 24.41±1.37 |
| 12 days | 24.0±1.2 | 23.2±1.3c | 8.3±0.9b | 12.0±0.7b | 15.4±0.9a | 23.81±1.23 |
These data indicate that the degree of mechanical
allodynia was relative to the methods of operation, and that SMIR
surgery could significantly increase the severity of mechanical
allodynia, as compared to the incision alone. It was also shown
that pinacidil could antagonize the SMIR-induced nociceptive
response, restoring it to a normal level, likely through the
activation of KATP channels.
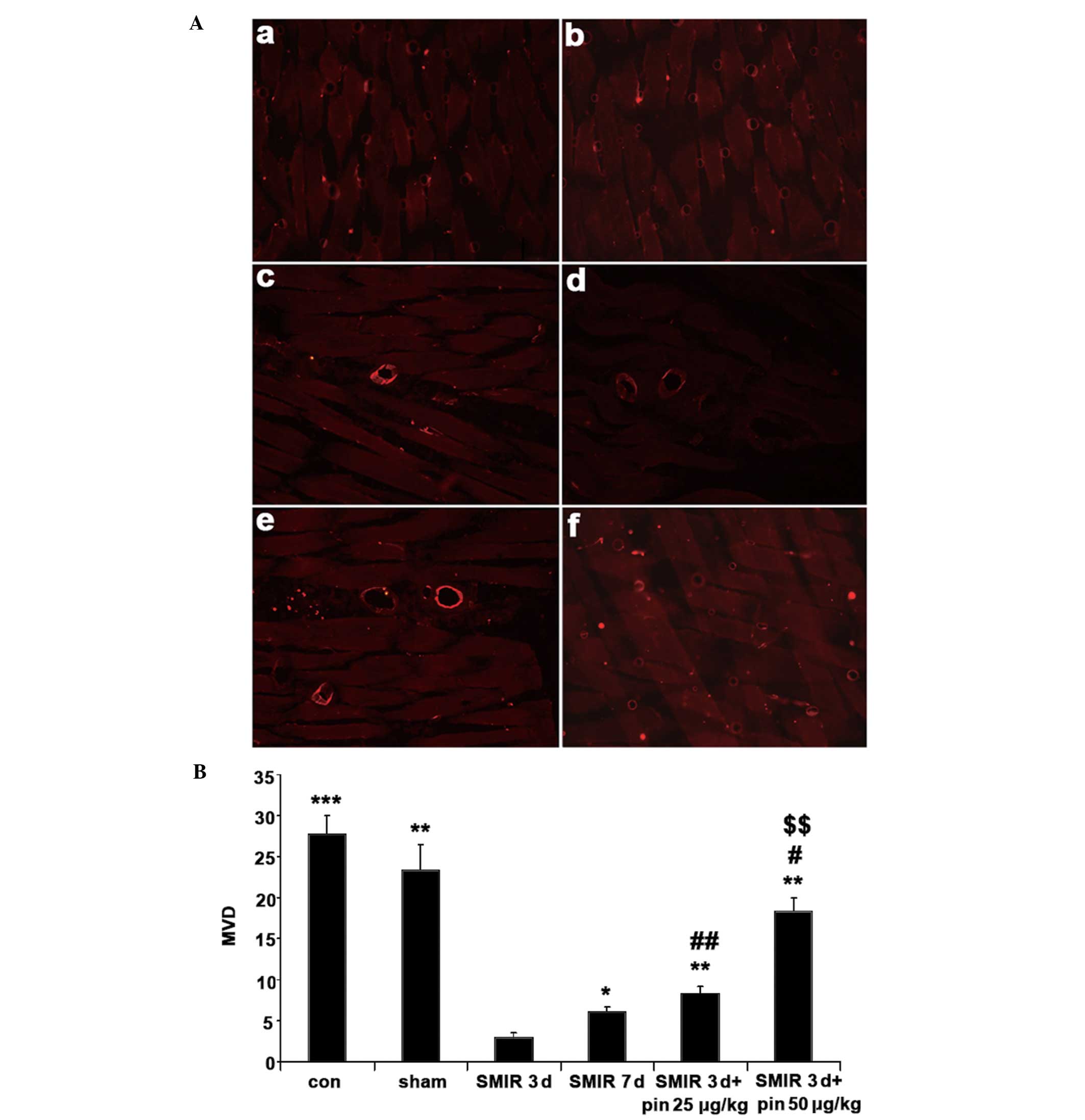
Changes in MVD around the incision
site
As shown in Fig. 2,
the values of MVD in the control and sham operation groups were
high at the start of the experiment, or three days after the sham
surgery, respectively, as compared with the MVD in the SMIR group.
In the SMIR group, the MVD value was significantly increased seven
days after the surgery as compared with three days after the
surgery (P<0.05). Additionally, the MVD values in the
middle-dose pinacidil and high-dose pinacidil groups were
significantly higher as compared with the value in the SMIR group 3
days after surgery.
Furthermore, three days after surgery, the MVD
values were shown to be significantly lower in the middle- and
high-dose pinacidil groups, as compared with the control group, and
the MVD value was significantly increased in the high-dose
pinacidil group as compared with the middle-dose pinacidil group
(P<0.01).
The results of the immunohistochemical staining
suggested that the SMIR surgery caused an imbalance between
apoptosis and proliferation of the vascular endothelial cells,
resulting in the dynamic changes in MVD. It is therefore suggested
that SMIR surgery may cause both microangiopathy and inflammation,
and that SMIR surgery was able to decrease MVD significantly, as
compared with incision alone. The present study also showed that
the administration of pinacidil, prior to surgery, resulted in a
dose-dependent increase in the MVD value and partly inhibited the
SMIR-induced reduction in MVD. Pinacidil may improve the
microenvironment around the incision site, thereby exerting its
inhibitory effects on both peripheral and central
sensitization.
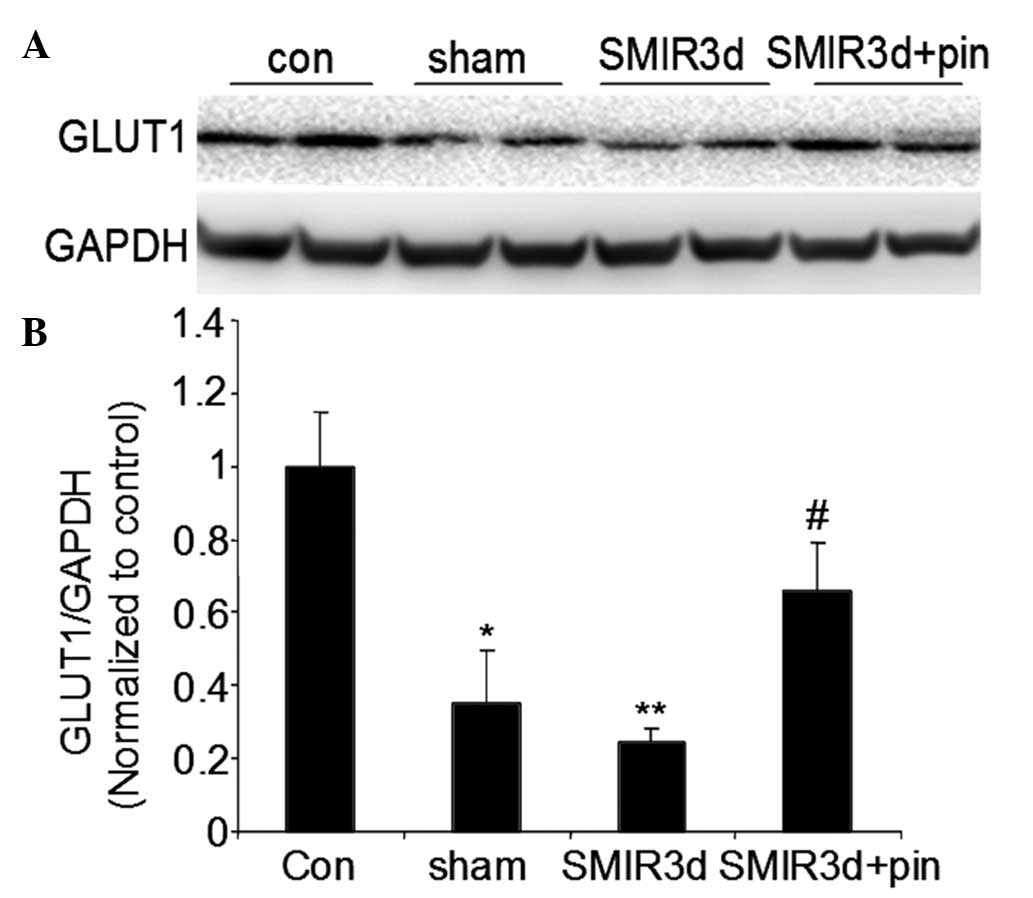
Changes in relative GLUT1 protein
expression levels around the incision site
As compared with the GLUT1 protein expression level
in the control group, the GLUT1 protein expression levels in the
sham operation and SMIR groups were significantly reduced three
days after surgery (P<0.05 and P<0.01, respectively).
Furthermore, the GLUT1 protein expression level in the middle-dose
pinacidil group three days after surgery was significantly higher,
as compared with that in the SMIR group (P<0.05) (Fig. 3).
These data suggest that the SMIR procedure was able
to significantly reduce the relative GLUT1 protein expression
level, as compared with the incision alone. It was also shown that
intraperitoneal administration of pinacidil prior to the SMIR
surgery, could inhibit the SMIR-induced reduction in GLUT1 protein
expression level and enhance local glucose utilization and basal
metabolism. In addition, pinacidil could improve the
microenvironment, and contribute to the inhibition of peripheral
and central sensitization.
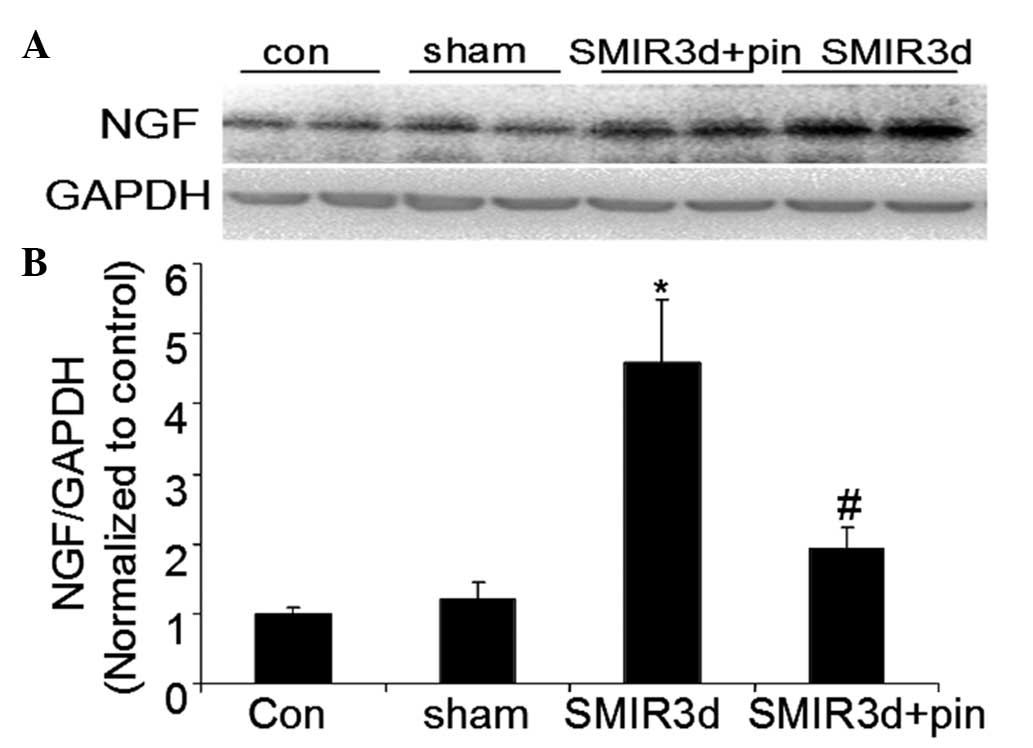
Changes in relative NGF protein
expression levels around the incision site
In the SMIR group, the NGF protein expression level
was significantly increased three days after surgery, as compared
with the NGF protein expression level in the control group
(P<0.01). There were no marked changes in the protein expression
levels of NGF in the sham operation group after surgery. The NGF
protein expression level in the middle-dose pinacidil group was
markedly lower as compared with that in the SMIR group, three days
after surgery (P<0.05) (Fig.
4).
These data indicate that the rats that underwent the
SMIR surgery had a significantly higher relative NGF protein
expression level, as compared with those treated with the incision
alone. It was also shown that intraperitoneal administration of
pinacidil prior to the SMIR surgery could inhibit the SMIR-induced
increase in NGF protein expression level and improve the
microenvironment, which may have an important contributory role in
the inhibition of peripheral sensitization.
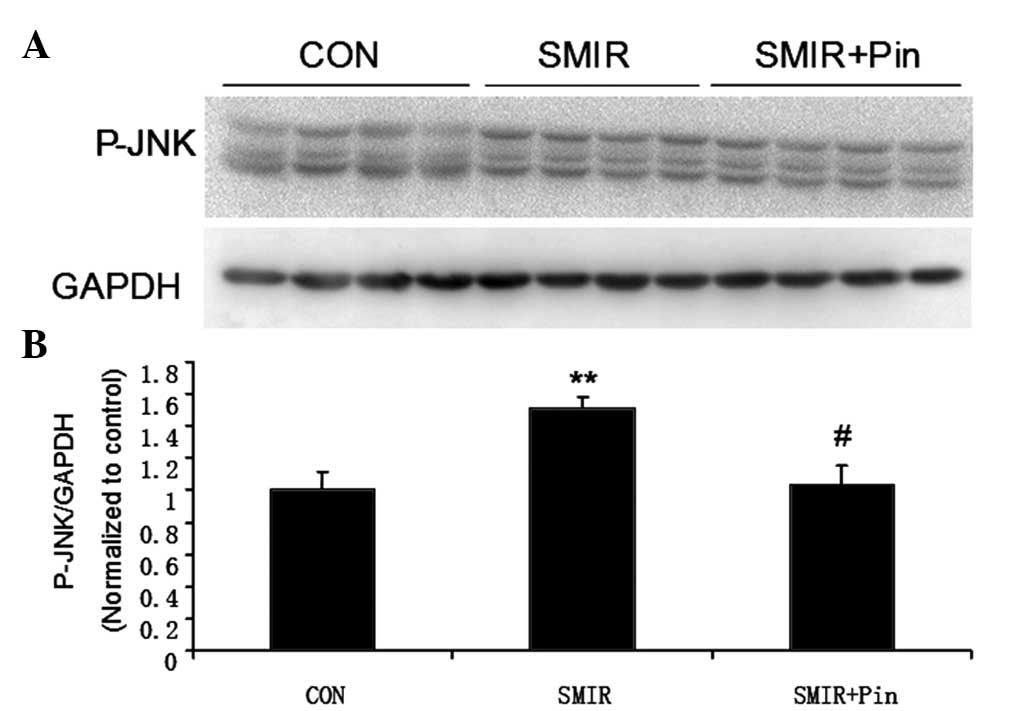
Changes in relative spinal JNK protein
expression levels around the incision site
Following surgery, the p-JNK protein expression
level was significantly increased in the SMIR group as compared
with the protein expression level in the control group (P<0.01).
Conversely, a significantly reduced level of p-JNK protein was
found in the middle-dose pinacidil group, as compared with that in
the SMIR group (P<0.05) (Fig.
5).
These data indicate that peripheral activation of
KATP channels, prior to SMIR surgery, could suppress the
SMIR-induced spinal JNK activation, and therefore inhibit central
sensitization.
Discussion
It has been previously reported that the
formalin-induced animal model of inflammatory nociception could not
simulate the microenvironment around the incision site (24,25).
The present study suggests that during SMIR surgery, prolonged
tissue retraction does not cause peripheral neuronal damage; thus,
the SMIR model can provide a more accurate reflection of the
microenvironment around an incision site. The results of the
present study determined that three days after SMIR surgery, the
MVD value was significantly decreased, as compared with the MVD
value of the control group, whereas a marked increased MVD value
was observed seven days after SMIR surgery. Therefore, it is
hypothesized that various pathological conditions associated with
an acute inflammatory response, including ischemia, anoxia and
ischemia/reperfusion, may be present around the incision site in
the SMIR model (26). Furthermore,
the relative GLUT1 protein expression level in the SMIR group,
three days after surgery, was significantly lower as compared with
that in the control group, likely resulting in a reduction in
glucose utilization and disorder of basal metabolism. In addition,
a significant elevation in the relative NGF protein expression
level was observed three days after surgery in the SMIR group as
compared with the control group. An increased NGF protein
expression level has previously been found to induce an
inflammatory response and promote the release of algogenic
substances (27–29). This would result in an increase to
the sensitivity and excitability of primary nociceptive neurons,
thereby resulting in peripheral sensitization following the SMIR
procedure. A more severe presentation of mechanical allodynia was
observed in the present study, three days after surgery in the SMIR
group, as compared with one day after surgery. These findings
indicate that SMIR surgery could induce peripheral and central
sensitization, and that the inflammatory responses around the site
of incision could promote excitation of the central nervous system,
which is considered to be the developmental phase of both
peripheral and central sensitization. The present study suggests
that SMIR-induced inflammation of primary nociceptive neurons in
the spinal cord could activate JNK and result in central
sensitization, which may markedly increase the severity of
mechanical allodynia. These data demonstrate that following SMIR
surgery, the inflammatory responses around the incision site, which
are induced by an imbalance between apoptosis and proliferation of
vascular endothelial cells and disorder of basal metabolism,
contribute to the development of peripheral and central
sensitization. Vascular endothelial cell apoptosis is implicated as
having a role in the generation of peripheral and central
sensitization. Hence, it may be speculated that peripheral and
central sensitization can be prevented by blocking the formation of
the inflammatory microenvironment around the incision site.
In the present study, the rats in the sham group
underwent a sham surgery involving a skin/muscle incision only. The
results showed that although a reduction in the MVD value was noted
in the sham operation group, there were no significant changes with
regards to the MVD three days after the surgery. The observations
also revealed that, as compared with the control group, the
relative GLUT1 protein expression level in the sham operation group
was significantly reduced three days after surgery; however, GLUT1
protein expression was further reduced in the SMIR group.
Furthermore, in the sham operation group, no significant
differences were observed in the relative protein expression of NGF
as compared with the expression level prior to the surgery. These
findings indicate that the microenvironment around the incision
site was not capable of persistent excitation of nociceptors,
resulting in peripheral and central sensitization. The rats that
underwent a skin/muscle incision did not show obvious mechanical
allodynia. MVD was therefore proven to be more favorable than GLUT1
for maintaining a balanced microenvironment. Collectively, the
results from the present study confirm that the microenvironment
around the incision site varied with the surgical methods used, and
the retraction procedure was shown to be more likely to cause
disorder of cell metabolism and deterioration of the
microenvironment, including an increase in NGF protein expression
and a reduction in GLUT-1 protein expression and MVD value. This
resulted in peripheral and central sensitization, thereby
increasing the severity of mechanical allodynia.
Previous studies have found that KATP channel
activators can protect against endothelial cell dysfunction
(30,31). Kir6.1/SUR2B is a subtype of the
vascular KATP channels (32), and
it has been suggested that SUR2 may be a therapeutic target of
pinacidil (33). The results from
the present study revealed that the administration of pinacidil
increased the MVD value around the incision site, in a
dose-dependent manner. It was also shown to partly inhibit the
SMIR-induced reduction of MVD, resulting in a large increase in the
low peripheral resistance to nutritional blood flow. Additionally,
intraperitoneal administration of pinacidil prior to SMIR surgery
inhibited the SMIR-induced reduction of GLUT1 protein expression
levels, which can contribute to the maintenance of basal metabolism
and glucose utilization. Pinacidil was also shown to exert an
inhibitory effect on the SMIR-induced increase in NGF protein
expression levels, and therefore prevent peripheral and central
sensitization. These findings suggest that there were no
inflammatory responses present in the primary sensory neurons
around the incision site, due to inhibition of the formation of an
inflammatory microenvironment by pinacidil. Furthermore, there were
no marked changes in the spinal JNK protein expression levels in
rats treated with pinacidil after surgery, indicating that
pinacidil can inhibit central sensitization, and reduce the degree
of mechanical allodynia in a dose-dependent manner. These findings
suggest that persistent excitement of nociceptors was not elicited
around the surgical site when KATP channels were activated prior to
the activation of nociceptors. Thus, pinacidil can exert an
inhibitory effect on peripheral and central sensitization.
In conclusion, the present study demonstrated that
SMIR-evoked postoperative pain can be considered a combined process
of microangiopathy and ischemia/reperfusion. It was also
demonstrated that the microenvironment around the incision site can
affect the development of peripheral and central sensitization.
Furthermore, pinacidil demonstrated an inhibitory effect on the
formation of the inflammatory microenvironment through activation
of the KATP channels, thereby inhibiting peripheral and central
sensitization. Pinacidil may be a potential treatment option in
preemptive analgesia.
References
|
1
|
Burke S and Shorten GD: When pain after
surgery doesn’t go away…. Biochem Soc Trans. 37:318–322. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Coderre TJ and Bennett GJ: A hypothesis
for the cause of complex regional pain syndrome-type I (reflex
sympathetic dystrophy): pain due to deep-tissue microvascular
pathology. Pain Med. 11:1224–1238. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nesic O, Sundberg LM, Herrera JJ,
Mokkapati VU, Lee J and Narayana PA: Vascular endothelial growth
factor and spinal cord injury pain. J Neurotrauma. 27:1793–1803.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sawayama H, Ishimoto T, Watanabe M, et al:
High expression of glucose transporter 1 on primary lesions of
esophageal squamous cell carcinoma is associated with hematogenous
recurrence. Ann Surg Oncol. 21:1756–1762. 2014. View Article : Google Scholar
|
|
5
|
Bateman RM, Tokunaga C, Kareco T,
Dorscheid DR and Walley KR: Myocardial hypoxia-inducible
HIF-1alpha, VEGF, and GLUT1 gene expression is associated with
microvascular and ICAM-1 heterogeneity during endotoxemia. Am J
Physiol Heart Circ Physiol. 293:H448–H456. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Semaan A, Munkarah AR, Arabi H, et al:
Expression of GLUT-1 in epithelial ovarian carcinoma: correlation
with tumor cell proliferation, angiogenesis, survival and ability
to predict optimal cytoreduction. Gynecol Oncol. 121:181–186. 2011.
View Article : Google Scholar
|
|
7
|
Kolar P, Lach S, Gaber T, et al: Effects
of celecoxib on the expression of osteoprotegerin, energy
metabolism and cell viability in cultured human osteoblastic cells.
Clin Exp Rheumatol. 27:99–107. 2009.PubMed/NCBI
|
|
8
|
Bie B, Wang Y, Cai YQ, et al: Upregulation
of nerve growth factor in central amygdala increases sensitivity to
opioid reward. Neuropsychopharmacology. 37:2780–2788. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao J, Seereeram A, Nassar MA, et al:
Nociceptor-derived brain-derived neurotrophic factor regulates
acute and inflammatory but not neuropathic pain. Mol Cell Neurosci.
31:539–548. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lewin GR, Winter J and McMahon SB:
Regulation of afferent connectivity in the adult spinal cord by
nerve growth factor. Eur J Neurosci. 4:700–707. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Priyanka HP, Bala P, Ankisettipalle S and
ThyagaRajan S: Bacopa monnieri and L-deprenyl differentially
enhance the activities of antioxidant enzymes and the expression of
tyrosine hydroxylase and nerve growth factor via ERK 1/2 and NF-κB
pathways in the spleen of female wistar rats. Neurochem Res.
38:141–152. 2013. View Article : Google Scholar
|
|
12
|
Fukui Y, Ohtori S, Yamashita M, et al: Low
affinity NGF receptor (p75 neurotrophin receptor) inhibitory
antibody reduces pain behavior and CGRP expression in DRG in the
mouse sciatic nerve crush model. J Orthop Res. 28:279–283.
2010.
|
|
13
|
Gao YJ, Xu ZZ, Liu YC, Wen YR, Decosterd I
and Ji RR: The c-Jun N-terminal kinase 1 (JNK1) in spinal
astrocytes is required for the maintenance of bilateral mechanical
allodynia under a persistent inflammatory pain condition. Pain.
148:309–319. 2010. View Article : Google Scholar :
|
|
14
|
Manassero G, Repetto IE, Cobianchi S, et
al: Role of JNK isoforms in the development of neuropathic pain
following sciatic nerve transection in the mouse. Mol Pain.
8:392012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zoga V, Kawano T, Liang MY, et al: KATP
channel subunits in rat dorsal root ganglia: alterations by painful
axotomy. Mol Pain. 6:62010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Perimal EK, Akhtar MN, Mohamad AS, et al:
Zerumbone-induced antinociception: involvement of the
L-arginine-nitric oxide-cGMP-PKC-K+ ATP channel pathways. Basic
Clin Pharmacol Toxicol. 108:155–162. 2011. View Article : Google Scholar
|
|
17
|
Du X, Wang C and Zhang H: Activation of
ATP-sensitive potassium channels antagonize nociceptive behavior
and hyperexcitability of DRG neurons from rats. Mol Pain. 7:352011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xia H, Zhang D, Yang S, et al: Role of
ATP-sensitive potassium channels in modulating nociception in rat
model of bone cancer pain. Brain Res. 1554:29–35. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Romero TR, Guzzo LS, Perez AC, Klein A and
Duarte ID: Noradrenaline activates the NO/cGMP/ATP-sensitive K(+)
channels pathway to induce peripheral antinociception in rats.
Nitric Oxide. 26:157–161. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Flatters SJ: Characterization of a model
of persistent postoperative pain evoked by skin/muscle incision and
retraction (SMIR). Pain. 135:119–130. 2008. View Article : Google Scholar
|
|
21
|
Dixon WJ: Staircase bioassay: the
up-and-down method. Neurosci Biobehav Rev. 15:47–50. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Weidner N, Semple JP, Welch WR and Folkman
J: Tumor angio-genesis and metastasis - correlation in invasive
breast carcinoma. N Engl J Med. 324:1–8. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Memis A, Ozden C, Ozdal OL, Guzel O, Han O
and Seckin S: Effect of finasteride treatment on suburethral
prostatic microvessel density in patients with hematuria related to
benign prostate hyperplasia. Urol Int. 80:177–180. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brennan TJ, Zahn PK and Pogatzki-Zahn EM:
Mechanisms of incisional pain. Anesthesiol. Clin North America.
23:1–20. 2005.
|
|
25
|
Pogatzki-Zahn EM, Zahn PK and Brennan TJ:
Postoperative pain - clinical implications of basic research. Best
Pract Res Clin Anaesthesiol. 21:3–13. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
de Mos M, Laferrière A, Millecamps M, et
al: Role of NFkappaB in an animal model of complex regional pain
syndrome-type I (CRPS-I). J Pain. 10:1161–1169. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Freund-Michel V and Frossard N: The nerve
growth factor and its receptors in airway inflammatory diseases.
Pharmacol Ther. 117:52–76. 2008. View Article : Google Scholar
|
|
28
|
Mousa SA, Cheppudira BP, Shaqura M, et al:
Nerve growth factor governs the enhanced ability of opioids to
suppress inflammatory pain. Brain. 130:502–513. 2007. View Article : Google Scholar
|
|
29
|
Ansari N, Khodagholi F, Amini M and
Shaerzadeh F: Attenuation of LPS-induced apoptosis in
NGF-differentiated PC12 cells via NF-κB pathway and regulation of
cellular redox status by an oxazine derivative. Biochimie.
93:899–908. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang H, Long C, Duan Z, Shi C, Jia G and
Zhang Y: A new ATP-sensitive potassium channel opener protects
endothelial function in cultured aortic endothelial cells.
Cardiovasc Res. 73:497–503. 2007. View Article : Google Scholar
|
|
31
|
Minamino T and Hori M: Protecting
endothelial function: A novel therapeutic target of ATP-sensitive
potassium channel openers. Cardiovasc Res. 73:448–449. 2007.
View Article : Google Scholar
|
|
32
|
Shi W, Cui N, Wu Z, et al:
Lipopolysaccharides up-regulate Kir6.1/SUR2B channel expression and
enhance vascular KATP channel activity via NF-kappaB-dependent
signaling. J Biol Chem. 285:3021–3029. 2010. View Article : Google Scholar :
|
|
33
|
Fan LH, Tian HY, Wang J, et al:
Downregulation of Kir6.1/SUR2B channels in the obese rat aorta.
Nutrition. 25:359–363. 2009. View Article : Google Scholar
|