Introduction
Parkinson’s disease (PD) is a common
neurodegenerative disease, leading to cell death of dopaminergic
(DA) neurons in the substantia nigra (SN) (1). An approximate of 5–10% of PD can be
attributed to heritable genetic mutations; however, the cause of
more common PD and the mechanisms underlying the development of the
disease have remained elusive (2,3).
Several lines of evidence suggest that activated microglia have a
critical role in the pathogenesis of PD through producing
inflammatory mediators, including tumor necrosis factor (TNF)-α,
interleukin (IL)-1β and neurotoxin, including inducible nitric
oxide synthase (iNOS) and cyclooxygenase-2 (Cox-2) (4). Along with microglial activation, it
has been reported that the production of reactive oxygen species
(ROS) can accelerate the death of DA neurons (5). Increases of pro-inflammatory
cytokines, activation of the nuclear factor (NF)-κB signaling
pathway and oxidative stress were observed in the destroyed DA
neurons (6). Therefore, all these
factors can be regarded as potential targets for treating PD.
Somatostatin (SST) as an inhibitor of growth hormone
(GH) has been identified to be abundant throughout the central
nervous system (7). As a
neuromodulator, SST has a crucial role in memory and cognition
(8). In addition, the decreased
SST levels in the frontal and entorhinal cortex as well as the
hippocampus have been correlated to the cognitive deficits in
patients with PD (9). However,
increases in SST have been observed in the cerebrospinal fluid of
patients with early PD (10). Due
to the controversial data reported, the function and mechanism of
SST which participates in PD are still subject to extensive
examination. Accumulating clinical and experimental evidence
suggested that SST can provide potential therapy in
neurodegenerative disorders involving cognitive dysfunctions
(11). To date, little is known
about the effects of SST in DA neurons in the context of PD and its
mechanism of action. To investigate this matter, an animal model of
PD was generated by injecting lipopolysaccharide (LPS) into the SN
of rat brains. It was examined whether SST administered prior to
LPS treatment was able to protect nigral DA neurons from
LPS-induced neurotoxicity through inhibiting microglial activation
and reducing subsequent neuroinflammation as well as oxidative
stress. To clarify the functional mechanism of SST, the expression
of NF-κB p65 and downstream factors were investigated in the
present study.
Materials and methods
Reagents
The following reagents and kits were used in the
present study: Rabbit polyclonal anti-tyrosine hydroxylase (TH;
cat. no. 2792; Cell Signaling Technology, Inc., Boston, MA, USA),
rabbit polyclonal anti-OX-42 (cat. no. orb11009; Biorbyt,
Cambridge, UK), hydroethidine (Molecular Probes, Eugene, OR, USA),
LPS (Sigma-Aldrich, St. Louis, MO, USA), heparin (Qianhong
Bio-pharma Co., Ltd., Changzhou, China), SST (ProSpec, East
Brunswick, NJ, USA), biotinylated goat anti-rabbit secondary
antibody (cat. no. A0277) and horseradish peroxidase (HRP)-labeled
streptavidin (Beyotime, Shanghai, China), bicinchoninic acid (BCA)
kit (Beyotime), Rat TNF-α ELISA kit, Rat IL-1β ELISA kit and
Prostaglandin E2 ELISA kit (PGE2; USCN, Wuhan, Hubei, China).
Stereotaxic surgery and SST
administration
All experiments were performed according to the
approved animal protocols and guidelines established by Chung et
al (12). 144 female Sprague
Dawley rats were provided by the Experimental Animal Centre of
China Medical University (Shenyang, China). The animal study
protocol was approved by the Animal Experimental Committee of China
Medical University, and the mice received humane care according to
the Principles of Laboratory Animal Care. They were randomly
assigned to six experimental groups and received unilateral
administration of 3 μl phosphate-buffered saline (PBS), LPS
(5 μg in 3 μl PBS), 2 μl saline + LPS, 2
μl SST (20 μg/kg) + LPS, 2 μl SST (40
μg/kg) + LPS, 2 μl SST (40 μg/kg)
respectively, into the right SN as previously described by Chung
et al (10). 24 rats of
each treated group were separated into three different subgroups
for various analyses, including OX-42 and hydroethidine tests of
brains after 24 h of treatment, western blot and ELISA assays of SN
after 24 h of treatment, as well as immunohistochemical detection
of TH and Nissl in the SN after 7 days of treatment. SST was
injected 1 h prior to LPS treatment.
Tissue preparation and
immunohistochemistry
Brain tissues were prepared for immunohistochemical
staining as previously reported (12). Tissues were dehydrated by using a
graded ethanol series of 70% ethanol for 2 h, 80% overnight, 90%
for 2 h and 100% for 2 h. Brain tissues were then post-fixed in
dimethylbenzene (China National Medicines Corporation Ltd.,
Beijing, China) for 30 min and embedded in dimethylbenzene-paraffin
at 60°C for 2 h, after which samples were embedded in a metal
frame. Coronal sections (5 μm) cut by a sliding microtome
CM69001 (Leica Microsystems, Mannheim, Germany) were spread in warm
water and placed onto glass slides to dry in a 70°C-chamber for 40
min. Sections were dewaxed using ethanol and then boiled in antigen
retrieval solution for 10 min. The cooled sections were incubated
in 3% H2O2 (China National Medicines
Corporation Ltd.) for 15 min at room temperature and then blocked
with normal goat serum (Beijing Solarbio Science & Technology
Co., Ltd., Beijing, China) for 15 min. Sections were incubated
overnight at room temperature with rabbit anti-OX-42 (1:100; cat.
no. orb11009) for microglia and rabbit anti-TH (1:100; cat. no.
2792) for DA neurons. Following removal of unbound primary
antibodies by washing, biotinylated goat anti-rabbit secondary
antibody (1:200; cat. no. A0277) was added and incubated for 30 min
at 37°C. Sections were then washed with PBS and incubated with
HRP-labeled streptavidin for 30 min at 37°C. Finally, 100 μl
diaminobenzidine (Beijing Solarbio Science & Technology Co.,
Ltd.) was added for coloration and hematoxylin stain (Beijing
Solarbio Science & Technology Co., Ltd.) for counterstaining.
Hydroethidine (Molecular Probes) was used for in situ
detection of O2− and
O2− -derived oxidants. For Nissl staining, a
number of the SN tissue samples were stained in 0.5% cresyl violet
(China National Medicines Corporation Ltd.). Following washing with
water and dehydrating with ethanol as well as treating with
dimethylbenzene (China National Medicines Corporation Ltd.),
stained samples were analyzed under a stereo microscope (BX51;
Olympus Corporation, Tokyo, Japan) or viewed with a confocal laser
scanning microscope (FV1000S-SIM/IX81; Olympus Corporation).
Stereological estimation
The total number of TH-positive neurons was counted
in the various groups at seven days post-injection (LPS, PBS, SST
or a combination) using the stereo microscope BX51 (Olympus
Corporation). This unbiased stereological method of cell counting
according to a previously described method is not affected by
either the counted elements (neurons) or the size of the reference
volume (SN) (13).
Western blot analysis
For western blot analysis, protein was extracted
from the SN of eight rats from each group following 24 h of
treatment. Following determination of the protein concentration
using a BCA kit, 40 μg protein was boiled in PBS with 5X
loading buffer (Beijing Solarbio Science & Technology Co.,
Ltd.) for 5 min. The bands of protein separated by SDS-PAGE (8% gel
for iNOS, 10% gel for NF-κB p65 and NF-κB p-p65, and 12% gel for
Cox-2) were transferred onto polyvinylidene difluoride membranes
(Millipore, Billerica, MA, USA) using an electroblot apparatus
(DYCZ-40D, Beijing, China). Filters were blocked in 5% non-fat milk
and incubated separately overnight at 4°C with the following
primary antibodies: Rabbit polyclonal anti-iNOS (1:1,000; cat. no.
bs-2072R; Bioss, Beijing, China), goat polyclonal anti-Cox-2
(1:100; sc-1747), mouse monoclonal anti-NF-κB p-p65 (1:100; cat.
no. sc-166748) and rabbit polyclonal anti-NF-κB p65 (1:100; cat.
no. sc-372) obtained from Santa Cruz Biotechnology, Inc. (Dallas,
TX, USA). Membranes were then washed with Tris-buffered saline
containing Tween-20 (TTBS) (BioSharp, Hefei, China) and incubated
with the following corresponding HRP-conjugated secondary
antibodies: Goat anti-mouse immunglobulin (Ig)G-HRP (1:5,000; cat.
no. A0216), goat anti-rabbit IgG-HRP (1:5,000; cat. no. A0208) and
donkey anti-goat IgG-HRP (1:5,000; cat. no. A0181) (Beyotime,
Shanghai, China) for 45 min at 37°C. Following washing with TTBS,
protein bands were visualized using enhanced chemiluminescence
reagent (cat. no. E002-5; Qihai Biotec, Shanghai, China). Protein
levels were quantified by gray value analysis using
Gel-Pro-Analyzer (Media Cybernetics, Inc., Rockville, MD, USA).
Measurement of TNF-α, IL-1β and PGE2
The production of TNF-α, IL-1β and PGE2 from the SN
of rats was determined by ELISA. Proteins were extracted through
homogenizing tissues and quantitated using the BCA kit. ELISA was
then performed according to the manufacturer’s instructions.
Statistical analysis
Values are expressed as the mean ± standard
deviation. All raw data were analyzed by one-way analysis of
variance followed by the Bonferroni test for post hoc comparisons.
Statistical analyses were performed using GraphPad Prism 5.0
software (GraphPad Software Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference
between values.
Results
Neuroprotective effect of SST on the
LPS-treated SN
LPS injection into the SN was previously shown to
result in a considerable loss of TH- and Nissl-positive cells, as
well as alterations in the morphology of TH-positive neurons to
shrunken neuronal cell bodies (12,13).
To investigate the potential of SST to prevent LPS-induced
neurotoxicity of nigral neurons, SST was administered 1 h prior to
LPS injection. SST significantly attenuated the LPS-mediated loss
of TH-positive DA neurons in the SN and preserved normal neuronal
morphology, as evidenced by an increased number of healthy cell
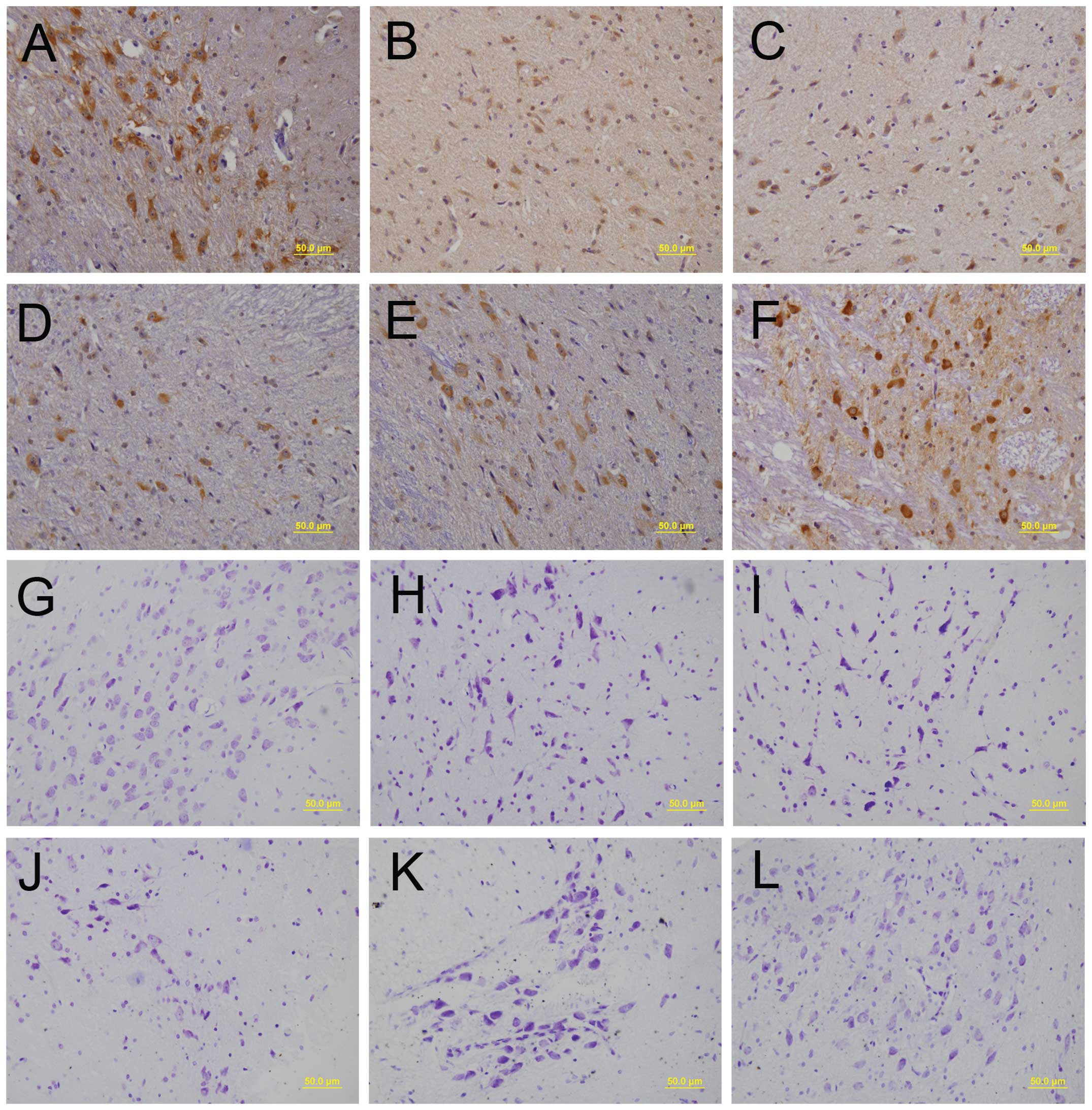
bodies and processes with enhanced staining intensity (Fig. 1A-F). As shown in Fig. 1G, the morphology of the neurons
were integrative and well-maintained. In Fig. 1H, the number of healthy neurons was
markedly decreased, and the number of dead neurons increased, as
characterized by the chromatolysis of Nissl bodies and the presence
of shrunken neuronal cell bodies with pyknotic nuclei surrounded by
a thin band of cytoplasm. In Fig.
1K, the above pathological damages were notably reduced,
following pretreatment with higher concentrations of SST. The
results of the LPS + saline group (Fig. 1I) were similar to those exhibited
in Fig. 1H. In addition, the
results of the LPS + 20 g/kg SST group (Fig. 1J) were similar, but not as obvious,
as those exhibited in Fig. 1K, and
the results of the 40 μg/kg SST group (Fig. 1L) were similar to the results shown
in Fig. 1G, thus indicating that
SST had no cytotoxic effects on the neurons. These results showed
that SST had neuroprotective effects on LPS-treated SN.
LPS-induced microglial activation is
inhibited by SST
It has been reported that LPS is able to activate
rat microglia and lead to neuronal death in vivo (14). Thus, the present study investigated
the effect of SST on LPS-induced microglial activation in the SN.
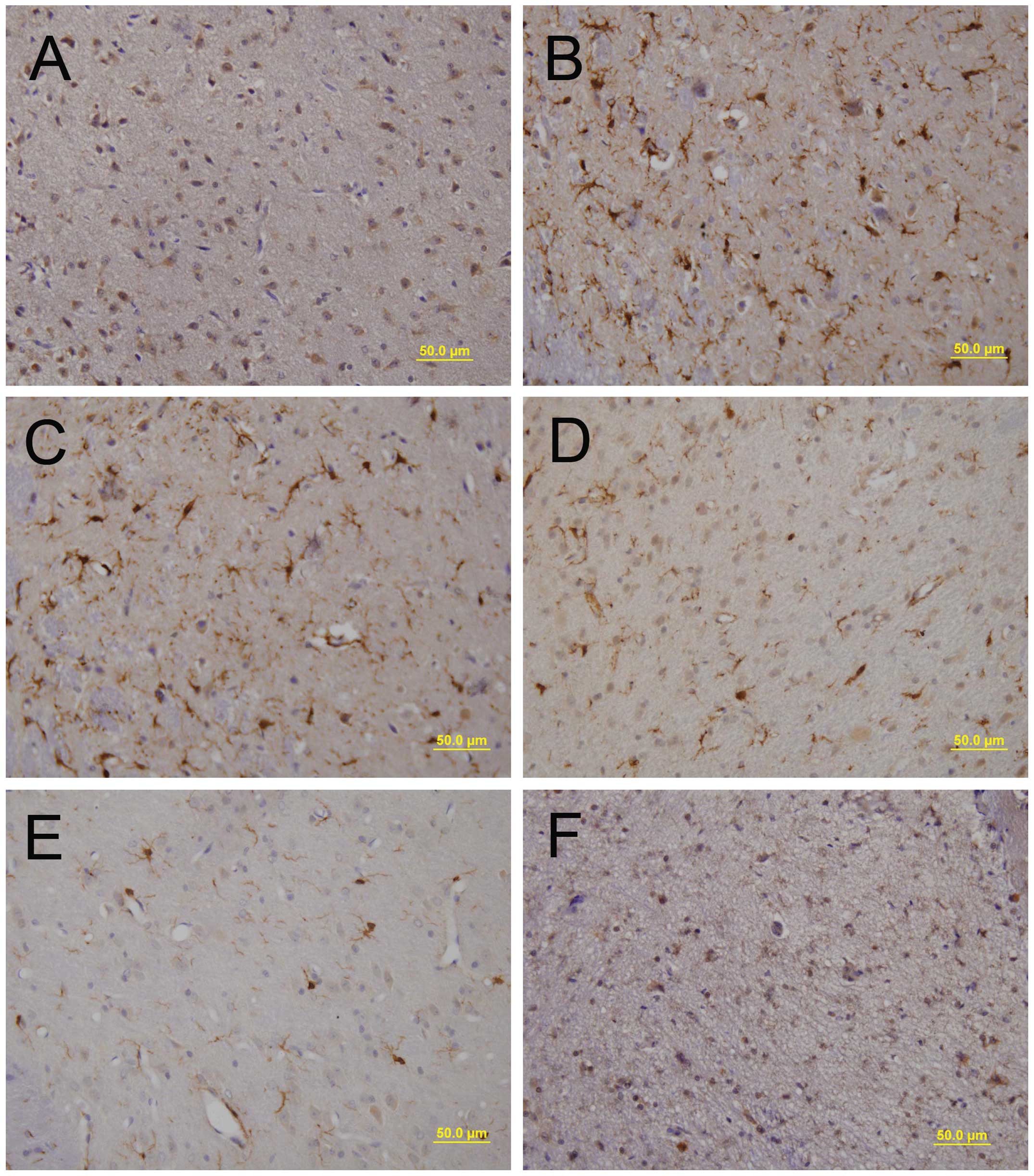
SN sections were prepared for immu-nohistochemical staining using
antibodies against OX-42 to detect microglial activation. The
majority of OX-42-positive microglia exhibited a resting morphology
in the PBS-injected SN (Fig. 2A),
whereas LPS-treated samples showed activated microglia with
enhanced staining intensity and larger cell bodies with short,
thick processes (Fig. 2B).
Pre-treatment with SST dramatically decreased the number of
activated microglia induced by LPS compared that in the
saline-pre-treated control (Fig.
2C-E), while SST alone had no effect on microglial activation
(Fig. 2F). These findings
suggested that SST inhibited the LPS-induced activation of
microglia.
LPS-induced ROS production is inhibited
by SST
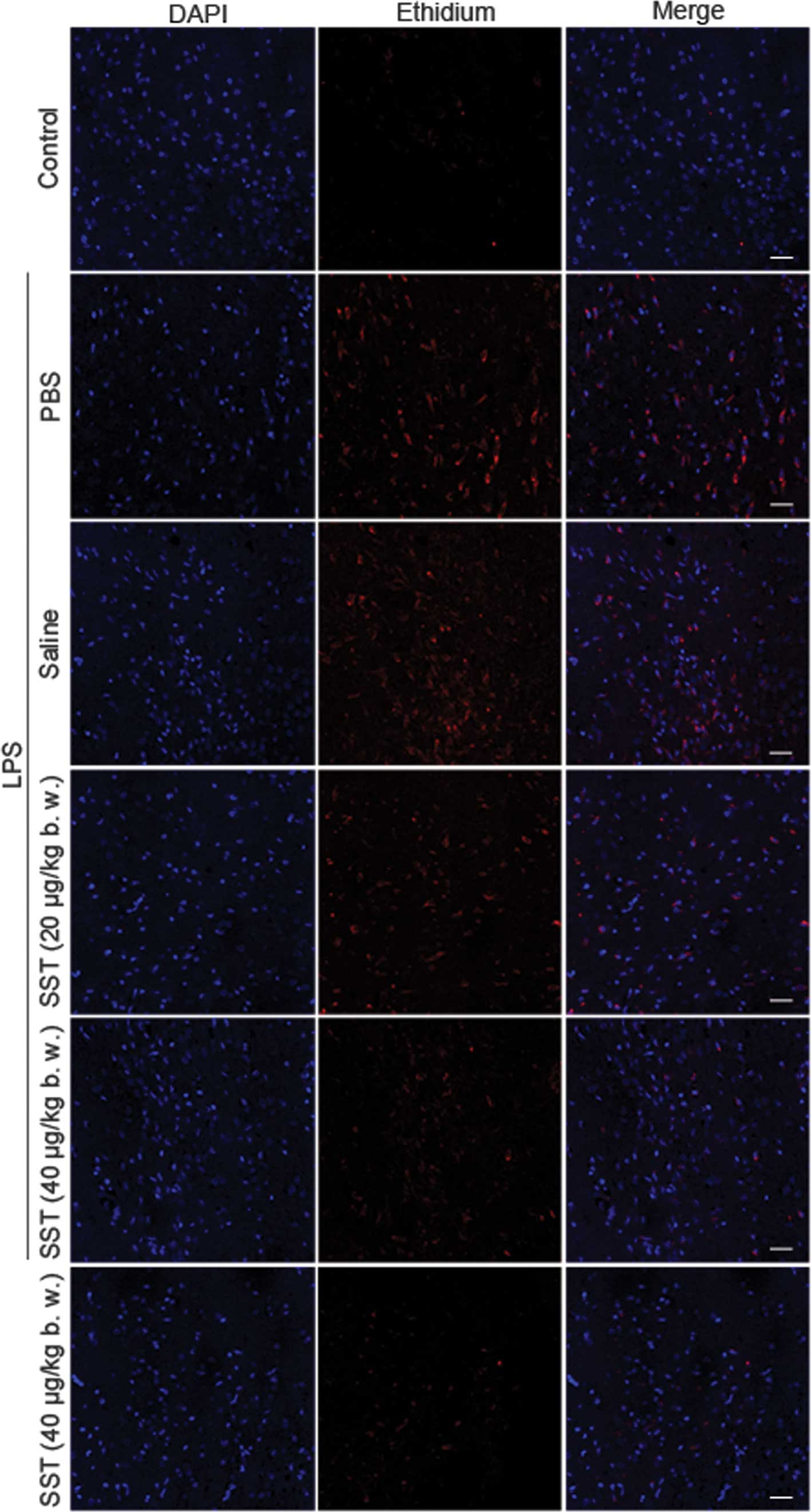
Recent studies have suggested that activated
microglia are able to produce O2− and
O2− -derived oxidants and they are thought to
mediate the loss of nigral DA neurons in vitro and in
vivo (15,16). Hence, the present study
investigated whether SST was able to enhance DA neuronal survival
by inhibiting LPS-induced production of ROS. Accumulation of
ethidium, the fluorescent product of oxidized hydroethidine, was
signifi-cantly increased at 48 h in the LPS-treated SN compared
with that in the PBS-injected controls, and the LPS-induced oxidant
production was dramatically decreased by SST (Fig. 3). These results showed that SST
inhibited LPS-induced ROS production.
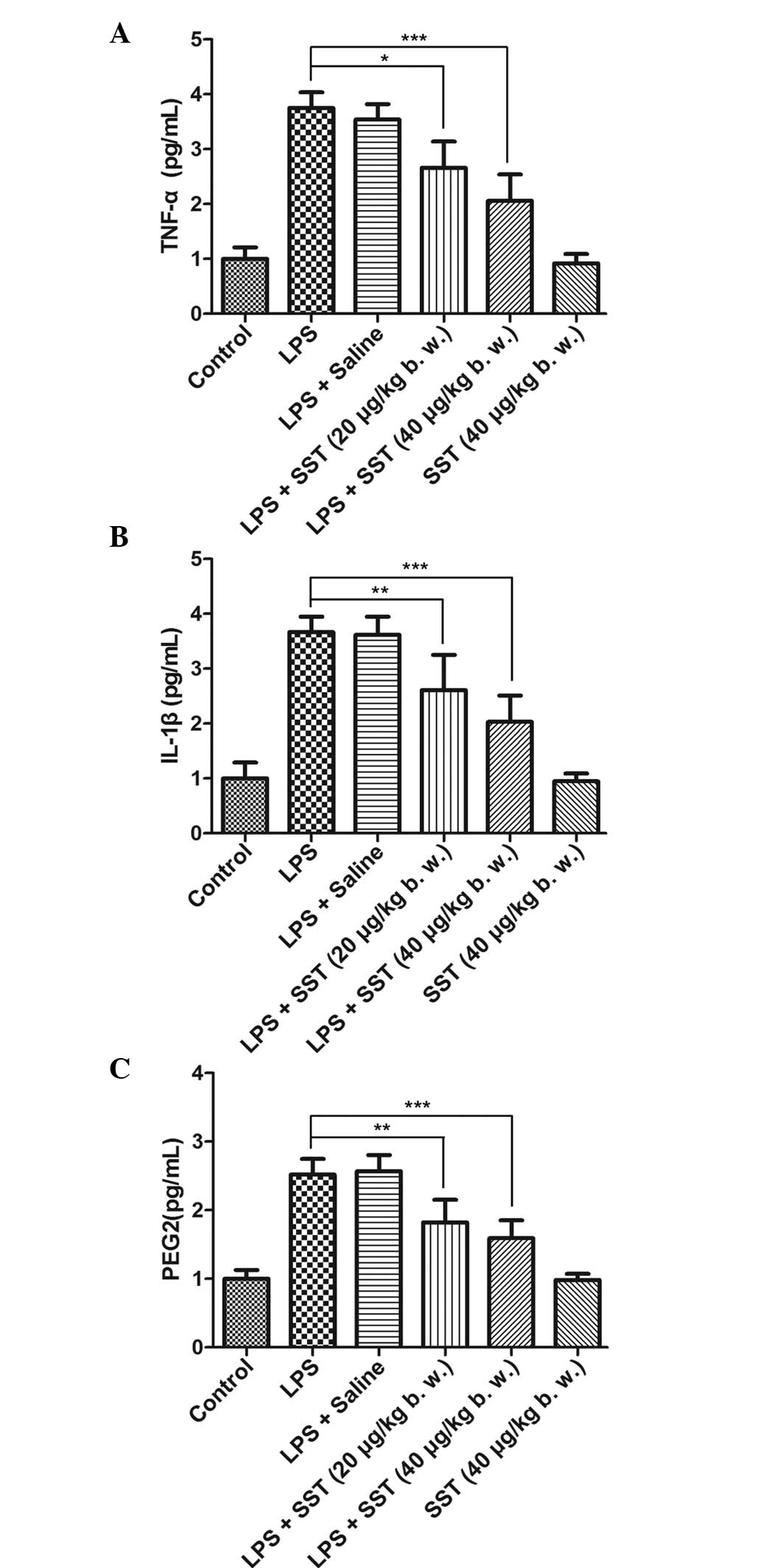
SST decreases LPS-induced production of
TNF-α, IL-1β and PGE2
Several studies have demonstrated that the
production of TNF-α, IL-1β and PGE2 are upregulated in LPS-injected
SN (14,17). Neuroinflammation is thought to
mediate DA neuronal death in the SN (18). Therefore, the present study
examined whether SST was able to decrease DA neuronal death by
regulating LPS-induced production of TNF-α, IL-1β and PGE2 in the
SN. When 20 μg/kg SST was administered prior to LPS
injection, the amount of TNF-α, IL-1β and PGE2 was significantly
reduced by 24.9% (Fig. 4A), 27.9%
(Fig. 4B) and 29.2% (Fig. 4C), respectively. The production of
TNF-α, IL-1β and PGE2 was decidedly decreased by 41.8, 43.9 and
41.6%, respectively, when 40 μg/kg SST was administered
prior to LPS treatment, while SST had no effect on the production
of TNF-α, IL-1β and PGE2 when it was used alone. This confirmed
that SST was able to decrease LPS-induced production of TNF-α,
IL-1β and PGE2.
SST decreases LPS-induced overexpression
of Cox-2, iNOS and NF-κB p-p65
Upregulation of Cox-2 and iNOS have been implicated
in DA neuronal cell death of patients with degenerative brain
diseases (19,20). Inhibition of the activation of
NF-κB p65 was able to prevent DA neuronal cell loss in a
1-methyl-4-phenyl-1,2,3,6-tetrahyropyridine-induced mouse model of
PD (21). Accordingly, the present
study examined the effect of SST on DA neuronal survival through
affecting LPS-induced expression of Cox-2, iNOS and the activation
of NF-κB in the SN. 24 hours following injection, LPS enhanced the
expression of Cox-2, iNOS and activated NF-κB p65 via
phosphorylation (Fig. 5).
Administration of 20 μg/kg SST prior to LPS injection
reduced the expression of Cox-2, iNOS and NF-κB p-p65 by 23.4, 26.1
and 35.9%, respectively. When 40 μg/kg SST was given prior
to LPS injection, the expression of Cox-2, iNOS and NF-κB p-p65 was
decreased by 40.5, 37.3 and 58.4%, respectively. SST alone had no
effects on Cox-2, iNOS and NF-κB p-p65 expression. It was therefore
demonstrated that SST was able to inhibit the expression of Cox-2,
iNOS and NF-κB p-p65.
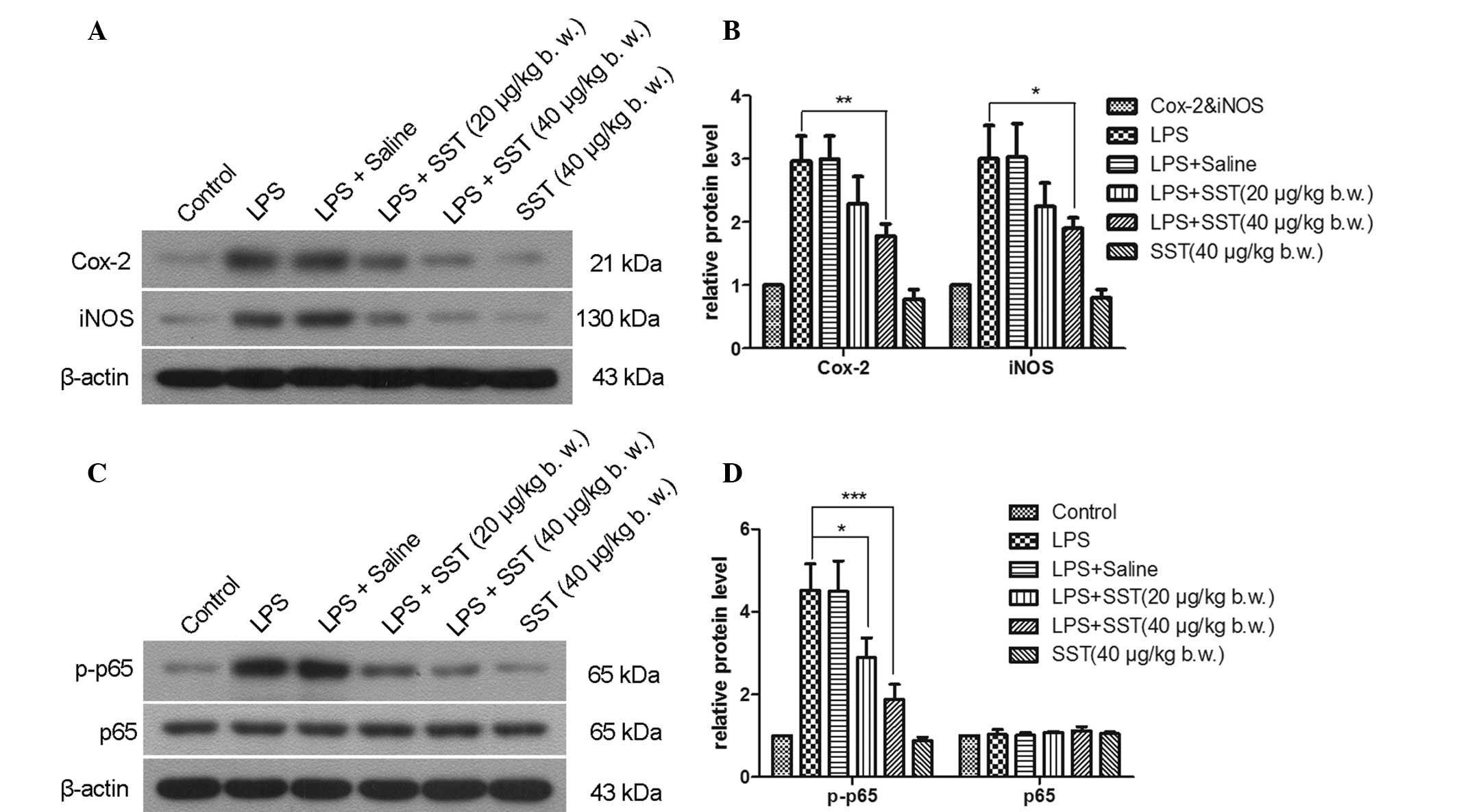 | Figure 5Effect of SST on LPS-induced
expression of Cox-2, iNOS and the activation of NF-κB p65. l. (A
and C) representative western blot gels showing Cox-2, iNOS, NF-κB
p65 and NF-κB p-p65 levels. Cox-2, iNOS and NF-κB p-p65 are
represented by the bands at 21, 130 and 65 kDa, respectively.
β-actin was used as an internal control. (B and D) Quantification
of A and C by gray value analysis. Values are presented as the mean
± standard error of the mean of eight animals per group.
*P<0.05, **P<0.01,
***P<0.001 compared with phosphate-buffered
saline-injected SN. LPS, lipopolysaccharide; SST, somatostatin; SN,
substantia nigra; COX, cyclooxygenase; iNOS, inducible nitric oxide
synthase; NF-κB, nuclear factor kappa B. |
Discussion
PD is a complex neurodegenerative disorder owing to
an aggravating process of neuronal loss within the SN (22). Microglial activation has been
reported to induce the death of DA neurons (23). This is due to activated microglia
being able to actively produce and secrete unfavorable toxic
substances, including pro-inflammatory cytokines and ROS (24). The present study demonstrated that
the neuroprotective effects of SST in the LPS-treated SN are based
on the ability of SST to suppress microglial activity and thereby
decrease the production of pro-inflammatory cytokines and ROS
generation. Immunohistochemical staining of OX-42 suggested that
SST inhibited microglial activation. Loss of nigral DA cells was
decreased by administration of SST prior to LPS injection compared
with LPS-treatment only, which was confirmed by immunohistochemical
staining for TH and Nissl staining of the SN. Together with the
results of a previous study (25),
the present study proved that downregulation of microglial activity
is able to improve survival of nigral DA neuronal cells. SST is
therefore suggested as a potential drug for PD treatment.
Increasing evidence suggested that activated
microglia are able to generate ROS, which results in oxidative
stress to DA neurons in the SN of PD patients (26). O2− and
O2− -derived oxidant molecules may exacerbate
neurotoxicity (27). The
accumulation of fluorescent oxidized hydroethidine showed that
LPS-induced production of ROS may be mitigated through SST
treatment prior to administration of LPS in the SN. Moreover, NO
generated by iNOS also contributes to the oxidative stress
associated with the neurotoxicity observed in PD (28). Importantly, neurodegeneration has
been reported to be associated with up-regulation of iNOS
expression in the SN (29). The
present study showed that LPS-induced overexpression of iNOS was
inhibited by treatment with SST. All these results suggested that
LPS-induced activation of microglia and oxidative stress were
prevented by SST, resulting in neuroprotection.
Neuroinflammation is an important feature in the
progression of neurodegenerative disease (30). The activated microglia expresses
pro-inflammatory cytokines including TNF-α, IL-1β and PGE2, which
lead to neuronal degeneration in the SN of PD patients (31). The results showed that LPS up
regulated production of TNF-α, IL-1β and PGE2 was inhibited by SST
application before LPS treatment in the SN. These findings are
similar to previous reports that fucoidan significantly inhibits
the release of TNF-α and prevents neurotoxicity in LPS-induced rat
model of PD (32). It suggests
that SST has the ability to inhibit the expression of
pro-inflammatory cytokines, and lead to neuroprotection as an
inhibitor of neuroinflammation.
In addition, a previous study showed that Cox-2 was
rapidly upregulated when inflammation occurred, which appeared to
be responsible for the formation of PGE2 and may have contributed
to the neurodegenerative process in PD (33). Meanwhile, NF-κB is thought to be a
transcriptional controller, which participates in the release of
Cox-2, following increased degeneration of DA neurons in an animal
model of PD (34). The results of
the present study showed that the expression of activated NF-κB
p65, Cox-2 and PGE2 in the SN was downregulated when SST was
administered prior to LPS treatment compared with that in the group
treated with LPS only. The NF-κB/Cox-2/PGE2 signaling pathway was
confirmed to participate in the neurodegeneration of the
LPS-induced PD model and the inflammatory responses of LPS-treated
P12 cells proceed via the same mechanism (17). As suppression of NF-κB is able to
attenuate the production of LPS-induced pro-inflammatory mediators
(35), the inhibition of NF-κB may
be a mechanism underlying the neuroprotective effect of SST. NF-κB,
Cox-2 and PGE2 may therefore be considered as targets for PD
treatment.
In conclusion, the findings of the present study
demonstrated that SST, particularly at high concentrations of SST
used in the SN, may inhibit ROS production, expression of
pro-inflammatory cytokines and the NF-κB pathway as well as the
activation of microglia, which may lead to increased neuronal
survival. These results suggest that SST offers great therapeutic
potential for treating neurodegenerative diseases, such as PD,
through inhibiting microglial activation.
Acknowledgments
This study was supported by grants from the National
Nature Science Foundation of China (no. 81371421) and the Social
Development Project of Liaoning Province (no. 2011225020).
References
|
1
|
Ramos-Moreno T, Castillo CG and
Martinez-Serrano A: Long term behavioral effects of functional
dopaminergic neurons generated from human neural stem cells in the
rat 6-OH-DA Parkinson’s disease model. Effects of forced expression
of BCL-X(L). Behav Brain Res. 232:225–232. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Golbe LI: Young-onset Parkinson’s disease:
A clinical review. Neurology. 41:168–173. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zanon A, Rakovic A, Blankenburg H, et al:
Profiling of Parkin-binding partners using tandem affinity
purification. PLoS One. 8:e786482013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Choi SH, Joe EH, Kim SU and Jin BK:
Thrombin-induced microglial activation produces degeneration of
nigral dopami-nergic neurons in vivo. J Neurosci. 23:5877–5886.
2003.PubMed/NCBI
|
|
5
|
Song DY, Yu HN, Park CR, et al:
Down-regulation of microglial activity attenuates axotomized nigral
dopaminergic neuronal cell loss. BMC Neurosci. 14:1122013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yuan L, Wu Y, Ren X, Liu Q, Wang J and Liu
X: Isoorientin attenuates lipopolysaccharide-induced
pro-inflammatory responses through down-regulation of ROS-related
MAPK/NF-κB signaling pathway in BV-2 microglia. Mol Cell Biochem.
386:153–165. 2014. View Article : Google Scholar
|
|
7
|
Epelbaum J, Guillou JL, Gastambide F,
Hoyer D, Duron E and Viollet C: Somatostatin, Alzheimer’s disease
and cognition: An old story coming of age? Prog Neurobiol.
89:153–161. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Viollet C, Lepousez G, Loudes C, Videau C,
Simon A and Epelbaum J: Somatostatinergic systems in brain:
Networks and functions. Mol Cell Endocrinol. 286:75–87. 2008.
View Article : Google Scholar
|
|
9
|
Epelbaum J, Ruberg M, Moyse E, Javoy-Agid
F, Dubois B and Agid Y: Somatostatin and dementia in Parkinson’s
disease. Brain Res. 278:376–379. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Espino A, Calopa M, Ambrosio S, Ortolà J,
Peres J and Navarro MA: CSF somatostatin increase in patients with
early parkinsonian syndrome. J Neural Transm Park Dis Dement Sect.
9:189–196. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tuboly G and Vécsei L: Somatostatin and
cognitive function in neurodegenerative disorders. Mini Rev Med
Chem. 13:34–46. 2013. View Article : Google Scholar
|
|
12
|
Chung ES, Chung YC, Bok E, et al:
Fluoxetine prevents LPS-induced degeneration of nigral dopaminergic
neurons by inhibiting microglia-mediated oxidative stress. Brain
Res. 1363:143–150. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chung ES, Bok E, Chung YC, Baik HH and Jin
BK: Cannabinoids prevent lipopolysaccharide-induced
neurodegeneration in the rat substantia nigra in vivo through
inhibition of microglial activation and NADPH oxidase. Brain Res.
1451:110–116. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu M and Bing G: Lipopolysaccharide
animal models for Parkinson’s disease. Parkinson’s Dis.
2011:3270892011.
|
|
15
|
Block ML, Zecca L and Hong JS:
Microglia-mediated neuro-toxicity: uncovering the molecular
mechanisms. Nat Rev Neurosci. 8:57–69. 2007. View Article : Google Scholar
|
|
16
|
Koppula S, Kumar H, Kim IS and Choi D-K:
Reactive oxygen species and inhibitors of inflammatory enzymes,
NADPH oxidase, and iNOS in experimental models of Parkinson’s
disease. Mediators Inflamm. 2012:8239022012. View Article : Google Scholar
|
|
17
|
Geng Y, Fang M, Wang J, et al: Triptolide
down-regulates COX-2 expression and PGE2 release by suppressing the
activity of NF-κB and MAP kinases in lipopolysaccharide-treated
PC12 cells. Phytother Res. 26:337–343. 2012.
|
|
18
|
Tansey MG and Goldberg MS:
Neuroinflammation in Parkinson’s disease: Its role in neuronal
death and implications for therapeutic intervention. Neurobiol Dis.
37:510–518. 2010. View Article : Google Scholar :
|
|
19
|
Minghetti L: Role of COX-2 in inflammatory
and degenerative brain diseases. Inflammation in the Pathogenesis
of Chronic Diseases. 42. Harris R, Bittman R, Dasgupta D, et al:
Springer; Netherlands: pp. 127–141. 2007
|
|
20
|
Broom L, Marinova-Mutafchieva L, Sadeghian
M, Davis JB, Medhurst AD and Dexter DT: Neuroprotection by the
selective iNOS inhibitor GW274150 in a model of Parkinson disease.
Free Radic Biol Med. 50:633–640. 2011. View Article : Google Scholar
|
|
21
|
Aoki E, Yano R, Yokoyama H, Kato H and
Araki T: Role of nuclear transcription factor kappa B (NF-kappaB)
for MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahyropyridine)-induced
apoptosis in nigral neurons of mice. Exp Mol Pathol. 86:57–64.
2009. View Article : Google Scholar
|
|
22
|
Dexter DT and Jenner P: Parkinson disease:
from pathology to molecular disease mechanisms. Free Radic Biol
Med. 62:132–144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim SR, Chung ES, Bok E, et al:
Prothrombin kringle-2 induces death of mesencephalic dopaminergic
neurons in vivo and in vitrovia microglial activation. J Neurosci
Res. 88:1537–1548. 2010.
|
|
24
|
Miller R, James-Kracke M, Sun G and Sun A:
Oxidative and inflammatory pathways in Parkinson’s disease.
Neurochem Res. 34:55–65. 2009. View Article : Google Scholar
|
|
25
|
Shen W, Qi R, Zhang J, et al: Chlorogenic
acid inhibits LPS-induced microglial activation and improves
survival of dopaminergic neurons. Brain Res Bull. 88:487–494. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Park ES, Kim SR and Jin BK: Transient
receptor potential vanilloid subtype 1 contributes to mesencephalic
dopaminergic neuronal survival by inhibiting microglia-originated
oxidative stress. Brain Res Bull. 89:92–96. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lull M and Block ML: Microglial activation
and chronic neuro-degeneration. Neurotherapeutics. 7:354–365. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hancock D, Martin ER, Vance JM and Scott
WK: Nitric oxide synthase genes and their interactions with
environmental factors in Parkinson’s disease. Neurogenetics.
9:249–262. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li M, Dai FR, Du XP, Yang QD and Chen Y:
Neuroprotection by silencing iNOS expression in a 6-OHDA model of
Parkinson’s disease. J Mol Neurosci. 48:225–233. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Miller JA, Trout BR, Sullivan KA, Bialecki
RA, Roberts RA and Tjalkens RB: Low-dose
1-methyl-4-phenyl-1,2,3,6-tetrahydro-pyridine causes inflammatory
activation of astrocytes in nuclear factor-κB reporter mice prior
to loss of dopaminergic neurons. J Neurosci Res. 89:406–417. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
More SV, Kumar H, Kim IS, Song SY and Choi
DK: Cellular and molecular mediators of neuroinflammation in the
pathogenesis of Parkinson’s disease. Mediators Inflamm.
2013:9523752013. View Article : Google Scholar
|
|
32
|
Cui YQ, Jia YJ, Zhang T, Zhang QB and Wang
XM: Fucoidan protects against lipopolysaccharide-induced rat
neuronal damage and inhibits the production of proinflammatory
mediators in primary microglia. CNS Neurosci Ther. 18:827–833.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Teismann P: COX-2 in the neurodegenerative
process of Parkinson’s disease. Biofactors. 38:395–397. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Phani S, Loike JD and Przedborski S:
Neurodegeneration and inflammation in Parkinson’s disease.
Parkinsonism Relat Disord. 18(Suppl 1): S207–S209. 2012. View Article : Google Scholar
|
|
35
|
Kang CH, Jayasooriya RG, Choi YH, Moon SK,
Kim WJ and Kim GY: β-Ionone attenuates LPS-induced pro-inflammatory
mediators such as NO, PGE2 and TNF-α in BV2 microglial cells via
suppression of the NF-κB and MAPK pathway. Toxicol In Vitro.
27:782–787. 2013. View Article : Google Scholar
|