Introduction
Atherosclerosis is a complex vascular disorder
involved in the pathogenesis of myocardial and cerebral infarction
(1). The atherosclerotic process
involves low-density lipoprotein (LDL) oxidation, oxidized LDL
(oxLDL) uptake and inflammatory response induction in macrophages,
the transformation of the lipid-laden macrophages into foam cells,
proliferation of vascular smooth muscle cells (VSMCs) as well as
foam cell accumulation resulting in fatty streaks and subsequent
plaque formation (2,3). Modulation of these key atherogenic
events may therefore be a promising therapeutic strategy for
atherosclerotic management.
Scutellariae Radix (SR), the root of
Scutellaria baicalensis, is one of the most widely used
types of Traditional Chinese Herbal Medicines. SR, whose Chinese
name is Huang-qin, forms the major ingredient of numerous herbal
preparations and is used to treat bacterial infections as well as
various inflammatory diseases. The principal constituents of SR
contain diverse flavonoids, such as baicalein, baicalin and wogonin
(4). In addition, previous studies
have demonstrated that SR may have various beneficial
pharmacological activities including antioxidative (5), anticancer (6,7) and
anti-inflammatory (8,9) effects.
Heme oxygenase (HO), a rate-limiting enzyme in heme
degradation (10), is an inducible
form of HO, which was reported to have cytoprotective effects
(11). These effects include
pro-oxidative heme degradation, which results in biliverdin release
and the subsequent conversion of biliverdin into bilirubin, both of
which have antioxidant properties (12). In addition, heme degradation
generates carbon monoxide, which has antiproliferative and
anti-inflammatory properties (13,14).
Numerous studies have suggested that HO-1 may be a potential
therapeutic target for the treatment of inflammatory diseases, such
as atherosclerosis.
In the present study, high-performance liquid
chromatography (HPLC) was used to analyze the flavonoid components
of SR. In addition, the present study investigated whether SR
exhibited inhibitory effects on LDL oxidation and the macrophage
inflammatory response, which are early events in the development of
atherosclerosis.
Materials and methods
Preparations of SR extract, standard and
sample solution
The roots of S. baicalensis were purchased in
April 2012 from Kwangmyungdang Medicinal Herbs (Ulsan, Korea) and
were confirmed taxonomically by Professor Je-Hyun Lee of Dongkuk
University (Gyeongju, Korea). A voucher specimen of SR
(SR-2012-EBM91) was deposited at the Herbal Medicine Formulation
Research Group, Korea Institute of Oriental Medicine (Daejeon,
Korea). Dried roots of S. baicalensis were extracted using
distilled water (1 liter) through reflux at 100°C for 2 h. The
extracted solution was filtered through filter paper, evaporated to
dryness and then freeze-dried (47.84 g). The yield of water extract
obtained was 47.84%. For HPLC analysis, the 100 mg sample powder
was dissolved in 100 ml 70% methanol (J.T. Baker, Phillipsburg, NJ,
USA) and the solution was passed through a 0.2 mm syringe filter
(Woongki Science Co. Ltd, Seoul, Korea) prior to HPLC injection.
Baicalin, baicalein and wogonin were purchased from Wako Pure
Chemical Industries, Ltd (Osaka, Japan). The purities of these
compounds were >98.0%, as determined using HPLC analysis.
Standard stock solutions of baicalin, baicalein and wogonin (all
1,000 μg/ml) were prepared in 100% methanol and stored at
4°C. Working standard solutions were prepared by serial dilution of
3.91–500 μg/ml baicalin, 2.34–300 μg/ml baicalein,
and 1.53–200.00 μg/ml wogonin, with 100% methanol.
Chromatographic system
Analysis was performed using a Shimadzu LC-10A HPLC
system (Shimadzu Corp., Kyoto, Japan), which was comprised of a
solvent delivery unit, an online degasser, an autosampler and a
photo-diode array (PDA) detector. The data processor used
LCsolution software (version 1.24; Shimadzu Corp.). The analytical
column used was a Gemini C18 (250×4.6 mm; particle size, 5 mm;
Phenomenex, Inc., Torrance, CA, USA). The mobile phases were (A)
1.0% acetic acid (Merck KGaA, Darmstadt, Germany) in aqueous
solution and (B) 1.0% acetic acid in acetonitrile-methanol (7:3;
J.T. Baker) solution. Gradient elution of the two mobile phases
were as follows: 25% B for 0–10 min, 25–45% B for 10–20 min, 45% B
for 20–24 min, 45–48% B for 24–35 min, 48–25% B for 35–40 min and
25% B for 40–50 min. Analysis was performed at a flow rate of 1.0
ml/min with PDA detection at 277 nm. The injection volume was 10
μl.
Determination of antioxidant
activity
The radical-scavenging activity of SR against
2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium
salt (ABTS; Sigma-Aldrich, St. Louis, MO, USA) was determined using
the method described by Re et al (15), with slight modifications. In brief,
ABTS radical cations (ABTS+) were produced by reacting 7
mM ABTS solution with 2.45 mM potassium persulfate (Sigma-Aldrich);
the solution was stored in the dark at room temperature for 16 h.
The solution was then diluted with phosphate-buffered saline (PBS;
pH 7.4; Bio-Rad Laboratories, Inc., Hercules, CA, USA) to an
absorbance of 0.7 at 734 nm using a microplate reader (Benchmark
Plus; Bio-Rad Laboratories, Inc.). A total of 100 ml diluted
ABTS+ solution was then added to a 96-well plate
containing the samples (20, 40, 80 and 160 mg/ml SR, or 2.5, 5 and
10 mg/ml AA). Following incubation for 5 min, absorbance was
measured at 734 nm using a Benchmark Plus microplate reader. The
extent of decolorization was calculated as the percentage reduction
in absorbance. The scavenging capacity of the test compounds was
calculated using the following equation: ABTS radical-scavenging
activity = (1-Asample/Acontrol) ×100, where
Acontrol is the absorbance of the negative control and
Asample is the absorbance of the standard antioxidant or
extract. RC50 values (the concentration required for 50%
reduction of ABTS radical) were calculated as the concentration at
which the absorbance was diminished by 50%.
The 2,2-diphenyl-2-picrylhydrazyl (DPPH;
Sigma-Aldrich) free radical-scavenging activity of SR was
determined according to the method described by Moreno et al
(16), with certain modifications.
In brief, 100 μl sample at various concentrations (20, 40,
80 and 160 mg/ml SR, or 5, 10 and 20 mg/ml AA) was added to 100
μl DPPH solution (0.15 mM in ethanol) in a 96-well plate.
Following incubation for 30 min in the dark at room temperature,
the absorbance was measured at 517 nm. The scavenging activity (%)
was calculated using the formula described in the preceding
paragraph. Ascorbic acid (AA), a positive control for the
antioxidant assay, was purchased from Sigma-Aldrich.
CuSO4-mediated LDL
oxidation
A CuSO4-mediated method (17) was used to induce the oxidation of
LDL. LDL samples (500 μg protein/ml; Biomedical
Technologies, Inc., Stoughton, MA, USA) were prepared at 37°C for 5
min in a medium containing 10 mM phosphate buffer (pH 7.4;
Sigma-Aldrich) with various sample concentrations. Oxidation was
then initiated by the addition of 25 μM CuSO4 at
37°C for 6 h. The oxidation, lipid peroxidation and electrophoretic
mobility of the LDL were then measured using the thiobarbituric
acid-reactive substance (TBARS) and relative electrophoretic
mobility (REM) assays, as described below.
Determination of TBARS
Lipid peroxidation of LDL was estimated by measuring
malondialdehyde (MDA) concentration using a TBARS assay kit
(BioAssay Systems, Hayward, CA, USA) according to the
manufacturer’s instructions (18).
In brief, following oxidation, 50 μg LDL was mixed with 200
μl thiobarbituric acid and incubated at 100°C for 30 min.
When the reaction was complete, the absorbance was measured at 535
nm using a Benchmark Plus microplate reader.
REM assay
The electrophoretic mobility of LDLs was measured
using agarose gel electrophoresis (0.8% agarose in
Tris-acetate-EDTA buffer; Bio-Rad Laboratories, Inc.) and Coomassie
brilliant blue R-250 (Bio-Rad Laboratories, Inc.) staining.
Electrophoresis was performed at 100 V for 30 min (Wide Mini-Sub
Cell GT systems; Bio-Rad Laboratory, Inc.). REM was defined as the
ratio of the distance migrated from the origin by oxLDL versus that
of native LDL (19).
Cell culture
A RAW264.7 murine macrophage cell line was purchased
from the American Type Culture Collection (Manassas, VA, USA).
Cells were incubated at 37°C in 5% CO2 in Dulbecco’s
modified Eagle’s medium (DMEM; Gibco-BRL, Grand Island, NY, USA)
supplemented with 100 U/ml penicillin, 100 μg/ml
streptomycin and 5.5% fetal bovine serum (Gibco-BRL).
In vitro cytotoxicity assay
Cells were seeded at 3×103 cells/well in
a 96-well plate and incubated with various concentrations of SR
(20, 40, 80 and 160 μg/ml) for 24 h. CCK-8 solution (Dojindo
Laboratories, Kumamoto, Japan) was added to cells, which were then
incubated at 37°C for 4 h. Following incubation, the absorbance was
measured at 450 nm using a Benchmark Plus microplate reader.
Measurement of nitric oxide (NO)
production
RAW264.7 cells were seeded at a density of
2.5×105 cells/well in a 48-well plate and incubated with
lipopolysaccharide (LPS; 1 μg/ml; Sigma-Aldrich) for 24 h in
the presence of various concentrations of SR (20, 40, 80 and 160
μg/ml). NG-Methyl-L-arginine acetate salt (NMMA),
a positive control for the NO assay, was purchased from
Sigma-Aldrich. Nitrite accumulated in the culture medium was
quantified as an indicator of NO production. In brief, 50 μl
cell culture medium was mixed with 100 μl Griess reagent (1%
sulfanilamide and 0.1% naphthylethylenediamine dihydrochloride in
2.5% phosphoric acid; Promega, Madison, WI, USA). The mixture was
incubated at room temperature for 30 min and absorbance was then
measured at 535 nm using a Benchmark Plus microplate reader. Fresh
culture medium was used as a blank control for each experiment. The
quantity of nitrite was determined from sodium nitrite standard
curves produced in the present study.
Western blot analysis
Following treatment, as mentioned previously, the
cells were washed twice with cold PBS and then lysed with 1X
Laemmli lysis buffer [60 mM Tris-Cl (pH 6.8), 2% SDS, 10% glycerol
and 0.01% bromophenol blue; Bio-Rad Laboratories, Inc.] and boiled
for 10 min. The protein content was measured using a bicinchoninic
acid protein assay reagent (Pierce Biotechnology, Inc., Rockford,
IL, USA). Reducing agent (2-mercaptoethanol; Sigma-Aldrich) was
added to the samples to obtain a final concentration of 355 mM
2-mercaptoethanol. Equal quantities (20 μg) of total
cellular protein were resolved using 10% SDS-polyacrylamide gel
electrophoresis (Mini-Protean® Tetra Cell systems;
Bio-Rad Laboratories, Inc.) and then transferred to a
nitrocellulose membrane (Bio-Rad Laboratories, Inc.). The
immunoblot was incubated with blocking solution (5% skimmed milk;
Bio-Rad Laboratories, Inc.) and then incubated overnight at 4°C
with the following primary antibodies: Anti-β-actin (1:1,000; cat.
no. sc-81178; Santa Cruz Biotechnology, Inc. Dallas, TX, USA),
anti-inducible NO synthase (iNOS; 1:1,000; cat. no. sc-7271; Santa
Cruz Biotechnology, Inc.) and anti-HO-1 (1:1,000; cat. no. ab13248;
Abcam, Boston, MA, USA). The blots were washed three times with
Tris-buffered saline containing Tween 20 (TBST; Bio-Rad
Laboratories, Inc.) and then incubated with a horseradish
peroxidase-conjugated secondary antibody (1:5,000; cat. no.
115-035-003; Jackson ImmunoResearch, West Grove, PA, USA) for 60
min at room temperature. The blots were washed three times with
TBST and then developed using an enhanced chemiluminescence kit
(cat. no. RPN2232; Amersham, GE Healthcare, Arlington Heights, IL,
USA). The bands were visualized using the ChemiDoc™ XRS Imaging
system (Bio-Rad Laboratories, Inc.).
Statistical analysis
Data were analyzed using the one-way analysis of
variance followed by the Dunnett’s multiple comparison tests using
GraphPad InStat 3.05 software (GraphPad Software Inc., La Jolla,
CA, USA). Values are presented as the mean ± standard error of the
mean. P<0.01 and P<0.05 were considered to indicate
statistically significant differences between values.
Results
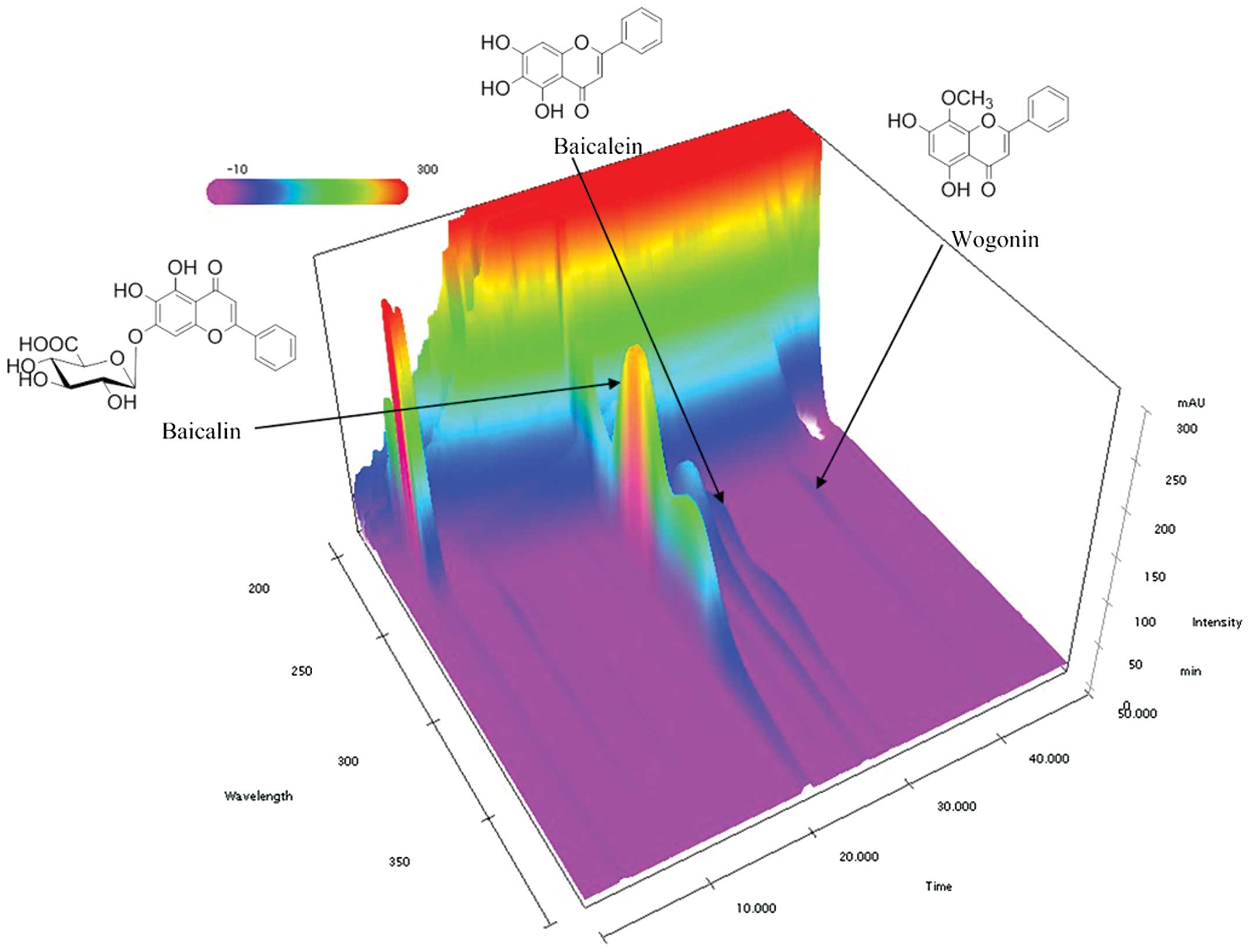
HPLC analysis
HPLC was used to confirm and quantify the standard
components of SR, including baicalin, baicalein and wogonin. HPLC
with a PDA detector were then used to produce a three-dimensional
chromatogram (Fig. 1). Using
optimized chromatography conditions, the three compounds were
eluted in <45 min of sample analysis using the gradient elution
of two mobile phases. The calibration curves of baicalin, baicalein
and wogonin were: y=37580.44x+6967.22 (3.91–500.00
μg/ml), y=55300.58x–31507.13 (2.34–300.00
μg/ml) and y=67938.69x+11675.10 (1.53–200.00
μg/ml), respectively. The correlation coefficients of the
three compounds were all 1.0000. The content of baicalin, baicalein
and wogonin in SR were: 110.16±0.41 mg/g, 14.04±0.11 mg/g and
3.90±0.05 mg/g, respectively, with relative standard deviations of
<1.50%.
Antioxidant activity of SR
In order to evaluate the antioxidant activity of SR,
the scavenging activities of SR were tested against ABTS and DPPH
radicals. The ABTS radical-scavenging activities of SR are
presented in Table I. The results
showed that the scavenging activities of SR increased in a
dose-dependent manner. The RC50 against ABTS radicals
was 51.53 μg/ml, whereas the RC50 of ascorbic
acid, as a positive control, was 5.66 μg/ml. The antioxidant
activities obtained using the DPPH method for SR are shown in
Table II, the results of which
were comparable to those of the ABTS assay. SR reduced the DPPH
radical formation in a concentration-dependent manner. The
RC50 of SR against DPPH radicals was 63.07 μg/ml,
whereas the RC50 of ascorbic acid was 8.56
μg/ml.
 | Table IScavenging effects of SR on
ABTS+. |
Table I
Scavenging effects of SR on
ABTS+.
| Agent | Concentration
(μg/ml) | Scavenging effect
(%) | RC50
(μg/ml) |
|---|
| SR | 20.0 | 23.95±2.31 | 51.53±0.81 |
| 40.0 | 44.30±0.71 | |
| 80.0 | 70.70±1.94 | |
| 160.0 | 95.43±0.82 | |
| AA | 2.5 | 20.44±0.59 | 5.66±0.04 |
| 5.0 | 44.39±1.26 | |
| 10.0 | 89.96±0.72 | |
 | Table IIScavenging effects of SR on DPPH. |
Table II
Scavenging effects of SR on DPPH.
| Agent | Concentration
(μg/ml) | Scavenging effect
(%) | RC50
(μg/ml) |
|---|
| SR | 20.0 | 16.54±1.48 | 63.07±3.00 |
| 40.0 | 37.70±1.87 | |
| 80.0 | 60.74±2.36 | |
| 160.0 | 78.68±1.64 | |
| AA | 5.0 | 15.54±0.43 | 8.56±0.04 |
| 10.0 | 63.66±0.25 | |
| 20.0 | 93.23±0.00 | |
Inhibitory effect of SR on
CuSO4-mediated LDL oxidation
The degree of LDL oxidation and lipid peroxidation
was evaluated using the TBARS assay to measure the concentration of
the degradation byproduct MDA (20). As shown in Fig. 2A, CuSO4 increased the
lipid peroxidation of LDL, which was then significantly inhibited
following treatment with 80 or 160 μg/ml SR (P<0.01).
Alteration in agarose gel electrophoretic mobility reflects an
increase in the negative charge of LDL particles, which occurs
during oxidation (19). In the
present study, oxidation of LDL performed in the presence of SR
significantly attenuated the increase in electrophoretic mobility
of oxLDL following CuSO4 treatment (P<0.01) (Fig. 2B and C).
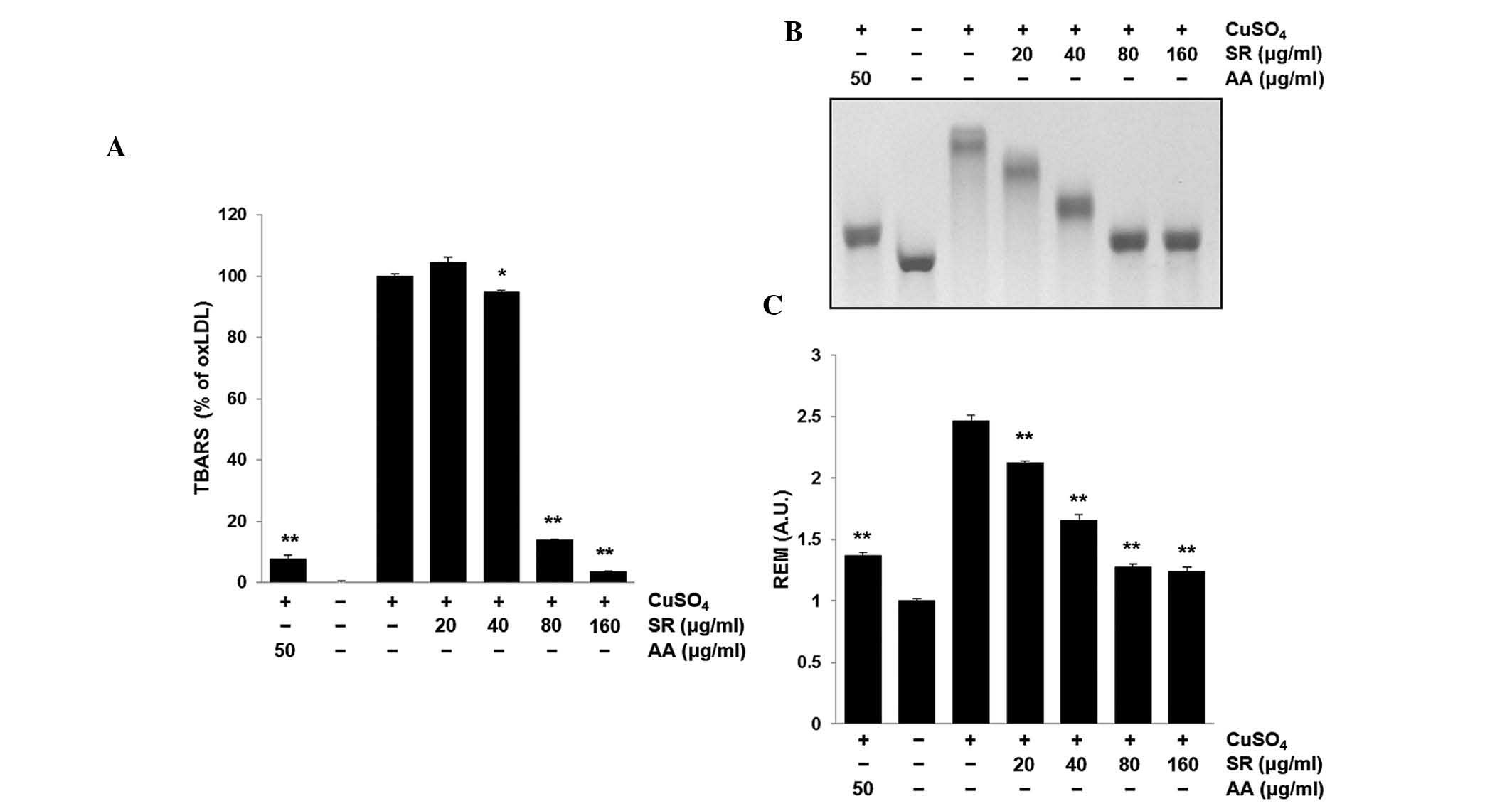 | Figure 2Effects of SR on
Cu2+-induced LDL oxidation. SR (20, 40, 80 and 160
μg/ml) or AA (50 μg/ml) in combination with LDL were
incubated with CuSO4 for 6 h at 37°C. (A) A TBARS assay
was used to quantify the lipid peroxidation levels of LDL. (B and
C) Representative gel and quantification of the electrophoretic
mobility of oxLDL, as determined using an REM assay. Values are
presented as the mean ± standard error of the mean of triplicate
experiments. *P<0.05 and **P<0.01 vs.
oxLDL (CuSO4 only) group. SR, Scutellariae Radix;
LDL, low-density lipoprotein; oxLDL, oxidized LDL; AA, ascorbic
acid; TBARS, thiobarbituric acid-reactive substance; REM, relative
electrophoretic mobility; A.U., absorbance units. |
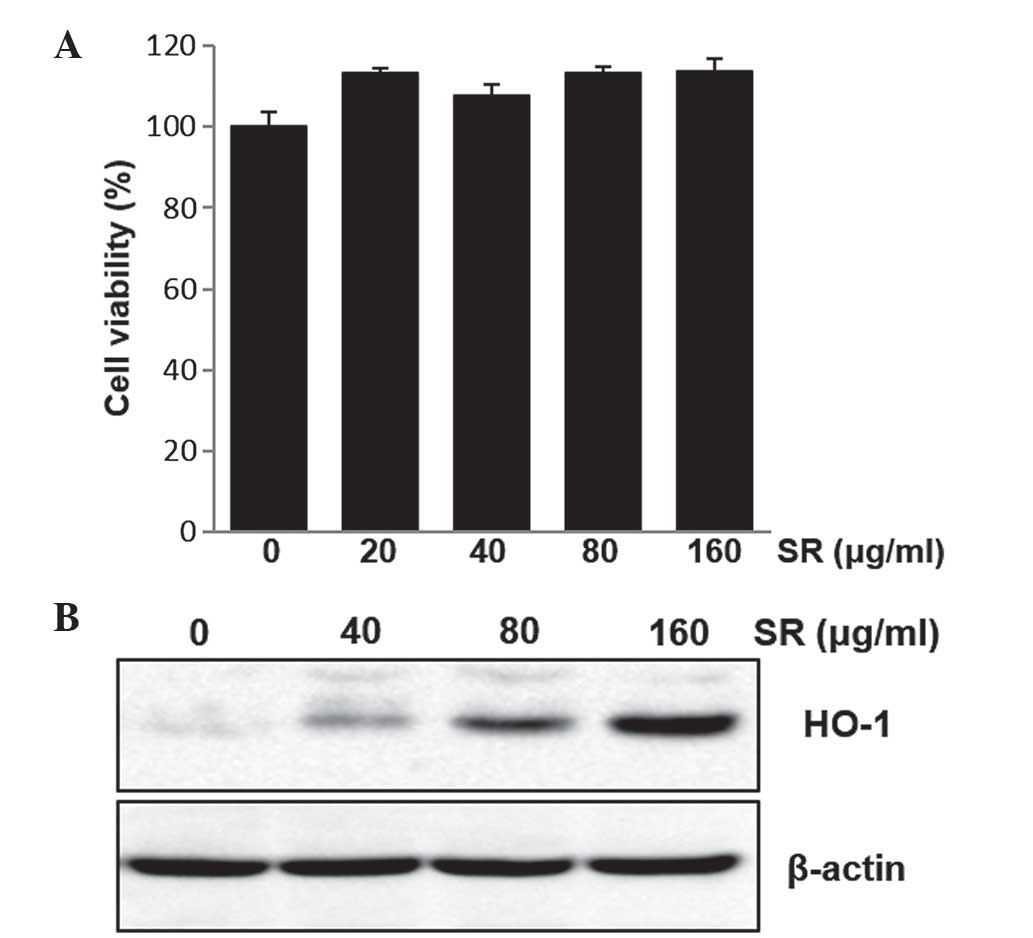
Cytotoxicity of SR in RAW264.7 cells
The cytotoxic effects of SR were investigated in
RAW264.7 cells in order to establish the appropriate concentration
range of SR treatment for subsequent experiments. The cytotoxicity
of SR in this murine macrophage cell line was evaluated using the
CCK-8 cell viability assay. RAW264.7 cells were exposed to various
concentrations of SR (20, 40, 80 and 160 μg/ml) for 24 h. As
shown in Fig. 3A, SR had no
significant cytotoxic effects on RAW264.7 cells. Therefore,
nontoxic concentrations of SR were used in all subsequent
experiments.
Effects of SR on HO-1 expression
In order to examine the effects of SR on the protein
expression of HO-1 in RAW264.7 cells, cells were treated with
various concentrations of SR (40, 80 and 160 μg/ml) for 24
h. Protein levels of HO-1 were then determined using western blot
analysis. The results showed that SR promoted HO-1 protein
expression in a concentration-dependent manner (Fig. 3B).
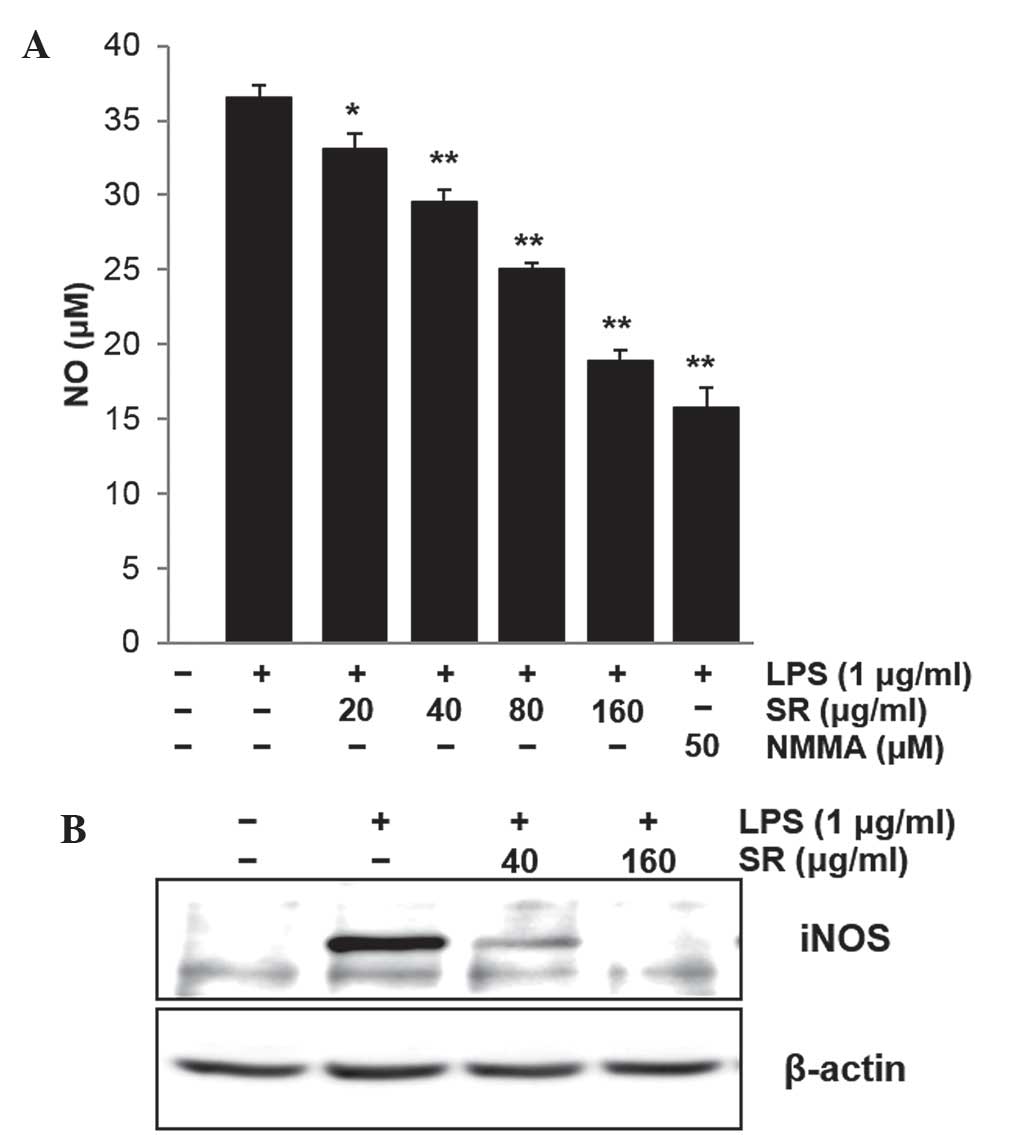
Effects of SR on NO production and iNOS
expression
RAW264.7 cells were treated with 1 μg/ml LPS
for 24 h in the presence of SR (20, 40, 80 and 160 μg/ml).
The quantity of NO produced was determined by measuring the amount
of nitrite converted from NO in the media. As shown in Fig. 4A, SR significantly suppressed
LPS-induced NO release in a concentration-dependent manner compared
with that LPS-treated cells without SR (P<0.05 and 0.01). In
addition, NMMA, an inhibitor of NO synthase, markedly reduced NO
release in RAW264.7 cells (P<0.01) (Fig. 4A). The inhibitory effects of SR on
NO release were 9.3, 18.58, 30.65 and 47.22% at 20, 40, 80 and 160
μg/ml, respectively. Furthermore, it was investigated
whether SR affects the protein expression of iNOS in RAW264.7
cells. As shown in Fig. 4B, iNOS
protein expression was markedly increased in LPS-stimulated cells
compared with that of the untreated control cells; however, this
increase was attenuated by the addition of SR at 40 or 160
μg/ml.
Discussion
Atherosclerosis comprises multiple events including
LDL oxidation (21), inflammation
at the injured area (22), foam
cell formation (23) as well as
VSMC proliferation and migration (24). Increasing evidence from in
vitro and in vivo studies has suggested that oxidative
modification of LDL, the major cholesterol carrier in the
bloodstream, may be involved in the formation of early
atherosclerotic lesions (22,25,26).
Atherosclerosis is widely accepted to be an inflammatory disease
(27); therefore, therefore it was
suggested that reducing the processes involved in LDL oxidation and
the macrophage inflammatory response may be crucial for protecting
against atherosclerosis. The current study investigated the effects
of SR, extracts of the root of S. baicalensis, on LDL
oxidation and LPS-induced inflammation.
The root of S. baicalensis is one of the
primary ingredients in numerous traditional medicines in China,
Japan and Korea (28–30). Studies have reported that
traditional herbal formulas containing SR have anti-atherosclerotic
activities (31–34). Ger-Gen-chyn-lian-tang was shown to
reduce atherosclerotic progression in an apolipoprotein
E−/− mouse model (31);
in addition, Dahuang Zhechong, in pill form, inhibited VSMC
proliferation and reversed the pathological changes observed in the
aorta of atherosclerotic rabbit models (32,33).
Furthermore, Huanglian-jie-du-tang was demonstrated to inhibit the
progression of atherosclerosis in diet-induced hypercholesterolemic
rabbits (34).
The LDL oxidation hypothesis of atherosclerosis
development suggested that inhibition of LDL oxidation may be a
potential target for anti-atherosclerotic therapy (25). Previous studies have reported that
SR inhibited lipid peroxidation in the rat liver (35,36).
In the present study, TBARS and REM assays were used to demonstrate
that SR reduced LDL oxidation in a dose-dependent manner. Lipid
hydroperoxides formation in LDL was previously reported to be an
early event in LDL oxidation (37). In the present study, it was
observed that SR decreased TBARS formation in
Cu2+-induced oxLDL. In the REM assay, oxLDL migrated
further compared with native LDL due to the increase in negative
change; in addition, oxLDL was less visible following Coomassie
blue staining due to partial degradation. In addition, SR inhibited
Cu2+-induced LDL oxidation in a dose-dependent manner.
These data suggested that SR possesses potent antioxidative
abilities, which may be mediated through the inhibition of LDL
oxidation.
In order to evaluate the anti-inflammatory effects
of SR, a LPS-induced NO production model was used in the present
study. iNOS expression and NO production were previously reported
to be increased in atherosclerotic lesions (38). In the present study, nitrite
concentrations in the culture medium were determined and the
results indicated that SR significantly inhibited NO production in
a concentration-dependent manner. In addition, western blot
analysis revealed that SR treatment markedly inhibited iNOS
expression. A previous study reported comparable anti-inflammatory
effects of SR (9). Kim et
al (9) reported that SR
displayed significant anti-inflammatory effects in vitro and
in vivo via suppression of mitogen-activated protein kinase
signaling. The induction of HO-1 has been widely reported to be
involved in the atherosclerosis process (39–41);
therefore, it is regarded as a major therapeutic target for the
prevention or treatment of atherosclerosis (40). The results of the current study
demonstrated that SR induced HO-1 expression and exhibited
anti-inflammatory properties. In addition, these results suggested
that the induction of HO-1 contributed to the anti-inflammatory
effects of SR and that SR may have potential therapeutic value in
the treatment or prevention of atherosclerosis.
In conclusion, the results of the present study
demonstrated that SR was able to reduce the oxidation of LDL and
suppress the macrophage inflammatory response. The antioxidant
properties and inhibitory effects of SR on macrophage inflammatory
responses suggested that SR may be effective in the prevention or
treatment of atherosclerosis; however, further studies are required
in order to confirm these results.
Acknowledgments
The present study was supported by grants from the
Korean Institute of Oriental Medicine (nos. K13030 and K12271).
References
|
1
|
Singh RB, Mengi SA, Xu YJ, et al:
Pathogenesis of atherosclerosis: A multifactorial process. Exp Clin
Cardiol. 7:40–53. 2002.PubMed/NCBI
|
|
2
|
Ross R: The pathogenesis of
atherosclerosis: a perspective for the 1990s. Nature. 362:801–809.
1993. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ross R: Atherosclerosis is an inflammatory
disease. Am Heart J. 138(5 Pt 2): S419–S420. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tong L, Wan M, Zhang L, et al:
Simultaneous determination of baicalin, wogonoside, baicalein,
wogonin, oroxylin A and chrysin of Radix scutellariae extract in
rat plasma by liquid chromatography tandem mass spectrometry. J
Pharm Biomed Anal. 70:6–12. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gao Z, Huang K, Yang X and Xu H: Free
radical scavenging and antioxidant activities of flavonoids
extracted from the radix of Scutellaria baicalensis Georgi. Biochim
Biophys Acta. 1472:643–650. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Park KI, Park HS, Kang SR, et al: Korean
Scutellaria baicalensis water extract inhibits cell cycle G1/S
transition by suppressing cyclin D1 expression and
matrix-metalloproteinase-2 activity in human lung cancer cells. J
Ethnopharmacol. 133:634–641. 2011. View Article : Google Scholar
|
|
7
|
Kumagai T, Müller CI, Desmond JC, et al:
Scutellaria baicalensis, a herbal medicine: anti-proliferative and
apoptotic activity against acute lymphocytic leukemia, lymphoma and
myeloma cell lines. Leuk Res. 31:523–530. 2007. View Article : Google Scholar
|
|
8
|
Jung HS, Kim MH, Gwak NG, et al:
Antiallergic effects of Scutellaria baicalensis on inflammation in
vivo and in vitro. J Ethnopharmacol. 141:345–349. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim EH, Shim B, Kang S, et al:
Anti-inflammatory effects of Scutellaria baicalensis extract via
suppression of immune modulators and MAP kinase signaling
molecules. J Ethnopharmacol. 126:320–331. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maines MD: The heme oxygenase system: a
regulator of second messenger gases. Annu Rev Pharmacol Toxicol.
37:517–554. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Origassa CS and Câmara NO: Cytoprotective
role of heme oxygenase-1 and heme degradation derived end products
in liver injury. World J Hepatol. 5:541–549. 2013.PubMed/NCBI
|
|
12
|
Jansen T and Daiber A: Direct antioxidant
properties of bilirubin and biliverdin. Is there a role for
biliverdin reductase? Front Pharmacol. 3:302012.
|
|
13
|
Otterbein LE, Kolls JK, Mantell LL, et al:
Exogenous administration of heme oxygenase-1 by gene transfer
provides protection against hyperoxia-induced lung injury. J Clin
Invest. 103:1047–1054. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ryter SW, Alam J and Choi AM: Heme
oxygenase-1/carbon monoxide: from basic science to therapeutic
applications. Physiol Rev. 86:583–650. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Re R, Pellegrini N, Proteggente A, et al:
Antioxidant activity applying an improved ABTS radical cation
decolorization assay. Free Radic Biol Med. 26:1231–1237. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Moreno MI, Isla MI, Sampietro AR and
Vattuone MA: Comparison of the free radical-scavenging activity of
propolis from several regions of Argentina. J Ethnopharmacol.
71:109–114. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Barnhart RL, Busch SJ and Jackson RL:
Concentration-dependent antioxidant activity of probucol in low
density lipoproteins in vitro: probucol degradation precedes
lipoprotein oxidation. J Lipid Res. 30:1703–1710. 1989.PubMed/NCBI
|
|
18
|
Buege JA and Aust SD: Microsomal lipid
peroxidation. Methods Enzymol. 52:302–310. 1978.PubMed/NCBI
|
|
19
|
Sparks DL and Phillips MC: Quantitative
measurement of lipoprotein surface charge by agarose gel
electrophoresis. J Lipid Res. 33:123–130. 1992.PubMed/NCBI
|
|
20
|
Wallin B, Rosengren B, Shertzer HG and
Camejo G: Lipoprotein oxidation and measurement of thiobarbituric
acid reacting substances formation in a single microtiter plate:
its use for evaluation of antioxidants. Anal Biochem. 208:10–15.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chisolm GM and Steinberg D: The oxidative
modification hypothesis of atherogenesis: an overview. Free Radic
Biol Med. 28:1815–1826. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Charo IF and Taub R: Anti-inflammatory
therapeutics for the treatment of atherosclerosis. Nat Rev Drug
Discov. 10:365–376. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li AC and Glass CK: The macrophage foam
cell as a target for therapeutic intervention. Nat Med.
8:1235–1242. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Andrés V: Control of vascular cell
proliferation and migration by cyclin-dependent kinase signalling:
new perspectives and therapeutic potential. Cardiovasc Res.
63:11–21. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Heinecke JW: Lipoprotein oxidation in
cardiovascular disease: Chief culprit or innocent bystander? J Exp
Med. 203:813–816. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Norata GD, Tonti L, Roma P and Catapano
AL: Apoptosis and proliferation of endothelial cells in early
atherosclerotic lesions: Possible role of oxidised LDL. Nutr Metab
Cardiovasc Dis. 12:297–305. 2002.
|
|
27
|
Libby P, Ridker PM and Maseri A:
Inflammation and atherosclerosis. Circulation. 105:1135–1143. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li C, Zhou L, Lin G and Zuo Z: Contents of
major bioactive flavones in proprietary traditional Chinese
medicine products and reference herb of Radix Scutellariae. J Pharm
Biomed Anal. 50(3): 298–306. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Akagiri S, Naito Y, Ichikawa H, et al:
Bofutsushosan, an Oriental herbal medicine, attenuates the weight
gain of white adipose tissue and the increased size of adipocytes
associated with the increase in their Expression ef uncoupling
protein 1 in high-fat diet-fed male KK/Ta mice. J Clin Biochem
Nutr. 42:158–166. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tanaka K, Nara K, Nishimura T, et al:
Fever of unknown origin successfully treated by oren-gedoku-to
(huanglian-jie-du-tang). Int J Gen Med. 6:829–832. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ho FM, Liao YH, Yang AJ, et al:
Anti-atherosclerotic action of Ger-Gen-Chyn-Lian-Tang and
AMPK-dependent lipid lowering effect in hepatocytes. J
Ethnopharmacol. 142:175–187. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ji YY, Liu JT, Wang ZD, et al: Study on
anti-atherosclerotic mechanisms of divided functional recipes of
dahuang zhechong pill in rabbits. China J Chin Mater Med.
32:1077–1081. 2007.In Chinese.
|
|
33
|
Ji YY, Liu JT and Li JL: Effect of the
disassembled recipes of dahuang zhechong pill on proliferation and
apoptosis of vascular smooth muscle cells in atherosclerotic
rabbits. Chin J Integr Tradit West Med. 26:913–917. 2006.In
Chinese.
|
|
34
|
Sekiya N, Kainuma M, Hikiami H, et al:
Oren-gedoku-to and Keishi-bukuryo-gan-ryo inhibit the progression
of atherosclerosis in diet-induced hypercholesterolemic rabbits.
Biol Pharm Bull. 28:294–298. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kimura Y, Kubo M, Kusaka K, et al: Studies
on Scutellariae radix. V Effects on ethanol-induced hyperlipemia
and lipolysis in isolated fat cells. Chem Pharm Bull (Tokyo).
30:219–222. 1982. View Article : Google Scholar
|
|
36
|
Kimura Y, Okuda H, Taira Z, et al: Studies
on Scutellariae radix. IX. New component inhibiting lipid
peroxidation in rat liver. Planta Med. 50:290–295. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Girotti AW: Lipid hydroperoxide
generation, turnover and effector action in biological systems. J
Lipid Res. 39:1529–1542. 1998.PubMed/NCBI
|
|
38
|
Napoli C and Ignarro LJ: Nitric oxide and
atherosclerosis. Nitric Oxide. 5:88–97. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Morita T: Heme oxygenase and
atherosclerosis. Arterioscler Thromb Vasc Biol. 25:1786–1795. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu D, He Z, Wu L and Fang Y: Effects of
induction/inhibition of endogenous heme oxygenase-1 on lipid
metabolism, endothelial function and atherosclerosis in rabbits on
a high fat diet. J Pharmacol Sci. 118:14–24. 2012. View Article : Google Scholar
|
|
41
|
Wu ML, Ho YC, Lin CY and Yet SF: Heme
oxygenase-1 in inflammation and cardiovascular disease. Am J
Cardiovasc Dis. 1:150–158. 2011.
|