Introduction
Radiotherapy is used widely in the treatment of
various types of cancer, including non-small cell lung cancer
(1). Together with chemotherapy
and surgery, radiotherapy has been demonstrated to be extremely
efficient. Fractionated radiation is a radiotherapy procedure,
which is applied clinically to assist in normal tissue recovery
(2). However, the incidence of
intrinsic and acquired radioresistance has increased in patients
with cancer, resulting in a reduction in treatment success
(3). Although efforts have been
made to investigate the mechanisms underlying radioresistance, and
to identify alternative treatment options, radioresistance remains
a significant obstacle in improving lung cancer treatment (4).
The capacity for cells to repair DNA, which is
damaged by ionizing radiation is a critical factor in determining
cellular radiosensitivity (5).
Ionizing radiation induces DNA double strand breaks (DSB), which is
considered the most harmful type of DNA damage (6). In mammalian cells, DNA-dependent
protein kinase (DNA-PK)-dependent non-homologous end joining (NHEJ)
is one of the predominant pathways for repairing DSBs (7). This pathway is initiated by
activation of ataxia telangiectasia mutated (ATM) kinase. DNA-PK is
a member of the phosphoinositide 3-kinase-like enzyme family, and
is a nuclear serine/threonine protein kinase, which is required for
repairing DSBs and for V(D)J recombination (8). Cells defend against DNA DSBs via
several repair pathways; among which, the end-joining pathway is
the most important (6). There are
four proteins, which are involved in this process: DNA-PK, Ku70 and
Ku80 (9,10) and XRCC4 (11). ATM is essential for controlling the
cellular response to DSB, since it can regulate apoptosis, cell
cycle checkpoints and DNA repair signaling processes (12). Following the induction of DSBs, ATM
is rapidly activated by intermolecular autophosphorylation
(13,14).
The H2AX histone protein is a substrate of ATM
kinase, which is required for recruiting DNA repair molecules to
sites of damange following exposure to ionizing radiation (15,16).
The transformation of histone H2AX at Ser139 to γ-H2AX has
previously been recognized as the initial signal step in the
response to DNA DSBs. γ-H2AX recruits other proteins to the sites
of DSBs and initiates the repair process (17). Suppression of DNA repair may lead
to an enhancement of radiosensitivity.
During the DNA repair process, DNA-PK is involved in
the NHEJ pathway, and in V(D)J recombination. DNA-PK acts as an
enzyme to activate numerous DNA damage-associated proteins and
coordinates with ataxia telangiectasia and Rad3 related (ATR) and
ATM to phosphorylate proteins associated with DNA damage
checkpoints. Previous studies have demonstrated that the
inactivation of DNA-PK restores the sensitivity of cells to
radiation (18,19). Therefore, an inhibitor targeting
DNA-PK has been commercially developed, to provide potential
clinical options for the treatment of cancer (20).
The mechanisms by which irradiated cells sense DNA
damage remain to be elucidated, however, it is likely that DNA-PK
and ATM are major signal transducers. Since the crucial DNA repair
enzymes are pivotal in cancer research, the discovery of their
inhibitors is of significant interest. The present study aimed to
investigate the effects of the novel specific DNA-PKcs inhibitor,
NU7026, which has been reported to act as a radiosensitizer in
vitro (21,22), combined with ionizing radiation, on
the activation of several components of the DNA damage and repair
response signaling pathway. In addition, the present study also
aimed to determine its effect on the induction of apoptosis in
acquired radioresistant cells.
Materials and methods
Cells and cell culture
The A549 human lung cancer cell line was purchased
from American Type Culture Collection (ATCC; Manassas, VA, USA).
The cells were cultured in 25 ml RPMI 1640 with modified Dulbecco’s
modified Eagle’s medium, supplemented with 20% fetal calf serum,
0.05% L-glutamine, 150 IU/ml penicillin and 50 μg/ml
streptomycin (all from Sigma-Aldrich, St. Louis, MO, USA), at 37°C
in a humidified atmosphere containing 5% CO2. The
culture medium was regularly replaced, and the cells were separated
once they had reached 80% confluence. A maximum of 20 passages were
used. To avoid mycoplasma contamination, the cell line was assessed
monthly using polymerase chain reaction (PCR) (23). For PCR, the ATCC Universal
Mycoplasma Detection Kit (ATCC) was used. In brief, 10 μl
reaction mixture with 10 ng sample was analyzed, using the
following primer sequences: Forward 5′-GGG AGC AAA CAG GAT TAG ATA
CCC T-3′ and reverse 5′-TGC ACC ATC TGT CAC TCT GTT AAC CTC-3′. The
PCR cycling conditions were as follows: 32 cycles of 4 sec at 95°C,
8 sec at 65°C and 16 sec at 72°C with the Applied Biosystems
GeneAmp 9600 thermal cycler (Applied Biosystems Life Technologies,
Foster City, CA, USA). The cell lines were authenticated using
Short Tandem Repeats (STR) analysis to avoid errors in
identification, which used the GenePrint 10 system (Promega
Corporation, Madison, WI, USA) to detect TH01, TPOX, vWA,
Amelogenin, CSF1PO, D16S539, D7S820, D13S317, D5S818 and D21S11
comparing against the ATCC STR database.
Development of acquired radioresistant
cells
A step-wise method (24) was used to produce the
radioresistant cell line. Parental A549 cells (1×106)
were plated in a 75 cm2 culture flask with 25 ml RPMI
1640. Once the cells had reached 70% confluence, the flask was
placed in a Theratron Cobalt-60 unit (Best Theratronics, Ontario,
Canada) and irradiated with 4 Gy γ-rays, at a dose rate of 1 Gy per
minute. The culture medium was replaced immediately following
irradiation, and regular medium replacement and separation of the
cells was performed when the cells had reached 90% confluence. To
separate the cells, they were washed with ice-cold
phosphate-buffered saline (PBS; Sigma-Aldrich), then 0.5 ml trypsin
(Sigma-Aldrich) was added into the culture flask, which was
incubated at 37°C in a humidified atmosphere with 5% CO2
for 5 min. The adherent cells were removed from the dish with a
cold plastic cell scraper and 5 ml RPMI 1640 was added. A total of
1 ml cell suspension was carefully added into a fresh flask with 24
ml RPMI 1640, which was incubated at 37°C in a humidified
atmosphere containing 5% CO2. Irradiation was performed
a total of 13 times (inreasing doses starting at 4 Gy, with a total
exposure of 60 Gy) within 6 months. The parental cells were
cultured in the same manner without irradiation.
In vitro γ-ray irradiation
The cells were counted using a BC TC20 Automatic
Cell Counter (BD Biosciences, San Jose, CA, USA) and adjusted to a
density of 1×106 cells/ml. The cells were then placed in
a 5% CO2 incubator at 37°C, and γ-ray irradiation was
performed using a Theratron Cobalt-60 treatment unit at a dose rate
of 1 Gy/min.
Colony formation assay
The cultured A549 parental and A549R radioresistant
cells (1×105) were irradiated with a single dose of 0,
4, 8 and 12 Gy. Following irradiation, the cells were plated into 6
cm dishes and cultured with 25 ml RPMI 1640 in an incubator at 37°C
with a humidified atmosphere containing 5% CO2 for 2
weeks with no additional treatment. Colony formation was visualized
by staining with 0.02% crystal violet solution (w/v in 75% ethanol;
Sigma-Aldrich), and the colonies were counted. The surviving
fraction was then calculated in the two groups, by first
calculating the plating efficiency [PE; (number of colonies
counted/number of cells plated)×100%] for the treatment and control
groups. The surviving fraction (SF) could then be calculated; SF =
(PE of treated group/PE of control group) ×100%.
Apoptosis assay
Subsequent to trypsinization and incubation at 37°C
for 24 h, the A549 and A549R cells (1×106) were seeded
in 10 mm dishes and incubated overnight at 37°C. Following
irradiation with 4, 8 and 12 Gy, the cells were treated with the
DNA-PK catalytic subunit (cs) inhibitor NU7026 (Sigma-Aldrich), at
a concentration of 1 μM for 24 h. Following 24 h incubation,
apoptotic analyses were performed using a flow cytometer(BD
FACSCalibur; BD Biosciences) with propidium iodide (PI) and annexin
V staining (Invitrogen Life Technologies, Carlsbad, CA, USA), as
described previously (19). In
brief, cells were washed in cold PBS then were treated with
annexin-binding buffer. Suspended cells were then fixed in 70% cold
ethanol and treated with 10 g/l RNase, then 5 μl Alexa Fluor
488 annexin V and 1 μl PI (100 μg/ml) were added per
100 μl cell suspension. Following incubation for 15 min at
room temperature, 400 μl annexin-binding buffer was added
with gentle mixing. Cells with early apoptotic signals (stained
with annexin V) and cells with late apoptotic signals (stained with
PI) were quantified and analyzed, and each assay was performed in
triplicate. The distributions of apoptotic cells were analyzed
using ModFit LT™ software, version 4.0 (Verity Software House,
Topsham, ME, USA).
γ-H2AX foci formation
The cells were trypsinized and resuspended following
fixation with 2% paraformaldehyde (Sigma-Aldrich) for 15 min, and
were subsequently permeabilized with 0.5% Triton X-100
(Sigma-Aldrich). The cells were then incubated with a mouse
monoclonal primary antibody specific for Ser 139 phosphorylation of
H2AX (ab11174; Abcam, Cambridge, MA, USA; 1:400) at 4°C overnight,
followed by incubation with the polyclonal goat anti-mouse Alexa
Fluor 488-labeled secondary antibody (A-10667; Life Technologies,
Grand Island, NY, USA; 1:1,000) for 2 h at room temperature. The
cells were collected and mounted on glass slides using
Vectashield® mounting medium (Vector Laboratories, Inc.,
Burlingame, CA, USA). The cells were visualized using the enhanced
chemiluminescence kit (Life Technologies) using a fluorescence
microscope (Axio Zoom. V16; Carl Zeiss Ltd., Cambridge, UK).
Western blotting
All procedures were conducted according to the
manufacturer’s instructions (Invitrogen Life Technologies). Equal
quantities of proteins (50 μg) were obtained from the two
cell groups using 0.5 ml ice-cold lysis buffer (Invitrogen Life
Technologies), maintaining constant agitation for 30 min at 4°C,
and centrifuging in a microcentrifuge at 4°C for 20 min at 14,000 ×
g. The protein samples were separated by SDS-PAGE (10% gel for the
upper chamber and 5% for the lower chamber; Invitrogen Life
Technologies), alongside molecular weight markers, and were then
electrotransferred onto nitrocellulose membranes (Life
Technologies). The membranes were blocked for 1 hour at room
temperature, or overnight at 4°C using 5% blocking solution (1X
Tris-buffered saline with Tween 20 and nonfat dried milk;
Invitrogen Life Technologies). The membranes were then incubated
with primary antibodies at 4°C overnight. Following washing with
buffer solution, the membranes were incubated with secondary
antibody overnight. The protein bands were visualized using Kodak
film (Eastman Kodak, Rochester, NY, USA) in a dark room.
Statistical analysis
Data are expressed as the mean ± standard error of
the mean, and all experiments were performed in triplicate. SPSS
13.0 software (SPSS Inc., Chicago, IL, USA) was used to perform all
statistical analyses. Student’s t-test and one-way analysis of
variance were used to analyze the differences between the groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
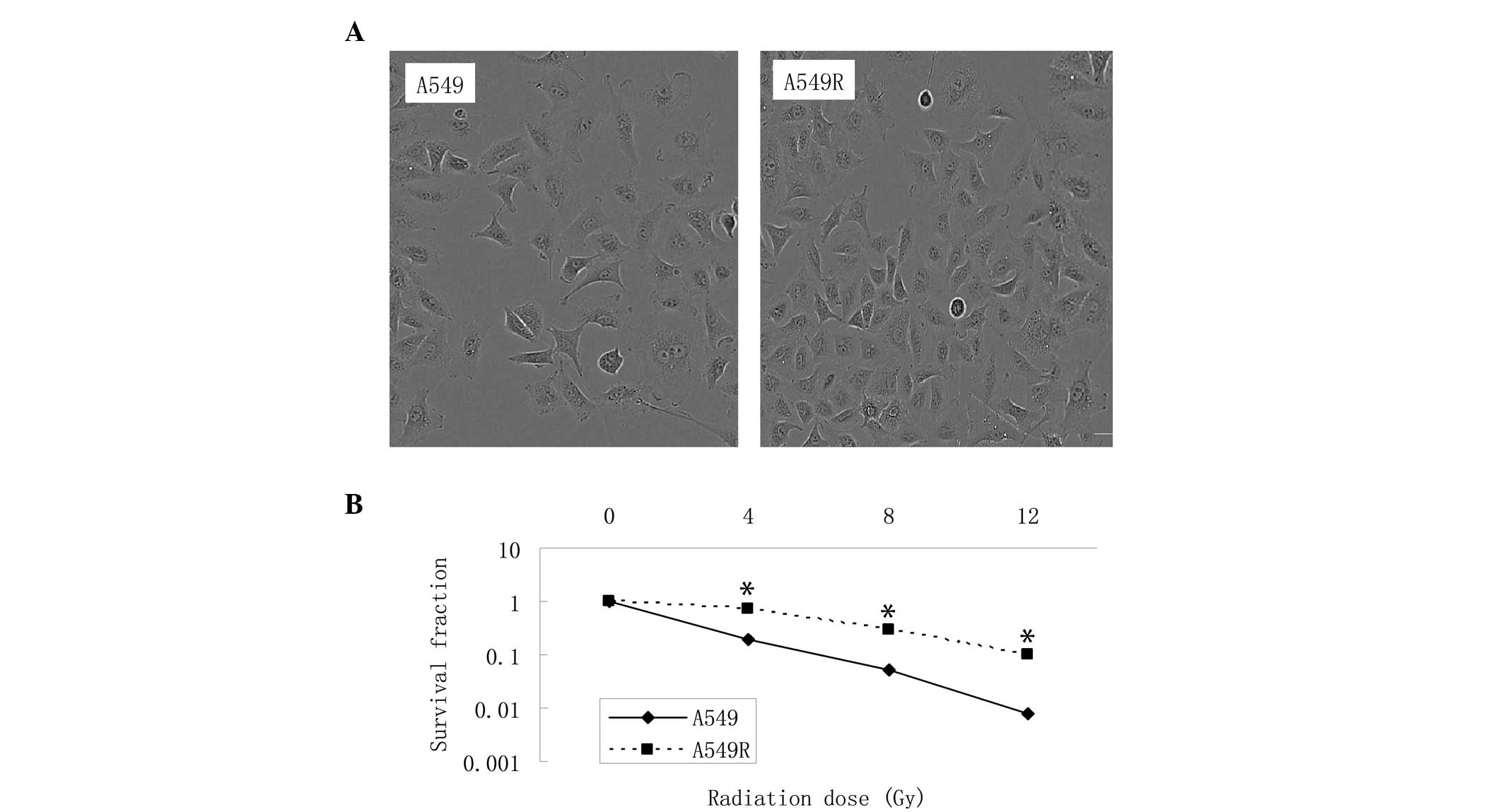
Identification of established
radioresistant A549 cells
To generate radioresistant cells, the parental A549
cell line was irradiated for 5 months using a step-wise method,
with an accumulated dose of 60 Gy. The A549R acquired resistance
cell line was then maintained in culture medium for 10 passages
without radiation. No significant changes in cell morphology were
observed in the A549R cells following irradiation, compared with
the parent cells (Fig. 1A), under
microscopy. The colony formation assay revealed that the A549R
cells exhibited higher rates of cell survival rate, compared with
the parental cells following irradiation (Fig. 1B).
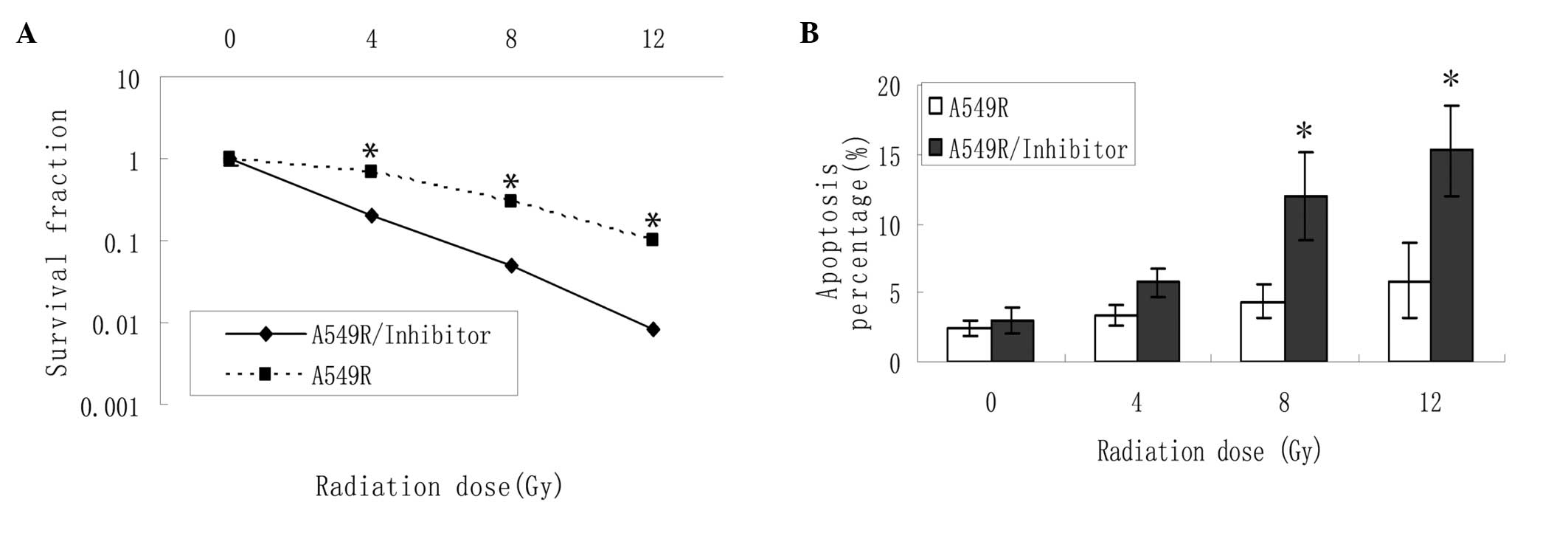
DNA-PKcs inhibitor treatment induces
apoptosis and reduces survival of radioresistant cells following
irradiation
The radiosensitivities of the A549R cells with or
without NU7026 were compared using a colony formation assay
(Fig. 2A). The survival fraction
was decreased in the A549R cells treated with NU7026 following
radiation therapy at different doses, indicating that the
radioresistance of the A549R cells was reversed following treatment
with the DNA-PKcs inhibitor. As shown in Fig. 2B, the percentage of
radiation-induced apoptotic cells was markedly increased in the
NU7026-treated cells, indicating the potential stimulatory effects
of NU7026 on apoptosis when used in conjunction with
irradiation.
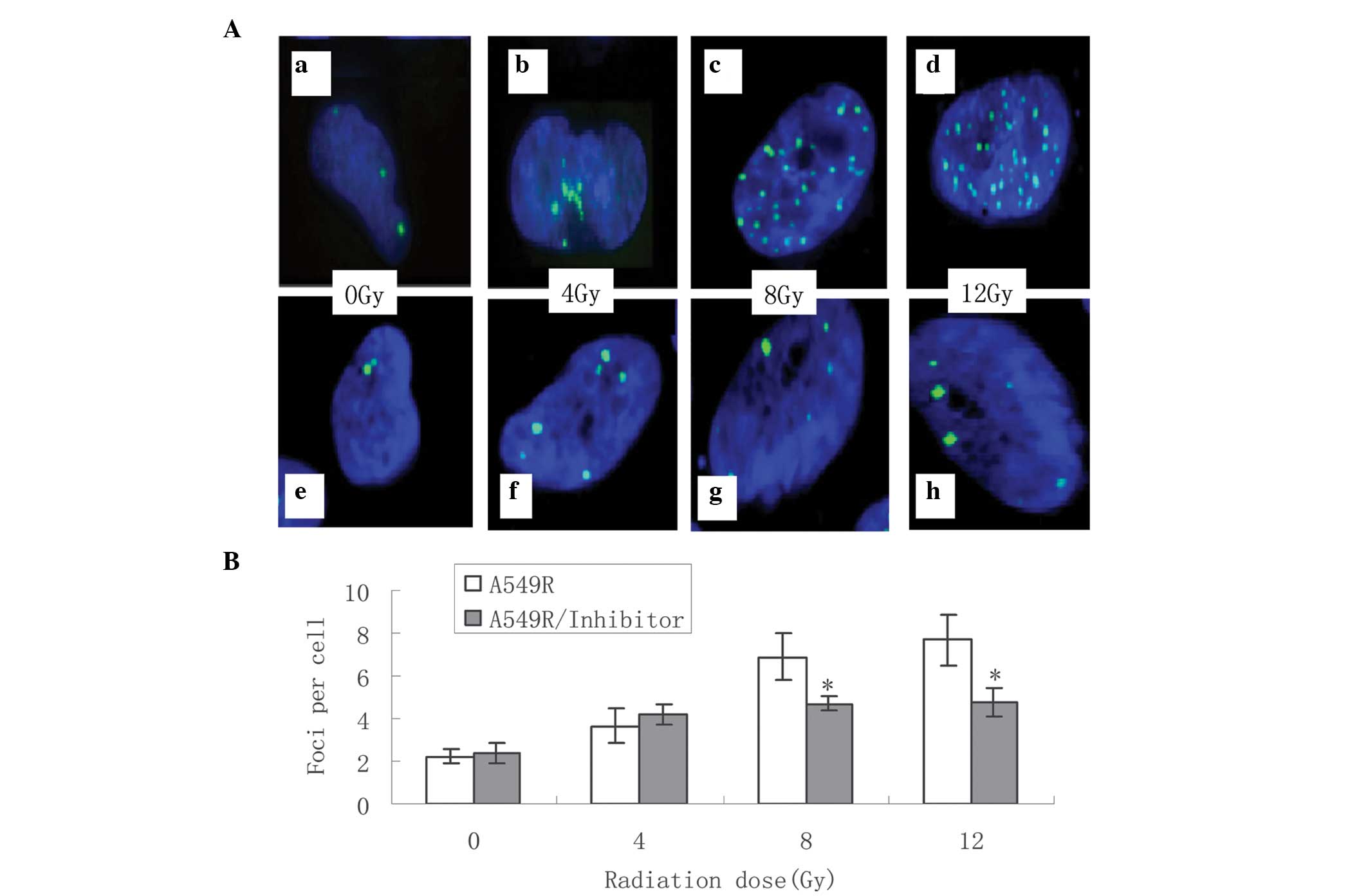
DNA damage signal is decreased following
treatment with a DNA-PKcs inhibitor
The effects of the NU7026 DNA-PKcs inhibitor were
evaluated on the cells with acquired radioresistance treated with
different doses of irradiation (0–12 Gy) within 24 h. The level of
DNA damage induced by irradiation was determined by counting the
number of histone γ-H2AX foci under fluorescence microscopy. A
total of 500 nuclei were counted in each group (Fig. 3A). The formation of γ-H2AX was
significantly downregulated following treatment with NU7026 and 8
Gy or 12 Gy irradiation (Fig. 3B).
These results suggested that treatment with NU7026 suppressed the
radiation-induced DNA repair signals in the A549R cells following
high dose irradiation.
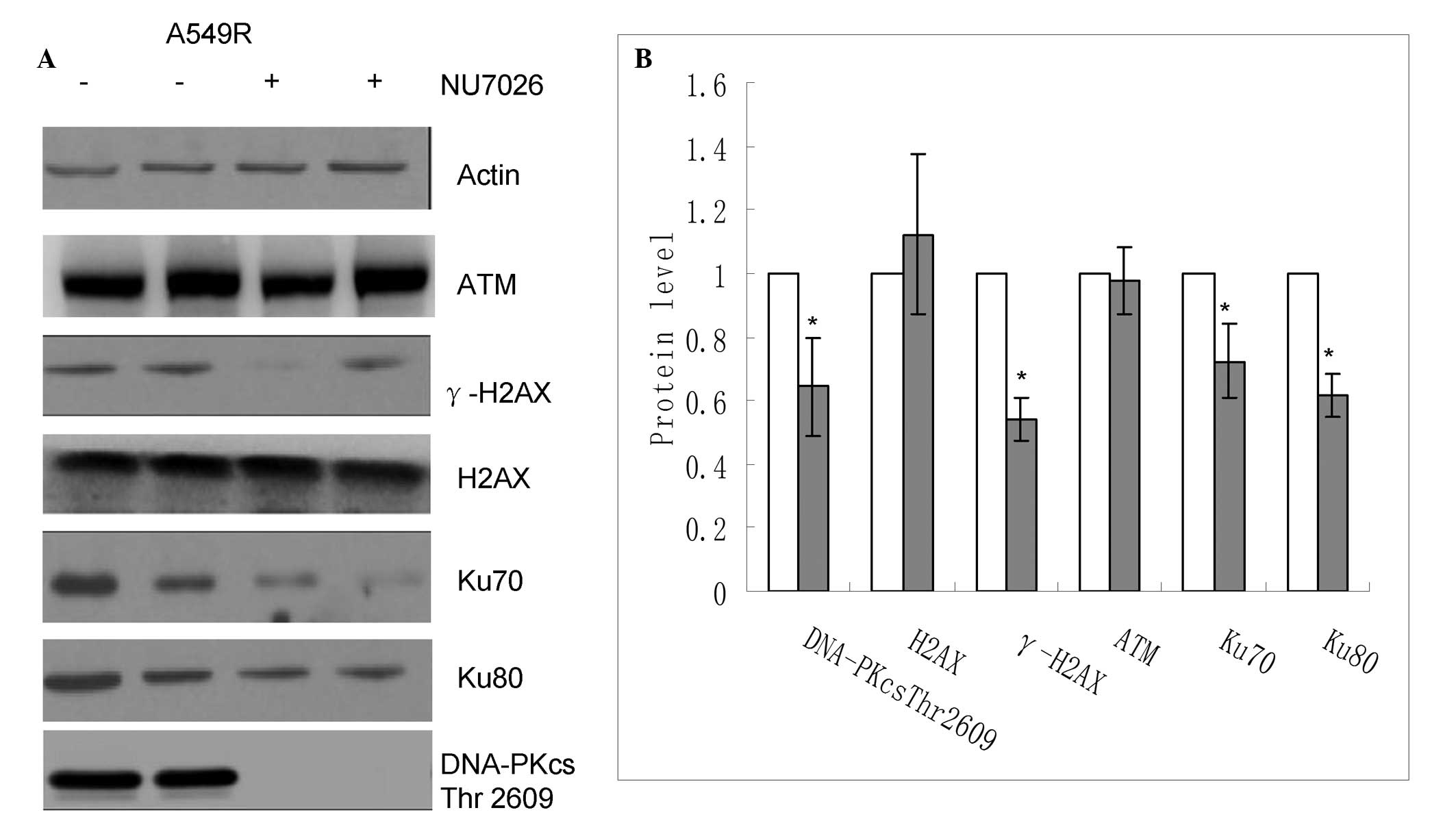
DNA-PKcs inhibitor treatment interferes
with the expression levels of NHEJ pathway-associated proteins in
radioresistant cells
The protein expression levels of various molecules
involved in DNA repair were compared between the radioresistant
cells, treated with or without the DNA-PKcs inhibitor, following
exposure to ionizing radiation. The protein expression levels of
DNA-PKcs Thr2609, γ-H2AX, Ku70 and Ku80 were suppressed in the
cells treated with the DNA-PKcs inhibitor, compared with the
untreated radioresistant cells, following irradiation (Fig. 4). Conversely, the expression levels
of ATM and H2AX demonstrated no significant change. These results
indicated that the restoration of radiosensitivity in the cells,
which were treated with the DNA-PKcs inhibitor, may have been
associated with a reduction in the capacity for DNA repair.
Discussion
The results of the present study provided evidence
for the radiosensitizing role of NU7026 in lung cancer cells
exhibiting acquired radioresistance. As expected, treatment with
the DNA-PKcs inhibitor directly attenuated the ability of the cells
to repair DNA, by inactivating radiation-induced NHEJ pathway
proteins, and further increased the levels of radiation-induced
apoptosis. This inhibitory effect was associated with initially
decreased levels of the γ-H2AX DNA repair factor and impaired the
recruitment of other repair proteins to the DNA DSB site. This
resulted in the restoration of radiosensitivity in the resistant
cells treated with irradiation, and a reduction in cell survival.
These results suggested the potential therapeutic application of
NU7026 in the treatment of radioresistant cancer cells.
Theoretically, the ability of a cell to repair
radiation-induced DNA damage determines the radiosensitivity of the
cell (25). The more proteins,
which are associated with DNA repair, and accumulate at the break
site, the less radiation-induced damage that occurs (11). Therefore, due to its ability to
recruit other cell repair proteins, γ-H2AX formation is regularly
used to assess the level of cellular DNA damage (26). γ-H2AX can be detected by either
flow cytometry or immunofluorescence, which quantify the percentage
of γ-H2AX formation. Increased levels of γ-H2AX lead to enhanced
DNA repair ability (27). The
present study demonstrated that treatment with NU7026 had an effect
on the levels of radiation-induced γ-H2AX formation. Decreased
levels of γ-H2AX resulted in a reduction in DSB signals, which led
to increased cell apoptosis and decreased cell viability following
irradiation.
DNA-PKcs is a key component of the NHEJ pathway, and
is important in DNA DSB repair, genomic integrity and maintenance
of telomere stability (28,29).
DNA-PKcs is upregulated in various types of cancer (30,31).
It has also been reported that increased expression of DNA-PKcs and
kinase activity are closely associated with radioresistance or
chemoresistance (32). Inhibitors
of DNA-PKcs, including NU7441, have been developed to enhance local
tumor control (33). The present
study demonstrated that adjuvant treatment with NU7026 overcame
radioresistance in lung cancer cells, by reducing the γ-H2AX
signal. The NU7026-mediated radiosensitization in lung cancer cells
was predominantly associated with delayed radiation-induced DSB
repair. Arevious study reported that a DNA-PKcs inhibitor was able
to enhance the glioma cell apoptosis percentage with irradiation
treatment (8).
The present study demonstrated markedly altered
expression levels of DNA repair pathway proteins in the
NU7026-treated cells following irradiation. As a key factor in DNA
repair, the phosphorylation of DNA-PKcs at Thr2609 inhibited the
recruitment of repair molecules localized in the DNA DSB region,
attenuating the cell repair defense system. In addition, two key
subunits of DNA-PKcs, Ku70 and Ku80, exhibited reduced protein
expression levels in cells treated with NU7026, in addition to the
inhibitory effect of NU7026 on the DNA-PKcs. These results
suggested that the effect of NU7026 on DNA-PKcs functions through
suppression of its inactivation (34).
In conclusion, treatment with a DNA-PKcs inhibitor
successfully restored radiosensitivity in lung cancer cells with
acquired radioresistance. According to the results of the present
study, the possible underlying mechanism may have been the
inactivation of NHEJ components by the DNA-PKcs inhibitor, which
may have subsequently impaired DNA repair ability, enhanced
apoptosis and decreased cell viability. These findings approved the
step-wise method as a useful technique to generate a resistant cell
model, and revealed the potential application of a DNA-PKcs
inhibitor on acquired radioresistance in lung cancer.
Acknowledgments
The present study was supported by Guizhou Province
Programs for Science and Technology Development (grant no.
gzwkj2011-1-054).
References
|
1
|
Reck M, Heigener DF, Mok T, Soria JC and
Rabe KF: Management of non-small-cell lung cancer: Recent
developments. Lancet. 382:709–719. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang HJ, Kim N, Seong KM, Youn H and Youn
B: Investigation of radiation-induced transcriptome profile of
radioresistant non-small cell lung cancer A549 cells using RNA-seq.
PloS One. 8:e593192013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gupta S, Koru-Sengul T, Arnold SM, Devi
GR, Mohiuddin M and Ahmed MM: Low-dose fractionated radiation
potentiates the effects of cisplatin independent of the
hyper-radiation sensitivity in human lung cancer cells. Mol Cancer
Ther. 10:292–302. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li J, Yang CX, Mei ZJ, et al: Involvement
of cdc25c in cell cycle alteration of a radioresistant lung cancer
cell line established with fractionated ionizing radiation. Asian
Pac J Cancer Prev. 14:5725–5730. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim YS, Kang MJ and Cho YM: Low production
of reactive oxygen species and high DNA repair: Mechanism of
radio-resistance of prostate cancer stem cells. Anticancer Res.
33:4469–4474. 2013.PubMed/NCBI
|
|
6
|
Vignard J, Mirey G and Salles B:
Ionizing-radiation induced DNA double-strand breaks: a direct and
indirect lighting up. Radiother Oncol. 108:362–369. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Beskow C, Skikuniene J, Holgersson A, et
al: Radioresistant cervical cancer shows upregulation of the NHEJ
proteins DNA-PKcs, Ku70 and Ku86. Br J Cancer. 101:816–821. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Daido S, Yamamoto A, Fujiwara K, Sawaya R,
Kondo S and Kondo Y: Inhibition of the DNA-dependent protein kinase
catalytic subunit radiosensitizes malignant glioma cells by
inducing autophagy. Cancer Res. 65:4368–4375. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Negroni A, Stronati L, Grollino MG,
Barattini P, Gumiero D and Danesi DT: Radioresistance in a tumour
cell line correlates with radiation inducible Ku 70/80 end-binding
activity. Int J Radiat Biol. 84:265–276. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vandersickel V, Mancini M, Slabbert J, et
al: The radiosensitizing effect of Ku70/80 knockdown in MCF10A
cells irradiated with X-rays and p(66)+Be(40) neutrons. Radiat
Oncol. 5:302010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang C and Lees-Miller SP: Detection and
repair of ionizing radiation-induced DNA double strand breaks: New
developments in nonhomologous end joining. Int J Radiat Oncol Biol
Phys. 86:440–449. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Khanna KK, Lavin MF, Jackson SP and
Mulhern TD: ATM a central controller of cellular responses to DNA
damage. Cell Death Differ. 8:1052–1065. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bakkenist CJ and Kastan MB: DNA damage
activates ATM through intermolecular autophosphorylation and dimer
dissociation. Nature. 421:499–506. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tichy A, Durisova K, Salovska B, et al:
Radio-sensitization of human leukaemic MOLT-4 cells by
DNA-dependent protein kinase inhibitor, NU7441. Radiat Environ
Biophys. 53:83–92. 2014. View Article : Google Scholar
|
|
15
|
Keogh MC, Kim JA, Downey M, et al: A
phosphatase complex that dephosphorylates gammaH2AX regulates DNA
damage checkpoint recovery. Nature. 439:497–501. 2006. View Article : Google Scholar
|
|
16
|
Leu JD, Chiu YW, Lo CC, et al: Enhanced
cellular radio-sensitivity induced by cofilin-1 over-expression is
associated with reduced DNA repair capacity. Int J Radiat Biol.
89:433–444. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
An J, Huang YC, Xu QZ, et al: DNA-PKcs
plays a dominant role in the regulation of H2AX phosphorylation in
response to DNA damage and cell cycle progression. BMC Mol Biol.
11:182010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Du L, Zhou LJ, Pan XJ, et al:
Radiosensitization and growth inhibition of cancer cells mediated
by an scFv antibody gene against DNA-PKcs in vitro and in vivo.
Radiat Oncol. 5:702010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xiong H, Lee RJ, Haura EB, Edwards JG,
Dynan WS and Li S: Intranuclear delivery of a novel
antibody-derived radiosensitizer targeting the DNA-dependent
protein kinase catalytic subunit. Int J Radiat Oncol Biol Phys.
83:1023–1030. 2012. View Article : Google Scholar
|
|
20
|
Zhou X, Zhang X, Xie Y, Tanaka K, Wang B
and Zhang H: DNA-PKcs inhibition sensitizes cancer cells to
carbon-ion irradiation via telomere capping disruption. PLoS One.
8:e726412013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Veuger SJ, Curtin NJ, Richardson CJ, Smith
GC and Durkacz BW: Radiosensitization and DNA repair inhibition by
the combined use of novel inhibitors of DNA -dependent protein
kinase and poly(ADP-ribose) polymerase-1. Cancer Res. 63:6008–6015.
2003.PubMed/NCBI
|
|
22
|
Veuger SJ, Curtin NJ, Smith GC and Durkacz
BW: Effects of novel inhibitors of poly(ADP-ribose) polymerase-1
and the DNA-dependent protein kinase on enzyme activities and DNA
repair. Oncogene. 23:7322–7329. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Uphoff CC and Drexler HG: Detecting
mycoplasma contamination in cell cultures by polymerase chain
reaction. Methods Mol Biol. 731:93–103. 2011.PubMed/NCBI
|
|
24
|
Bristow RG, Jang A, Peacock J, Chung S,
Benchimol S and Hill RP: Mutant p53 increases radioresistance in
rat embryo fibroblasts simultaneously transfected with HPV16-E7
and/or activated H-ras. Oncogene. 9:1527–1536. 1994.PubMed/NCBI
|
|
25
|
Maroschik B, Gürtler A, Krämer A, et al:
Radiation-induced alterations of histone post-translational
modification levels in lymphoblastoid cell lines. Radiat Oncol.
9:152014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Djuzenova CS, Elsner I, Katzer A, et al:
Radiosensitivity in breast cancer assessed by the histone γ-H2AX
and 53BP1 foci. Radiat Oncol. 8:982013. View Article : Google Scholar
|
|
27
|
Fernández MI, Gong Y, Ye Y, et al: γ-H2AX
level in peripheral blood lymphocytes as a risk predictor for
bladder cancer. Carcinogenesis. 34:2543–2547. 2013. View Article : Google Scholar
|
|
28
|
Lee SH and Kim CH: DNA-dependent protein
kinase complex: A multifunctional protein in DNA repair and damage
checkpoint. Mol Cells. 13:159–166. 2002.PubMed/NCBI
|
|
29
|
Hefferin ML and Tomkinson AE: Mechanism of
DNA double-strand break repair by non-homologous end joining. DNA
Repair (Amst). 4:639–648. 2005. View Article : Google Scholar
|
|
30
|
Gil del Alcazar CR, Hardebeck MC, et al:
Inhibition of DNA double-strand break repair by the dual PI3K/mTOR
inhibitor NVP-BEZ235 as a strategy for radiosensitization of
glioblastoma. Clin Cancer Res. 20:1235–1248. 2014. View Article : Google Scholar
|
|
31
|
Tonotsuka N, Hosoi Y, Miyazaki S, et al:
Heterogeneous expression of DNA-dependent protein kinase in
esophageal cancer and normal epithelium. Int J Mol Med. 18:441–447.
2006.PubMed/NCBI
|
|
32
|
Ryu JS, Um JH, Kang CD, et al:
Fractionated irradiation leads to restoration of drug sensitivity
in MDR cells that correlates with down-regulation of P-gp and
DNA-dependent protein kinase activity. Radiat Res. 162:527–535.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ciszewski WM, Tavecchio M, Dastych J and
Curtin NJ: DNA-PK inhibition by NU7441 sensitizes breast cancer
cells to ionizing radiation and doxorubicin. Breast Cancer Res
Treat. 143:47–55. 2014. View Article : Google Scholar
|
|
34
|
Qu YY, Hu SL, Xu XY, et al: Nimotuzumab
enhances the radiosensitivity of cancer cells in vitro by
inhibiting radiation-induce d DNA damage repair. Plos one.
8:e707272013. View Article : Google Scholar
|