Introduction
A migraines is a multifactorial primary headache
disorder, which is characterized by severe unilateral throbbing
pain (1). Although its
pathogenesis remains to be fully elucidated, progress has been
made. Vasogenic, neurogenic and trigeminovascular theories have
been suggested (2), changing the
understanding of migraines from a vascular disease to a widely
accepted neurovascular disease. Several animal models have been
used in investigations of pathogens and the development of novel
drugs, including a contracting model of the carotid arteriovenous
anastomosis and the external carotid artery, and models of cortical
spreading depression, neurogenic dural inflammation, nitroglycerin
and the depletion of 5-hydroxytryptamine (5-HT) (3,4),
which have revealed different pathophysiological characteristics of
migraines. Previous studies have found that the signs and symptoms
of the nitroglycerin and 5-HT depletion animal models are similar
to traditional Chinese medicine (TCM) syndromes, suggesting that
these animal models are applicable for TCM investigations. The
signs and symptoms of the model of nitroglycerin are similar to
those of the hyperactivity of liver-yang and the upward disturbance
of wind-fire in migraines, whereas the signs and symptoms observed
in the model of 5-HT depletion are similar to those observed in
colds, blood stasis and yang deficiency in migraines (5). Therefore, according to the Wuzhuyu
decoction characteristics of warming middle-jiao and tonifying
deficiency, models of 5-HT depletion were selected for
pharmacodynamic investigations in the present study.
At present, triptans, corticosteroids, non-steroidal
anti-inflammatory drugs and anti-epileptic drugs are predominantly
used for the treatment of clinical migraines, however, the
application of these drugs can cause several side effects (6). Therefore, investigations into the use
of TCM treatments for migraines has received increasing attention.
Evodia, ginseng, ginger and jujube are four commonly used TCMs,
contained in the Chinese Pharmacopoeia (7). Evodia contains a variety of
ingredients, including alkaloids, limonoids and flavonoids
(8). Previous pharmacological
studies have demonstrated that evodiamine (Ev) and rutaecarpine
(Ru) promote the secretion of catecholamines (9) and Ev inhibits the activity of
monoamine oxidase (10); transient
receptor potential vanilloid type 1 (TRPV1) promotes the
phosphorylation of endothelial nitric oxide synthase (NOS) and
induces the generation of nitric oxide (NO) (11); and limonin (Li) has a significant
neuroprotective effect (12) and
inhibits calcium influx and excessive NO production (13). The predominant chemical ingredients
of ginseng are a variety of ginsenosides (14), including Rg1, which can protect
dopaminergic (DA) neurons (15),
improve NO levels in myocardial tissues and promote myocardial
angiogenesis (16) and Rb1, which
is involved in increasing the levels of monoamine neurotransmitter
(17) and anti-oxidative stress
(18). The main chemical
ingredients of ginger are gingerols (19), including 6 gingerol (6 Gi), which
is involved in inhibiting gastric acid secretion (20) angiotensin converting enzyme
activity (21) and the
lipopolysaccharide (LPS) induced production of NO (22). These four herbs constitute a
classical Chinese herbal formula, termed Wuzhuyu decoction, and
clinical and experimental investigations have confirmed its
efficacy in the treatment of migraines (23).
Although the chemical ingredients of Wuzhuyu
decoction and its pharmacological effects for the treatment of
migraine have been investigated (24), the ingredients of Chinese herbal
formulae are complicated, and their chemical composition does not
produce traditional pharmacological effects, therefore, the active
ingredients in treating migraines remain to be elucidated thus
affecting efficacy and quality control. To resolve these issues,
previous studies have used the method of serum pharmacochemistry to
analyze the chemical composition following intragastric
administration of Wuzhuyu decoction (25). This method revealed that the
ingredients of Wuzhuyu decoction, including isorhamnetin-3-O-β-D
glucosyl(6′-1′)-α-L rhamnoside (Irs), dehydroevodiamine (Deh), Ev,
Ru and ginsenoside Rd (Rd), Rg1, Re, Rb1, are absorbed into the
bloodstream. However, whether these are the active ingredients
remain to be fully elucidated. In the present study, 10 specific
chromatograms of Wuzhuyu decoction and models of reserpine induced
migraine were established. The 10 ingredients of the Wuzhuyu
decoction were analyzed quantitatively and semi-quantitatively, and
the levels of monoamine neurotransmitter, NO and the activity of
NOS were determined. Finally, correlation analyses between the
ingredients of Wuzhuyu decoction and various pharmacodynamic
indices were performed using a partial least squares regression
(PLSR) method. The effective ingredients of the Wuzhuyu decoction
for treating deficiency-cold type migraines were detected to
control the quality of Wuzhuyu decoction. Spectral efficiency
association was used to determine the effective ingredients of the
Wuzhuyu decoction in treating migraines, and those significantly
involved in improving the pharmacodynamics indices were determined.
PLSR, which is most suitable for Chinese herbal formulae, was
applied to determine the spectral efficiency of the Chinese herbal
formula. The results demonstrated for the first time, to the best
of our knowledge, that Rv exhibited a regulatory effect on
monoamine neurotransmitters.
Materials and methods
Experimental design and location
Spectral efficiency association analysis was
performed on the components of the Wuzhuyu decoction to determine
the efficacy of the herbal formula.
The investigations, including Wuzhuyu decoction high
performance liquid chromatography (HPLC) analysis, preparation of
the migraine model and determination of the NOS activity and NO
content, were performed at the School of Traditional Chinese
Medicine, Capital Medical University (Beijing, China). The
determination of neurotransmitters was performed in the Central
Laboratory, Xuanwu Hospital of Capital Medical University (Beijing,
China). The investigations were performed between February and May
2013.
Drugs and reagents
Chemical reference substances of Rg1, Re, Rb1, Li,
6-Gi, Ev and Ru were purchased from the National Institutes for
Food and Drug Control (Beijing, China) and had purities of
>88.5%. These were used as reference substances for content
determination. Limocitrin-3-O-β-D-glucoside (Lcs) was produced in
Beijing Key Lab of TCM Collateral Disease Theory Research (Beijing,
China), with a purity of >95%. An NO assay kit, NOS assay kit
and coomassie blue were purchased from Nanjing Jiancheng
Bioengineering Institute (Nanjing, China). Reserpine was provided
by Guangdong Bangmin Pharmaceutical Co, Ltd. (Jiangmen, China; cat.
no. 100509). Sumatriptan succinate tablets were provided by Tianjin
Huajin Pharmaceutical Co, Ltd. (Tianjin, China; cat. no. 2F69151)
and HPLC grade methanol and acetonitrile were provided by Thermo
Fisher Scientific (Waltham, MA, USA).
Instruments
Determination of the content of Wuzhuyu decoction
was performed using an Agilent 1100 series HPLC system (Agilent
technologies, Santa Clara, CA, USA), which was equipped with a
quaternary low-pressure infusion pump, inline degasser,
autosampler, column oven and diode array detector (DAD). The
determination of the monoamine neurotransmitter content was
completed using an ESA HPLC system (IKA, Staufen, Germany), which
was equipped with a binary low pressure infusion pump, inline
degasser, autosampler, column oven and diode array detector. A
Silent Crusher M homogenizer (Heidolph, Germany), 3K15 low
temperature refrigerated centrifuge (Sigma-Aldrich, St. Louis, MO,
USA) and DV215CD analytical balance (Sartorius, Shanghai, China)
were also used.
Experimental animals
ICR mice (SPF grade; n=168), at 7–8 weeks old,
weighing 28–32 g, were purchased from Beijing Vital River
Laboratory Animal Technology Co., Ltd. (Beijing, China; License no.
SCXK (Beijing) 2012–0001). The mice were bred in a standard barrier
environment, and the breeding rooms maintained at a constant
temperature (25±2°C) and constant humidity (40–60%). The mice were
provided with nutritional pellet feeds and unlimited access to
water. The lighting was maintained on a 12 h light/dark cycle. All
animal procedures were approved by Beijing Capital Medical
University Animal Ethics Committee (Beijing, China) and were
performed according to the Guidance Suggestions for the Care and
Use of Laboratory Animals promulgated by the Ministry of Science
and Technology of the People’s Republic of China. All animal
procedures were approved by the Animal Ethics Committee of Beijing
Capital Medical University (Beijing, China).
Medicinal materials
The sources of the medicinal materials used are
shown in Table I. The medicinal
materials were appraised by Professor Luo Rong of the School of
Traditional Chinese Medicine, Capital Medical University, and were
used in accordance with the requirements for each medicine in
Chinese Pharmacopeia 2010 Edition (7). The compositions and crude drug
concentrations of the 10 Wuzhuyu decoctions are shown in Table II.
 | Table INumbers and sources of four medicinal
constituents of Wuzhuyu decoction. |
Table I
Numbers and sources of four medicinal
constituents of Wuzhuyu decoction.
| Evodia (no.) | Source | Ginger (no.) | Source | Jujube (no.) | Source | Ginseng (no.) | Source |
|---|
| WZY 1 | Zhejiang | SJ 1 | Hebei | DZ 1 | Sinkiang | RS 1 | Wanliang, Jilin |
| WZY 2 | Zhejiang | SJ 2 | Shandong | DZ 2 | Unknown | RS 2 | Wanliang, Jilin |
| WZY 3 | Jiangxi
(Zhonghua) | SJ 3 | Shandong | DZ 3 | Sinkiang | RS 3 | Fusong, Jilin |
| WZY 4 | Jiangxi
(Chunzhonghua) | SJ 4 | Shandong | DZ 4 | Beijing | RS 4 | Fusong, Jilin |
| WZY 5 | Jiangxi | SJ 5 | Shandong | DZ 5 | Hebei | RS 5 | Fusong, Jilin |
| WZY 6 | Hunan | | | DZ 6 | Shanxi | RS 6 | Jilin |
| WZY 7 | Guangxi | | | | | RS 7 | Jilin |
| RS 8 | Jilin |
| RS 9 | Jilin |
| RS 10 | Weikang |
 | Table IIDetermination results of the
ingredients, mass concentrations and contents of 10 Wuzhuyu
decoctions (mg 0.2 ml−1). |
Table II
Determination results of the
ingredients, mass concentrations and contents of 10 Wuzhuyu
decoctions (mg 0.2 ml−1).
| Decoction | Constituent
| Mass concentration
(g/ml) | Ingredient content
| Rv (Peak area) | X(Peak area) |
|---|
| Evodia | Ginseng | Ginger | Jujube | Lcs | Rg1 | Re | Rb1 | Li | 6-Gi | Ev | Ru |
|---|
| 1 | WZY 7 | RS 1 | SJ 1 | DZ 1 | 2.205 | 0.085 | 0.247 | 0.141 | 0.328 | 0.069 | 0.010 | 0.021 | 0.014 | 518.4 | 640.6 |
| 2 | WZY 6 | RS 4 | SJ 3 | DZ 6 | 1.470 | 0.014 | 0.071 | 0.039 | 0.107 | 0.223 | 0.018 | 0.032 | 0.005 | 1,595.3 | 792.3 |
| 3 | WZY 7+WZY 1 | RS 5 | SJ 1 | DZ 3 | 0.735 | 0.013 | 0.044 | 0.018 | 0.024 | 0.106 | 0.004 | 0.005 | 0.002 | 81.8 | 67.2 |
| 4 | WZY 5 | RS 10 | SJ 4 | DZ 5 | 0.735 | 0.009 | 0.051 | 0.010 | 0.048 | 0.158 | 0.007 | 0.006 | 0.001 | 799.6 | 290.2 |
| 5 | WZY 3 | RS 3 | SJ 3 | DZ 6 | 2.205 | 0.057 | 0.150 | 0.060 | 0.172 | 0.505 | 0.031 | 0.063 | 0.013 | 4,798.0 | 2,370.1 |
| 6 | WZY 1 | RS 8 | SJ 5 | DZ 3 | 1.470 | 0.102 | 0.143 | 0.066 | 0.156 | 0.067 | 0.017 | 0.029 | 0.013 | 414.0 | 525.8 |
| 7 | WZY 6 | RS 2 | SJ 4 | DZ 5 | 0.735 | 0.009 | 0.042 | 0.026 | 0.030 | 0.091 | 0.009 | 0.017 | 0.002 | 601.8 | 327.6 |
| 8 | WZY 5 | RS 7 | SJ 5 | DZ 4 | 2.205 | 0.071 | 0.171 | 0.110 | 0.327 | 1.020 | 0.060 | 0.036 | 0.008 | 498.1.9 | 2,075.5 |
| 9 | WZY 4 | RS 9 | SJ 2 | DZ 4 | 1.470 | 0.022 | 0.069 | 0.049 | 0.138 | 0.332 | 0.010 | 0.012 | 0.003 | 1,561.0 | 442.0 |
| 10 | WZY 2 | RS 6 | SJ 2 | DZ 1 | 1.470 | 0.024 | 0.070 | 0.053 | 0.094 | 0.135 | 0.014 | 0.031 | 0.010 | 1,257.9 | 653.3 |
Preparation of Wuzhuyu decoction
Dry pastes of Wuzhuyu decoction were produced in
Beijing Key Lab of TCM Collateral Disease Theory Research (Beijing,
China), according to a previously reported technique (25). The crude drugs (280 g) were weighed
and two extractions were performed. For the initial extraction,
water (2,000 ml) was added to the drugs, which were soaked at room
temperature for 30 min, and the extraction duration was 15 min. For
the second extraction, 1,600 ml water was added and the extraction
duration was 12 min. The extracted solutions were combined
following filtration using eight layers of gauze (Piaoan, Xinxiang,
China), and the solution was decompressed and concentrated into dry
pastes at 65°C using a rotary evaporator (Eyela N 1100V W, Tokyo,
Japan). Vaccume drying was perfomed in a VO400 oven (Memmert,
Schwabach, Bavaria, Germany) The composition and concentration of
each Wuzhuyu decoction are shown in Table II.
Preparation of the standard and test
solutions
To prepare the standard solutions, 15.2 mg Lcs, 4.4
mg Ru and 9.5 mg Ev were dissolved in 50 ml methanol as stock
solution 1. Subsequently, 16.8 mg Rg1, 5.4 mg Re, 11.3 mg Rb1 and 9
mg 6-Gi were dissolved in 5 ml stock solution 1, and 5 ml methanol
was added to the volumetric flask of 10 ml to make the reference
solution A. Methanol was added to 3.33 ml reference solution A to a
volume of 5 ml, to produce the reference solution B. The serial
dilution of the reference solution is identical.
To prepare the test solution, Wuzhuyu decoction dry
paste (~3.5 g crude drug) was used and 20 ml analytical grade
methanol was added prior to being treated with ultrasound (KQ500E;
Kunshan, Suzhou, China) for 30 min and filtered. The filtrate was
evaporated to dryness and the residue was dissolved in HPLC-grade
methanol, (5 ml). The solution was filtered using a 0.45 μm
filter membrane (Jinteng, Tianjin, China) to determine the contents
of the 10 types of Wuzhuyu decoction. All solutions were stored at
4°C and they were equilibrated to room temperature prior to
measurements.
Chromatographic conditions
A Kromasil 100-5 C18 chromatographic column (250×4.6
mm; 5 μm; Kromasil, Shanghai, China) was used for analysis,
with a detection temperature of 30°C. The mobile phase consisted of
methanol-water-acetonitrile, and the gradient programme is shown in
Table III. The elution rate was
maintained at 1 ml/min, the DAD detecting wavelength was set to 203
nm and the chromatograms were recorded. The injection volumes of
the reference and test samples were 10 μl.
 | Table IIIGradient program of high performance
liquid chromatography analysis. |
Table III
Gradient program of high performance
liquid chromatography analysis.
| Time (min) | Methyl alcohol
(%) | Acetonitrile
(%) | Water (%) |
|---|
| 0 | 1 | 16 | 83 |
| 5 | 1 | 16 | 83 |
| 10 | 1 | 19 | 80 |
| 45 | 4 | 19 | 77 |
| 55 | 4 | 25 | 71 |
| 65 | 4 | 34 | 62 |
| 110 | 4 | 34 | 62 |
Preparation of animal migraine models and
drug administration
The mice were randomly divided into 14 groups (12
mice in each group) and experiments were initiated 4 days after
acclimatization. From the beginning of the experiment, the delivery
mode of the preventative and treatment drugs for mice in each
Wuzhuyu decoction group was 0.2 ml/10 g body weight, perfused
orally with the appropriate quantity of Wuzhuyu decoction. Each
group was administered one of 10 different Wuzhuyu decoctions with
different contents of ingredients (Table II). The mice in the model group,
sham group and normal control group were perfused orally with an
equal volume of distilled water. The mice in the positive drug
group were only treated with drugs and they were perfused orally
with an equal dose of sumatriptan from the sixth day. The
experiment was performed for a total of 20 days. Mouse models were
generated between the 6th and 13th day, as described previously
(26). The mice in each Wuzhuyu
decoction group were injected subcutaneously once with reserpine at
0.17 mg/kg body weight. The mice were anesthetized using 3% chloral
hydrate (Tianjin Institute of Fine Chemicals retrocessio, Tianjin,
China) at 450 mg/kg body weight on the 14th day, their scalps were
cut and a hole was drilled 2 mm under the anterior fontanelle, 2 mm
to the right side of the sagittal suture. The autologous blood was
collected by cutting the mouse tail, and 2 μl autologous
blood was injected into the cerebral cortex through the hole at a
depth of 1 mm. Following surgery, bone wax (Johnson & Johnson,
New Brunswick, NJ, USA) was used to stop bleeding, penicillin (100
U/mouse; once per mouse following surgery; Nesco Medical Co.,
Qingdao, China) was used to treat infections, and the wound was
sutured. Between the 15th day and 20th day, oral drug
administrations were performed, as described above.
Sampling
The mice were anesthetized using 3% chloral hydrate
on the 21st day and 0.5–1 ml mixed blood was extracted
by inserting a syringe needle into the apex of the heart, which was
transferred into a 1.5 ml centrifuge tube and stored at 4°C for 2
h. The blood was centrifuged at 2,296 x g for 10 min at 4°C, and
the serum was collected and stored at −20°C for the NO content and
NOS activity assays. The mice were sacrificed by cervical
dislocation, and their brains were removed. The telencephalons were
removed by cutting along the needle marks and were stored at −80°C
for the NO content and NOS activity assays. The remaining brain
tissues were stored separately for the determination of
neurotransmitters.
Content determination of monoamine
neurotransmitters
The chromatographic conditions used were as follows:
ODB-C18 column (4.6×150 mm; 5 μm; Agilent Technologies) and
methanol-buffered saline solution, containing sodium acetate (70
mmol/l), citric acid (50 mmol/l), EDTA (100 μmol/l) and
octane sulfonate (200 μmol/l, pH 4.1), which was used for
gradient elution. The flow rate was 1 ml/min, the column
temperature was 30°C and the injection volume was 20 μl.
Perchloric acid homogenate (0.4%) was prepared, as
described previously (27),
containing Na2EDTA (0.5 mmol/l), L cysteine (0.83
mmol/l) and 2,5 dihydroxybenzoic acid (internal standard substance;
0.36 μmol/l). The brain tissues were removed from the −80°C
freezer and 0.4% perchloric acid homogenate was added at 1:4 at
4°C. Tissue homogenization was performed in an ice bath. Brain
homogenate was placed in a refrigerated centrifuge at 4°C and
centrifuged twice at 18,001 x g for 10 min each time. The
supernatant (20 μl) was obtained for the determination of
the monoamine neurotransmitter content.
NO content and NOS activity assay
To determine the NO content and NOS activity in the
serum, the sera were thawed at room temperature, and 30 μl
and 100 μl were used for determination of NO and NOS,
respectively. Each reaction reagent kit (Jiancheng Company,
Nanjing, China) was added, according to the manufacturer’s
instructions, and the reaction was allowed to continue at 37°C for
a certain time (60 min for NO, 15 min for NOS and 10 min for
protein meaurements). The absorbance was determined using an
ultraviolet spectrophotometer (UV2450; Shimadzu, Kyoto, Japan) at a
wavelength of 203 nm and the absorbance values were recorded to
calculate the NO content and NOS activity, according to the
manufacturer’s instructions.
To determine the NO content and NOS activity of the
brain tissues, the brain tissues were thawed at room temperature
and physiological saline (Sigma-Aldrich, Shanghai, China) at 4°C
was added at a 1:9 ratio. Homogenization was performed in an ice
bath and the brain homogenate was placed in a refrigerated
centrifuge at 4°C, and centrifuged at 574 x g for 10 min.
Supernatant (50 and 500 μl) were collected for the
determination of NOS activity and NO content. To determine the
protein quantities, mouse brain homogenate (10 μl) was
dissolved in physiological saline to a volume of 1 ml for analysis.
Commassie blue (10 ml; Jiancheng Company) was diluted with
deionized water to a volume of 50 ml. A protein solution of 0.563
g/l was used as standard solution and physiological saline was used
as a blank. Diluted commassie blue (3 ml) was added to 50μl
diluted brain samples, standard substance and physiological saline.
Following incubation for 10 min at room temperature, the absorbance
values were recorded at 595 nm using a UV-spectrophotometer. The
protein content in sample was calculated by the equation: protein
(g/l) =
(Abstandard−Abblank)/(Absample−Abblank)
x 0.563 g/l. These results were recorded to calculate the NO
content and NOS activity using the formula, according to the
manufacturer’s instructions.
Statistical analyses
The experimental data were analyzed using SPSS 19.0
statistical software (SPSS, Inc., Chicago, IL, USA). Measurement
data is expressed as the mean ± standard deviation. The data
conformed to a normal distribution and one-way analysis of variance
was used to compare between the groups. P<0.05 was considered to
indicate a statistically significant difference.
Analytical methods for determining
spectral efficiency
Using the PLSR method in SIMCA-P+12.0 Demo, the data
on the components and pharmaceutical effects of the Wuzhuyu
decoctions were analyzed for spectral efficiency. The principal
analysis of the components was performed automatically by the
software and, by adding the factor numbers manually, the
explanatory abilities of the predominant components for independent
variables and dependent variables were >90% and the regression
coefficients were recorded. The predominant components of Wuzhuyu
decoction affecting the pharmacodynamics indices were identified
according to the sizes of the regression coefficients.
Results
Quantitative analysis of the experimental
animal model
The ICR mice were randomly divided into 14 groups
(12 mice in each group), including a normal control group, sham
group, model group, nos. 1–10 Wuzhuyu decoction groups and a
positive drug group. Blood was collected from the heart, the sera
was obtained and the NO content and NOS activity were determined.
In addition, the neurotransmitters, NO content and NOS activity in
brain tissue homogenates were determined. Analysis of the final
results included 168 mice (12 mice in each group). Characteristics
indicative of a successful mouse models were symptoms of
continuously decreasing weight and body temperature (≥3 days),
lassitude, being curled up, inactive and exhibiting limb tremor,
and the levels of 5-HT in the brain tissue were significantly
decreased. In the present study, the modeling success rate was
90.8%
Investigation methodology
The HPLC analysis conditions, linear associations
and sensitivity investigations revealed that the regression
equation coefficients of all the components measured were
>0.9995. The lowest limit of detection and the lowest limit of
quantification were <9.62 μg/ml andv 32.06 μg/ml,
respectively (Table IV). The same
mixed reference solution was injected six times continuously and
all relative standard deviation (RSD) values of the contents of the
measured components were <3%. Sample preparation was performed
repeatedly six times for each Wuzhuyu decoction, the samples were
injected and the RSD values of the component contents were <5%.
The same sample of Wuzhuyu decoction was injected five times in 12
h (0, 3, 6, 9 and 12 h), and all the RSD values of the component
contents were <5%, with recovery rates between 97.03 and 106.65%
(Table V). These findings
demonstrated that the HPLC method was stable and feasible.
 | Table IVResults of the analyses of linearity
of calibration curves and sensitivities for eight ingredients in
Wuzhuyu decoction. |
Table IV
Results of the analyses of linearity
of calibration curves and sensitivities for eight ingredients in
Wuzhuyu decoction.
| Ingredient | Regression
equation | Regression
coefficient (r) | Linear range
(mg/ml) | LOD
(μg/ml) | LOQ
(μg/ml) |
|---|
| Lcs | y=33742 ×
95.59 | 0.9997 | 0.0190–0.152 | 2.88 | 9.61 |
| Rg1 | y=2737 ×
+32.69 | 0.9996 | 0.2106–1.682 | 9.62 | 32.06 |
| Re | y=2999 × 10.01 | 0.9997 | 0.0679–0.542 | 7.89 | 26.31 |
| Rb1 | y=2460. ×
+12.81 | 0.9995 | 0.1420–1.134 | 8.44 | 28.12 |
| Li | y=6970 × 32.03 | 0.9996 | 0.0578–1.846 | 2.38 | 7.95 |
| 6-Gi | y=66623 ×
153.0 | 0.9996 | 0.0141–0.2582 | 0.77 | 2.55 |
| Ev | y=41984 ×
18.26 | 0.9996 | 0.0115–0.092 | 0.66 | 2.19 |
| Ru | y=75447 ×
65.67 | 0.9997 | 0.0055–0.044 | 1.22 | 4.08 |
 | Table VRepeatabilities, stabilities and
recoveries of eight ingredients in Wuzhuyu decoction. |
Table V
Repeatabilities, stabilities and
recoveries of eight ingredients in Wuzhuyu decoction.
| Ingredient | Degree of precision
(n=6)
| Repeatability (n=6)
| Stability (0 12 h)
| Recovery (n=5)
|
|---|
| Mean ± SD
(mg·ml−1) | RSD (%) | Mean ± SD
(mg·ml−1) | RSD (%) | Mean ± SD
(mg·ml−1) | RSD (%) | Mean (%) |
|---|
| Lcs | 0.2526±0.0037 | 1.48 | 0.1502±0.0051 | 3.40 | 0.5312±0.0103 | 1.95 | 100.13 |
| Rg1 | 0.6290±0.0062 | 0.98 | 0.3250±0.0156 | 4.80 | 1.0456±0.0416 | 3.97 | 100.76 |
| Re | 0.7372±0.0159 | 2.15 | 0.0805±0.0029 | 3.63 | 0.2763±0.0102 | 3.69 | 104.35 |
| Rb1 | 0.8771±0.0269 | 1.66 | 0.3536±0.0107 | 3.03 | 1.2522±0.0574 | 4.58 | 100.43 |
| Li | 0.4402±0.0062 | 1.41 | 0.7569±0.0177 | 2.33 | 2.5837±0.0259 | 1.00 | 98.97 |
| 6-Gi | 0.1160±0.0017 | 1.46 | 0.0221±0.0009 | 3.93 | 0.0744±0.0035 | 4.63 | 99.65 |
| Ev | 0.0611±0.0010 | 1.75 | 0.0240±0.0008 | 3.50 | 0.0872±0.0024 | 2.72 | 97.03 |
| Ru | 0.0311±0.0007 | 2.30 | 0.0112±0.0006 | 4.92 | 0.0378±0.0014 | 3.64 | 106.65 |
HPLC analyses of the 10 types of Wuzhuyu
decoction
Samples of the 10 types of Wuzhuyu decoction and
mixed reference substance were examined, according to definite HPLC
analysis conditions. The chromatograms of Wuzhuyu decoctions and
mixed reference substance were obtained (Fig. 1). A total of 10 common peaks with
stable presence and higher response values were observed. Compared
with the chromatograms of the mixed reference substances, purity
assessment using the DAD detector and LC-MSn structure confirmation
revealed that the main ingredients were Lcs (1), ginsenoside Rg1 (2), ginsenoside Re (3), ginsenoside Rb1 (4), rutaevine (Rv) (5), limonin (Li) (6), 6-gingerol (6-Gi) (7), evodiamine (Ev) (8), ingredient X (9) and rutaecarpine (Ru) (10). From the identified ingredients,
eight were analyzed quantitatively using the regression equation
(Table IV), and the decoction
numbers 5 and 9 were analyzed semi-quantitatively without reference
substances as peak areas, as shown in Table II.
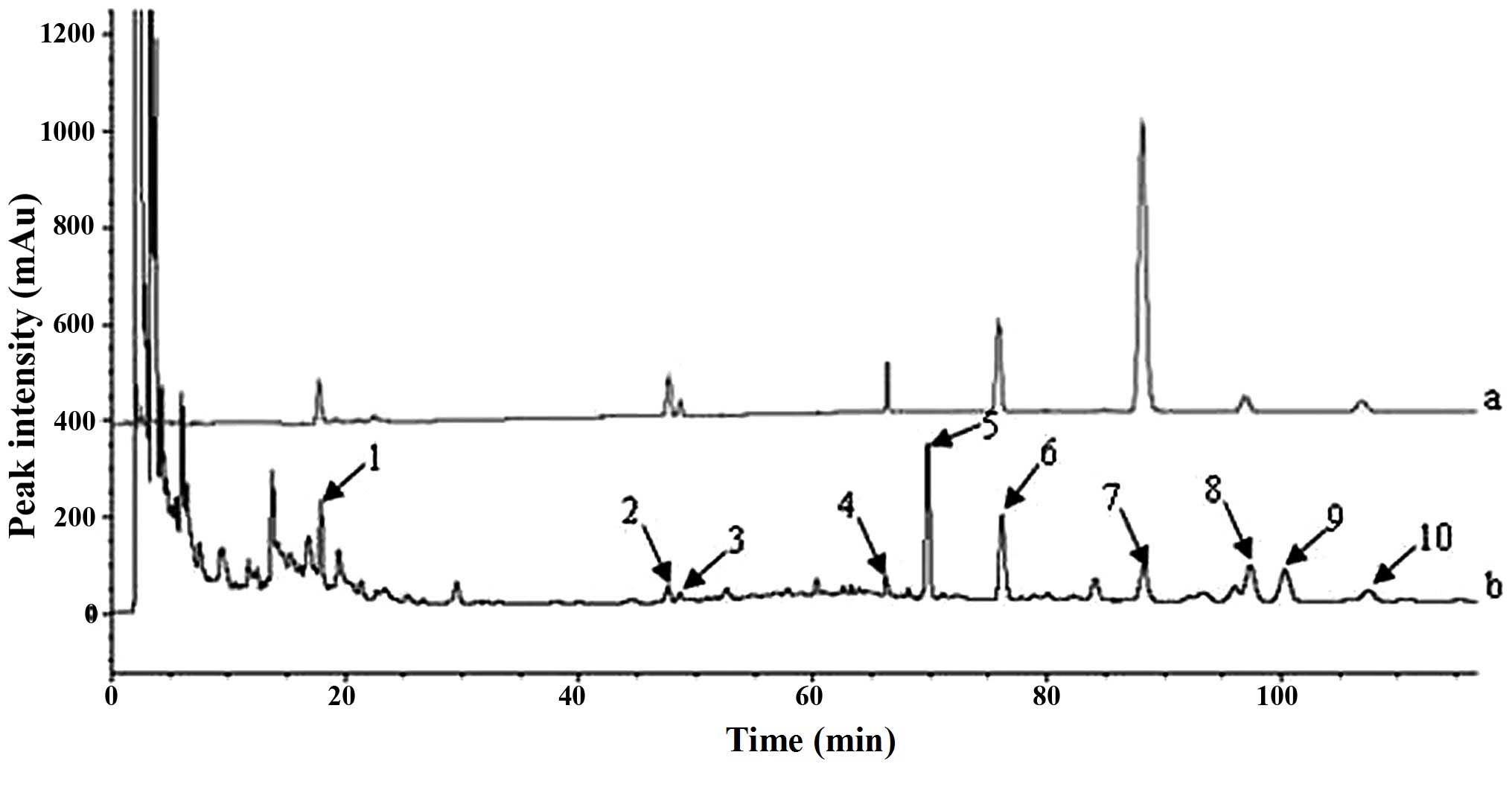 | Figure 1Chromatograms of the (a) mixed
reference substances and (b) Wuzhuyu decoction sample. The numbers
1 10 indicated at the peaks indicate an identified ingredient: 1,
limocitrin-3-O-β-D-glucoside; 2, Rg1; 3, Re; 4, Rb1; 5, rutaevin;
6, limonin; 7, 6-Gi; 8, evodiamine; 9, X (awaiting identification);
10, rutaecarpine. |
Effects of Wuzhuyu decoction on monoamine
neurotransmitters in mouse brain tissues and on NO and NOS in brain
tissues and sera
The statistical results demonstrated that the
contents of 5 HT, NE and DA in the brain tissues of the model mice
were reduced to 337.785±84.504, 171.173±65.172 and 242.075±158.621
mg/g, respectively. The NO content in the brain tissue increased to
0.425±0.184 μmol/mg protein and the NOS activity in the
brain tissue and sera increased to 0.719±0.477 and 50.688±8.132
U/ml, respectively. The above indices were compared with those in
the normal control and sham groups, and all differences were
statistically significant (P<0.01). Following treatment with the
different types of Wuzhuyu, the 5-HT content of the mouse brain
tissues were all significantly increased, and NE contents in the
brain tissues sera were significantly increased. The DA content was
increased compared with the model group, but no statistically
significant differences were observed between the groups. The NO
contents and NOS activity in the brain tissues and sera were
significantly decreased, as shown in Table VI.
 | Table VIContents of monoamine
neurotransmitters and NO and NOS activities in the brain tissues
and sera of mice in different treatment groups. |
Table VI
Contents of monoamine
neurotransmitters and NO and NOS activities in the brain tissues
and sera of mice in different treatment groups.
| Group | n | Brain tissue
(ng·g−1 brain tissue)
| Serum
|
|---|
| 5-HT content | NE content | DA content | NO content
(μmol·g−1 protein) | NOS activity
(U·mg−1 protein) | NO content
(μmol·l−1) | NOS activity(U·ml
1) |
|---|
| Sham | 14 |
643.110±107.014 | 439.004±57.942 |
386.223±128.718 | 0.204±0.090 | 0.274±0.176 | 6.541±3.020 | 41.382±3.429 |
| Normal control | 14 | 664.526±63.861 | 442.259±46.713 | 391.039±94.181 | 0.206±0.094 | 0.257±0.220 | 8.992±5.197 | 39.895±4.912 |
| Model | 12 |
337.785±84.5041b |
171.173±65.172a,b |
242.075±158.621a,b |
0.425±0.184a,b |
0.719±0.477a,b | 3.705±3.098 |
50.688±8.132a,b |
| Positive drug | 12 |
417.810±92.491d | 188.654±25.649 |
259.308±104.721 | 0.252±0.137c | 0.442±0.215d | 3.770±3.163 |
42.934±5.046d |
| Decoction 1 | 13 |
427.344±86.341c |
222.491±37.219c | 238.002±83.673 | 0.264±0.111d | 0.301±0.216d |
47.712±28.203d |
41.957±6.928d |
| Decoction 2 | 14 |
526.862±95.044d |
225.873±40.351d |
282.070±115.021 | 0.252±0.087d | 0.357±0.237d |
48.057±25.602d |
43.453±5.200c |
| Decoction 3 | 13 |
443.495±96.816c | 177.888±35.760 | 234.888±83.549 | 0.334±0.218 | 0.301±0.212d | 8.146±6.227 |
42.906±4.442d |
| Decoction 4 | 14 |
443.097±63.413c | 196.890±43.600 |
244.404±126.079 | 0.233±0.117d | 0.295±0.194d | 4.750±4.414 | 47.639±10.730 |
| Decoction 5 | 12 |
496.664±152.423d |
224.429±47.589d |
267.604±132.124 | 0.264±0.082d | 0.416±0.244d |
17.935±6.520c |
40.065±6.370d |
| Decoction 6 | 12 |
492.962±78.320c | 195.196±46.363 | 256.052±84.982 | 0.242±0.102d | 0.255±0.181d |
16.952±12.444c |
39.848±2.443d |
| Decoction 7 | 12 |
433.650±107.179c | 191.963±33.028 |
338.159±141.715 | 0.343±0.159 | 0.320±0.218d | 13.106±10.452 |
44.307±6.753c |
| Decoction 8 | 11 |
502.575±133.067d |
240.470±55.478d |
301.118±129.806 | 0.279±0.137d | 0.361±0.436d |
25.621±16.644d |
45.440±7.591c |
| Decoction 9 | 13 |
486.512±72.174d |
239.249±53.514d |
298.839±104.626 | 0.215±0.103d | 0.408±0.275d |
20.727±16.112d | 46.103±5.701 |
| Decoction 10 | 12 |
496.519±123.627d |
215.693±82.047c |
305.776±107.064 | 0.274±0.169d | 0.322±0.301d |
16.545±11.290c |
42.852±6.026d |
PLSR analysis
The contents of 10 ingredients, monoamine
neurotransmitter and NO contents, and the NOS activities were
analyzed by PLSR, and the regression coefficients of the
ingredients against different pharmacodynamics indices were
obtained (Table VII). Positive
numbers indicated that the ingredient contents had positive
correlations with the pharmacodynamics indices, and vice versa for
negative numbers. The regression coefficients were combined with
the indices of improved migraine, of which, those of the contents
of 5-HT, NE and DA and serum NO contents were either positive,
indicating positive effects on migraine improvement, or negative,
indicating negative effects on migraine improvement, and vice versa
for NO content in the brain tissues and NOS activity in the brain
tissues and sera. Ingredients with greater impacts on the indices
(with the first three absolute values of the correlation
coefficients) were selected and, following normalization, images
were captured (Fig. 2).
Ingredients, which had effects on multiple pharmacodynamics indices
(≥4) were Rg1, Re, Rb1, Rv, Li, Ev, X and Ru, of which those with
the most positive effects were Rb1, Ev, Ru and Rv, and those with
the most negative effects were Rg1, Re, Li and X. The contents of
the above eight ingredients exhibited regulatory effects on the
pharmacodynamics indices, therefore, may be the predominant
pharmacodynamic substances of Wuzhuyu decoction in treating
migraines. These results were confirmed by rewinding and two
pharmacodynamic verifications, which produced reproducible and
reliable results.
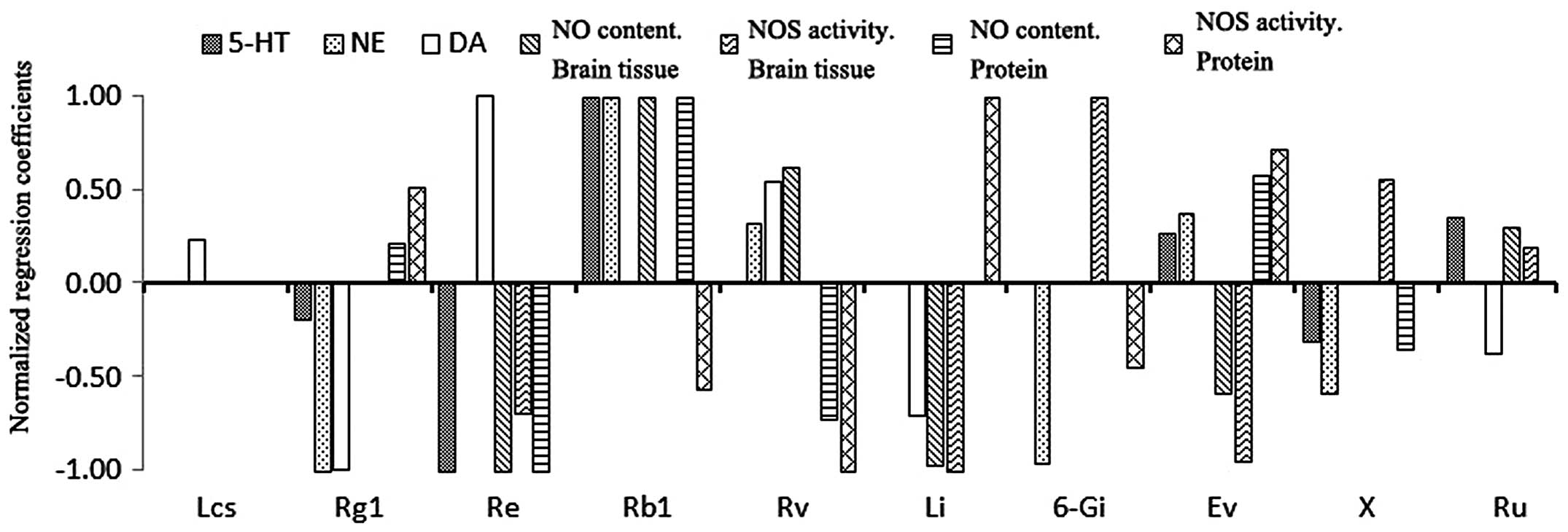 | Figure 2Normalized regression coefficients
between the contents of the 10 ingredients in the Wuzhuyu
decoctions and the values of pharmacodynamic indices. According to
the results in Table VII, the
ingredients with the greatest effects on the indices (. with the
first three absolute values of the correlation coefficients) were
selected, their regression coefficients were normalized and results
were obtained. Positive numbers indicated that the ingredient
contents had positive correlations with the pharmacodynamic indices
and vice versa for negative numbers. The ingredients with
significant regulatory effects on four or more pharmacodynamic
indicees were Rg1, Re, Rb1, Rv, Li, Ev, X and Ru, of which Rb1, Rv,
Ev and Ru had the greatest positive effects. 5-HT,
5-hydroxytryptamine; NE, noradrenaline; DA, dopamine; NO, nitric
oxide; NOS, nitric oxide synthase; Lcs,
limocitrin-3-O-β-D-glucoside; Li, limonin; Ev, evodiamine; Ru,
rutaecarpine; Rv, rutaevin; Ev, evodiamine; X (awaiting
identification). |
 | Table VIIRegression coefficients of partial
least squares regression between the contents of the 10 Wuzhuyu
decoction ingredients and the values of the pharmacodynamic
indices. |
Table VII
Regression coefficients of partial
least squares regression between the contents of the 10 Wuzhuyu
decoction ingredients and the values of the pharmacodynamic
indices.
| Ingredient | 5-HT | NE | DA | Brain NO
content | Brain NOS
activity | Serum NO
content | Serum NOS
activity |
|---|
| Lcs | −0.490 | 0.103 | 1.827 | 0.507 | 0.142 | −0.827 | −1.031 |
| Rg1 | −0.842 | 1.491 | −6.441 | 3.012 | −0.145 | 0.897 | −2.494 |
| Re | −4.370 | 0.143 | 7.861 | 5.502 | 1.633 | −2.844 | 0.079 |
| Rb1 | 4.588 | 2.548 | −2.061 | −8.160 | −0.003 | 4.166 | 2.470 |
| Li | 1.046 | 0.504 | −4.553 | 5.360 | 2.360 | 0.063 | −4.833 |
| 6-Gi | −0.016 | 1.427 | 1.156 | −0.678 | −2.845 | 0.353 | 1.963 |
| Ev | 1.236 | 0.956 | 0.441 | 3.223 | 2.230 | 2.409 | −3.439 |
| Ru | 1.624 | 0.004 | −2.464 | −2.445 | −1.333 | −0.732 | 0.513 |
| Rv | −0.839 | 0.808 | 4.247 | −5.067 | −0.320 | −2.075 | 4.410 |
| unknown X | −1.353 | 0.877 | −0.233 | −0.357 | −0.560 | −0.994 | 0.972 |
Discussion
The pathogenesis of migraines remains to be fully
elucidated, and animal models of migraine, which are developed
according to the different pathophysiological characteristics, have
provided support for investigation of the disease and drug
development. In the present study, the migraine model used
reserpine, resulting in low levels of 5-HT, and placement of
autologous blood clots in the cerebral cortices, stimulated
respectively by the reduced 5-HT (28), and the noxious stimulation of
internal and external factors on the meninges during migraine
attacks (29). Previous
investigations have induced headaches in patients with migraines
using a method of subcutaneous injection of reserpine, and the
association between catecholamine substances and migraine attacks
were investigated by examining the metabolic products of 5-HT,
adrenaline (A) and NE, including 5-hydroxyindoleacetic acid and
3-methoxy-4-hydroxy mandelic acid, as indicators. It was observed
that reserpine-induced migraine headache attacks are associated
with excessive catecholamine metabolism (30), therefore, a disturbance of
catecholamine metabolism is important in migraines. In addition,
meningeal noxious stimulation activates the primary sensory
trigeminal fibers, resulting in the release of calcitonin gene
related peptide, and endogenous NO is involved in this process
(31). The catecholamines, 5-HT,
NE, DA, and vasoactive substances, NO, NOS are associated with the
modeling mechanism and can visually reflect the pathophysiological
characteristics of models, thus, they are selected as
pharmacodynamic indices. Sumatriptan is a triptan drug, which is a
highly selective 5-HT1B/1D receptor agonist. It is a first-line
drug in the treatment of acute migraine attacks (6) and, since its functional
characteristics are consistent with the those of model animals, it
is used as a positive control drug.
Few studies have reported the correlation between
the predominant ingredients and pharmacodynamic effects of Wuzhuyu
decoction, limiting its quality control and development. In the
present study, the ingredients of Wuzhuyu decoction were associated
with their pharmacodynamic effects for the treatment of migraines
using a spectral efficiency association method, to detect the
predominent effective ingredients affecting the efficacy of Wuzhuyu
decoction. The spectral efficiency association method used in the
present study is a novel approach suggested on the basis of
fingerprint studies. The fingerprints are combined with efficacy
results using mathematical methods, for the quality control of TCMs
and identifying effective substances with improved specificity
compared with simple fingerprints. However, these investigations
use predominantly multivariate regression analysis or multivariate
correlation analysis, which are not applicable in sample sizes less
than or equal to the number of independent variables, or when there
is multicollinearity between independent variables. In these
situations, the PLSP method may be selected, the use of which has
been limited in previous studies and has only been applied to
investigate the formula of a single herb (32).
In the present study, using the spectral efficiency
association method, the regression coefficients of the various
ingredients in Wuzhuyu decoctions with different pharmacodynamics
indices were obtained, and, following normalization, results were
obtained (Fig. 2). It was revealed
that Rg1, Re, Rb1, Rv, Li, Ev, X and Ru had significant regulatory
effects on the majority of the pharmacodynamics indices and,
therefore, they may be active substances of Wuzhuyu decoction in
treating migraines. Four ingredients, Rb1, Ev, Ru and Rv, had an
important positive effect on index improvement. These results
provided references for determining the effective substances of
Wuzhuyu decoction in treating migraines and in selecting the
quality control indices.
At present, the pharmacodynamic indices selected
following analysis of spectral efficiency remain relatively simple
and do not include deeper pathophysiological changes in migraine
attacks, including the gene expression of c-fos, release of
endogenous opioid peptides and lack of animal behavioral indices.
These issues require consideration and improvement in future
investigations. The present study also demonstrated that the
effects of the variety of ingredients of a compound on
pharmacodynamics indices are complicated. The same ingredient may
exhibit different effects on different indices and different
ingredients can have a synergistic effect on the same indices. This
indicates that, when a variety of ingredients in a compound are
important and multiple mechanisms are activated simultaneously,
close attention is required on the effects of active constituents
and their proportions on their efficacy and safety. Therefore,
optimization of the ingredients of Wuzhuyu decoction in treating
migraines requires further investigation.
Acknowledgments
This study was supported by the National Natural
Sciences Foundation of China (no. 81473360), the Science and
Technology Development Program of Beijing Municipal Commission of
Education (no. KM20121002501) and the Capital Key Research Program
of Traditional Chinese Medicine (no. 13ZY02).
References
|
1
|
Headache classification subcommittee of
the international headache society: The international
classification of headache disorders. Cephalalgia. 24:9–160.
2004.
|
|
2
|
Arulmozhi DK, Veeranjaneyulu A and
Bodhankar SL: Migraine: Current concepts and emerging therapies.
Vascul Pharmacol. 43:176–187. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arulmani U, Gupta S, VanDenBrink AM,
Centurión D, Villalón CM and Saxena PR: Experimental migraine
models and their relevance in migraine therapy. Cephalalgia.
26:642–659. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Supornsilpchai W, Sanguanrangsirikul S,
Maneesri S and Srikiatkhachorn A: Serotonin depletion, cortical
spreading depression and trigeminal nociception. Headache.
46:34–39. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang LL and Fan JP: The use of the model
animals with migraine in studying of traditional Chinese medicine.
Chin Arch Trad Chin Med. 25:760–762. 2007.
|
|
6
|
Gelfand AA and Goadsby PJ: A neurologist’s
guide to acute migraine therapy in the emergency room.
Neurohospitalist. 2:51–59. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
China Pharmacopoeia Committee: China
Pharmacopoeia. 1. China Medical Science Press; Beijing, China: pp.
8pp. 21pp. 93pp. 1602010
|
|
8
|
Wang YX, Gong MX, Wang ZM, Zhang QW, Gao
HM and Song YF: A summary of the studies on the chemical
constituents in plants of Evodia J.R.et G. Forst. Chinese
Pharmaceutical Journal. 45:641–646. 2010.
|
|
9
|
Zhao FR, Mao HP, Zhang H, Hu LM, Wang H,
Wang YF, Yanagihara N and Gao XM: Antagonistic effects of two herbs
in Zuojin Wan, a traditional Chinese medicine formula, on
catecholamine secretion in bovine adrenal medullary cells.
Phytomedicine. 17:659–668. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee SS, Hwang BY, Ro JS and Lee MK:
Inhibition of monoamine oxidase by evodiamine. Saengyak Hakhoech.
37:320–323. 2006.
|
|
11
|
Ching LC, Chen CY, Su KH, Hou HH, Shyue
SK, Kou YR and Lee TS: Implication of AMP-activated protein kinase
in transient receptor potential vanilloid type 1-mediated
activation of endothelial nitric oxide synthase. Mol Med.
18:805–815. 2012. View Article : Google Scholar
|
|
12
|
Yoon JS, Sung SH and Kim YC:
Neuroprotective limonoids of root bark of dictamnus dasycarpus. J
Nat Prod. 71:208–211. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoon JS, Yang H, Kim SH, Sung SH and Kim
YC: Limonoids from dictamnus dasycarpus protect against
glutamate-induced toxicity in primary cultured rat cortical cells.
J Mol Neurosci. 42:9–16. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim DH: Chemical diversity of Panax
ginseng, Panax quinquifolium and Panax notoginseng. J Ginseng Res.
36:1–15. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu H, Jiang H, Wang J and Xie J: Rg1
protects iron-induced neurotoxicity through antioxidant and iron
regulatory proteins in 6-OHDA-treated MES23.5 cells. J Cell
Biochem. 111:1537–1545. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang QY, Chen YP, Liu F and Chen X:
Ginsenoside-Rg1 promotes angiogenesis in rats with acute myocardial
ischemia. Third Military Medical University Journals (in press)
Pharmacol Therapeut. 20:482–486. 2013.
|
|
17
|
Lee SH, Hur J, Lee EH and Kim SY:
Ginsenoside Rb1 modulates level of monoamine neurotransmitters in
mice frontal cortex and cerebellum in response to immobilization
stress. Biomol Ther (Seoul). 20:482–486. 2012. View Article : Google Scholar
|
|
18
|
Kim S, Na JY and Song KB: Protective
effect of ginsenoside Rb1 on hydrogen peroxide-induced oxidative
stress in rat articular chondrocytes. J Ginseng Res. 36:161–168.
2012. View Article : Google Scholar
|
|
19
|
Chrubasika S, Pittlerc MH and Roufogalisb
BD: Zingiberis rhizoma: A comprehensive review on the ginger effect
and efficacy profiles. Phytomedicine. 12:684–701. 2005. View Article : Google Scholar
|
|
20
|
Okumi H, Tashima K, Matsumoto K, Namiki T,
Terasawa K and Horie S: Dietary agonists of TRPV1 inhibit gastric
acid secretion in mice. Planta Med. 78:1801–1806. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liang C, Yun NY, Jung SW, Kim DS, Lee YJ
and Ma JY: Analysis of the ingredients of Guibitang and fermented
Guibi-tang and their ability to inhibit angiotensin-converting
enzyme. Nat Prod Sci. 17:363–366. 2011.
|
|
22
|
Hong SS and Oh JS: Phenylpropanoid ester
from Zingiber officinale and their inhibitory effects on the
production of nitric oxide. Arch Pharm Res. 35:315–320. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gong MX, Wang YX, Zou ZD and Wang ZM:
Regularity of Syndrome and medication of wuzhuyu decoction in
modern clinical application. China Journal of Experimental
Traditional Medical Formulae. 15:84–86. 2009.
|
|
24
|
Yang XW, Xiao SY, Yang Z, Du LJ, Bi KS,
Chen DW, Chen DF, Wang ZM and Zou YH: Studies on chemical
constituents of the on chemical constituents of the refined wuzhuyu
capsule. Journal of Peking University (Health Sciences).
33:280–282. 2001.
|
|
25
|
Gong MX, Wang YX, Sun J, Zhang QW and Wang
ZM: Dynamic analysis of 10 ingredients of the Chinese herbal
compound Wuzhuyu-tang absorbed into rat plasma:. Neural Regen Res.
6:2594–2599. 2011.
|
|
26
|
Du LJ, Sun H, Li M, Su XL, Wang ZM and
Xiao SY: Study of the effect of the refined Wuzhuyu Capsule in a
mouse model of migraine. Pharmacology and Clinics of Traditional
Chinese Medicine. 15:3–5. 1999.
|
|
27
|
Yang S, Zhang DK and Su ZT: Effect of
different proportion and different dosage forms of combination of
Chuanxiong rhizoma and Angelicae dahuricae radix on migraine in
animal models. Chinese Journal of Experimental Traditional Medical
Formulae. 17:225–228. 2011.
|
|
28
|
Curzon G, Barrie M and Wilkinson MI:
Relationships between headache and amine changes after
administration of reserpine to migrainous patients. J Neurol
Neurosurg Psychiatry. 32:555–561. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nozaki K, Boccalini P and Moskowitz MA:
Expression of c-fos-like immunoreactivity in brainstem after
meningeal irritation by blood in the subarachnoid space.
Neuroscience. 49:669–680. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Juhasz G, Zsombok T, Modos EA, Olajos S,
Jakab B, Nemeth J, Szolcsanyi J, Vitrai J and Bagdy G: NO-induced
migraine attack: strong increase in plasma calcitoningene-related
peptide (CGRP) concentration and negative correlation with platelet
serotonin release. Pain. 106:461–470. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hibino T, Yuzurihara M, Kanno H, Kase Y
and Takeda A: Goshuyuto, a traditional japanese medicine and
aqueous extracts of Evodiae Fructus constrict isolated rat aorta
via adrenergic and/or serotonergic receptors. Biol Pharm Bull.
32:237–241. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang HW, Wu ZB and Meng J: Partial least
squares regression linear and nonlinear methods. Beijing: National
Defense Industry Press; pp. 22006
|
















