Introduction
MicroRNAs (miRNAs) are a class of small non-coding
RNAs, which can downregulate gene expression through binding to the
3′-untranslated region of target mRNAs, resulting in mRNA
degradation and translation inhibition (1). They have crucial roles in the control
of several physiological processes, including development,
differentiation, apoptosis, proliferation and metabolism, however,
abnormally expressed miRNAs are involved in various human diseases,
including cancer (2). miRNAs
located in fragile sites or aberrant genomic regions have oncogenic
or suppressive roles in cancer (3). Notably, different expression levels
of the same miRNA can occur in various human diseases. For example,
the expression levels of miRNA (miR)-125a-5p in liver fibrosis and
hepatocellular carcinoma (HCC) may be different (4,5).
miR-125a is located at 19q13, which is frequently deleted in types
of human cancer. A previous study demonstrated that the expression
of miR-125a-5p was decreased in HCC tissues and cell lines, and was
associated with aggressive pathological features (4). However, the results differed in liver
fibrosis tissues. Higher levels of miR-125-5p were observed in the
liver of patients with a histological activity index (HAI) >6
and a fibrosis score >2, compared with patients with lower
scores (5). In addition, the
expression of liver miR-125-5p was lowest at HAI=0, fibrosis
score=0. Additionally, the liver expression of miR-125-5p
correlated with plasma hepatitis B virus (HBV)-DNA. From these
previous studies, it is evident that miR-125a-5p is a useful marker
in liver diseases.
However, obtaining liver tissue is a significant
challenge, as liver biopsy is causes pain to the patients, miRNAs
in the tissues are unsuitable as diagnostic markers, therefore, a
non-invasive marker for liver diseases is required. Previous
studies have demonstrated that circulating miRNAs are detectable in
the serum and plasma in a form, which is sufficiently stable to
serve as a biomarker (6–9). Since the levels of miRNAs in the
serum and plasma are markedly correlated, either the serum or
plasma can be used for the investigation of blood-based biomarkers
(8).
Several investigations have reported the role of
miR-125a-5p in liver fibrosis and HCC, however, the significance of
serum miR-125a-5p in patients with chronic hepatitis B (CHB) and
HCC remains to be elucidated. HBV chronically infects >250
million individuals worldwide and is one of the leading causes of
hepatitis, cirrhosis and HCC (10). In China, ~7.8% of the population
are HBV carriers of HBV, which is two-thirds of the total number of
carriers worldwide (11).
Therefore, as a predominant etiological factor, HBV infection is a
major cause of liver disease in China. With the development of
chronic HBV infection, the risk of cirrhosis and HCC is increased.
This risk is associated with the level of HBV-DNA and the
persistence of HBeAg (12).
Therefore, the association between serum levels of miR-125a-5p and
different stages of liver diseases, including liver fibrosis and
HCC, require further elucidation.
The present study investigated the expression of
serum miR-125a-5p in patients with CHB and HCC to evaluate its
clinical significance in different liver diseases.
Materials and methods
Study subjects
Blood samples (5 ml) were obtained from patients
attending the First Affiliated Hospital of Wenzhou Medical
University (Wenzhou, China) and the Second Yinzhou Hosptial
(Ningbo, China) between 2007 and 2011 (Table I) through median cubital vein. The
present study comprised a total of 164 healthy controls, with
normal liver biochemistry, no history of liver disease or alcohol
abuse, and no viral hepatitis, 91 patients with CHB and 120
patients with HCC. Demographic and clinical information was
obtained, and a blood sample was collected from each patient. The
patients with CHB were naïve to nucleos(t)ide analogues and
interferon therapy, and exhibited no marker of hepatitis C,
hepatitis D or human immunodeficiency virus infection, no history
of alcohol intake and no clinical or biochemical signs of liver
cirrhosis. The blood sample was obtained for each patient on the
day which they underwent a diagnostic liver biopsy. The diagnosis
of HCC was based on histopathological assessment (13). For patients with HCC, the blood
samples were obtained at the time of initial consultation, prior to
definitive surgical intervention and/or adjuvant therapy. The
follow-up period was used for calculating HCC patient survival
rate. The follow-up period was defined as the time from the date of
surgery to the date of patient mortality or the last follow-up
point. Follow up was completed on January 1, 2013. Informed consent
was obtained from all individuals for the use of their blood
samples in the present study. The study was approved by the Ethics
Committee of the First Affiliated Hospital of Wenzhou Medical
University.
 | Table ICharacteristics of the patients
enrolled. |
Table I
Characteristics of the patients
enrolled.
| Group | Median age; range
(years) | Gender
(male/female) | Etiology | Fibrosis stage |
|---|
| Healthy control
(n=164) | 48; 16–81 | 98/66 | | |
| Liver fibrosis
(n=91) | 49; 22–56 | 52/39 | HBV (n=91) | F0 (n=0) |
| F1 (n=15) |
| F2 (n=24) |
| F3 (n=18) |
| F4 (n=21) |
| F5 (n=3) |
| F6 (n=10) |
| HCC (n=120) | 60; 25–83 | 75/45 | HBV (n=102) | |
Liver histology
Liver biopsy was performed using a 16-gauge Menghini
needle. The samples were fixed in 10% neutral-buffered formalin
(Changfeng, Wenzhou, China), embedded in paraffin (Wenzhou
Changfeng Biotechnology Co., Ltd., Wenzhou, China) and stained with
1 ml hematoxylin and eosin (Beyotime Institute of Biotechnology,
Haimen, China) for 5 min. The samples from 91 CHB patients were
reviewed by experienced hepatopathologists in a blinded manner. The
HAI and fibrosis stages (F0=no fibrosis-F6=cirrhosis) were
assessed, according to the Ishak scoring system (14).
Blood sample processing
The blood samples were centrifuged at 3,400 × g for
7 min at room temperature using a micro 17R centrifuge (Thermo
Fisher Scientific, Fair Lawn, NJ, USA), within 4 h of being
obtained. The sera were subsequently transferred into Eppendorf
tubes (Eppendorf AG, Hamburg, Germany) and centrifuged at 12,000 ×
g for 10 min at 4°C to remove the remaining cells. The serum
samples were stored at −80°C pending RNA extraction.
Detection of miR-125a-5p by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the serum samples using
an miRNeasy Mini kit (Qiagen, Carlsbad, California, USA), according
to the manufacturer’s instructions. To remove any contaminating DNA
in the total RNA, 1 μl 2 U/μl DNase (Qiagen) was used
and the final elution volume was 20 μl. Prior to the RT
reaction, the serum RNA preparations were quantified on a Nanodrop
1000 (Nanodrop, Wilmington, Delaware, USA). The RT reaction was
performed in a 20 μl reaction volume using a TaqMan MicroRNA
Reverse Transcription kit (Applied Biosystems, Foster City, CA,
USA). For the synthesis of cDNA, the reaction mixtures were
sequentially incubated at 16°C for 30 min, 42°C for 30 min and 85°C
for 5 min. The miR-125a-5p was then quantified in triplicate by
qPCR using TaqMan MicroRNA Assay kits (Applied Biosystems). qPCR
was performed in an ABI 7500 Real-Time PCR system (Applied
Biosystems), according to the standard TaqMan MicroRNA assay
instructions, with the following cycles: 95°C for 10 min, followed
by 40 cycles of 95°C for 15 sec and 60°C for 60 sec. Each
amplification reaction was performed in a final volume of 20
μl containing 2 μl of the cDNA (100 ng/reaction), 10
μl TaqMan 2X Universal PCR Master mix without AmpErase
Uracil N-glycosylase, 1 μl miRNA specific TaqMan probes, 2
μl 10X primers and 5 μl RNase free water (Assay ID
000448; Applied Biosystems). The cycle threshold (Ct) values were
calculated using SDS 2.0.1 software (Applied Biosystems). The
relative expression levels of miR-125a-5p were calculated and
normalized against that of U6 small nuclear RNA (Applied
Biosystems), using the comparative ΔCt method with the equation
2−ΔΔCt, as described previously (15).
Serum hepatitis marker and liver fibrosis
marker analysis
For the patients with CHB, the HBV-DNA was
quantified using an Artus HBV QS-RGQ kit (Qiagen), with a lower
detection limit of 10.2 IU/ml. Analyses of the levels of serum
hyaluronic acid (HA), laminin (LN), type III procollagen protein
(PCIII) and type IV collagen (IV-C) were performed using a Magnetic
Immunity Chemiluminescence assay (Shenzen New Industries Biomedical
Engineering Co., Ltd., Shenzhen, China).
Statistical analysis
Statistical analyses were performed using SPSS 13.0
software (SPSS, Inc., Chicago, IL, USA). Differences between the
different groups were determined using a Mann-Whitney U test. Data
are presented as the median (range). P<0.05 was considered to
indicate a statistically significant difference. Box plots were
constructed, with vertical lines indicating the range and
horizontal boundaries of the boxes indicating the first and third
quartile. The correlation coefficients (r) were calculated using
Spearman’s correlation. Survival curves were plotted using the
Kaplan-Meier method and were analyzed using the log-rank test.
Results
Patient population
A total of 375 subjects were recruited into the
present study (Table I),
comprising 164 healthy individuals, 91 patients with liver fibrosis
and 120 patients with HCC. In the present study, HBV was the
underlying etiology of liver disease in 100% of the patients in the
liver fibrosis group and 85% of patients in the HCC group.
Expression levels of serum miR-125a-5p in
the different groups
The serum levels of miR-125a-5p were detected in
different categories (Table II).
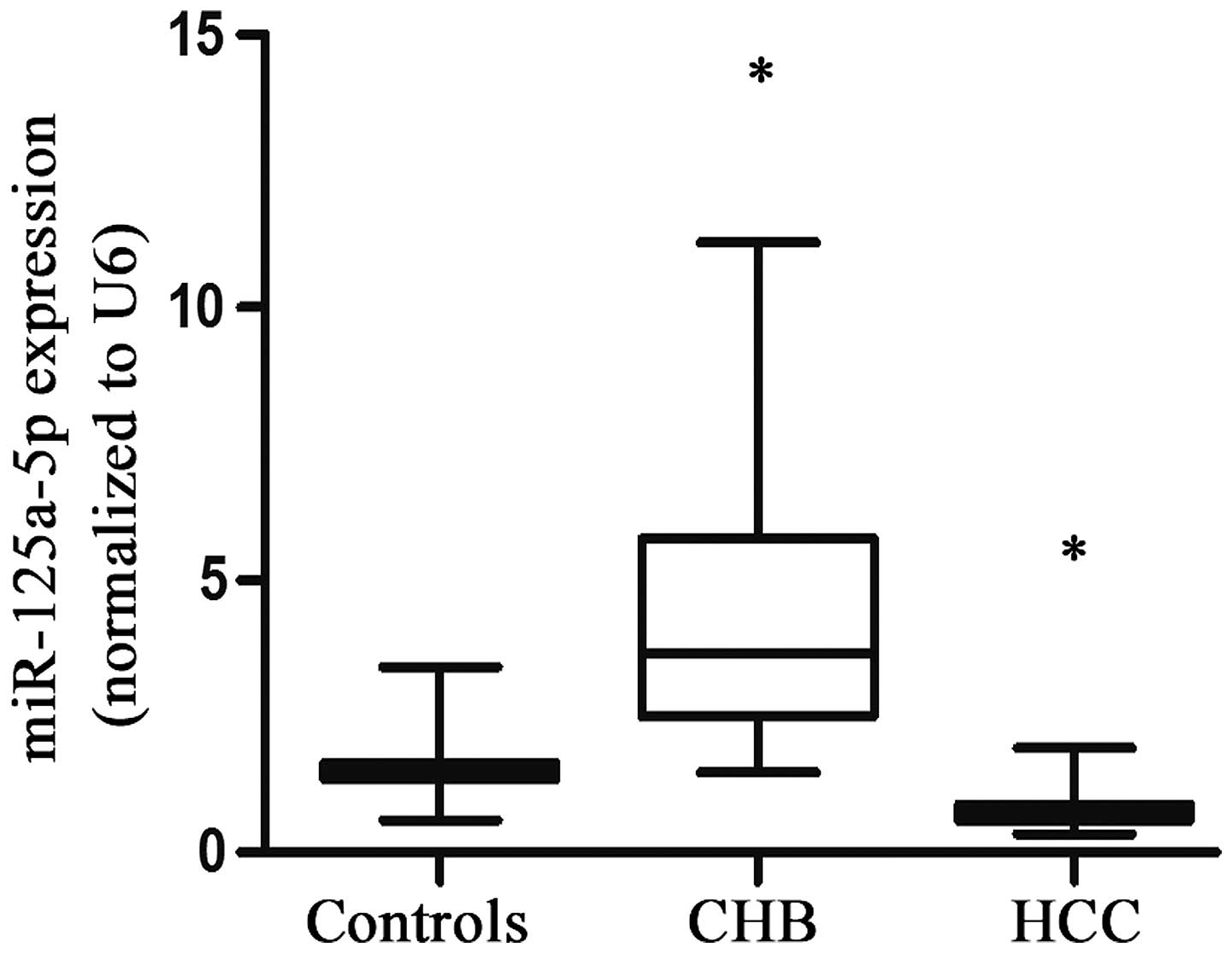
The median levels of serum miR-125a-5p were 1.44 in the healthy
individuals, 3.66 in the liver fibrosis group and 0.68 in the HCC
group. These results demonstrated that the levels of serum
miR-125a-5p were markedly increased in the liver fibrosis group and
significantly reduced in the HCC group, compared with the controls
(P<0.05; Fig. 1).
 | Table IIExpression levels of serum miR-125a-5p
in different groups. |
Table II
Expression levels of serum miR-125a-5p
in different groups.
| Group | n | Median | Data range
(miR-125A-5p/U6)
| Data range
(miR-125A-5p/U6)
|
|---|
| 25% | 75% | 5% | 95% |
|---|
| Healthy control | 164 | 1.44 | 1.34 | 1.64 | 0.79 | 1.92 |
| Liver fibrosis | 91 | 3.66 | 2.50 | 5.76 | 1.65 | 9.70 |
| F1 | 15 | 2.14 | 1.93 | 2.32 | 1.56 | 2.62 |
| F2 | 24 | 2.78 | 2.05 | 3.17 | 1.47 | 4.14 |
| F3 | 18 | 4.22 | 3.49 | 5.32 | 2.50 | 6.47 |
| F4 | 21 | 4.70 | 3.56 | 6.74 | 2.66 | 8.81 |
| F5 | 3 | 5.47 | 5.34 | 5.87 | 5.34 | 5.87 |
| F6 | 10 | 9.35 | 8.57 | 10.55 | 7.90 | 11.20 |
| HCC | 120 | 0.68 | 0.60 | 0.88 | 0.46 | 1.42 |
Expression levels of serum miR-125a-5p
and quantitative HBV-DNA in liver fibrosis
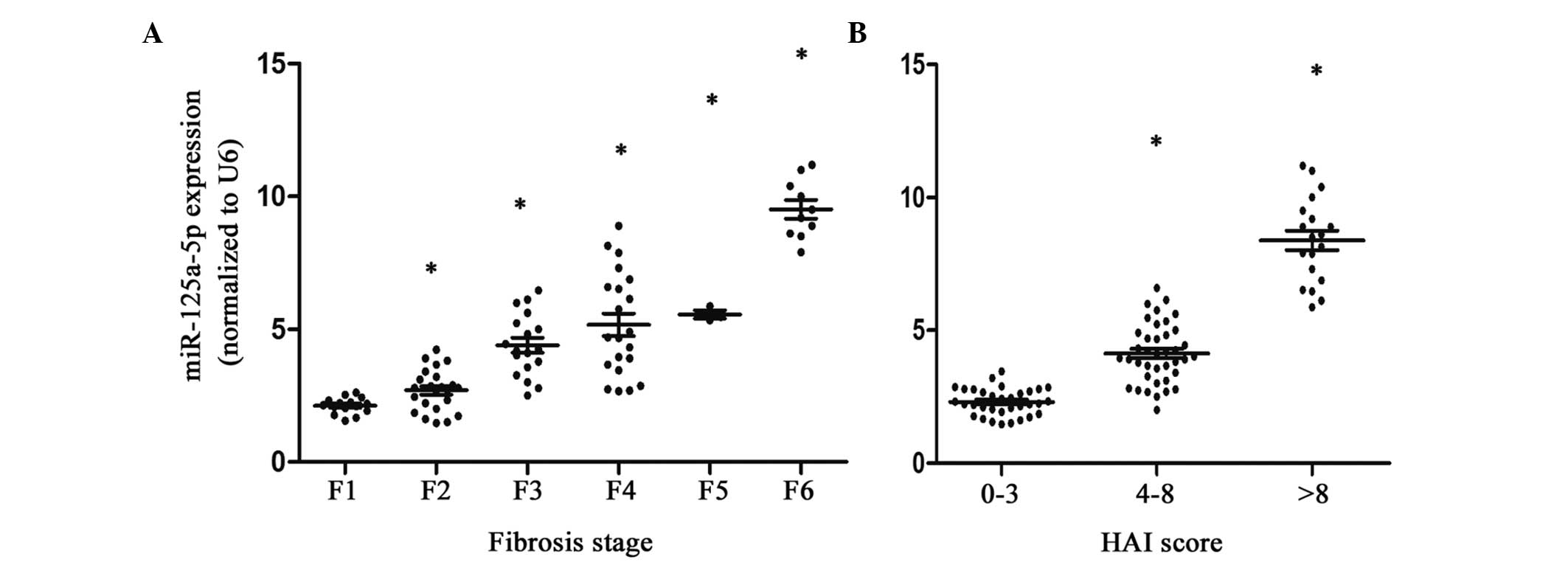
The results revealed that the expression levels of
miR-125a-5p in the different liver fibrosis stages were higher in
the liver fibrosis and HCC groups compared with the healthy
controls (Table II). Notably,
with the development of liver fibrosis, there were significant
increases in the expression levels of miR-125a-5p (P<0.05;
Fig. 2A). In terms of the HAI
scores, higher expression levels of miR-125a-5p were observed in
the high HAI score patient group (P<0.05; Fig. 2B). However, the levels of HBV-DNA
were not correlated with the stages of fibrosis or the HAI scores
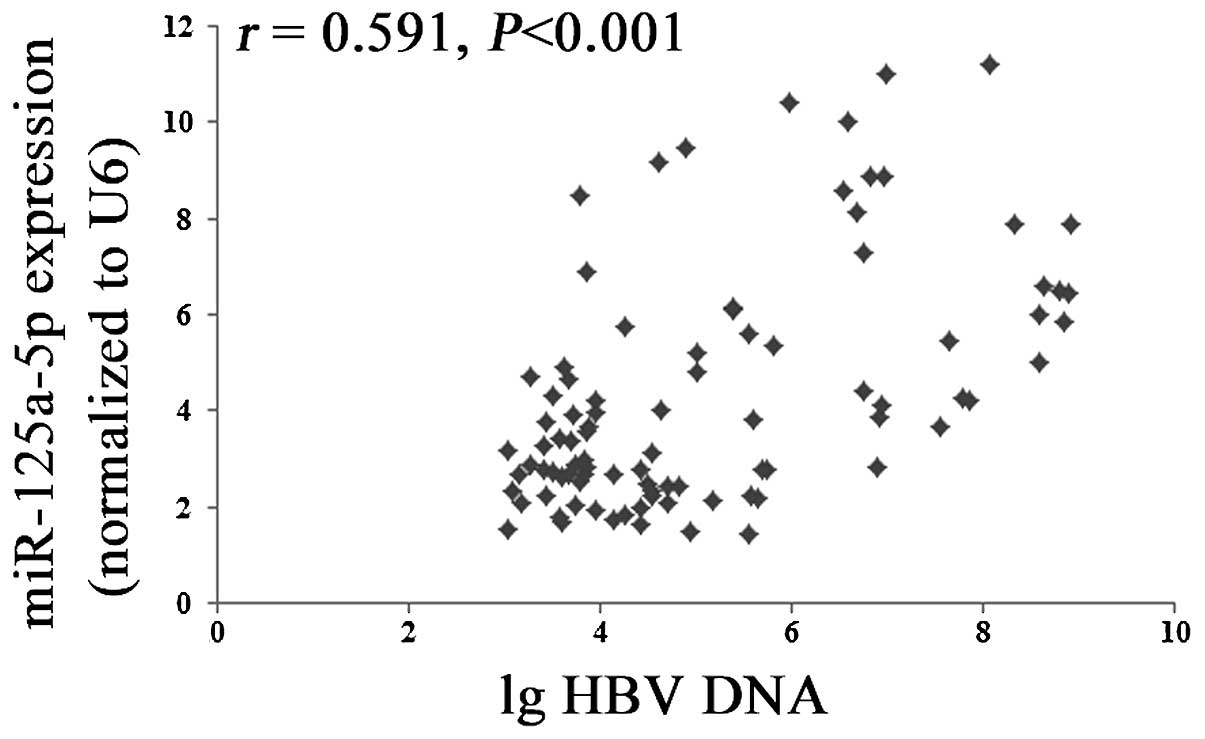
(P>0.05; Table III; Fig. 3A and B). Furthermore, the present
study demonstrated that there was a significant positive
correlation between the expression levels of miR-125a-5p and the
levels of HBV-DNA (P<0.001; Fig.
4).
 | Table IIILevels of hepatitis B virus DNA in
the liver fibrosis group. |
Table III
Levels of hepatitis B virus DNA in
the liver fibrosis group.
| Group | n | Median | Data range (IU/ml)
| Data range (IU/ml)
|
|---|
| 5% | 95% | 5% | 95% |
|---|
| Liver fibrosis | 91 |
3.40×104 |
5.50×103 |
3.90×106 |
1.46×103 |
5.00×108 |
| F1 | 15 |
9.00×103 |
3.90×103 |
5.10×104 |
1.10×103 |
4.40×105 |
| F2 | 24 |
2.60×104 |
6.95×103 |
3.90×105 |
1.12×103 |
2.83×107 |
| F3 | 18 |
1.75×105 |
7.05×103 |
6.30×107 |
2.60×103 |
7.90×108 |
| F4 | 21 |
6.80×103 |
3.45×103 |
5.20×106 |
1.45×103 |
6.00×108 |
| F5 | 3 |
4.40×107 |
6.50×105 |
7.10×108 |
6.50×105 |
7.10×108 |
| F6 | 10 |
3.65×106 |
6.80×104 |
3.71×107 |
6.00×103 |
8.10×108 |
Expression levels of serum miR-125a-5p
and other markers of liver fibrosis
The expression levels of miR-125a-5p were markedly
increased in liver fibrosis, compared with the healthy individuals.
In addition, the expression levels of other markers associated with
liver fibrosis were analyzed (Table
IV). The findings demonstrated that there was a significant
positive correlation between the expression levels of miR-125a-5p
and the expression levels of HA, LN, PCIII and IV-C (P<0.001;
Table V).
 | Table IVExpression levels of serum
miR-125a-5p and other markers in the liver fibrosis group. |
Table IV
Expression levels of serum
miR-125a-5p and other markers in the liver fibrosis group.
| Marker | Median | Data range
(μg/l)
| Data range
(μg/l)
|
|---|
| 25% | 75% | 5% | 95% |
|---|
| HA | 277.00 | 163.10 | 438.00 | 67.81 | 856.60 |
| LN | 85.20 | 66.65 | 135.50 | 24.83 | 191.70 |
| PCIII | 78.50 | 67.50 | 126.70 | 18.31 | 174.30 |
| IV-C | 82.40 | 45.45 | 114.40 | 17.50 | 158.40 |
 | Table VSpearman’s correlation coefficients
of the correlation between serum miR-125a-5p and markers of liver
fibrosis in the liver fibrosis group. |
Table V
Spearman’s correlation coefficients
of the correlation between serum miR-125a-5p and markers of liver
fibrosis in the liver fibrosis group.
| Marker | r-value | P-value |
|---|
| HA | 0.931 | <0.001 |
| LN | 0.211 | <0.001 |
| PCIII | 0.763 | <0.001 |
| IV-C | 0.728 | <0.001 |
Expression levels of serum miR-125a-5p in
HCC
The expression levels of serum miR-125a-5p were
reduced in the patients with HCC compared with the healthy
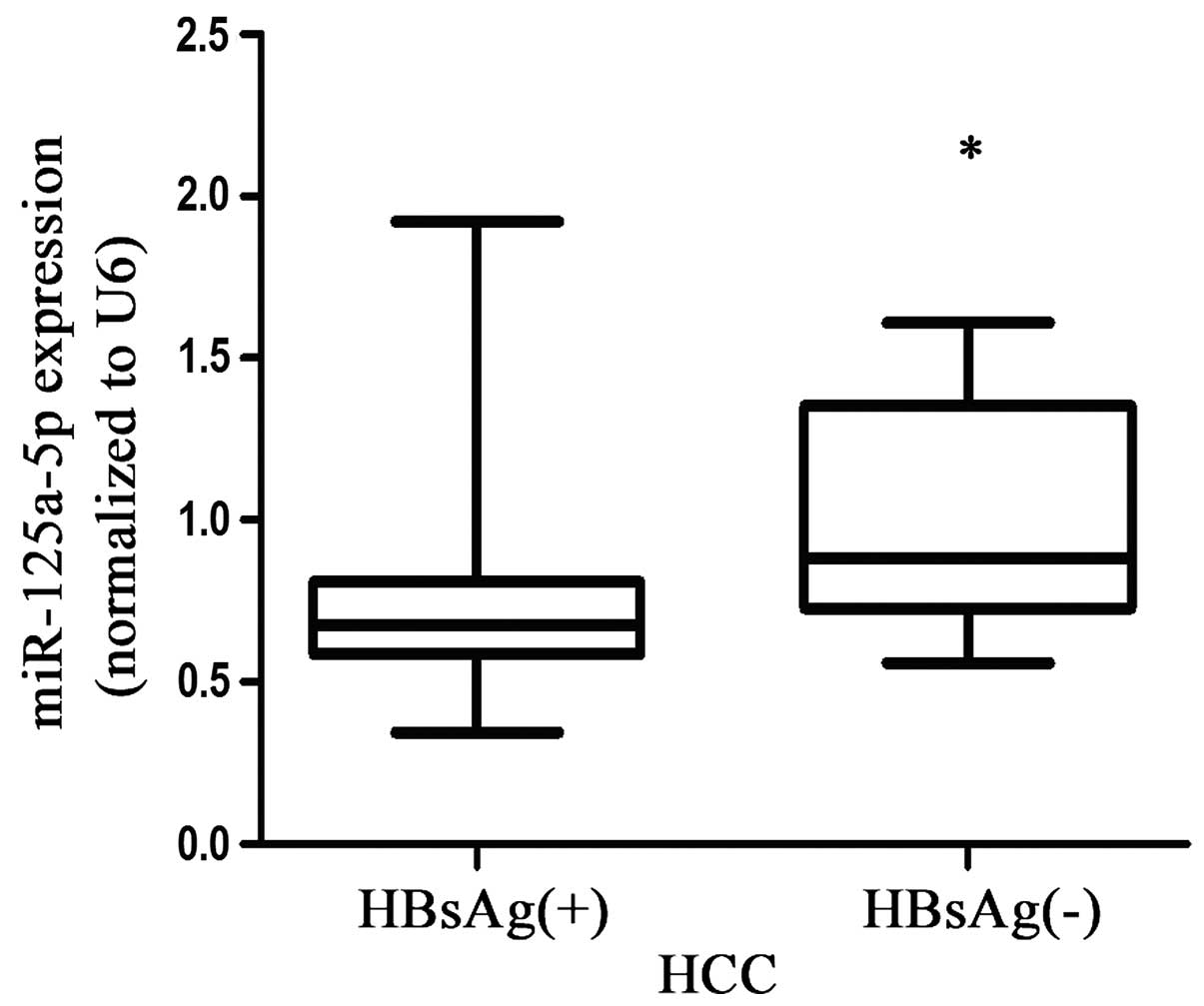
individuals or the liver fibrosis group (P<0.05; Fig. 1A). In addition, the expression
levels of miR-125a-5p in patients with HBsAg (+) HCC were higher
compared with the patients with HBsAg (−) HCC (P<0.05; Fig. 5). Notably, the expression levels of
miR-125a-5p differed between the HCC and liver fibrosis groups,
indicating that serum miR-125a-5p may be suitable for use as a
diagnostic biomarker in HCC. Additionally, the association between
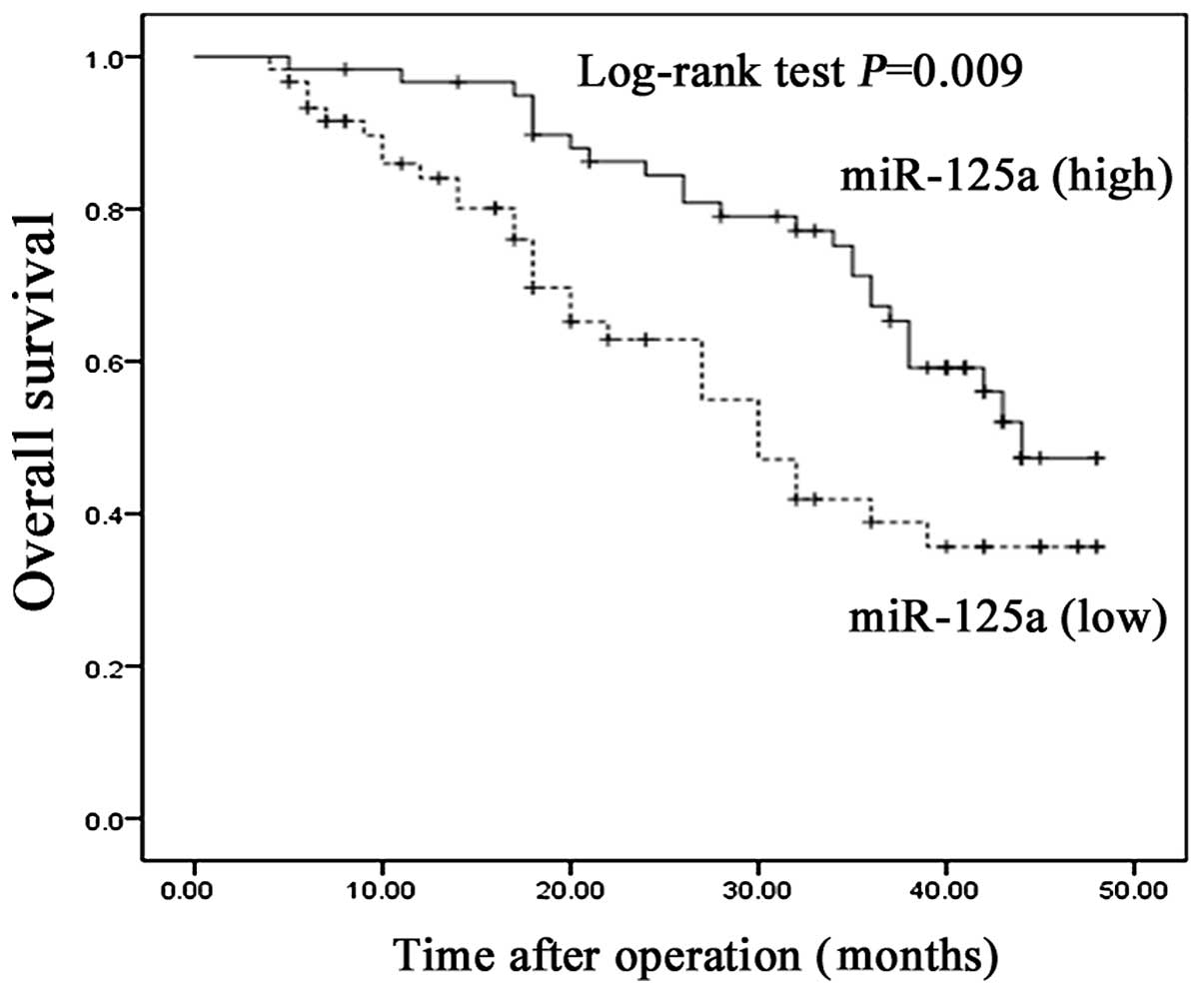
miR-125a-5p and the prognosis in patients with HCC was analyzed by
Kaplan-Meier survival analysis. The patients with HCC were divided
into low and high expression groups, based on the median expression
levels of miR-125a-5p (0.68). The results indicated that patients
with HCC exhibiting low expression levels of miR-125a-5p had a
lower survival rate compared with those with exhibiting high
expression levels of miR-125a-5p, with median survival rates of
30.66 and 39.04 months, respectively; P=0.009; Fig. 6).
Discussion
In China, CHB is a significant public health
problem, due to the huge number of infected individuals and high
costs of treatment (16). Chronic
HBV infection induces a series of liver diseases, ranging between a
clinically asymptomatic carrier state and the development of
cirrhosis-associated complications and HCC (17,18).
The predominant goal of clinical antiviral therapy is to inhibit
the progression of liver disease. In the present study, significant
increases in the expression levels of serum miR-125a-5p were
observed in different states of liver fibrosis, between F1 and F6.
This indicated that increased serum miR-125a-5p was correlated with
disease progression. A similar result was observed in the
correlation between expression levels of serum miR-125a-5p and HAI
scores. However, no correlation was observed between the levels of
HBV-DNA and the stage of liver fibrosis stages, nor was there any
correlation between the levels of HBV-DNA and the HAI score. This
indicated that the levels of HBV-DNA may not provide a good
biomarker for the development of liver fibrosis. However, liver
miR-125a-5p, identified as an independent predictor of disease
progression by multivariate logistic regression analysis, has been
associated with increased severity of disease progression (5). The results of the present study were
in accordance with this previous study.
It has been reported that high levels of HBV-DNA can
increase the risk of cirrhosis and HCC (12). One of criteria for antiviral
therapy is that HBV-DNA levels are >2,000 IU/ml (19). miRNAs are important in regulating
virus-host interactions (20). For
example, with regards to HBV, miR-125a-5p can target a viral
sequence within overlapping polymerase and surface antigen coding
regions (21). Another previous
study confirmed the effect of miR-125a-5p on the gee expression of
HBV in the cultured hepatocytes (22). In the present study, the
correlation between serum miR-125a-5p and viral replication was
analyzed. The results demonstrated that increased expression levels
of serum miR-125a-5p were correlated with higher levels of HBV-DNA
levels. This finding was similar to the correlation between the
liver miR-125a-5p and HBV-DNA plasma levels (5).
Liver fibrosis is characterized by an excessive
accumulation of extracellular matrices and is a common feature of
chronic liver injury (23).
Confirmation of the stages of liver fibrosis and the inflammatory
grades is crucial for estimating disease progression, treatment
selection and monitoring of the disease (24). With the exception of liver biopsy,
the levels of serum HA, LN, PCIII and IV-C are often analyzed to
estimate the degree of liver fibrosis, as fluctuation in their
concentrations is consistent with the stages of liver fibrosis
(25–27). In the present study, the
concentrations of serum HA, LN, PCIII and IV-C were examined in the
liver fibrosis group. Notably, the expression levels of serum
miR-125a-5p were correlated with these markers, indicating that it
may be used as a potential marker for liver fibrosis.
HCC accounts for 90% of the cases of primary liver
cancer and represents the third most common cause of
cancer-associated mortality worldwide (28). HCC is associated with hepatitis
virus infection (29) and one of
the major risk factors is HBV. Previous studies reported that serum
microRNAs may be used as biomarkers for HCC (30). The present study demonstrated that
serum miR-125a-5p was significantly reduced in the HCC group
compared with the healthy control and liver fibrosis groups. In
addition, higher expression levels of serum miR-125a-5p were
observed in the patients with HBsAg (+) HCC, indicating a
correlation between serum miR-125a-5p and hepatitis virus
infection. The present study also demonstrated that lower
expression levels of serum miR-125a-5p were correlated with a poor
prognosis, which was consistent with previous observations in
tissue samples (4). These results
indicated that serum miR-125a-5p may be used as a prognostic marker
for HCC. In addition, Bi et al demonstrated that
overexpression of miR-125a inhibits the proliferation and
metastasis of HCC cells by targeting matrix metalloproteinase 11
and vascular endothelial growth factor A (4), indicating that miR-125a may be
important as a suppressor of HCC proliferation and metastasis.
In the present study, fluctuations in the expression
levels of miR-125a-5p in the serum were similar to those in liver
tissue, indicating that serum miR-125a-5p may be released from the
liver tissue, as the expression levels of serum miR-125a-5p were
higher in the liver fibrosis group and lower in the HCC group. The
levels of serum miR-125a-5p were significantly different in these
two groups, indicating that serum miR-125a-5p may be a useful
marker for liver diseases. However, the reason for the marked
change in expression levels of serum miR-125a-5p in the liver
fibrosis and HCC requires further investigation.
In conclusion, thee results of the present study
demonstrated that the expression levels of serum miR-125a-5p
differed in liver diseases, including liver fibrosis and HCC, and
were correlated with disease progression. These results suggested
that serum miR-125a-5p may be used as a marker for liver
diseases.
Acknowledgments
The study was supported by the National Natural
Science Foundation of China (nos. 81000176/H0317 and
81100292/H0317), the Zhejiang Provincial Natural Science Foundation
of China (nos. Y2090326, Y2110634 and Y12H200010), the Department
of Science and Technology of Zhejiang Province (no. 2013C37006) and
the Wenzhou Municipal Science and Technology Bureau (no.
Y20120127).
References
|
1
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li L, Guo Z, Wang J, Mao Y and Gao Q:
Serum miR-18a: a potential marker for hepatitis B virus-related
hepatocellular carcinoma screening. Dig Dis Sci. 57:2910–2916.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ding J, Huang S, Wu S, et al: Gain of
miR-151 on chromosome 8q24.3 facilitates tumour cell migration and
spreading through downregulating RhoGDIA. Nat Cell Biol.
12:390–399. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bi Q, Tang S, Xia L, et al: Ectopic
expression of MiR-125a inhibits the proliferation and metastasis of
hepatocellular carcinoma by targeting MMP11 and VEGF. PloS One.
7:e401692012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Coppola N, Potenza N, Pisaturo M, et al:
Liver microRNA has-miR-125a-5p in HBV chronic infection:
correlation with HBV replication and disease progression. PLoS One.
8:e653362013. View Article : Google Scholar
|
|
6
|
Chen X, Ba Y, Ma L, et al:
Characterization of microRNAs in serum: a novel class of biomarkers
for diagnosis of cancer and other diseases. Cell Res. 18:997–1006.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fang C, Zhu DX, Dong HJ, et al: Serum
microRNAs are promising novel biomarkers for diffuse large B cell
lymphoma. Annals Hematol. 91:553–559. 2012. View Article : Google Scholar
|
|
8
|
Gilad S, Meiri E, Yogev Y, et al: Serum
microRNAs are promising novel biomarkers. PLoS One. 3:e31482008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mitchell PS, Parkin RK, Kroh EM, et al:
Circulating microRNAs as stable blood-based markers for cancer
detection. Proc Natl Acad Sci USA. 105:10513–10518. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim KH, Kim ND and Seong BL: Discovery and
development of anti-HBV agents and their resistance. Molecules.
15:5878–5908. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu X, Wan X, Li Z, Lin C, Zhan Y and Lu
X: Golgi protein 73 (GP73), a useful serum marker in liver
diseases. Clin Chem Lab Med. 49:1311–1316. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen CJ, Yang HI, Su J, et al: Risk of
hepatocellular carcinoma across a biological gradient of serum
hepatitis B virus DNA level. JAMA. 295:65–73. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
2004 guidelines for surgical treatment of
primary hepatocellular carcinoma. Chinese Journal of Hepatology.
13:329–330. 2005.PubMed/NCBI
|
|
14
|
Ishak K, Baptista A, Bianchi L, et al:
Histological grading and staging of chronic hepatitis. J Hepatol.
22:696–699. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yao J, Liang L, Huang S, et al:
MicroRNA-30d promotes tumor invasion and metastasis by targeting
Galphai2 in hepatocellular carcinoma. Hepatology. 51:846–856.
2010.PubMed/NCBI
|
|
16
|
Lau DT and Bleibel W: Current status of
antiviral therapy for hepatitis B. Therap Adv Gastroenterol.
1:61–75. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bayram A, Erkilic S, Ozkur A, Bayram M and
Sari I: Quantification of intrahepatic total hepatitis B virus DNA
in chronic hepatitis B patients and its relationship with liver
histology. J Clin Pathol. 61:338–342. 2008. View Article : Google Scholar
|
|
18
|
Yuen MF, Ng IO, Fan ST, et al:
Significance of HBV DNA levels in liver histology of HBeAg and
Anti-HBe positive patients with chronic hepatitis B. Am J
Gastroenterol. 99:2032–2037. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
European Association For The Study Of The
Liver: EASL Clinical Practice Guidelines: management of chronic
hepatitis B. J Hepatol. 50:227–242. 2009. View Article : Google Scholar
|
|
20
|
Russo A and Potenza N: Antiviral effects
of human microRNAs and conservation of their target sites. FEBS
Lett. 585:2551–2555. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Potenza N, Papa U, Mosca N, Zerbini F,
Nobile V and Russo A: Human microRNA hsa-miR-125a-5p interferes
with expression of hepatitis B virus surface antigen. Nucleic Acids
Res. 39:5157–5163. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park SO, Kumar M and Gupta S: TGF-beta and
iron differently alter HBV replication in human hepatocytes through
TGF-beta/BMP signaling and cellular microRNA expression. PloS One.
7:e392762012. View Article : Google Scholar
|
|
23
|
Sekiya Y, Ogawa T, Yoshizato K, Ikeda K
and Kawada N: Suppression of hepatic stellate cell activation by
microRNA-29b. Biochem Biophys Res Commun. 412:74–79. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vizzutti F, Arena U, Rega L and Pinzani M:
Non invasive diagnosis of portal hypertension in cirrhotic
patients. Gastroenterol Clin Biol. 32:80–87. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xie SB, Yao JL, Zheng RQ, Peng XM and Gao
ZL: Serum hyaluronic acid, procollagen type III and IV in
histological diagnosis of liver fibrosis. Hepatobiliary Pancreat
Dis Int. 2:69–72. 2003.PubMed/NCBI
|
|
26
|
Parsian H, Rahimipour A, Nouri M, et al:
Serum hyaluronic acid and laminin as biomarkers in liver fibrosis.
J Gastrointestin Liver Dis. 19:169–174. 2010.PubMed/NCBI
|
|
27
|
Lydatakis H, Hager IP, Kostadelou E,
Mpousmpoulas S, Pappas S and Diamantis I: Non-invasive markers to
predict the liver fibrosis in non-alcoholic fatty liver disease.
Liver Int. 26:864–871. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ahmed F, Perz JF, Kwong S, Jamison PM,
Friedman C and Bell BP: National trends and disparities in the
incidence of hepatocellular carcinoma, 1998–2003. Prev Chronic Dis.
5:A742008.
|
|
29
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qi P, Cheng SQ, Wang H, Li N, Chen YF and
Gao CF: Serum microRNAs as biomarkers for hepatocellular carcinoma
in Chinese patients with chronic hepatitis B virus infection. PLoS
One. 6:e284862011. View Article : Google Scholar : PubMed/NCBI
|