Introduction
Acute kidney injury (AKI) is a relatively common
complication in hospitalized patients with an overall mortality
rate of ~30% (1,2). AKI is associated with a high
mortality rate and may lead to increased hospital costs (3). Previous studies have demonstrated
that 5–10% of patients admitted to the intensive care unit (ICU)
developed AKI and required renal replacement therapy (RRT)
(4,5). Although the mortality rate of AKI has
been reduced since the application of RRT, its mortality rate
remains as high as 40–70% (6).
Among all the internal organs, the lung is most easily damaged by
ischemic injuries due to the extensive vascular network in the lung
mesenchyme (7). It is well
established that cardiac and non-cardiac acute lung injuries (ALI)
are the main causes of mortality in patients with AKI (8). Although the exact pathophysiology of
AKI-induced ALI remains to be elucidated, the general mechanisms
underlying AKI-induced ALI are well understood, including
inflammation, activation of soluble and cellular factors as well as
neurohumoral and hemodynamic alterations (9).
Asymmetric dimethylarginine (ADMA) is an endogenous
nitric oxide (NO) synthase inhibitor, which may affect the
biological activity of NO (10).
ADMA is a naturally occurring amino acid that is found in tissues
and cells, circulates in the plasma and is expelled in the urine
(11). ADMA is eliminated through
metabolic degradation and renal excretion (12). Increased blood concentrations of
ADMA may contribute to endothelial dysfunction, thereby linking to
the development and progression of chronic and acute kidney
diseases (13). Activation of the
p38 mitogen-activated protein kinase (MAPK)/heat shock protein 27
(HSP27) pathway by elevated ADMA has been verified, and is involved
in endothelial dysfunction and abnormal permeability (10,14).
Thus, the excessive accumulation of ADMA accompanied by elevated
HSP27 levels provides a potential explanation for the occurrence of
AKI-induced ALI. The aim of the present study was to establish a
rat model of acute ischemic kidney injury by continually occluding
the bilateral renal artery and renal veins. In addition, the roles
of ADMA in the development of AKI-induced ALI were examined.
Materials and methods
Study approval
The animal use protocol was reviewed and approved by
the Institutional Animal Care and Use Committee of the First
Affiliated Hospital of China Medical University (Shenyang,
Liaoning, China). The present study was performed strictly in
accordance with the recommendations of the Guide for the Care and
Use of Laboratory Animals of the National Institutes of Health
(Bethesda, MA, USA).
Animal models
In total, 45 male Wistar rats, weighing 300–320 g,
were provided by the Experimental Animal Center of the China
Medical University. A solution of 5% chloral hydrate (300 mg/kg)
was used to anesthetize the rats followed by tracheotomy. The
arteria cervicalis and jugular vein of the rats were punctured and
a catheter was embedded. Respiratory frequency, heart rate,
systolic pressure, diastolic pressure, mean arterial blood pressure
and central venous pressure were monitored. The rats were allocated
at random into three groups (n=15) 30 min after attaining a stable
state (Table I).
 | Table IExperimental interventions performed
on the rats in each group (n=15). |
Table I
Experimental interventions performed
on the rats in each group (n=15).
| Group | Experimental
method |
|---|
| Control | A midline abdominal
incision was performed, bilateral renal artery and renal veins were
separated without ligation; all rats received 2 ml saline by
intraperitoneal injection. |
| AKI | A midline abdominal
incision was performed, bilateral renal artery and renal veins were
separated with continuous occlusion; all rats received 2 ml saline
by intraperitoneal injection. |
| AKI + SB203580 | A midline abdominal
incision was performed, bilateral renal artery and renal veins were
separated with continuous occlusion; all rats received 2 ml (2
mg/kg) SB203580 (p38 mitogen-activated protein kinase inhibitor) by
intraperitoneal injection. |
Specimen collection and preservation
Following establishment of the models, all the rats
were sacrificed 24 h after initiation of the experiment. For each
rat, the venous blood sample was collected to detect ADMA levels.
Then, the chest was opened to expose the lungs. Following ligation
of the right hilum, the upper-right lung was harvested and fixed
with 4% paraformaldehyde. Hematoxylin and eosin (H&E) staining
of plastic-embedded tissue sections was examined by light
microscopy (Motic BA 400; Motic, Xiamen, China).
Detection of plasma ADMA levels by enzyme
linked immunosorbent assay (ELISA)
The double sandwich ELISA method was used to measure
plasma ADMA levels and was conducted according to the
manufacturer’s instructions (Shanghai Senxiong Biotech Industry
Co., Ltd., Shanghai, China).
Detection of p38 MAPK/HSP27 by western
blot analysis
Protein was extracted from the right lung lobe and
preserved at −80°C following detection of the protein
concentration. The protein sample (100 µg) was
electrophoresed in 12% sodium dodecyl sulfate polyacrylamide gel
electrophoresis (JY-JX5L; Beijing Jun Yi Huaxin Technology Co.,
Ltd., Beijing, China) and then transferred onto polyvinylidene
fluoride membranes (Kynar K760; Inc., Philadelphia, PA, USA) using
a constant voltage (120 V) at 4°C. The membranes were sealed with
5% skimmed milk powder at room temperature (22°C) for 1 h.
The polyclonal p38MAPK (threonine 180 and tyrosine
182; 1:1,000; cat. no. AM065) and polyclonal p-HSP27 primary
antibodies (1:1,000 dilution; cat. no. AH728) and GAPDH (an
internal standard) primary antibody (1:500 dilution; cat. no.
AG019). All antibodies were purchased from Beyotime Institute of
Biotechnology (Shanghai, China) were mixed with the solution.
Following overnight incubation at 4°C, horseradish
peroxidase-labeled rabbit anti-mouse secondary antibody
(Sigma-Aldrich, St. Louis, MO, USA) at a dilution of 1:1,000 was
added into the mixture, which caused a color change following
incubation at room temperature for 1 h. Images were captured using
photographic photometry on an Exilim EX-FC100 digital camera
(Casio, Shibuya, Japan) and the experimental results were analyzed
using Quantity One Software (Bio-Rad Laboratories, Hercules, CA,
USA). The optical density value was scanned and presented as the
ratio of protein-to-internal standard GAPDH.
Immunofluorescent detection of globular
actin (G-actin) and filamentous actin (F-actin)
Following incubation for 2 h, dewaxing in xylene
(Sigma-Aldrich) and rehydrating in ethanol (Sigma-Aldrich), the
sections were rinsed three times (5 min each time) with 30 ml
phosphate-buffered saline (PBS; pH 7.4; Gibco-BRL, Carslbad, CA,
USA) with 1 mg/ml glucose at 25°C. Microwave antigen retrieval was
conducted subsequently using a microwave oven, microwave with
pressure cooker and 0.01 M sodium citrate buffer (pH 6.0), followed
by incubation for 1 h with blocking solution (buffer 1 containing
0.3% Triton X-100/2% goat serum; Sigma-Aldrich) after rinsing three
times with PBS. G-actin was stained with deoxyribonuclease I (DNase
I; 0.3 µM DNase I; Sigma-Aldrich). For F-actin staining,
coverslips were incubated in a 10 U/ml solution (0.165 M) of
fluorescein isothiocyanate-labeled phalloidin (Sigma, St. Louis,
MO, USA). After rinsing with PBS, tissues were bathed in 200
µl 4′, 6-diamidino-2-phenylindole (DAPI; Roche Diagnostics,
Mannheim, Germany) for at least 5 min. Green and red fluorescence
protein indicated G-actin and F-actin, respectively. The nucleus
showed blue fluorescence. The samples were investigated and images
were captured using a fluorescence microscope (Motic BA 400T). The
images were processed using digital analysis software Motic Fluo
1.0.
Statistical analysis
Continuous variables were expressed as the mean ±
standard deviation. Categorical data were presented as the
frequencies and percentages. Differences among the groups were
compared using the two-tailed, non-paired Student’s t-test or
one-way analysis of variance for continuous variables, where
appropriate. Comparisons of categorical variables among groups were
performed using the χ2 test. All tests of statistical
significance were two-sided, with P<0.05 being considered to
indicate a statistically significant difference. Statistical
analyses were performed using SPSS version 17.0 (SPSS, Inc.,
Chicago, IL, USA).
Results
Plasma ADMA levels and pulmonary
permeability index (PI)
Compared with the control group (0.69±0.13
µmol/l), the AKI (1.93±0.17 µmol/l) and AKI +
SB203580 (1.33±0.09 µmol/l) groups demonstrated significant
increases in plasma ADMA levels (all P<0.05). There was also a
significant difference in plasma ADMA levels between the AKI and
AKI + SB203580 groups (P<0.05; Fig.
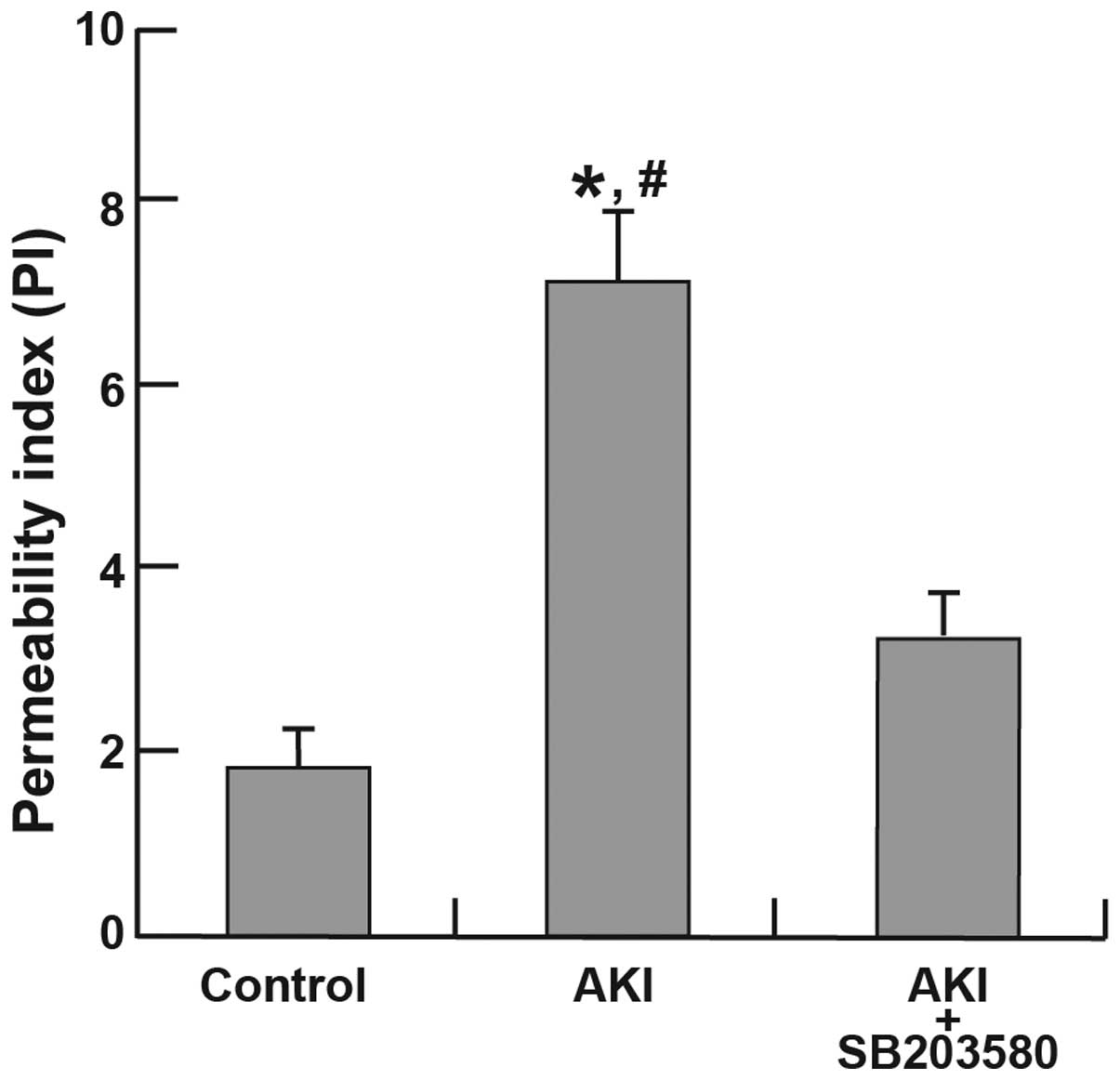
1). The increase in the PI in the AKI group (6.54±0.42) was
significantly higher than that in the control group (1.91±0.16;
P<0.001). Significant differences in permeability were also
found between the AKI group (6.54±0.42) and the AKI + SB203580
group (3.24±0.1; P=0.027). However, no significant difference in PI
was identified between the control group and the AKI + SB203580
group (P=0.130; Fig. 2).
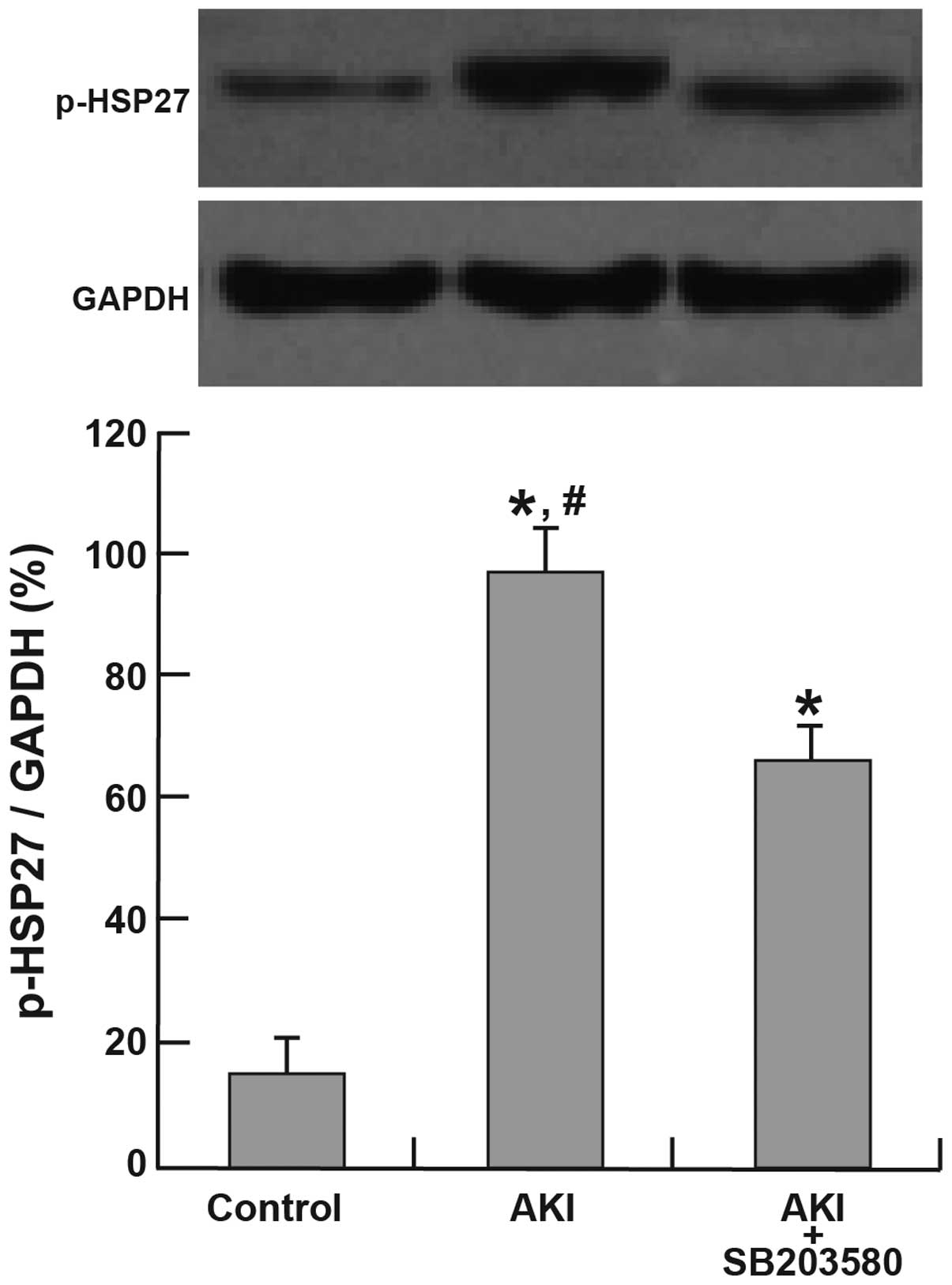
Activation of p38 MAPK and HSP27
The expression of p-p38 and p-HSP27 was
significantly higher in the AKI and AKI + SB203580 groups compared
with the control group (all P<0.05). The AKI group also
demonstrated a higher expression of p-p38 and p-HSP27 than the AKI
+ SB203580 group (all P<0.05; Figs.
3 and 4).
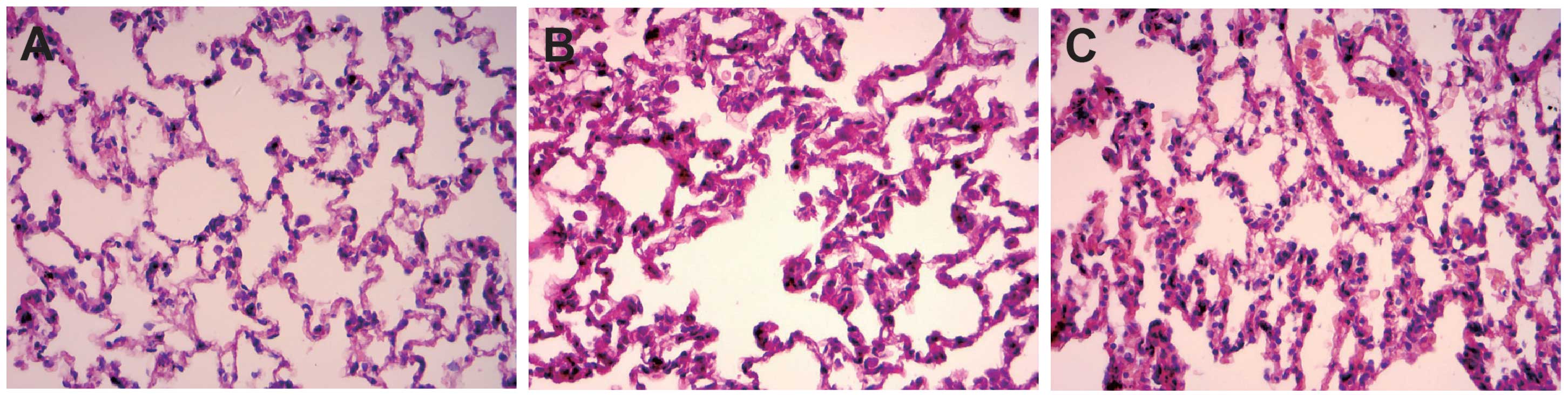
Pathological changes of rat lung
tissue
In the control group, rats exhibited good alveolar
structural integrity without clear intra-alveolar exudation and
interstitial pulmonary edema (Fig.
5A). By contrast, rats in the AKI group showed epithelial
swelling, marked widening of alveolar walls due to capillary
proliferation and congestion, which led to clear interstitial
pulmonary edema with exudations of inflammatory cells, red blood
cells and associated proteins, and conversely causing inflammatory
cell infiltration (Fig. 5B). Small
airway injury and alveolar structural disorder were also dientified
in the AKI group. All the described symptoms demonstrate the
pathological changes of acute lung injury (ALI). In the AKI +
SB203580 group, rats also had exudations of inflammatory cells, red
blood cells and associated proteins. However, compared with the AKI
group, these rats demonstrated mitigations of edema (Fig. 5C).
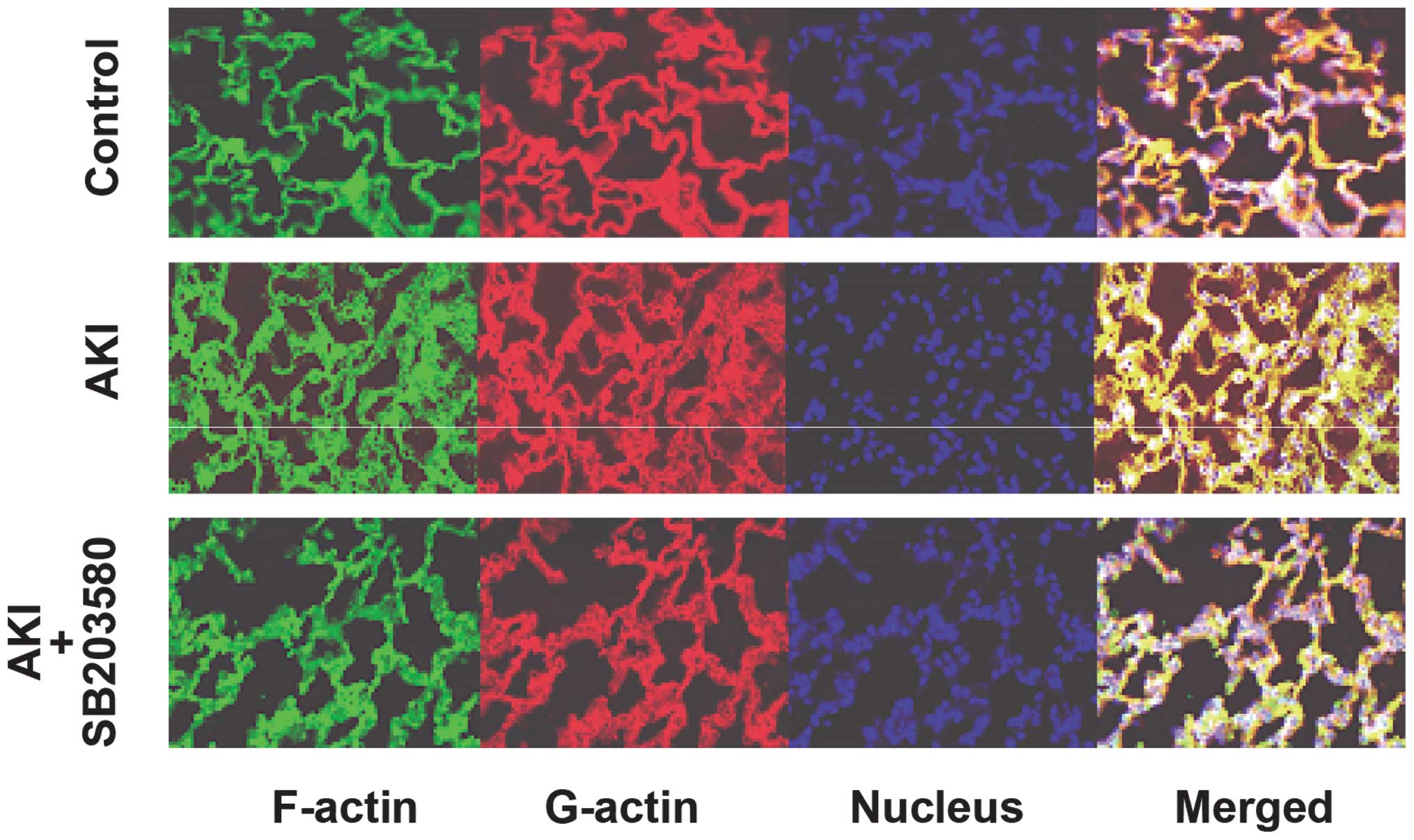
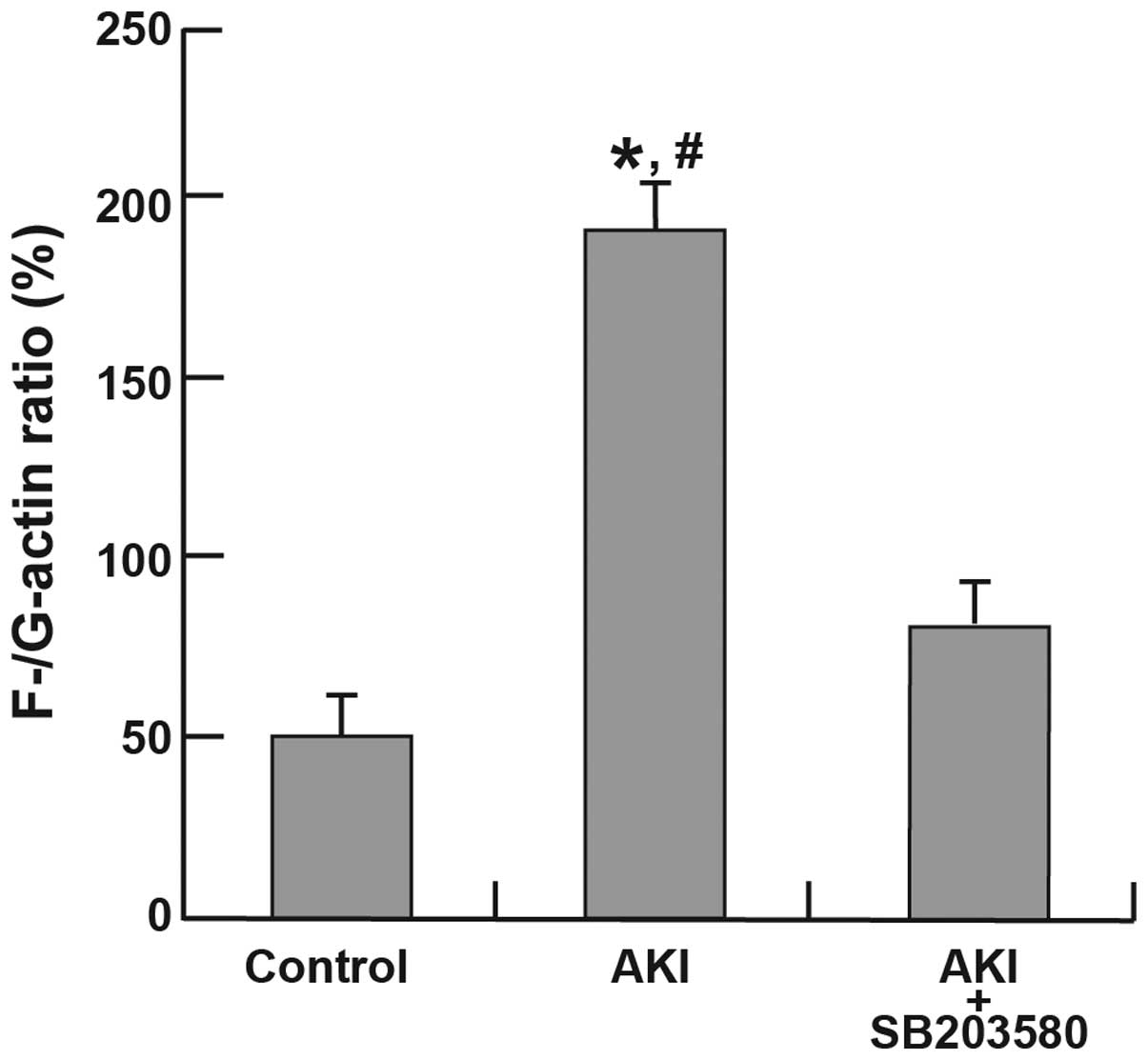
Alterations in the F-/G-actin ratio
Immunofluorescence analyses of F-actin and G-actin
are shown in Fig. 6. The
expression of F-actin in the AKI group was clearer than that in the
control and AKI + SB203580 groups. The F-/G-actin ratio in the AKI
group was also higher compared with that of the control and AKI +
SB203580 groups (all P<0.05; Fig.
7).
Discussion
AKI is a multifactorial syndrome and may be
accompanied by multiple organ injury, including ALI or acute
respiratory distress syndrome, particularly in critically ill
patients (15,16). Patients with AKI who are treated
with RRT have a high mortality rate of 50–60% (17). It is well established that AKI is
associated with a high mortality rate and distant organ
dysfunctions. The current theory is that AKI induces pathological
alterations of the lung, which predispose patients to a poor
prognosis (2). The lung is the
most easily damaged by ischemic injury due to the extensive
vascular network in the lung mesenchyme (7). Findings of previous studies have
indicated that ICU patients with AKI-induced ALI have a mortality
rate of ~80% (18). In a previous
study, it was found that ALI usually occurs during the early stages
of AKI, characterized by inflammatory cell infiltration, hyperemia
in alveolar space and alterations in the structure of pulmonary
alveoli (19). Microscopically,
evidence of pulmonary endothelial injury was observed, including
endothelial cell swelling, widening of interendothelial junctions
and an increased number of pinocytotic vesicles.
Impaired epithelial barrier integrity and function
are important features of ALI (20). It has been demonstrated that
endothelial cell structure is maintained by the cyto-skeleton,
which is possibly responsible for maintaining and restoring normal
cell morphology and functions (21). The actin microfilaments, which are
mainly composed of F-actin and actin-binding proteins, are a major
component of the cytoskeleton and are intricately involved
mechanically and biochemically in various cell processes, including
cell division and motility (22).
It is well established that the polymerization-depolymerization
dynamics of actin is a crucial process in a wide variety of
cellular functions (23). HSP27,
the F-actin polymerization modulator, is important in the
reorganization of the actin cytoskeleton network of cells (24). It has been demonstrated that
non-phosphorylated HSP27 was able to promote the reduction of
F-actin in cells, thereby inhibiting F-actin polymerization
(25). Activation of p38 caused
the activation of MAPK-activated protein kinase-2/3 and
phosphorylation of HSP27, leading to the F-actin polymerization and
extension, and thereby inducing the recombination of the actin
cytoskeleton and increase in intracellular space (26). These alterations may ultimately
lead to the impairment of the barrier function of pulmonary
endothelium and cause AKI.
In the present study, it was found that increased
p-HSP27 may be associated with increases in the F/G-actin ratio and
the degree of lung injury. It has been demonstrated that p-HSP27
was not able to inhibit F-actin polymerization and extension, which
may promote the impairment of the integrity and dysfunction of the
pulmonary endothelial and epithelial barrier (27). The present study also found that
the expression levels of p-HSP27 demonstrated an expected positive
correlation with p-p38 MAPK. The SB203580, an inhibitor of the p38
MAPK pathway, may decrease the expression levels of p-HSP27,
indicating that p38 MAPK regulates the expression of p-HSP27.
The present study has demonstrated that
ischemia-induced AKI rats exhibited significantly higher plasma
ADMA levels than those in the control group. H&E staining
showed the typical pathological alterations of ALI, including
alveolar inflammation, impairment of alveolar integrity and cell
infiltration. Furthermore, p-p38MAPK, p-HSP27 and F-actin also
progressively increased. Nevertheless, SB203580 was able to inhibit
the expression of p-HSP27 and F-actin followed by reciprocal
alleviation of ALI. A possible explanation may be that the
activation of p38 MAPK downregulated the activity of HSP27, thereby
inducing pulmonary vascular endothelial cell regeneration and
reorganization. These alterations may affect cell permeability. The
results suggested that the p38 MAPK/HSP27 signaling pathway is
important in the development of AKI-induced ALI.
In conclusion, the results from the present study
indicate that the plasma levels of ADMA may be a good biomarker for
AKI-induced ALI. Furthermore, the p38 MAPK/HSP27 pathway is
important in modulating the levels of ADMA.
Acknowledgments
The present study was supported by the Fund for
Scientific Research of The First Hospital Of China Medical
University (fsfh1103) and the Science and Technology project of
Shenyang (F11-264-1-52).
References
|
1
|
Liu KD, Himmelfarb J, Paganini E, et al:
Timing of initiation of dialysis in critically ill patients with
acute kidney injury. Clin J Am Soc Nephrol. 1:915–919. 2006.
View Article : Google Scholar
|
|
2
|
Himmelfarb J and Ikizler TA: Acute kidney
injury: changing lexicography, definitions, and epidemiology.
Kidney Int. 71:971–976. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Akcan-Arikan A, Zappitelli M, Loftis LL,
et al: Modified RIFLE criteria in critically ill children with
acute kidney injury. Kidney Int. 71:1028–1035. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mehta RL, Kellum JA, Shah SV, et al: Acute
Kidney Injury Network: report of an initiative to improve outcomes
in acute kidney injury. Crit Care. 11:R312007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schwenger V, Weigand MA, Hoffmann O, et
al: Sustained low efficiency dialysis using a single-pass batch
system in acute kidney injury – a randomized interventional trial:
the REnal Replacement Therapy Study in Intensive Care Unit
PatiEnts. Crit Care. 16:R1402012. View
Article : Google Scholar
|
|
6
|
Martin A and Acierno MJ: Continuous renal
replacement therapy in the treatment of acute kidney injury and
electrolyte disturbances associated with acute tumor lysis
syndrome. J Vet Intern Med. 24:986–989. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kuchnicka K and Maciejewski D:
Ventilator-associated lung injury. Anaesthesiol Intensive Ther.
45:164–170. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hassoun HT, Grigoryev DN, Lie ML, et al:
Ischemic acute kidney injury induces a distant organ functional and
genomic response distinguishable from bilateral nephrectomy. Am J
Physiol Renal Physiol. 293:F30–F40. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
White LE, Chaudhary R, Moore LJ, et al:
Surgical sepsis and organ crosstalk: the role of the kidney. J Surg
Res. 167:306–315. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Böger RH: Asymmetric dimethylarginine, an
endogenous inhibitor of nitric oxide synthase, explains the
‘L-arginine paradox’ and acts as a novel cardiovascular risk
factor. J Nutr. 134:2842S–2847S. 2004.
|
|
11
|
Vallance P and Leiper J: Cardiovascular
biology of the asymmetric dimethylarginine:dimethylarginine
dimethyl-aminohydrolase pathway. Arterioscler Thromb Vasc Biol.
24:1023–1030. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Palm F, Onozato ML, Luo Z, et al:
Dimethylarginine dimethyl-aminohydrolase (DDAH): expression,
regulation, and function in the cardiovascular and renal systems.
Am J Physiol Heart Circ Physiol. 293:H3227–H3245. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fliser D, Kronenberg F, Kielstein JT, et
al: Asymmetric dimethyl arginine and progression of chronic kidney
disease: the mild to moderate kidney disease study. J Am Soc
Nephrol. 16:2456–2461. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang LY, Zhang DL, Zheng JF, et al:
Apelin-13 passes through the ADMA-damaged endothelial barrier and
acts on vascular smooth muscle cells. Peptides. 32:2436–2443. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chertow GM, Burdick E, Honour M, et al:
Acute kidney injury, mortality, length of stay, and costs in
hospitalized patients. J Am Soc Nephrol. 16:3365–3370. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Togel F, Weiss K, Yang Y, et al:
Vasculotropic, paracrine actions of infused mesenchymal stem cells
are important to the recovery from acute kidney injury. Am J
Physiol Renal Physiol. 292:F1626–F1635. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hoste EA and Schurgers M: Epidemiology of
acute kidney injury: how big is the problem? Crit Care Med.
36:S146–S151. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Coca SG, Yusuf B, Shlipak MG, et al:
Long-term risk of mortality and other adverse outcomes after acute
kidney injury: a systematic review and meta-analysis. Am J Kidney
Dis. 53:961–973. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ma T and Liu Z: Functions of aquaporin 1
and alpha-epithelial Na+ channel in rat acute lung
injury induced by acute ischemic kidney injury. Int Urol Nephrol.
45:1187–1196. 2013. View Article : Google Scholar
|
|
20
|
Vadasz I, Raviv S and Sznajder JI:
Alveolar epithelium and Na,K-ATPase in acute lung injury. Intensive
Care Med. 33:1243–1251. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Banan A, Choudhary S, Zhang Y, et al:
Oxidant-induced intestinal barrier disruption and its prevention by
growth factors in a human colonic cell line: role of the
microtubule cytoskeleton. Free Radic Biol Med. 28:727–738. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rosenthal KS, Perez R and Hodnichak C:
Inhibition of herpes simplex virus type 1 penetration by
cytochalasins B and D. J Gen Virol. 66:1601–1605. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fujiwara I, Takahashi S, Tadakuma H, et
al: Microscopic analysis of polymerization dynamics with individual
actin filaments. Nat Cell Biol. 4:666–673. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Weber NC, Toma O, Wolter JI, et al:
Mechanisms of xenon- and isoflurane-induced preconditioning – a
potential link to the cytoskeleton via the MAPKAPK-2/HSP27 pathway.
Br J Pharmacol. 146:445–455. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Loktionova SA and Kabakov AE: Protein
phosphatase inhibitors and heat preconditioning prevent Hsp27
dephosphor-ylation, F-actin disruption and deterioration of
morphology in ATP-depleted endothelial cells. FEBS Lett.
433:294–300. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rousseau S, Houle F, Landry J, et al: p38
MAP kinase activation by vascular endothelial growth factor
mediates actin reorganization and cell migration in human
endothelial cells. Oncogene. 15:2169–2177. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xiong Z, Wang Y, Gong W, et al: Expression
of Hsp27 correlated with rat detrusor contraction after acute
urinary retention. Mol Cell Biochem. 381:257–265. 2013. View Article : Google Scholar : PubMed/NCBI
|