Introduction
The number of individuals with a high-fat diet and
sedentary lifestyle have increased worldwide. A high-fat diet
causes severe damage to organs and leads to serious complications
(1). The excessive intake of
calories in the diet and lack of exercise lead to a predisposition
towards obesity (2). Obesity is a
key risk factor for a decrease in insulin sensitivity, also
referred to as IR (3), in which
the sensitivities of organs or tissues to insulin are compromised
and can be lost, and the uptake and utilization efficiency of blood
glucose are decreased (4). IR may
trigger type 2 diabetes, metabolic syndrome, uarthritis or
cardiovascular diseases (1).
However, the mechanism of IR remain to be fully elucidated. A
previous study indicated that the phosphatidylinositol 3-kinase
(PI3K)/Akt signaling pathway, particularly the activation of Akt,
may be important in the development of IR (5). However, the exact role of these
proteins in the pathogenesis of IR remains to be elucidated.
Calcium-sensing receptor (CaSR) is an integral membrane protein,
which belongs to the G protein-coupled receptor 3 family (6). CaSR is expressed predominantly in the
liver, but is also expressed in the muscle, placenta and brain.
CaSR has been observed to be closely associated with the PI3K/Akt
pathway in cardiovascular and bone systems (7,8).
Another study reported that a CaSR antagonist inhibited Akt
activity, while its agonist significantly enhanced the activation
of Akt (9). Therefore, CaSR may be
involved in the patho-physiology of IR by affecting Akt. In the
present study, the possible correlation between the expression of
CaSR and the fat emulsion-induction of IR in rats was
investigated.
Materials and methods
Fat emulsion preparation
A 100 ml fat emulsion, containing lard (20%;
Lihongde Co., Ltd., Tianjin, China), propylthiouracil (1%;
Sigma-Aldrich, St. Louis, MO, USA), cholesterol (5%), sodium
glutamate (1%), fructose (5%) (Shanghai Huishi Biochemical Co.
Ltd., Shanghai, China), sucrose (5%; Tianjin Basifu Chemical Co.,
Ltd., Tianjin China) and edible salt (6%; China National Salt
Industry Co., Ltd., Beijing, China) in Tween 80 (20%; Tianjin Jin
Feng Chemical Co., Ltd., Tianjin, China) and propylene glycol (30%;
Tianjin Kemiou Chemical Reagent Co., Ltd., Tianjin China), was
prepared by adding 7 ml distilled water to 100 ml, as previously
reported (4), and stored at 4°C
until used to feed the animals. The procedures involving the use of
animals were approved by the Animal Experimental Ethical Committee
of Heilongjiang University of Chinese Medicine (Harbin, China).
Animals and rat IR model
Adult, 2-month-old male Wistar rats were purchased
from Yisi Lab Animal Technology Co. (Changchun, China), and were
housed individually in an air-conditioned facility with a 12-h
light/dark cycle at 25±1°C and 50 ± 5% humidity, and supplied with
food and water ad libitum. A total of 40 Wistar rats were
randomly divided into a control group (n=20) and an IR model group
(n=20). The rats in the control group were further divided into
four subgroups: 2 week control (n=5), 4 week control (n=5), 6 week
control (n=5) and 8 week control (n=5). Accordingly, the rats in
the IR model group were divided into four corresponding subgroups
with the same durations and numbers of animals as the control
groups. The rats in the IR model group were administered with a fat
emulsion (10 ml/kg) via gavage every day for 2, 4, 6 or 8 weeks,
whereas the control rats were administered with the same quantity
of distilled water, at the same time-points. Following 12 h
fasting, blood samples (1.5 ml) were collected from the abdominal
aorta of the rats to examine the concentrations of insulin and
blood glucose. Glucose meter determination (Accu-Chek; Roche,
Dublin, Ireland) was used to measure the levels of fasting blood
glucose (FBG), according to the manufacturer’s instruction. An
enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems,
Inc., Minneapolis, MN, USA) was used to investigate the levels of
fasting insulin (FINS). The rat insulin sensitivity index (ISI) was
calculated using the following equation: ISI = ln [1 / (FBG ×
FINS)]. At the end of the experiments, the animals were sacrificed
by cervical dislocation under 1% pentobarbital (Sigma-Aldrich) and
liver and skeletal muscle samples were harvested from all animals
and maintained in liquid nitrogen.
Immunofluorescence
The fresh liver and skeletal muscle tissues were
embedded in Optimum Cutting Temperature compound (Thermo Fisher
Scientific, Waltham, MA, USA). A freezing microtome (Thermo Fisher
Scientific) was used to prepare frozen sections of skeletal muscle
and liver. The frozen tissue sections were then fixed with cold
acetone (Luoyang Haohua Chemical Reagent Co., Ltd., Henan, China)
for 10 min. Following rinsing with phosphate-buffered saline (PBS;
GE Healthcare Life Sciences, Beijing, China), blocking with 0.1%
Triton X-100 (Solarbio, Beijing, China) and 10% bovine serum
albumin (Thermo Fisher Scientific) for 30 min at room temperature,
the sections were incubated with rabbit polyclonal CaSR antibody
(1:1,000; ab137408; Abcam, MA) at room temperature for another 1 h.
Following three 10 min washes with PBS, the sections were then
incubated with fluorescein isothiocyanate (FITC)-labeled second
antibody at room temperature for a further 1 h. Subsequently,
4,6-diamidino-2-phenylindole (Solarbio) was used to label the cell
nuclei, and the sections were then washed, mounted and examined
using a fluorescence microscope (IX51; Olympus, Tokyo, Japan).
RNA isolation
The liver and skeletal muscle mRNA was extracted
using a TRIzol kit (Invitrogen Life Technologies, Carlsbad, CA,
USA), according to the manufacturer’s instructions. Subsequently,
the total mRNA from all the samples was quantified using an
ultraviolet and visible spectrophotometer (BioSpecnano; Shimadzu
Corporation, Kyoto, Japan).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The total mRNAs from liver and skeletal muscle were
reversely transcribed to cDNA using AccuPower RocketScript RT
Premix (Bioneer Corporation, Daejeon, Korea). qPCR was then
performed in duplicate using an AccuPower GreenStar qPCR master mix
(Bioneer Corporation), according to the manufacturer’s
instructions. CaSR primers (forward 5′-ttctttgaacctggacgacgagt-3′
and reverse 3′-gcgaggaaggatttgtac-5′) were used for amplifying the
fragments. qPCR was performed using a q-PCR device from Bio-Rad
Laboratories (Hercules, CA, USA) using the following steps: 95°C
for 10 min, followed by 40 two-step cycles of 95°C for 15 sec and
47°C for 1 min. β-actin (forward primer
5′-gtcaggtcatcactatcggcaat-3′ and reverse
3′-agaggtctttacggatgtcaacgt-5′) was used as an internal control,
The relative expression values were calculated using the
2−∆∆Ct method. The qPCR products also were visualized
via agarose gel electrophoresis (AGE) to identify non-specific
bands.
Western blot analysis
Protein samples (50 µg) were separated on a 10% SDS
gel (Beyotime Institute of Biotechnology, Jiangsu, China) and
transferred onto a polyvinylidene (PVDF; Invitrogen Life
Technologies) membrane. The nonspecific proteins were blocked using
5% non-fat dry milk at 37°C for 1 h. Subsequently, each PVDF
membrane was incubated with the following primary antibodies at 4°C
for 12 h: Rabbit polyclonal anti-CaSR (1:1,000; ab137408; Abcam,
Cambridge, MA, USA); rabbit polyclonal protein kinase B (1:1,000;
ab106693; Abcam), rabbit monoclonal phosphorylated (phospho)-Akt at
Ser473 (1:1,000; ab81283; Abcam) and rabbit monoclonal phospho-Akt
at T308 (1:1,000; Cell Signaling Technology, Inc. Danvers, MA,
USA). The membranes were then incubated for 2 h with the goat
anti-rabbit horseradish peroxidase-conjugated affiniPure secondary
IgG antibody (1:1,000; ab6721; Abcam) and then visualized using
chemiluminescence (Solarbio).
Statistical analysis
The results are presented as the mean±standard error
of the mean (SEM), and the data were analyzed with SPSS, version
19.0 (IBM SPSS, Armonk, NY, USA). The data were analyzed using
Student’s t-tests P<0.05 was considered to indicate a
statistically significant difference.
Results
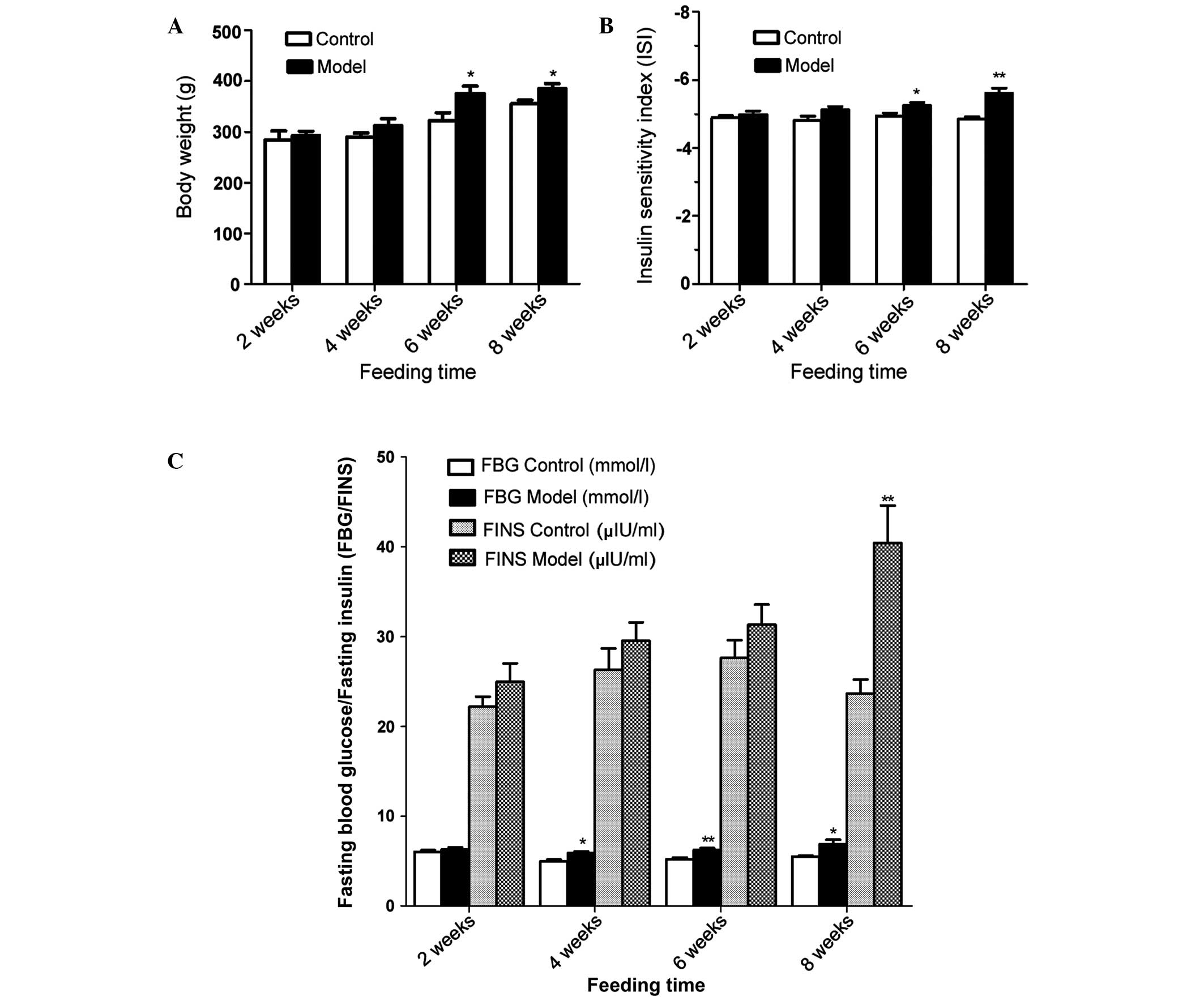
Body weight, FBG, FINS and ISI in rats
with fat emulsion-induced IR
The results demonstrated that a high-fat diet
induced a significantly increased body weight in the rats with IR,
and there were significant differences between the group fed for 6
weeks and that fed for 8 weeks (Fig.
1A). The ISI value decreased between −4.98 and −5.60 following
8 weeks on a high fat diet in the rats with IR, and there was a
significant difference in the ISI between the group fed for 6 weeks
and that fed for 8 weeks (Fig.
1B). In addition, as shown in Fig.
1C, the FBG values in the rats with IR were markedly higher
following 4, 6 and 8 weeks high-fat gavage-feeding compared with
those in the control group. Following 8 weeks on the high-fat diet,
the FINS was significantly increased in the IR group.
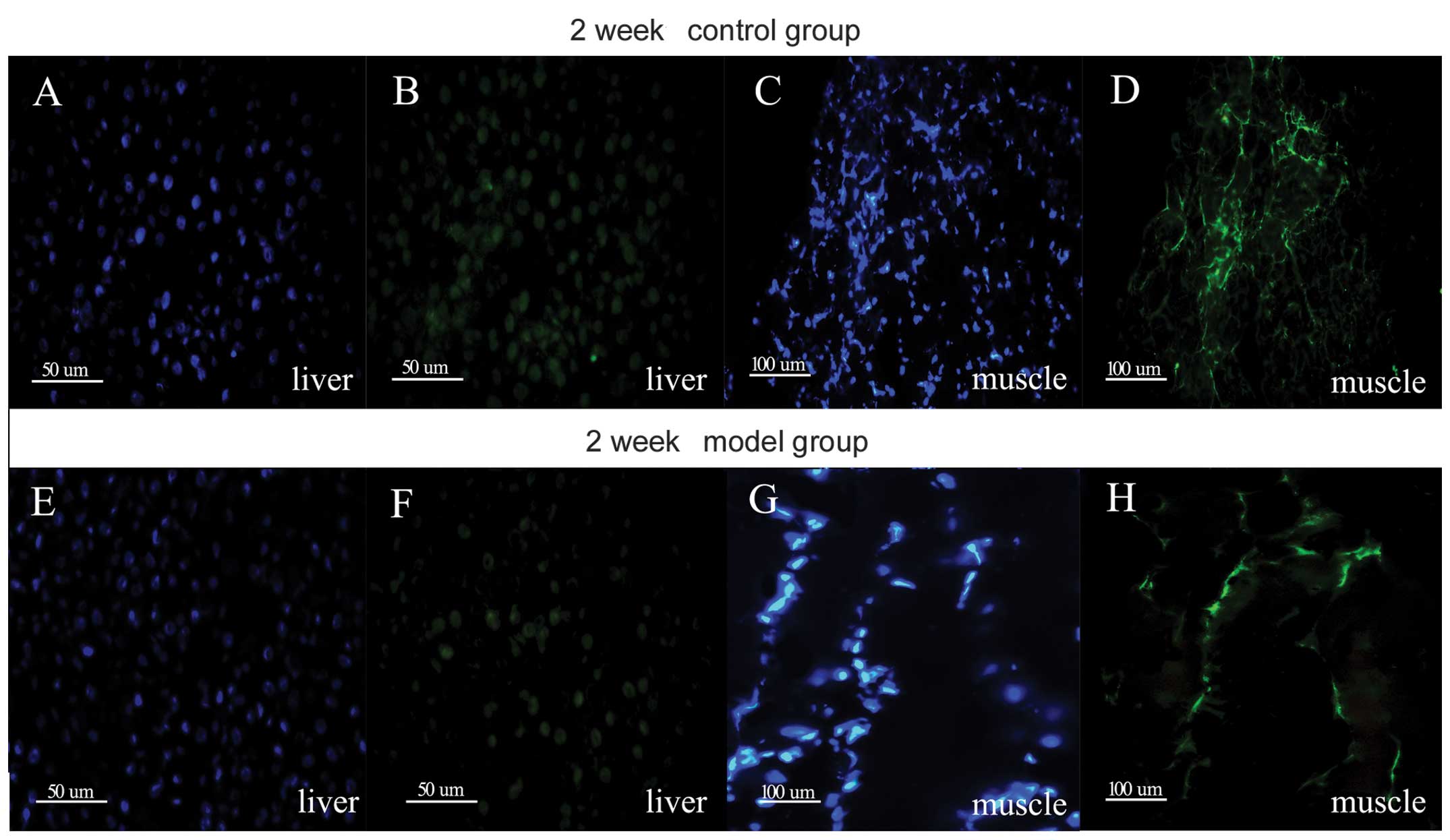
Protein expression of CaSR in the liver
and skeletal muscle of rats
To confirm the expression of CaSR in the rat liver
and skeletal muscle, immunostaining was performed on the liver and
skeletal muscle sections of rats in the control and IR groups (2
weeks). As shown in Fig. 2, the
liver and skeletal muscle demonstrated positive staining for the
expression of CaSR.
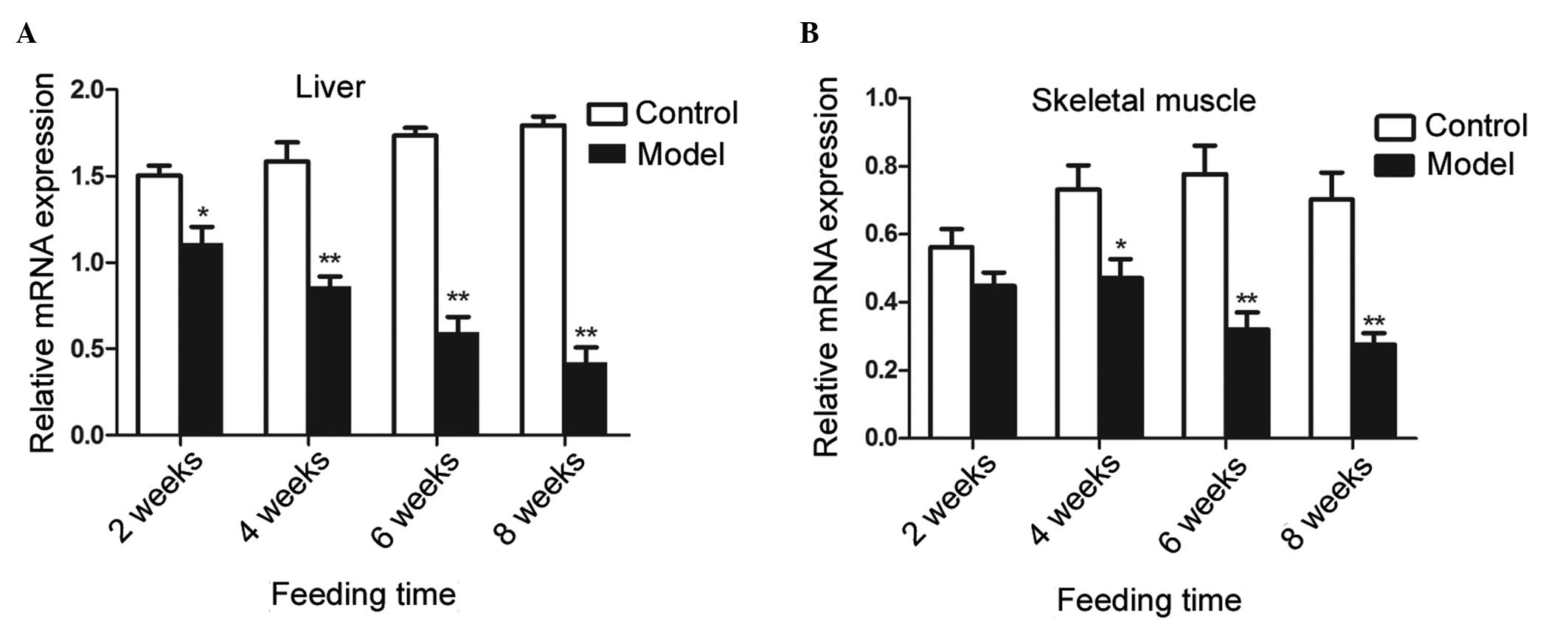
Expression of CaSR and activity of Akt in
the skeletal muscle and liver tissues of rats with or without a
high-fat diet
The present study subsequently examined whether CaSR
was regulated in response to a high-fat diet in rats. The mRNA
expression levels of CaSR in the liver and skeletal muscles were
downregulated in the IR group compared with the control group
(Fig. 3). The mRNA expression of
CaSR was significantly reduced in the IR group following 2, 4, 6
and 8 weeks a high-fat diet. In the muscle tissues, although no
significant difference in the mRNA expression levels of CaSR were
observed between the two groups following 2 weeks of feeding, the
mRNA level of CaSR was significantly reduced following 4, 6 and 8
weeks of feeding.
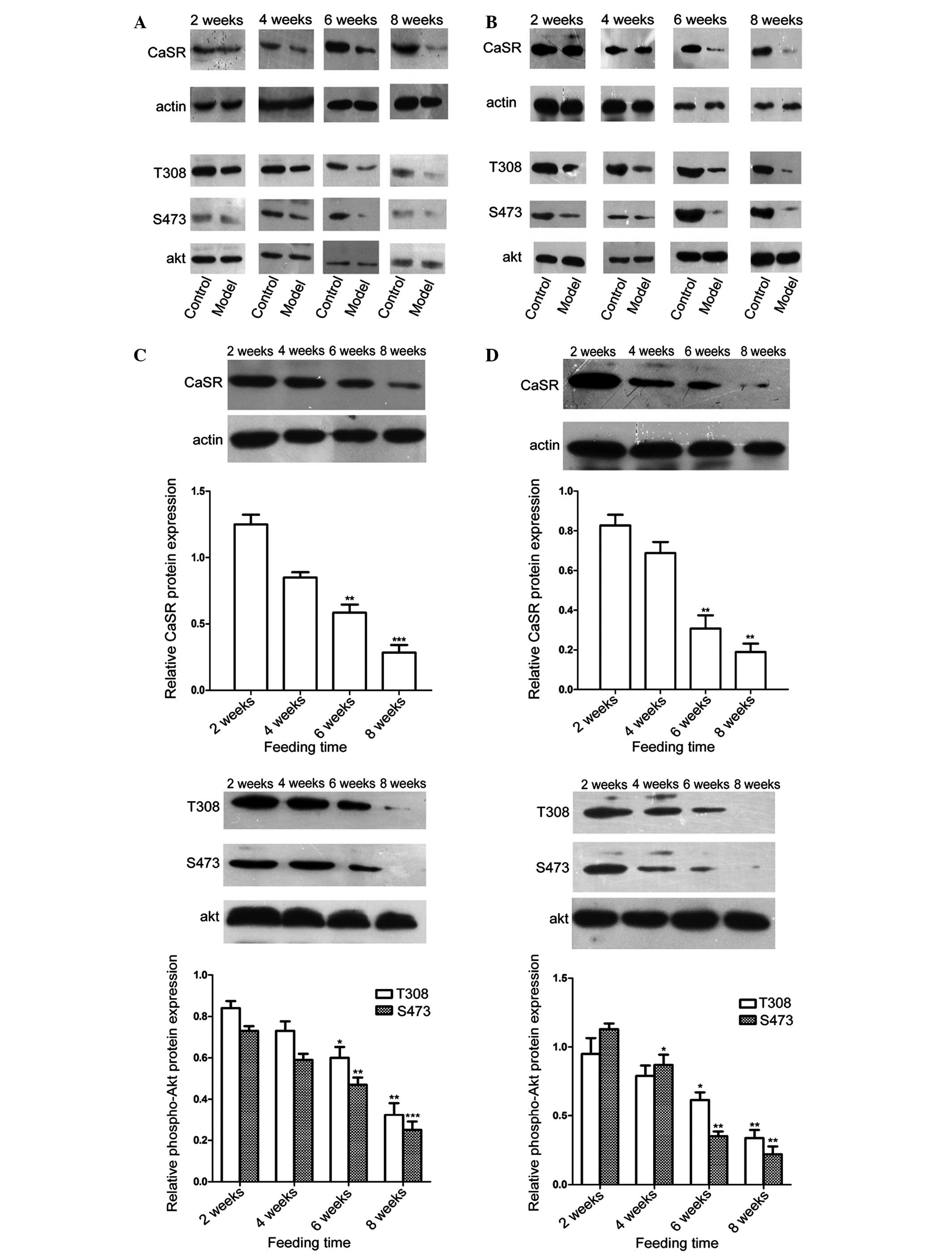
The protein expression levels of CaSR in the two
groups were also investigated using western blot analysis (Fig. 4). A notable decrease in the protein
expression of CaSR was observed in the IR group, compared with the
control group. The present study then assessed whether there was an
association between the protein expression of CaSR and Akt
activity. As shown in Fig. 4, the
expression levels of phospho-Akt (Ser473 and Thr308) in the liver
and skeletal muscles were also significantly lower in the IR group
than in the control group following 6 and 8 weeks of a high-fat
diet.
Discussion
The aim of the present study was to understand the
molecular mechanism responsible for high-fat diet-induced IR in a
rat model. At present, several methods are used to establish an IR
model, including genetic modification and feeding a high-fat diet
(10). In the present study, an IR
model was established by feeding rats with a fat emulsion via
gavage, which enabled control of the daily diet. The rats in the
control group were fed distilled water by gavage, in order to mimic
possible damage to the esophagus, which may occur due to gavage
feeding in the fat emulsion group. Using this animal model, the
present study aimed to clarify whether CaSR was involved in the
pathogenesis of fat emulsion-induced IR. The results demonstrated
that feeding rats fat emulsion for 6 weeks resulted in significant
differences in the ISI, FINS, FBG and body weights between the
control and IR groups. In addition, the high-fat diet had
time-dependent effects on IR in the rats fed a high fat diet.
IR refers to the loss of the sensitivity of body
target organs or tissues to insulin and the failure of insulin to
promote glucose uptake (3). The
liver and skeletal muscles are important in bodily glucose uptake
(11) and are considered to be the
major target tissues of insulin (12,13).
A previous study reported that CaSR knockdown compromised
receptor-induced insulin secretion in a genetically modified animal
model (14). Therefore, CaSR may
be a potential factor in increasing glucose-induced insulin
secretion. Homozygous genetic mutations and mitochondrial DNA
mutations of CaSR can lead to episodes of type 2 diabetes (15). All these previous findings suggest
that the CaSR may be involved in IR. However, the exact role of
CaSR in these processes remains to be fully elucidated.
In the present study, the expression levels of CaSR
were examined in the skeletal muscle and liver tissues of rats
using immunofluorescent staining, which provided a foundation for
further investigation. Using RT-qPCR, marked decreases in the gene
expression of CaSR were observed in the liver and skeletal muscle
tissues of rats with high-fat diet-induced IR, which was negatively
correlated with IR severity in these animals.
Several studies have demonstrated that the PI3K/Akt
pathway is a key factor during the pathological changes of IR,
particularly the activity of certain downstream kinases of Akt,
including protein kinase B and Rac (5). It has been established that the
mechanism by which growth factors and insulin increase Akt activity
involve the phosphorylation of the Thr308 and Ser473 sites of Akt
(5). Phosphorylation at these two
sites is an important step for the function of Akt in survival and
glycogenesis (16–18). Akt is also involved in cell cycle
regulation and may be involved in the regulation of insulin in
glucose transport (19,20). In the liver and muscles, insulin
induces the phosphorylation of Akt at Thr308 and Ser473 via the
activation of PDK1 (21). In the
present study, immunofluorescence staining revealed that the
protein expression of CaSR was decreased in the IR group following
2 weeks on a high-fat diet. The decreased expression of CaSR was
confirmed using western blot analysis. In addition, a significant
decrease was observed in the phosphorylation of Akt in the IR group
following 2, 4, 6 and 8 weeks of high-fat diet feeding. These
results suggested that the PI3K/Akt pathway was inhibited following
feeding with the high-fat diet. A significant reduction was also
observed in the protein expression of CaSR in the IR group, with a
notable increase in the levels of IR in these animals. These data
suggested that CaSR may have been responsible for the development
of IR in this high-fat animal model by inhibiting the activity of
Akt in the PI3k/Akt pathway.
In conclusion, the present study demonstrated the
expression of CaSR in the liver and skeletal muscles in rats. A fat
emulsion diet caused a significant decrease in the expression of
CaSR in the IR rat model. The data also suggested that the
expression of CaSR was closely associated with the activity of the
PI3K/Akt pathway, which is an important signaling pathway leading
to IR. These findings may offer a novel area of investigation of
the underlying molecular mechanisms by which IR occurs in animals
fed a high-fat diet.
Acknowledgments
The authors would like to thank Dr Shaohong Fang
from the Key Laboratory of Myocardial Ischemia, Harbin Medical
University of the Chinese Ministry of Education for her assistance
in technical support. This study was supported by grants from the
National Natural Science Foundation of China (no. 81273650),
Chinese Ministry of Science and Technology (no.
2012ZX09103201-018), Natural Science Foundation of Heilongjiang
province (no. LC2011C03); Harbin Science and Technology Bureau of
Heilongjiang Province (no. 2011RFLXS024); the ‘Excellent creative
talents support program’ of Heilongjiang University of Chinese
Medicine (no. 2012RCD19) and the Key Laboratory of Myocardial
Ischemia, Harbin Medical University, Chinese Ministry of Education
(no. KF201319).
References
|
1
|
Ai J, Wang N, Yang M, Du ZM, Zhang YC and
Yang BF: Development of Wistar rat model of insulin resistance.
World J Gastroenterol. 11:3675–3679. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Haslam DW and James WPT: Obesity. Lancet.
366:1197–1209. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Langeveld M and Aerts JM:
Glycosphingolipids and insulin resistance. Prog Lipid Res.
48:196–205. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Panzer C, Lauer MS, Brieke A, Blackstone E
and Hoogwerf B: Association of fasting plasma glucose with heart
rate recovery in healthy adults: a population-based study.
Diabetes. 51:803–807. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shepherd PR, Withers DJ and Siddle K:
Phosphoinositide 3-kinase: The key switch mechanism in insulin
signalling. Biochem J. 333:471–490. 1998.PubMed/NCBI
|
|
6
|
Justinich CJ, Mak N, Pacheco I, et al: The
extracellular calcium-sensing receptor (CaSR) on human esophagus
and evidence of expression of the CaSR on the esophageal epithelial
cell line (HET-1A). Am J Physiol Gastrointest Liver Physiol.
294:G120–G129. 2008. View Article : Google Scholar
|
|
7
|
Li GW, Xing WJ, Bai SZ, et al: The
calcium-sensing receptor mediates hypoxia-induced proliferation of
rat pulmonary artery smooth muscle cells through MEK1/ERK1,2 and
PI3K pathways. Basic Clin Pharmacol Toxicol. 108:185–193. 2011.
View Article : Google Scholar
|
|
8
|
Dvorak MM, Siddiqua A, Ward DT, et al:
Physiological changes in extracellular calcium concentration
directly control osteoblast function in the absence of calciotropic
hormones. Proc Natl Acad Sci USA. 101:5140–5145. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li HX, Kong FJ, Bai SZ, et al: Involvement
of calcium-sensing receptor in oxLDL-induced MMP-2 production in
vascular smooth muscle cells via PI3K/Akt pathway. Mol Cell
Biochem. 362:115–122. 2012. View Article : Google Scholar
|
|
10
|
King AJ: The use of animal models in
diabetes research. Br J Pharmacol. 166:877–894. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vangipurapu J, Stančáková A, Kuulasmaa T,
et al: Association between liver insulin resistance and
cardiovascular risk factors. J Intern Med. 272:402–408. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
El-Serag HB, Tran T and Everhart JE:
Diabetes increases the risk of chronic liver disease and
hepatocellular carcinoma. Gastroenterology. 126:460–468. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Walker KS, Deak M, Paterson A, Hudson K,
Cohen P and Alessi DR: Activation of protein kinase B beta and
gamma isoforms by insulin in vivo and by
3-phosphoinositide-dependent protein kinase-1 in vitro: comparison
with protein kinase B alpha. Biochem J. 331:299–308.
1998.PubMed/NCBI
|
|
14
|
Leech CA and Habener JF: Regulation of
glucagon-like peptide-1 receptor and calcium-sensing receptor
signaling by L-histidine. Endocrinology. 144:4851–4858. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ohkubo E, Aida K, Chen J, et al: A patient
with type 2 diabetes mellitus associated with mutations in calcium
sensing receptor gene and mitochondrial DNA. Biochem Biophys Res
Commun. 278:808–813. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Franke TF, Kaplan DR and Cantley LC: PI3K:
downstream AKTion blocks apoptosis. Cell. 88:435–437. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Burgering BM and Coffer PJ: Protein kinase
B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction.
Nature. 376:599–602. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Franke TF, Yang SI, Chan TO, et al: The
protein kinase encoded by the Akt proto-oncogene is a target of the
PDGF-activated phosphatidylinositol 3-kinase. Cell. 81:727–736.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hajduch E, Litherland GJ and Hundal HS:
Protein kinase B (PKB/Akt) - A key regulator of glucose transport?
FEBS Lett. 492:199–203. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Diehl JA, Cheng M, Roussel MF and Sherr
CJ: Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis
and subcellular localization. Genes Dev. 12:3499–3511. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Alessi DR, Andjelkovic M, Caudwell B, et
al: Mechanism of activation of protein kinase B by insulin and
IGF-1. EMBO J. 15:6541–6551. 1996.PubMed/NCBI
|