Introduction
Epilepsy is a relatively common neurologic disorder
in children (1). Although
conventional anti-epileptic drugs (AEDs) control the disorder in
the majority of patients, they fail to provide therapeutic benefit
in 20–25% of patients (2). In
addition, the serious adverse effects of AEDs on brain development,
particularly on cognitive function, has provoked further research
on non-AED management modalities. A number of previous studies have
investigated alternative treatments for epilepsy, including the use
of melatonin, a ketogenic diet, vitamins or biofeedback (3,4).
Among these, melatonin has been used as an adjunct treatment for
pediatric epilepsy.
Melatonin is a hormone, which is made in the pineal
gland. Apart from its well-recognized roles as an anti-aging
substance, sleep aid and antioxidant, clinical data suggests that
it is effective in the adjunctive therapy of osteoporosis, sepsis,
jet-lag syndrome, neurodegenerative diseases, certain types of
isomnia and even cancer (3). As
for epilepsy, melatonin may have clinical benefits. In a previous
study, 10 paediatric patients suffering from severe epileptic
disorders were administered a nightly dose of 3 mg melatonin. The
results demonstrated regained periodic plasma melatonin levels and
improved control in convulsive episodes (5). Baseline melatonin levels may be low
in childhood refractory epilepsy and febrile seizures, and levels
increase markedly following seizures (6–8).
Another study demonstrated that treatment with melatonin (10 mg
daily at night) decreased diurnal seizures in 10 patients aged
between 9 and 32 years with intractable epilepsy (9). The majority of experimental data also
indicates the anticonvulsant properties of the hormone in maximal
electroshock, amygdale kindling, pentylenetetrazole (PTZ),
pilocarpine, penicillin or kainic- and quinolinic acid-induced
animal models of epilepsy (10).
However, the suggestion that melatonin has anticonvulsant
properties remains controversial. For example, oral melatonin (5
mg) in neurologically disabled children was observed to result in
increased seizures 13 days following the onset of the therapy. The
proconvulsant effects disappeared immediately following treatment
discontinuation (11). In a
hippocampal slice seizure model, induced using low Mg2+
or bicuculline, the pharmacological concentration of melatonin
enhanced the frequency of epileptiform activity, whereas this
effect was suppressed by luzindole, a melatonin antagonist
(12). These results suggest that
further investigation is required prior to establishing melatonin
as a potential drug candidate for adjunctive therapy in children
with epilepsy.
In the majority of experimental seizure models, the
anticonvulsant action of melatonin has been observed in adult
animals in rats, mice, hamster and guinea pigs. However, there has
been little investigation of the effects of melatonin in
developmental animals. Using a phenobarbital-induced neonatal
seizure rat model, Forcelli et al observed that melatonin
(0–80 mg/kg), prior to PTZ, potentiated the anticonvulsant efficacy
of phenobarbital, however, it did not exert anticonvulsant effects
alone. This previous study did not further investigate the
underlying molecular mechanisms (13). Our previous study investigated the
dynamic expression pattern of mossy fiber sprouting-associated
genes in the rat brain using a flurothyl-induced recurrent neonatal
seizure model. Furthermore, this model, as described extensively,
has been widely used to evaluate the neuroprotective efficacy of
compounds, including autophagy inhibitor 3-MA, CBI and E-64d
(14–16). The important function of melatonin
in epilepsy, and the requirement to elucidate its role in neonatal
seizure-induced long-term excitotoxicity, prompted the present
study to further examine whether pretreatment with melatonin
alleviates the deleterious changes in the hippocampus. These
changes are indicated by hippocampal mossy fiber sprouting and
metabolic-associated genes, which are integral components of
developmental brain injury-induced epileptogenesis (14,15).
Materials and methods
Animal preparation
Sprague-Dawley rats were treated in accordance with
the guidelines set by the National Institutes of Health for the
humane treatment of animals. The litters were randomly assigned to
an experimental group and the animals were weaned on postnatal day
21, and following this age they were housed in a standard
light-dark cycle. The present study was approved by the ethics
committee of Soochow University Affiliated Children’s Hospital
(Suzhou, China). Attempts were made to minimize the number of
animals used. A total of 48 Sprague-Dawley neonatal rats at
postnatal day 6 (P6) were randomly divided into four groups:
Control (Cont), melatonin-treated control (Mel), recurrent neonatal
seizure (RS) and melatonin and RS combined treatment (Mel+RS). The
procedure of seizure induction was described in detail previously
(16). In brief, the neonatal rats
were placed into a transparent plastic airtight box and liquid
volatile flurothyl (bis-2,2,2-triflurothyl ether; Sigma-Aldrich,
St. Louis, MO, USA) was added, using a syringe, onto filter paper
in the center of the container, for agent evaporation. The rats
were immediately removed from the chamber on observation of tonic
extension of the forelimbs and hindlimbs. The experimental rats
received 45 induced seizures during over nine consequetive days,
between P6 and P14. The rats had five seizures/day over this
duration, with 30 min intervals between each seizure. The control
rats were placed into the container for an equal duration without
exposure to the flurothyl. In the two melatonin-treated groups,
each rat was pretreated with an intraperitoneal (i.p) injection of
melatonin prior to seizure induction (55 mg/kg, Sigma-Aldrich, St.
Louis, MO, USA) (17,18).
For molecular investigations, the animals were
sacrificed by decapitation on P35 for subsequent Timm staining and
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) analyses.
Timm staining
A total of six rats from each group were
anesthetized using chloropent (3 ml/kg; i.p; Sinopharm Chemical
Reagent Shanghai Co., Ltd., Shanghai, China). The method used has
been described in detail previously (19). Rats from each group were perfused
through the heart with 0.9% saline, followed by 0.375% sodium
sulfide and 4% paraformaldehyde in PBS. The brains were sectioned
into 30 μm-thick coronal tissue sections from the septal
area, where the two blades of the hippocampal dentate and pyramidal
CA3 region were equal. The processing solutions consisted of 30%
gum Arabic, 3.825% citric acid, 3.525% sodium citrate, 3.525%
hydroquinone and 25.5% silver nitrate. Timm staining was analyzed
under a ×10 objective on a light microscope.
RT-qPCR
A total of six rats from each group were
anesthetized using chloropent (3 ml/kg; i.p.). The method has been
described in detail previously (19). The total RNA was extracted from
each fresh hippocampal sample using TRIzol reagent (Invitrogen Life
Technologies, Carlsbad, CA, USA). The concentration, purity and
quantitiy of the total isolated RNA was determined by ultraviolet
spectrophotometry, which were of high quality and purity by
measuring the optical density at 260 and 280 nm. The total RNA (2
μg) was reverse transcribed into cDNA using random primers,
200 units of MMLV reverse transcriptase (Invitrogen Life
Technologies), 0.5 mM dNTPs, 10 mM dithiothreitol and 25 unitsof
RNase inhibitor (Invitrogen Life Technologies). RT reaction (40
μl) was performed at 37°C for 60 min, then at 95°C for 5
min. RT-qPCR was performed using TaqMan probe-based chemistry
(Applied Biosystems, Foster City, CA, USA). The primers and probes
of the 19 genes were designed against GenBank-published sequences
using Primer Express 2.0 software (Applied Biosystems Life
Technologies, Inc., Foster City, CA, USA). The sequences are listed
in the subsequent figures. The 19 genes were as follows: Kcnj11,
Lepr, Drd2, Mc4r and CCK, which are involved in regulation of
energy metabolism; Apoa1, Oprk1, Pdk4, Cyp46a1, ACAT1, nSMase and
Cathepsin-E, which are involved in regulation of lipid metabolism;
NR1, NR2B, GABA-A-α1, CaMKII alpha and beta, which are involved in
the regulation of neural excitability; ZnT-1 and MT-1, which are
involved in the regulation of zinc metabolism. The RT-qPCR
threshold cycle (CT) of the target mRNAs and the internal control
β-actin was determined and the ΔCT method of relative
quantification was used to determine the fold changes in
expression. The ratios of the target gene to β-actin were
calculated as follows: 2CT(target) − CT(β-actin). ΔCT =
CT target − CT β-actin). The fold change in expression was then
obtained using the 2−ΔCT method (20).
The gene expression levels (2−ΔCT) were
compared with post-hoc comparisons using a Bonferroni test
following analysis of variance using SAS 8.0 statistical software.
Data are presented as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Results
Timm staining
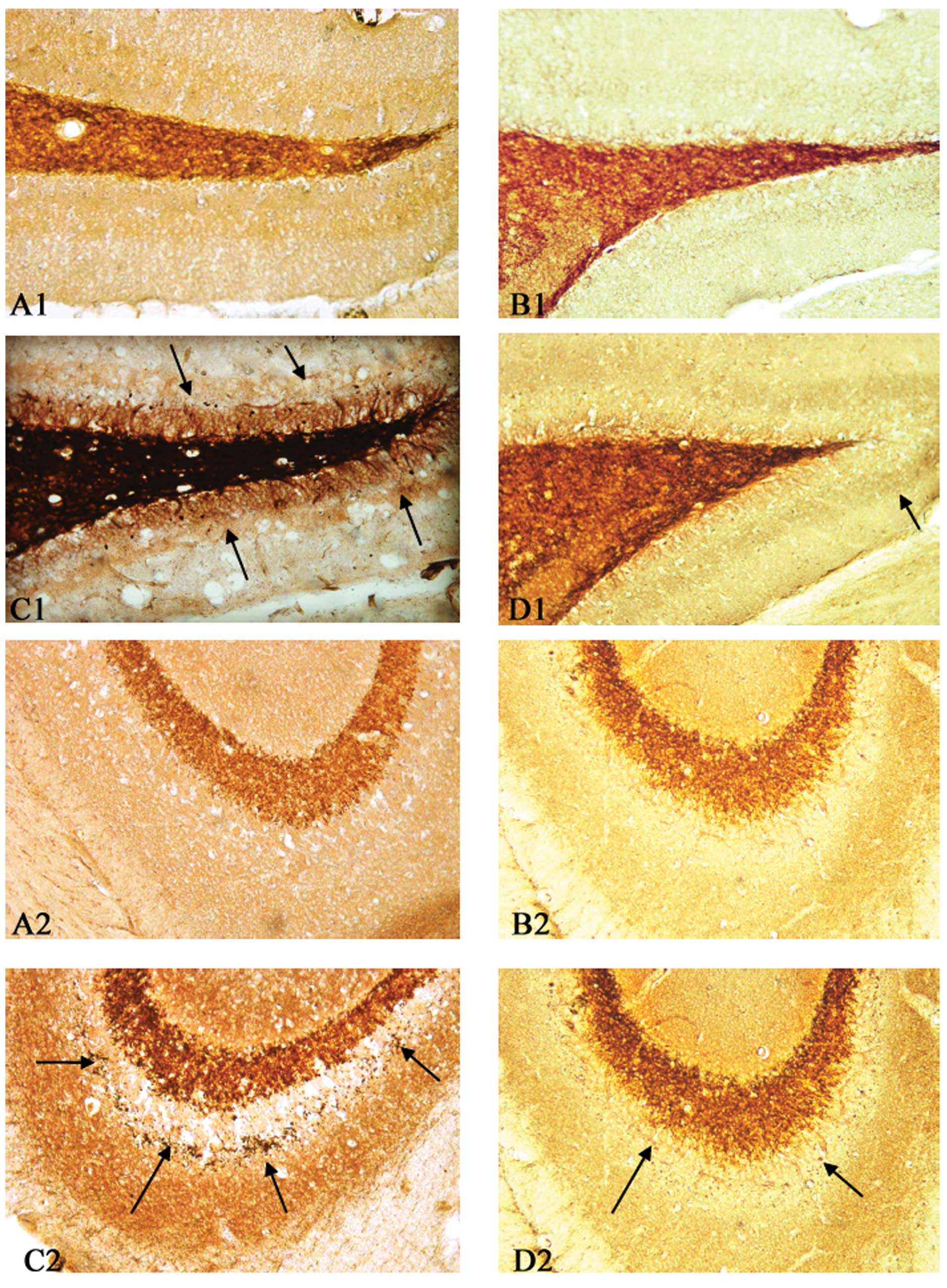
The results of the Timm staining revealed a
prominent aggregation of mossy fiber terminals in the supragranular
region of the dentate gyrus (Fig.
1C1) and CA3 subfield (Fig.
1C2). The aberrant mossy fiber sprouting in the dentate gyrus
(Fig. 1D1) and in the stratum
pyramidale of the CA3 subfield of the hippocampus (Fig. 1D2) was significantly inhibited
following pretreatment with melatonin. No mossy fiber terminals
were observed in the two control hippocampi (Fig. 1A and B).
RT-PqCR analysis
From the 19 genes examined, four energy
metabolism-associated genes (Kcnj11, Lepr, Drd2 and Mc4r; Fig. 2), four lipid metabolism-associated
genes (Apoa1, Oprk1, Pdk4 and Cyp46a1; Fig. 3), and ZnT-1, nSMase and Cathepsin-E
(Fig. 4), were markedly
downregulated in the Mel group and in the RS and Mel+RS groups,
compared with those in the Cont group. However, a significant
upregulation was observed in the mRNA expression levels of CaMKIIα,
ACAT1, ZnT-1, MT-1, nSMase and Cathepsin-E in the hippocampus of
the rats in the Mel+RS group, compared with those in the RS group
(Figs. 4 and 5).
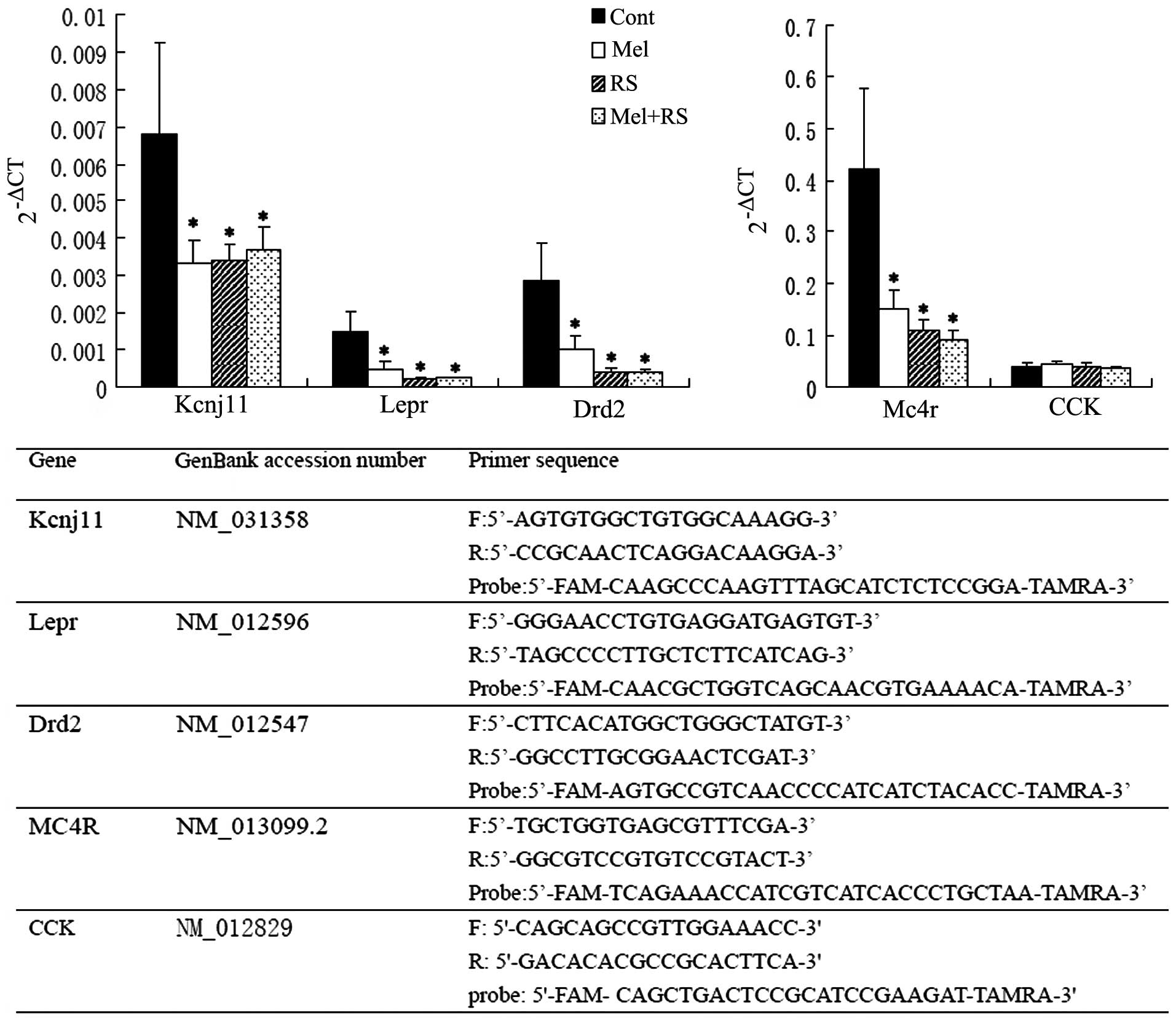 | Figure 2RT-qPCR analysis of hippocampal energy
metabolism-associated genes (Kcnj11, Lepr, Drd2, Mc4r and CCK). On
comparing the mRNA levels between the Cont group and the three
treatment groups, Kcnj11, Lepr, Drd2 and Mc4r were significantly
different (*P<0.05, compared with the Cont group).
Data are presented as the mean ± standard deviation. The lower part
of the chart shows the oligonucleotide primers used for the RT-qPCR
analysis. RT-qPCR, reverse transcription-quantitative polymerase
chain reaction; Cont, untreated control; Mel, melatonin treatment;
RS, recurrent neonatal seizure; Kcnj11, adenosine
triphosphate-sensitive potassium channel subunit; Lepr, leptin
receptor; Drd2, dopamine receptor D2; Mc4r, melanocortin 4
receptor; CCK, cholecystokinin; CT, threshold cycle. |
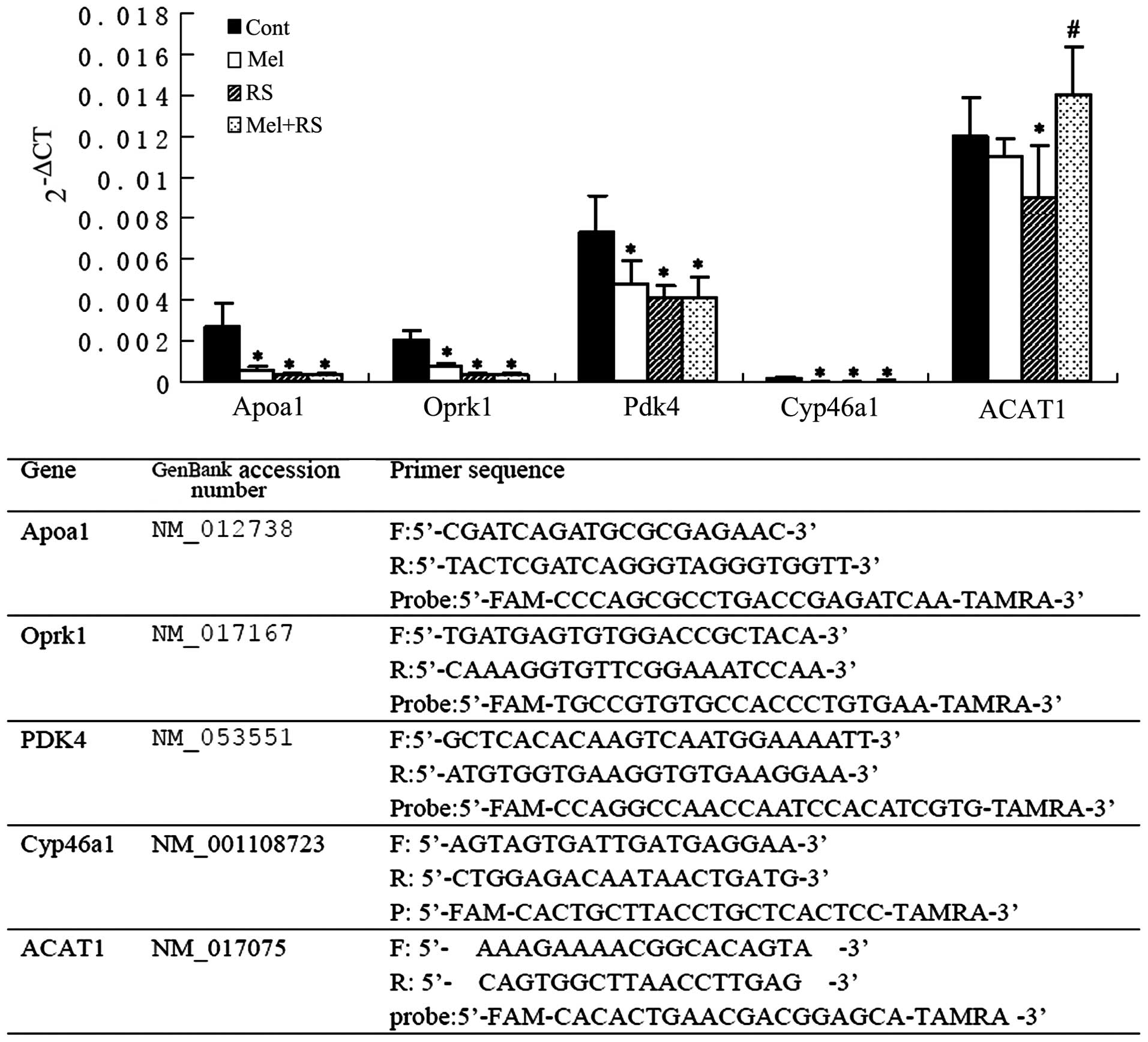 | Figure 3RT-qPCR analysis of hippocampal lipid
metabolism-associated genes (Apoa1, Oprk1, Pdk4, Cyp46a1 and
ACAT1). On comparing the mRNA levels between the Cont group and the
other three groups, significant differences were observed in the
expression levels of Apoa1, Oprk1, Pdk4 and Cyp46a1
(*P<0.05, compared with the Cont group). Notably, the
expression of ACAT1 in the RS group was significantly
downregulated, compared with the Cont group,, however, the mRNA
level was increased in the Mel+RS group, compared with the RS group
(#P<0.05). Data are presented as the mean ± standard
deviation. The lower part of the chart shows the oligonucleotide
primers used for real-time RT-qPCR analysis. RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; Cont,
untreated control; Mel, melatonin treatment; RS, recurrent neonatal
seizure. Apoa1, apolipoprotein A-I; Oprk1, opioid receptor κ 1;
Pdk4, dehydrogenase kinase, isozyme 4; Cyp46a1, cytochrome P450,
family 46, subfamily a, polypeptide 1; ACAT1, acetyl-Coenzyme-A
acetyltransferase 1; CT, threshold cycle. |
 | Figure 4RT-qPCR analysis of hippocampal
zinc/lipid-associated genes (ZnT-1, MT-1, nSMase and Cathepsin-E).
Notably, the expression levels of the four genes in the RS group
were significantly downregulated, compared with those in the Cont
group (*P<0.05), however, the mRNA levels of the four
genes were increased in the Mel+RS group, compared with those in
the RS group (#P<0.05). Data are presented as the
mean ± standard deviation. The lower part of the chart shows the
oligonucleotide primers used for RT-qPCR analysis. RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; Cont,
untreated control; Mel, melatonin treatment; RS, recurrent neonatal
seizure; ZnT-1, zinc transporter 1; MT-1, metallothionein 1;
nSMase, neutral sphingomyelinase; CT, threshold cycle. |
 | Figure 5RT-qPCR analysis of hippocampal
neural excitability-associated genes (CaMKIIα and β, NR1, NR2B and
GABA-A-α1). Notably, the expression of CaMKIIα in the RS group was
significantly downregulated, compared with the Cont group
(*P<0.05), however, the mRNA level was increased in
the Mel+RS group, compared with the RS group
(#P<0.05). Data are presented as the mean ± standard
deviation. The lower part of the chart shows the oligonucleotide
primers used for RT-qPCR analysis. RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; Cont,
untreated control; Mel, melatonin treatment; RS, recurrent neonatal
seizure; CaMK II calcium/calmodulin-dependent protein kinase II;
NR1, N-methyl-D-aspartate receptor; NR2B, GABA-A-α1, GABA-A
receptor α 1; NR2B, N-methyl D-aspartate 2B; CT, threshold
cycle. |
Discussion
The results of the present study indicated that the
animals which underwent pretreatment with melatonin exhibited a
minor grade of mossy fiber sprouting in the hippocampus, compared
with those in the RS group. In addition to this morphological
change, the gene expression analysis of a range of hippocampal
metabolism-associated genes demonstrated that pretreatment with
melatonin led to a significant upregulation of the mRNA expression
levels of CaMKIIα, ACAT1, ZnT-1, MT-1, nSMase and Cathepsin-E in
the hippocampi of rats in the Mel+RS group, compared with the RS
group.
Hippocampal signaling pathways have been extensively
investigated and are considered the predominant target of melatonin
for preventing and treating neurological diseases and injuries,
including autism, cerebral ischemia and cognitive impairment
(21–25). In terms of epilepsy, several
independent studies have investigated the effects of melatonin on
biochemical alterations in experimental epilepsy models. In a
kainate (KA) model of temporal lobe epilepsy, Tchekalarova et
al demonstrated that melatonin reduces neuronal damage in the
CA1 area of the hippocampus and piriform cortex, and recovers the
decrease in hippocampal levels of serotonin (5-HT) in rats with
epilepsy (26). Jain et al
provided evidence that KA and melatonin-treated animal groups
exhibit reduced numbers of nicotinamide adenine dinucleotide
phosphate reduced diaphorase positive neurons in the dentate gyrus,
hilus, CA1 and CA3 areas of the hippocampus and a decline in
cytosolic Ca2+ concentrations compared with
treatment with KA alone, suggesting enhanced levels of cytosolic
Ca2+ and nitric oxide (NO) in KA-induced
excitotoxicity (27). Mareš et
al demonstrated that flurothyl-induced single tonic-clonic
seizures caused significant increases in hydroxyl and nitroxyl
radicals 60 min following the seizure, which was inhibited
following pretreatment with melatonin prior to seizure induction,
compared with animals without pretreatment (28). In addition, Atanasova et al
observed that pretreatment with melatonin (10 mg/kg per day for 14
days) attenuates the KA-induced increase in the level of lipid
peroxidation, superoxide dismutase/CuZn production and expression
of heat shock protein 72 in the hippocampus (29). Taken together, the above
observations suggest that the efficacy of melatonin exposure prior
to seizures may be associated with the expression of genes in the
hippocampus, particularly oxidative stress-associated genes.
However, the key signaling molecules underlying its efficacy remain
to be elucidated. Due to the anticonvulsant properties of melatonin
and that hippocampal mossy fiber sprouting is an integral component
of brain injury-induced epileptogenesis, the biochemical processes
of melatonin may be elucidated by analyzing the expression of
sprouting-associated genes in the hippocampus following
seizures.
In our previous study, the abnormal expression of
zinc/lipid transporter-associated genes in hippocampus was
observed, which may be associated with mossy fiber sprouting
following penicillin-induced developmental seizures. The results
revealed upregulated expression levels of ACAT1, clusterin and
ApoE, and the downregulated expression of ZnT-1 following
developmental seizures, compared with the control animals. In
addition, the upregulation of ApoE and Clusterin was inhibited
following pretreatment with E-64d antophagy inhibitor (15). However, whether melatonin exerts
its anticonvulsant effects by regulating lipid/zinc
metabolism-associated pathway in the hippocampus has not been
previously investigated.
The results of the present study demonstrated, by
analyzing the expression pattern of a number of energy/lipid
metabolism-associated genes, that the transcriptional regulation of
several metabolism-associated molecules, including
ACAT1/nSMase/Cathepsin-E and ZnT-1/MT-1, and with CaMKIIα, may be
important for the neuroprotective effect of melatonin following
neonatal seizures. Using a flurothy-induced prolonged neonatal
seizure model, in which the rats inhaled flurothyl continuously for
30 min/day for six consecutive days, and semi-quantitative PCR, our
previous study revealed the downregulated expression levels of
ZnT-1 and CaMKIIα in the hippocampus, which may be associated with
long-term cognitive deficits and hippocampal mossy fiber sprouting
(30). The results of the present
study are in accordance with these findings, however, there are
several differences between the two studies. In the present study
used a recurrent, rather than prolonged, neonatal seizure model,
the experimental rats received 45 induced seizures during nine
consecutive days (five seizures/day, minimum 30 min interval).
Secondly, RT-qPCR and the 2−ΔCT methods were used in the
present study, rather than semi-quantitative PCR. In addition, the
present study further evaluated the intervention effects of
melatonin on hippocampal mossy fiber sprouting and associated
metabolism-associated genes. The results of the present study
demonstrated for the first time, to the best of our knowledge that
the ZnT-1, CaMKIIα and MT-1 genes associated with zinc and calcium
transduction, and ACAT1, nSMase and Cathepsin-E, which function as
modulators of lipid metabolism, may be of particular importance for
the inhibitory effects of melatonin on hippocampal mossy fiber
regenerative sprouting. It has been established that a ketogenic
diet, in which >90% of calories are derived from fat, is an
effective treatment for lipid metabolism-associated neurological
diseases, including epilepsy (31). Notably, all three of the
above-mentioned molecules may be targets for the inhibitory effects
of the ketogenic diet on epilepsy. Acetyl-co A acetyltransferase
(ACAT1) is a mitochondrial enzyme involved in ketogenic pathway
metabolism. ACAT1 performs the final step in ketolysis during the
processing of fats. It converts one acetoacetyl-CoA into two
molecules of acetyl CoA during ketolysis. Coincidentally, it has
been hypothesized that ketone bodies contribute to the
anticonvulsant and antiepileptic effects of a ketogenic diet
(32). Therefore, the present
study hypothesized that the downregulation of ACAT1 mRNA observed
in the present study can lead to decreased ketolysis. Therefore,
the resulting increase in the level of ketones may trigger the
compensatory anticonvulsant effects. In parallel with the
dowregulated expression of ACAT1, the present study also observed
downregulated expression levels of Cathepsin-E and nSMase.
Cathepsin-E is an aspartic proteinase. Abnormal levels of
cathepsin-E have been observed in tumor, senile plaques of
Alzheimer’s disease and KA-injected rat brains (33–35).
Notably, using microarray data and subsequent RT-PCR experiments,
Jeong et al demonstrated that the hippocampal expression of
cathepsin E was modulated by a ketogenic diet in a KA-induced
seizure model (4).
Sphingomyelinase (SMase) is an enzyme responsible for ceramide,
ceramide 1-phosphate, sphingosine and sphingosine 1-phosphate
production in the brain by catabolizing glycosphingolipids. These
metabolites modulate the activity of phospholipase A(PLA) (3), and exogenous PLA (3) can increase ketogenesis, while a
ketogenic diet inhibits brain ganglioside GM2 accumulation
(36,37). This cross talk between metabolites
of glycerophospholipid and sphingolipid metabolism suggests an
important role of SMase in the processes of ketogenic diet-mediated
neuroprotection following developmental- seizure-induced
neuropathology.
Ruiz et al (38) examined the expression levels of
energy metabolism-associated genes in the hippocampus of
age-matched control and chronic epileptic animals. The results
demonstrated that Kcnj11 was significantly upregulated 24 h, 1
month and 2 months post-SE. The results of the present study also
revealed downregulated expression levels of Kcnj11, and of Lepr,
Drd2 and Mc4r in the hippocampi of rats in the RS group compared
with those in the Cont group. Kcnj11 is a subunit of the
KATP channel, which is involved in neuroexcitability and
cognitive function, and mutations in the KATP channel in
humans have been linked to developmental delay, epilepsy and
neonatal diabetes syndrome (39).
Mice lacking this channel are prone to seizures when subjected to
brief periods of hypoxia (40).
Mc4r, leptin and Drd2 are present in the brain and hypothalamus,
which are involved in regulating food intake and energy metabolism.
Studies have demonstrated that the three genes can have
antiepileptic effects in the brain (41–43).
Therefore, the downregulated expression levels of Mc4r, Drd2 and
leptin in the RS group in the present study may be associated with
seizure propagation during epileptogenesis.
In a previous study by Ueda et al, genes
associated with lipid metabolism, Apoa1, Gh, Mc4r, Oprk1 and Pdk4,
in the hippocampus were temporarily upregulated in the sub-chronic
phase in a rat model of posttraumatic epilepsy (44). In the present study, abnormal
expression patterns were also observed in the Apoa1, Oprk1, Pdk4
and Cyp46a1 lipid metabolism-associated genes, however, these genes
were markedly downregulated in developmental seizures (RS and
Mel+RS groups), compared with the Cont group. This discrepancy may
be due to the different animal models used or the different
time-points analyzed. Ueda et al used adult animals (5 weeks
of age) and detected the gene expressions 15 days following
amygdale injection (sub-chronic phase of injury), while the present
study used a neonatal recurrent seizure model, in which the gene
expression levels were detected 30 days following the initial
attack (chronic phase of injury).
Notably, while no clear adverse effects were
observed in the melatonin-treated animals, certain energy/lipid
metabolism-associated genes were markedly downregulated by
melatonin, compared with that in the control. Due to a limit number
of hippocampal samples, the present study did not further evaluate
the changes in the protein expression levels. However, our
preliminary investigation revealed that normal rats fed a ketogenic
diet had long-term adverse effects on neurobehavioral functions,
detected using an open-field assessment (data not shown).
Therefore, whether melatonin has long-term adverse effects on brain
development and function in normal animals requires further
investigation using a variety of toxicology and neurobehavioral
methods.
This study was supported by the National Natural
Science Foundation of China (nos. 81271458 and 81471337), the
Jiangsu Province’s Key Provincial Talents Program (no. RC2011113)
and a Project Funded by the Priority Academic Program Development
of Jiangsu Higher Education Institutions.
References
|
1
|
Tolaymat A, Nayak A, Geyer JD, Geyer SK
and Carney PR: Diagnosis and management of childhood epilepsy. Curr
Probl Pediatr Adolesc Health Care. 45:3–17. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Anovadiya AP, Sanmukhani JJ and Tripathi
CB: Epilepsy: Novel therapeutic targets. J Pharmacol Pharmacother.
3:112–117. 2012.PubMed/NCBI
|
|
3
|
Banach M, Gurdziel E, Jędrych M and
Borowicz KK: Melatonin in experimental seizures and epilepsy.
Pharmacol Rep. 63:1–11. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Uberos J, Augustin-Morales MC, Molina
Carballo A, Florido J, Narbona E and Muñoz-Hoyos A: Normalization
of the sleep-wake pattern and melatonin and 6-sulphatoxy-melatonin
levels after a therapeutic trial with melatonin in children with
severe epilepsy. J Pineal Res. 50:192–196. 2011.
|
|
5
|
Banach M, Gurdziel E, Jedrych M and
Borowicz KK: Melatonin in experimental seizures and epilepsy.
Pharmacol Rep. 63:1–11. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jain S and Besag FM: Does melatonin affect
epileptic seizures? Drug Saf. 36:207–215. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ardura J, Andres J, Garmendia JR and
Ardura F: Melatonin in epilepsy and febrile seizures. J Child
Neurol. 25:888–891. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Paprocka J, Dec R, Jamroz E and Marszał E:
Melatonin and childhood refractory epilepsy-a pilot study. Med Sci
Monit. 16:CR389–396. 2010.PubMed/NCBI
|
|
9
|
Goldberg-Stern H, Oren H, Peled N and
Garty BZ: Effect of melatonin on seizure frequency in intractable
epilepsy: A pilot study. J Child Neurol. 27:1524–1528. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sheldon SH: Pro-convulsant effects of oral
melatonin in neurologically disabled children. Lancet.
351:12541998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Banach M, Gurdziel E, Jedrych M and
Borowicz KK: Melatonin in experimental seizures and epilepsy.
Pharmacol Rep. 63:1–11. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Musshoff U and Speckmann EJ: Diurnal
actions of melatonin on epileptic activity in hippocampal slices of
rats. Life Sci. 73:2603–2610. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Forcelli PA, Soper C, Duckles A, Gale K
and Kondratyev A: Melatonin potentiates the anticonvulsant action
of phenobarbital in neonatal rats. Epilepsy Res. 107:217–223. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ni H, Jiang YW, Xiao ZJ, Tao LY, Jin MF
and Wu XR: Dynamic pattern of gene expression of ZnT-1, ZnT-3 and
PRG-1 in rat brain following flurothyl-induced recurrent neonatal
seizures. Toxico Lett. 194:86–93. 2010. View Article : Google Scholar
|
|
15
|
Ni H, Ren SY and Zhang LL: Expression
profiles of hippocampal regenerative sprouting-related genes and
their regulation byE-64 d in a developmental rat model of
penicillin-induced recurrent epilepticus. Toxico Lett. 217:162–169.
2013. View Article : Google Scholar
|
|
16
|
Ni H, Yan JZ, Zhang LL, Feng X and Wu XR:
Long-term effects of recurrent neonatal seizures on neurobehavioral
function and related gene expression and its intervention by
inhibitor of cathepsin B. Neurochem Res. 37:31–39. 2012. View Article : Google Scholar
|
|
17
|
Moezi L, Shafaroodi H, Hojati A and
Dehpour AR: The interaction of melatonin and agmatine on
pentylenetetrazole-induced seizure threshold in mice. Epilepsy
Behav. 22:200–206. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mareš J, Pometlová M, Deykun K, Krýsl D
and Rokyta R: An isolated epileptic seizure elicits learning
impairment which could be prevented by melatonin. Epilepsy Behav.
23:199–204. 2012. View Article : Google Scholar
|
|
19
|
Ni H, Jiang YW, Tao LY, Cen JN and Wu XR:
Effects of penicillin-induced developmental epilepticus on
hippocampal regenerative sprouting, related gene expression and
cognitive deficits in rats. Toxico Lett. 188:161–166. 2009.
View Article : Google Scholar
|
|
20
|
Johnson MR, Wang K, Smith JB, Heslin MJ
and Diasio RB: Quantitation of dihydropyrimidine dehydrogenase
expression by real-time reverse transcription polymerase chain
reaction. Anal Biochem. 278:175–184. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tian Y, Yabuki Y, Moriguchi S, Fukunaga K,
Mao PJ and Hong LJ: Melatonin reverses the decreases in hippocampal
protein serine/threonine kinases observed in an animal model of
autism. J Pineal Res. 56:1–11. 2014. View Article : Google Scholar
|
|
22
|
Koh PO: Melatonin regulates the
calcium-buffering proteins, parvalbumin and hippocalcin, in
ischemic brain injury. J Pineal Res. 53:358–365. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cetinkaya M, Alkan T, Ozyener F, Kafa IM,
Kurt MA and Koksal N: Possible neuroprotective effects of magnesium
sulfate and melatonin as both pre- and post-treatment in a neonatal
hypoxic-ischemic rat model. Neonatology. 99:302–310. 2011.
View Article : Google Scholar
|
|
24
|
Sánchez-Barceló EJ, Mediavilla MD and
Reiter RJ: Clinical uses of melatonin in pediatrics. Int J Pediatr.
2011:8926242011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zamorskii II, Sopova IY and Khavinson VKh:
Effects of melatonin and epithalamin on the content of protein and
lipid peroxidation products in rat cortex and hippocampus under
conditions of acute hypoxia. Bull Exp Biol Med. 154:51–53. 2012.
View Article : Google Scholar
|
|
26
|
Tchekalarova J, Petkova Z, Pechlivanova D,
Moyanova S, Kortenska L and Mitreva R: Prophylactic treatment with
melatonin after status epilepticus: effects on epileptogenesis,
neuronal damage and behavioral changes in a kainate model of
temporal lobe epilepsy. Epilepsy Behav. 27:174–187. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jain A, Sharma D, Suhalka P, Sukhwal P and
Bhatnagar M: Changes in the density of nitrergic neurons in the
hippocampus of rats following kainic acid and melatonin
administration. Physiol Res. 62:197–203. 2013.
|
|
28
|
Mareš J, Stopka P, Nohejlová K and Rokyta
R: Oxidative stress induced by epileptic seizure and its
attenuation by melatonin. Physiol Res. 62(Suppl 1): 67–74.
2013.
|
|
29
|
Atanasova M, Petkova Z, Pechlivanova D,
Dragomirova P, Blazhev A and Tchekalarova J: Strain-dependent
effects of long-term treatment with melatonin on kainic
acid-induced status epilepticus, oxidative stress and the
expression of heat shock proteins. Pharmacol Biochem Behav.
111:44–50. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ni H, Jiang YW, Tao LY, Jin MF and Wu XR:
ZnT-1, ZnT-3, CaMK II, PRG-1 expressions in hippocampus following
neonatal seizure-induced cognitive deficit in rats. Toxicol Lett.
184:145–150. 2009. View Article : Google Scholar
|
|
31
|
Adibhatla RM and Hatcher JF: Altered lipid
metabolism in brain injury and disorders. Subcell Biochem.
49:241–268. 2008.PubMed/NCBI
|
|
32
|
McNally MA and Hartman AL: Ketone bodies
in epilepsy. J Neurochem. 121:28–35. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zaidi N, Hermann C, Herrmann T and
Kalbacher H: Emerging functional roles of cathepsin E. Biochem
Biophys Res Commun. 377:327–330. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bernstein HG and Wiederanders B: An
immunohistochemical study of cathepsin E in Alzheimer-type dementia
brains. Brain Res. 667:287–290. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tominaga K, Nakanishi H, Yasuda Y and
Yamamoto K: Excitotoxin-induced neuronal death is associated with
response of a unique intracellular aspartic proteinase, cathepsin
E. J Neurochem. 71:2574–2584. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chihara M, Nomura T, Tachibana M, Nomura
H, Nomura Y and Hagino Y: Effects of exogenous phospholipase
enzymes, arachidonic acid and 1-oleoyl-2-acetyl-sn-glycerol on
ketogenesis in isolated rat hepatocytes. Biochim Biophys Acta.
1012:5–9. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Farooqui AA, Horrocks LA and Farooqui T:
Interactions between neural membrane glycerophospholipid and
sphingolipid mediators: a recipe for neural cell survival or
suicide. J Neurosci Res. 85:1834–1850. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ruiz N, Pacheco LF, Farrell B, Cox CB,
Ermolinsky BS, Garrido-Sanabria ER and Nair S: Metabolic gene
expression changes in the hippocampus of obese epileptic male rats
in the pilocarpine model of temporal lobe epilepsy. Brain Res.
1426:86–95. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Koster JC, Cadario F, Peruzzi C, Colombo
C, Nichols CG and Barbetti F: The G53D mutation in Kir6.2 (KCNJ11)
is associated with neonatal diabetes and motor dysfunction in
adulthood that is improved with sulfonylurea therapy. J Clin
Endocrinol Metab. 93:1054–1061. 2008. View Article : Google Scholar
|
|
40
|
Yamada K, Ji JJ, Yuan H, Miki T, Sato S,
Horimoto N, Shimizu T, Seino S and Inagaki N: Protective role of
ATP-sensitive potassium channels in hypoxia-induced generalized
seizure. Science. 292:1543–1546. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu ZL, He B, Fang F, Tang CY and Zou LP:
Analysis of single nucleotide polymorphisms in the melanocortin-4
receptor promoter in infantile spasms. Neuropediatrics. 38:304–309.
2007. View Article : Google Scholar
|
|
42
|
Dunleavy M, Provenzano G, Henshall DC and
Bozzi Y: Kainic acid-induced seizures modulate Akt (SER473)
phosphorylation in the hippocampus of dopamine D2 receptor knockout
mice. J Mol Neurosci. 49:202–210. 2013. View Article : Google Scholar :
|
|
43
|
Jayaram B, Khan RS, Kastin AJ, Hsuchou H,
Wu X and Pan W: Role of astrocytic leptin signaling against
excitotoxicity. J Mol Neurosci. 49:523–530. 2013. View Article : Google Scholar :
|
|
44
|
Ueda Y, Kitamoto A, Willmore LJ and Kojima
T: Hippocampal gene expression profiling in a rat model of
posttraumatic epilepsy reveals temporal upregulation of lipid
metabolism-related genes. Neurochem Res. 38:1399–1406. 2013.
View Article : Google Scholar : PubMed/NCBI
|