Introduction
Helicobacter pylori (H. pylori) is a
gram-negative, microaerophillic, flagellated bacteria, which
affects >50% of the world’s population (1–3).
Gastric cancer is a leading malignant disease in numerous
countries, including China, Korea and Japan (4). H. pylori is ranked as a class
I carcinogen by the International Agency for Research on Cancer
(Lyon, France). H. pylori colonizes to the gastric mucosa
and adheres to gastric epithelial cells; therefore, it is of
interest to use human cells in the study of H. pylori
infection (5). However, the
availability of human gastric normal and cancer cell lines is
limited and it is difficult to successfully culture gastric
epithelial cells (5,6).
Gastric biopsy tissues are widely used in gastric
cancer research, but have various drawbacks (7). A significant limitation of using
tissues from endoscopic biopsies is the lack of sufficient cell
numbers for plating. For the successful growth and differentiation
of epithelial cells a certain cell planting density and the use of
the appropriate culture media is required (8). Gastric cell lines established from
human gastric surgical tissues may prove more useful in the study
of gastric infectious diseases (4). At present, gastric epithelial biology
research relies on primary cultures generated from fresh surgical
tissues (6). There are numerous
advantages to this technique, including the maintenance of
characteristics of the original tissue and low interference of
stromal components. Surgical tissues are therefore suitable for use
in the study of cell morphology, genetic characteristics, cell
differentiation, invasion, metastasis and gastric cancer therapy
(7).
Several methods have been reported for the isolation
of cells from the gastric mucosa for cell culture (8). However, the successful isolation and
subsequent culture of human gastric mucous epithelial cells has
remained difficult due to numerous factors (8). The production of various gastric
stromal factors, including fibroblasts, interferes with the
analysis of gastric cancer cells. Furthermore, too great a number
of passages of the cells altered the protein expression and
mutation behavior of the cultured cells compared to those in
vivo (7,8).
Therefore, the establishment of human gastric cell
lines exhibiting a true epithelial phenotype is an important step
in the study of gastric epithelial cells (9). The present study therefore aimed to
develop a continuous human gastric epithelial cell culture from
gastric surgical tissue, which may be used in the study of H.
pylori gastric infections.
Materials and methods
Cell culture reagents and media
RPMI-1640, fetal bovine serum (FBS), trypsin/EDTA
and penicillin/streptomycin were purchased from Invitrogen Life
Technologies (Carlsbad, CA, USA). Polyclonal rabbit anti-H.
pylori was purchased from Dako North America, Inc.
(Carpinteria, CA, USA). Mouse anti-carbohydrate antigen 724
(CA724), amphotericin B, fibronectin and horseradish peroxidase
(HRP)-conjugated anti-rabbit and anti-mouse immunoglobulin G were
purchased from Santa Cruz Biotechnology Inc. (Dallas, TX, USA).
Rabbit anti-proliferating cell nuclear antigen (PCNA), cytokeratin
18 and granulin (GRN) were purchased from ProteinTech Group, Inc.
(Chicago, IL, USA). Cytokeratin 19 was purchased from ZSGB-BIO
(Beijing, China). Tissue culture plates and flasks were purchased
from Corning-Costar (Corning, New York, NY, USA). Hoechst 3358 was
obtained from Beyotime Institute of Biotechnology (Haimen, China)
and periodic acid was purchased from Sinopharm Group Co., Ltd
(Beijing, China).
Preparation of fibronectin-coated
flask
Fibronectin was dissolved in cell culture medium at
a concentration of 100 μg/ml. Subsequently, sufficient
fibronectin solution (3 ml) to provide a coating was added to a
cell-culture flask and cell-culture plate and incubated at 37°C for
1–2 h. The fibronectin solution was aspirated and rinsed with
media, prior to storage at 4°C.
Preparation of media
RPMI-1640 media was supplemented with 20% FBS, 100
U/ml penicillin, 100 μg/ml streptomycin, 25 mM hepes buffer
(Tocris Bioscience, Bristol, UK), 20 mM sodium bicarbonate (Tianjin
KaiXin chemical industry, Tianjin, china) and 0.5 U/ml insulin
(Sigma-Aldrich, St. Louis, MO, USA). The transport medium did not
contain serum and was supplemented with 2 μg/ml amphotericin
B (Sigma-Aldrich).
Human gastric surgical tissue
procurement
Gastric tissues (n=45) were obtained from patients
(age, 35–85) undergoing surgical gastrectomy. Gastric cancer
tissues were obtained at the Dalian Medical First and Second
Affiliated Hospitals (Dailan, China) between 2012 and 2013.
Surgical tissues were removed from the tumor sections, adjacent
tissue and normal gastric mucosa of 45 different patients. The
specimens were collected in transport medium and transported to the
research laboratory. All gastric tissue samples collected from the
patients and the research protocols were performed in accordance
with the Institutional Review Board of Dalian Medical University
(Dailan, China).
Tissue processing and plating
Gastric tissues were placed in 100-mm cell culture
plates containing transport media and the fat, connective and
necrotic sections of the tissue were removed. The remaining tissue
was finely cut into 1-mm3 sections. For the primary
tumor culture, invasive areas were selected from the serosal
surface, whenever possible, to decrease the chance of microbial
contamination. Normal and cancerous tissue sections from each
patient were arranged in fibronectin-coated culture flasks (n=45),
with 0.5-cm spacing between each tissue and incubated at 37°C with
5% CO2. Initially, no media was added so that the tissue
edges dried and adhered to the surface. Following three hours of
incubation without media, when the edges of the tissue were
sufficiently dry, the tissue was supplemented with sufficient trace
medium (1–2 ml) to cover the bottom of the flask. The media was
replaced every 24 h. Prior to replacing the media, the flask was
stood vertically and following the change of media, the flask was
carefully laid down in order to avoid the detachment of tissue
pieces. Following six times of culture, tissues which had epidermal
cells that were identifiable under a microscope were marked.
Unmarked tissues were removed by curettage when replacing the
culture medium. Any remaining fibroblasts were also scratched off,
which resulted in a significant reduction in the number of
fibroblast cells in the culture. Epithelial cells were confirmed by
cytokeratin 18 and 19 antigen expression and gastric cancer cells
were identified using GRN and CA724 markers. Cell proliferation was
determined by immunocyto-chemical analysis of PCNA expression.
Gastric cell growth rate
A suspension of l×l05 cells was seeded in
35-mm plastic dishes in the culture medium. The number of cells was
counted in triplicate at 24-h intervals for seven days using a
hemocytometer (Sysmex, Kobe, Japan). The doubling times of the cell
populations were estimated during the exponential growth phase.
Periodic acid-Schiff (PAS) staining
Cells were trypsinized and seeded onto cover slips.
The sections were rinsed in phosphate-buffered saline (PBS),
oxidized for 5 min in 0.5% periodic acid (Sinopharm Group Co.,
Ltd), rinsed once in PBS prior to the addition of Schiff’s reagent
(Sigma-Alrich) for 15 min. The Schiff’s reagent was subsequently
removed and the sections were rinsed with tap water for 10 min.
Finally, the sections were counterstained with hematoxylin (Santa
Cruz Biotechnology, Inc.) and cover-slips were mounted with
mounting media for microscopic visualization (Olympus IX71; Olympus
Corp., Tokyo, Japan).
Immunocytochemistry
Cells, which had been continuously passaged until
they were pure epithelial cells, were grown on glass coverslips,
fixed with 4% paraformaldehyde (Sigma-Aldrich) for 20 min and
subsequently treated with 0.1% Triton-PBS (Merck, Darmstadt,
Germany) for 10 min. Following being blocked with goat serum (Santa
Cruz Biotechnology, Inc.) for 2 h, cells were incubated with
polyclonal rabbit anti-H. pylori (1:100), rabbit
anti-cytokeratin 18 (1:100), rabbit anti-cytokeratin 19 (1:100),
rabbit anti-PCNA (1:100), rabbit anti-GRN (1:100) or mouse
anti-CA724 (1:100), at 4°C overnight. The next day, the sections
were rinsed with PBS and subsequently incubated with their
respective secondary antibody (HRP-conjugated anti-rabbit and
anti-mouse IgG for 30 min at room temperature. Immunocytochemical
staining was performed using an avidin-biotin peroxidase complex
kit (ZSGB-BIO, Beijing, China). Samples were then mounted with
mounting medium containing DAPI (Santa Cruz Biotechnology, Inc.
Images were captured with an inverted microscope (Olympus IX71;
Olympus Corp., Tokyo, Japan).
Determination of mycoplasmic
contamination using the Hoechst method
Cells were trypsinized, seeded on cover slips and
fixed in 4% paraformaldehyde for 15 min, prior to the addition of
100% cold methanol for 20 min at room temperature. Cells were
subsequently rinsed three times with PBS and stained with Hoechst
(5 μg/ml) for 15 min at room temperature. Finally, cells
were rinsed with PBS and visualized under a fluorescence microscope
(Olympus IX71; Olympus Corp.).
Statistical analysis
Statistical differences between test groups were
analyzed using independent and paired Student’s t-test and GraphPad
Prism 5.03 software was used to statistical analyses (GraphPad
Software, Inc., La Jolla, CA, USA). P<0.05 was considered to
indicate as statistically significant difference between
values.
Results
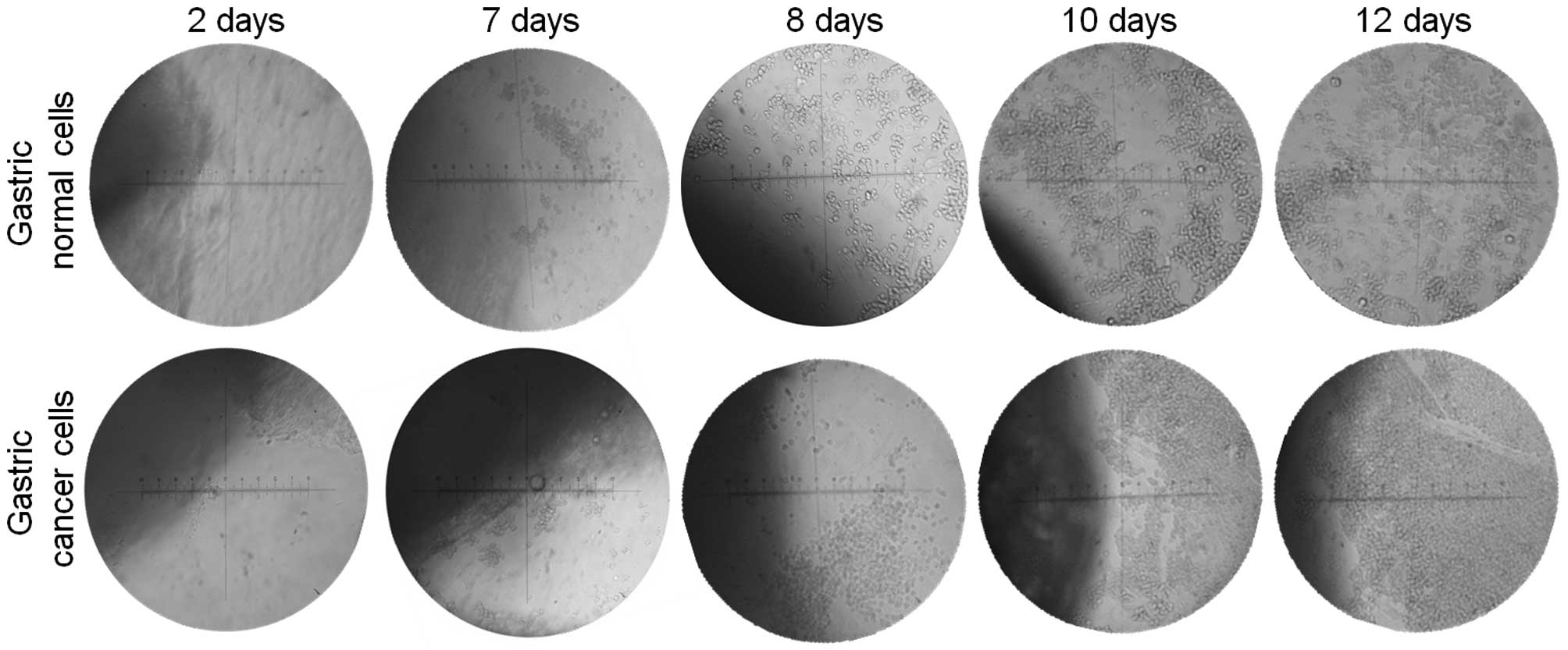
Gastric primary cell culture
Following seven days of culture of the explants of
gastric tissue, epithelial cells began their outward migration of
growth from the explants and started to increase their cell number
with time. The cells reached high numbers at days 10–12 of culture
(Fig. 1). Pure epithelial cells
were cultured by the removal of fibroblast cells by curettage. The
number of fibroblast cells in culture was significantly decreased
by repeatedly scraping the tissue cultures until no fibroblast
cells were detected. The passage number was dependent on the growth
rate of tumor cells, which varied among patients. Cells were
cultured for numerous months to maintain the cell morphology and
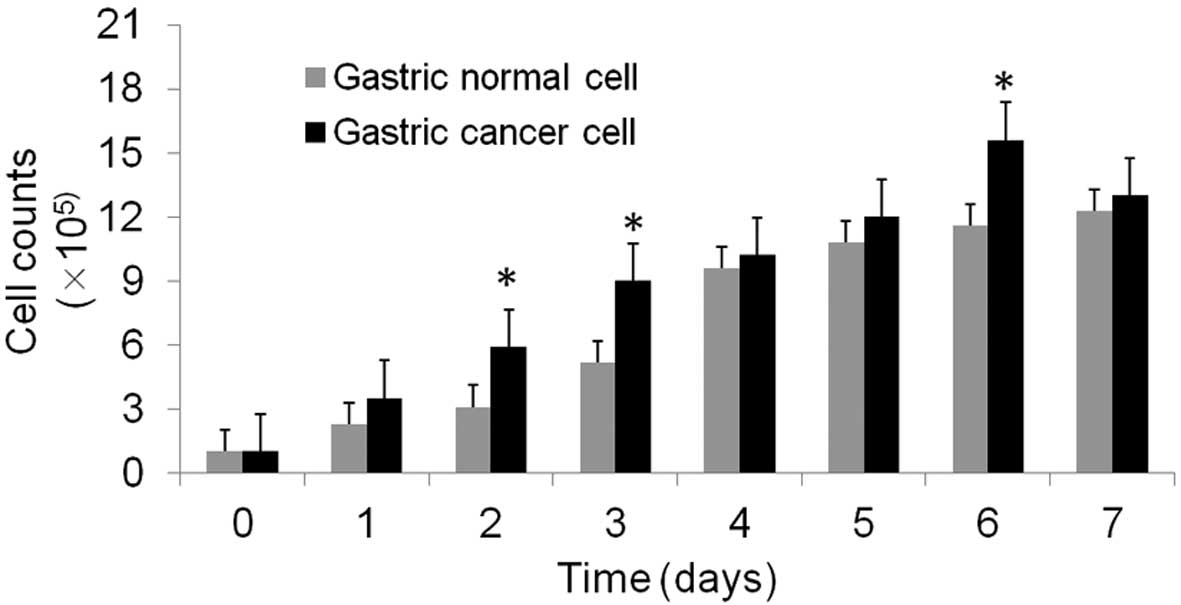
growth rate. The cancer cells grew rapidly with an estimated
doubling time of 13–52 h (P<0.05), which was significantly
increased compared with the doubling time of normal cells, which
was 20–53 h (P<0.05; Fig. 2 and
Table I). In addition, the gastric
cancer had a significantly higher cell count compared with the
normal gastric cells at 2, 3 and 6 days (P<0.05). The cells were
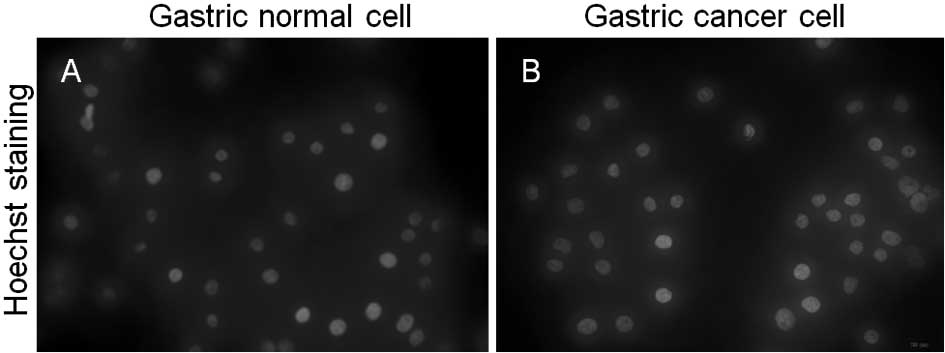
found to be free of contamination with mycoplasma (Fig. 3).
 | Table IDoubling time of gastric cancer and
normal cells at various time-points. |
Table I
Doubling time of gastric cancer and
normal cells at various time-points.
Normal cells
| Cancer cells
|
|---|
| Cell count | Days [n, (h)] | Doubling time
(h) | Cell count | Days [n, (h)] | Doubling time
(h) |
|---|
|
1.0×l05 | 0 | – |
l.0×l05 | 0 | – |
|
2.3×l05 | 1 (24) | 20 |
3.5×l05 | 1 (24) | 13 |
|
3.1×l05 | 2 (48) | 29 |
5.9×l05 | 2 (48) | 19 |
|
5.2×l05 | 3 (72) | 30 |
9.0×l05 | 3 (72) | 23 |
|
9.6×l05 | 4 (120) | 37 |
10.2×l05 | 4 (120) | 36 |
|
10.8×l05 | 5 (144) | 42 |
12.0×l05 | 5 (144) | 40 |
|
11.6×l05 | 6 (168) | 48 |
15.6×l05 | 6 (168) | 42 |
|
12.3×l05 | 7 (192) | 53 |
13.0×l05 | 7 (192) | 52 |
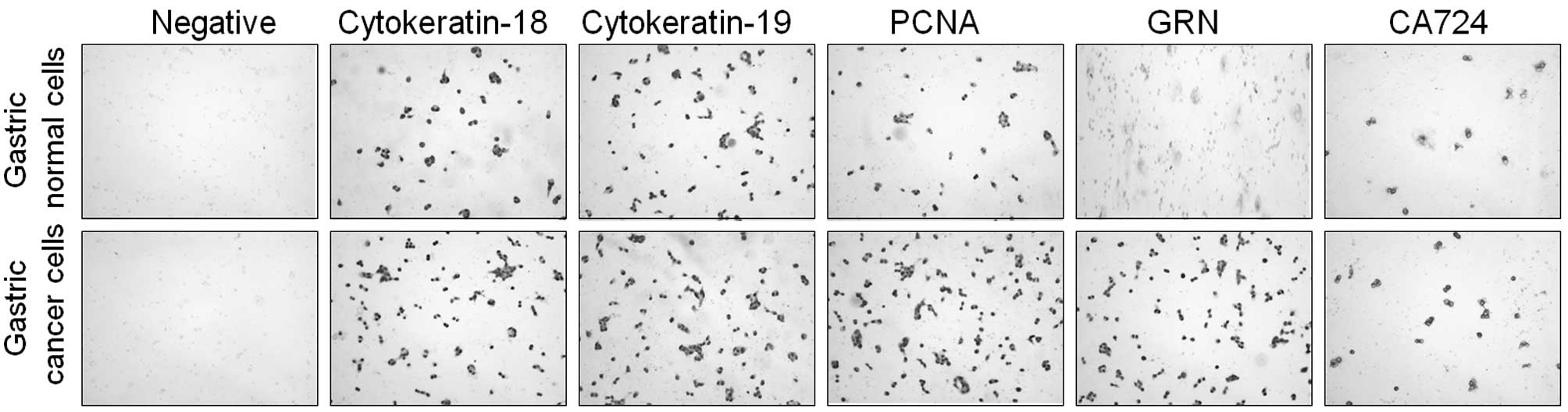
Expression of cytokeratin and neutral
mucin demonstrates the gastric epithelial status of the primary
culture cells
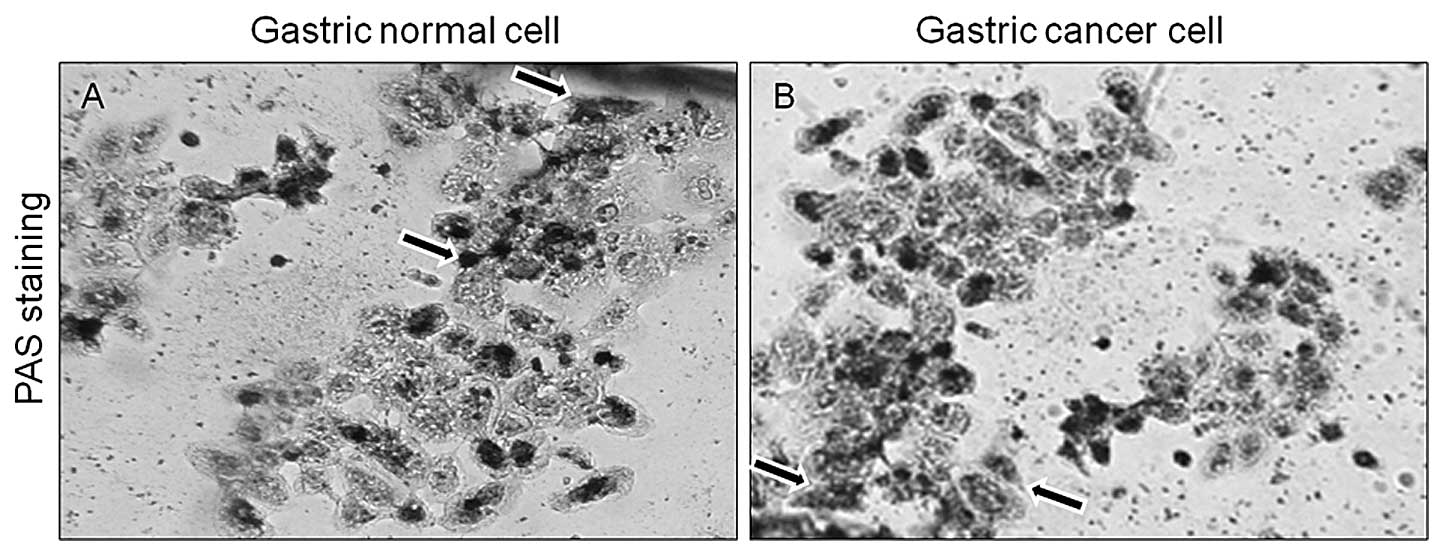
Primary gastric epithelial cells were stained with
PAS reagent to determine the presence of neutral mucin and confirm
the gastric epithelial origin of the cells. Gastric epithelial
cells stained positive for neutral mucin by exhibiting a purple
color (Fig. 4). The expression of
antigens associated with gastric epithelial cells and gastric tumor
markers was also evaluated. High expression levels of cytokeratin
18 and 19 were detected, which further confirmed the epithelial
status of the gastric cancer and normal cells. To differentiate the
gastric cancer from gastric normal cells, GRN and CA724 markers
were used. The expression levels of GRN and CA724 were markedly
higher in gastric cancer cells than those in normal cells.
Furthermore, the high expression levels of PCNA in gastric cancer
culture cells indicated evidence of high cell proliferation
(Fig. 5).
Discussion
Gastric cancer is a multifactorial disease, caused
by complex interactions between genetics, lifestyle and
environmental factors (10,11).
There is a high prevalence of gastric cancer in China, which
accounts for 42% of gastric cancer cases worldwide (12). Epidemiological studies conducted in
Japan and China identified H. pylori infection as a
significant risk factor for the development of gastric cancer
(13). H. pylori infection
is a major cause of gastritis and gastric ulcers, as well as
gastric carcinoma (1). To date,
studies have used gastric primary cell cultures to analyze H.
pylori gastric infection, which colonizes to the gastric mucosa
and adheres to gastric epithelial cells. It is difficult to
establish gastric cell line cultures which exhibit a pure
epithelial phenotype due to gastric stromal factor interference in
the epithelial growth rate. A method for generating a gastric
primary cell culture with high growth of gastric epithelial cells
was therefore required. In the present study, fresh gastric
surgical tissue was used to establish an effective method for
culturing gastric epithelial from surgical gastric tissues
(5,7).
The selection of tissue, and specifically the
gastric section of the tissue, is important for the successful
development of a primary culture. Fresh gastric surgical tissues
from patients <60 years of age have lower chances of bacterial
contamination than those of patients aged ≥60 years, which may have
hypochlorhydria (5). In the
present study, high growth of gastric epithelial cells with low
contamination was found in specimens from younger patients
(5). Furthermore, in gastric
sections of the tissue, the tumor edges or junctions between the
tumor and normal tissues were selected due to their previously
reported high metabolic activity and ease of adherence (7). Ruttenl et al (8) reported an increased rate of growth of
gastric epithelial cells from surgical specimens, compared with
that of other specimens. Surgical tissues have numerous advantages,
including the ease of removal from smooth muscle and that they are
relatively simple to process (8).
In order to avoid contamination, amphotericin B was added to the
transport media to prevent fungal growth. Gastric primary cells
were also tested for mycoplasmic contamination using Hoechst 33258,
and no contamination was detected. Therefore, fresh gastric
surgical tissue from patients of lower age was recommended to
produce cultures of high growth rate and with low risk of
contamination. Cell adherence to the flask surface is another
important factor for the establishment of a successful primary
culture (8). Fibronectin increases
the rate of attachment and growth of the gastric epithelial cells.
A previous study reported that fibronectin was a suitable substrate
for mediating cell attachment (8).
During primary gastric culture, there are frequently
problems with gastric stromal tissue components, including
fibroblasts, which have potential cytotoxic effects and perturb the
growth of pure gastric epithelial cells (14). In the present study, when cells
surrounding the tissue began to appear following seven days of
culture, no fibroblast cells were observed as the tissue was
cultured in starting media, which contained no serum. Fibroblast
growth depends upon the presence of serum; however, this is not a
requirement for the growth of gastric cancer cells (14). For this reason, only gastric
epithelial cell growth rather than fibroblast growth was observed
in the cultured tissues. At a later stage, conventional media was
used to promote rapid cell growth, and concurrent slow growth of
fibroblast cells was detected (7).
When the epithelial cell growth was high and had increased the
number of epithelial cells, the media containing 20% FBS was
changed and fibroblast cells were removed by mechanical scraping.
This method of primary culture was advantageous as it significantly
inhibited the growth of fibroblast cells and a culture comprising
100% pure gastric epithelial cells was generated (7,14).
The culture medium may modulate the biological behavior of cultured
cells (15). The appropriate use
of media is an important factor for the successful growth and
differentiation of gastric epithelial cells (8). It is also recognized that the
majority of cells in vivo secrete endogenous growth factors
to stimulate their own proliferation (8). Therefore, similar conditions may be
achieved by using the appropriate media conditions during cell
culture. In the present study, RPMI-1640 media, which is
characterized by a combination of richness in trace elements, amino
acids and high nutrient concentration, was used. Sodium bicarbonate
and hepes buffer were also used for their abilities to maintain the
pH of the media (15).
The characterization and investigation of
cytokeratin expression by gastric cells is a simple method of
determining epithelial nature (5).
Cytokeratin markers were therefore used in the present study, to
confirm that the primary gastric cells in culture were free from
fibroblasts and comprised a pure epithelial gastric cell
population. High levels of staining for cytokeratin 18 and 19 were
identified in gastric normal and cancer primary cells, which
confirmed the epithelial nature of the gastric culture cells. The
cells that did not stain with cytokeratin 18 or 19 were presumed to
be fibroblast cells (5). Mucin,
which is found within the cells, may also be used to characterize
gastric primary cultures (9). The
PAS staining method was used to determine mucin expression within
the gastric cells. In the present study, purple cytoplasmic
staining was detected, which indicated the presence of neutral
mucin within the gastric epithelial cells. This combination of
neutral mucin and cytokeratin 18 and 19 expression demonstrated
that the primary cultures were comprised of mucin-secreting gastric
epithelial cells (5).
In order to differentiate gastric cancer cells from
gastric normal cells, the expression of CA724 and GRN, which are
associated with gastric tumor cells, was evaluated. High levels of
CA724 and GRN staining are detected in gastric cancer cells,
compared to those in normal gastric cells (16). CA724 is a specific gastric cancer
marker used for the diagnosis of gastric diseases (17). Chen et al (18) demonstrated that CA724 was the most
correlative and specific tumor biomarker for gastric cancer in the
Chinese population. GRN is also a gastric cancer marker, which is
highly expressed in gastric cancer and promotes cell proliferation,
migration and invasion (19).
Determination of the gastric cell proliferation rate aids the
elucidation of the replicative ability of the primary cell culture.
In order to determine the gastric culture cell proliferation rate,
immunocytochemical staining was performed using PCNA antibodies.
The results showed that difference in replication rate between
gastric cancer and normal cells lies in the S-phase progression of
the cell cycle. The replication rate of gastric cancer cells was
higher than that of normal gastric cells. These results indicated
that primary gastric cells had an active DNA synthesis and
possessed the potential to continue gastric epithelial cell
replication. Cell growth was examined using a trypan blue exclusion
assay. Gastric cancer cell growth was markedly higher (13–52 h)
than that of normal gastric cells (20–53 h). This result indicated
that gastric cancer cells grow more rapidly than normal gastric
cells (5).
In conclusion, the present study provided a method
for the primary culture of gastric epithelial cells from fresh
gastric surgical tissue. The advantage of the gastric primary
culture method outlined is that the human tissue remained in medium
and kept its activity intact with sufficient nutrition and
adherence to the flask, which provided a suitable environment for
continuous cell growth. The growth rate of gastric epithelial cells
using this protocol was high and cultures were free from fibroblast
cells. These cultured gastric epithelial cells may therefore be
used to investigate the effects of H. pylori attachment to
gastric epithelial cells and the therapeutic potential of various
drugs against H. pylori infection.
Acknowledgments
The present study was supported by the China 973
grant (no. 2012CB822100) and the National Natural Science
Foundation of China Research grant (nos. 30672753 and
31270866).
References
|
1
|
Aziz F, Sherwani SK, Akhtar SS and Kazmi
SU: Development of an in-house enzyme-linked immunosorbent assay
based on surface whole cell antigen for diagnosis of Helicobacter
pylori infection in patients with gastroduodenal ulcer disease.
World J Microbiol Biotechnol. 30:305–315. 2014. View Article : Google Scholar
|
|
2
|
Suerbaum S and Michetti P: Helicobacter
pylori infection. N Engl J Med. 347:1175–1186. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Torres J, Leal-Herrera Y, Perez-Perez G,
et al: A community-based seroepidemiologic study of Helicobacter
pylori infection in Mexico. J Infect Dis. 178:1089–1094. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Park JG, Frucht H, LaRocca RV, et al:
Characteristics of cell lines established from human gastric
carcinoma. Cancer Res. 50:2773–2780. 1990.PubMed/NCBI
|
|
5
|
Smoot DT, Sewchand J, Young K, et al: A
method for establishing primary cultures of human gastric
epithelial cells. Methods Cell Sci. 22:133–136. 2000. View Article : Google Scholar
|
|
6
|
Chailler P and Ménard D: A new approach to
primary culture of human gastric epithelium. Methods Mol Med.
107:217–236. 2005.
|
|
7
|
Liu G, Chai Y, Zhu X and Zhang Q: Explants
culture of gastric tissue continuously in a small amount of medium.
Cancer Res Prev Treat. 2:147–148. 2008.In Chinese.
|
|
8
|
Rutten MJ, Campbell DR, Luttropp CA, et
al: A method for the isolation of human gastric mucous epithelial
cells for primary cell culture: A comparison of biopsy vs surgical
tissue. Methods Cell Sci. 18:269–281. 1996. View Article : Google Scholar
|
|
9
|
Chailler P and Ménard D: Establishment of
human gastric epithelial (HGE) cell lines exhibiting barrier
function, progenitor, and prezymogenic characteristics. J Cell
Physiol. 202:263–274. 2005. View Article : Google Scholar
|
|
10
|
Luk GD: Tumors of the stomach. Sleisenger
and Fordtran’s Gastrointestinal and Liver Disease:
Pathophysiology/Diagnosis/Management. Feldman M, Sleisenger MH and
Scharschmidt B: 1. 6th. Saunders Co; Philadelphia: pp. 733–757.
1998
|
|
11
|
Peek RM and Blaser MJ: Helicobacter pylori
and gastrointestinal tract adenocarcinomas. Nat Rev Cancer.
2:28–37. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Parkin DM, Bray FI and Devesa SS: Cancer
burden in the year 2000. The global picture. Eur J Cancer. 37(Suppl
8): S4–S66. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fock KM, Talley NJ, Fass R, et al:
Asia-Pacific consensus on the management of gastroesophageal reflux
disease: update. J Gastroenterol Hepatol. 23:8–22. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chew CS, Ljungström M, Smolka A and Brown
MR: Primary culture of secretagogue-responsive parietal cells from
rabbit gastric mucosa. Am J Physiol. 256(1 Pt 1): G254–G263.
1989.PubMed/NCBI
|
|
15
|
Wu X, Lin M, Li Y, Zhao X and Yan F:
Effects of DMEM and RPMI 1640 on the biological behavior of dog
periosteum-derived cells. Cytotechnology. 59:103–111. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Isaka K, Nishi H, Nakada T, et al:
Establishment and characterization of a new human cell line (EJ)
derived from endometrial carcinoma. Hum Cell. 15:200–206. 2002.
View Article : Google Scholar
|
|
17
|
Başoğlu M, Kiziltunç A, Akçay F, et al:
Increased serum CA 72-4 levels in patients with gastrointestinal
carcinoma. Turk J Med Sci. 28:259–263. 1998.
|
|
18
|
Chen XZ, Zhang WK, Yang K, Wang LL, et al:
Correlation between serum CA724 and gastric cancer: multiple
analyses based on Chinese population. Mol Biol Rep. 39:9031–9039.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Loei H, Tan HT, Lim TK, et al: Mining the
gastric cancer secretome: identification of GRN as a potential
diagnostic marker for early gastric cancer. J Proteome Res.
11:1759–1772. 2012. View Article : Google Scholar
|