Introduction
The use of herbs for the prevention and treatment of
disease has been practiced for thousands of years in various
countries, including China, Japan and Korea (1,2).
Particularly in cancer treatment, polyherbal Chinese traditional
medicine (TCM) formulas are widely used as an adjuvant therapy to
surgery, chemotherapy and radiotherapy (3,4).
Certain TCM herbs and formulas have previously been reported to
target cancer by regulating immune function, inhibiting
angiogenesis, inducing apoptosis and reversing the multi-drug
resistance of tumor cells (5–7).
Since the identification of increasingly clarified molecular
mechanisms, and the low toxicity of medicinal herbs, TCM has
recently gained increased global attention.
The regulation of apoptosis in malignant cells is an
area of extensive investigation in cancer research. Apoptosis may
be induced by either intrinsic stimuli, such as cytokine
deprivation and DNA damage; or extrinsic stimuli, such as death
ligand-receptor engagement. Intrinsic and extrinsic apoptotic
signaling eventually leads to the activation of cysteine-dependent
aspartate-directed proteases, which are known as caspases, and
nucleases, resulting in cellular destruction (8,9). The
mitochondrial pathway is the main intrinsic apoptotic pathway, and
is responsible for mitochondrion-dependent apoptosis. Pro-apoptotic
and anti-apoptotic proteins regulate the permeability of the
mitochondrial outer membrane (MOM) (10,11).
Numerous plant-derived compounds, including matrine, quercetin and
glaucocalyxin, have been shown to induce apoptosis via the
mitochondria-mediated pathway in various cancer cell lines
(12–14). In addition, unidentified components
in the aqueous extracts of Bryonia dioica, Hedyotis
diffusa Willd and Prunella have also been reported to
induce apoptosis of tumor cells via the mitochondrion-dependent
pathway (15–17). Furthermore, certain TCM antitumor
formulas, such as Yi Guan Jian, Chan-Yu-Bao-Yuan-Tang and
Ge-Jee-Bok-Ryung-Hwan, have been shown to induce apoptosis
(18–20). Therefore, promoting cell apoptosis
by regulating the mitochondrial pathway is an important antitumor
mechanism of TCM.
Jiedu Xiaozheng Yin (JXY) is a formula that contains
four anti-inflammatory and detoxification herbs: Hedyotis
diffusa Willd (HDW) 30 g, Sophora flavescens (SF) 15 g,
Psuedobulbus cremastrae (PC) 15 g and Spica prunellae
15 g. Jiedu means clearing heat and detoxification; Xiaozheng means
removing the mass (tumor); and Yin means water solution or
decoction. According to the TCM theory, the accumulation of
carcinogens and heat (fever symptoms) are key causative factor of
tumorigenesis; therefore, it is concordant that anti-inflammation
and detoxification are main principles in anticancer treatment
(21). A previous study by our
group demonstrated that the ethyl acetate fraction of JXY (EE-JXY)
can inhibit the growth of HepG2 hepatoma cancer by arresting cells
at G1 phase of the cell cycle, and inhibiting
angiogenesis by downregulating the expression levels of vascular
endothelial growth factor (VEGF)-A and VEGFR-2 in vivo and
in vitro (22,23). However the precise mechanisms
regarding its antitumor activity remain largely unknown. Therefore,
the present study aimed to investigate the molecular mechanisms of
JXY-induced apoptosis in the HepG2 hepatoma cell line.
Materials and methods
Materials and reagents
Dulbecco’s modified Eagle’s medium (DMEM), fetal
bovine serum (FBS), penicillin-streptomycin, trypsin-EDTA,
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-benz-imidazol-carbocyanine
iodide (JC-1), iBlot® Western Detection
Stack/iBlot® Dry Blotting system, and caspase-3 and -9
colorimetric protease assay kits were purchased from Invitrogen
Life Technologies (Carlsbad, CA, USA). Rabbit anti-human monoclonal
antibodies targeting Bcl-2 (cat. no. 2876) and Bax (cat. no. 2774)
were obtained from Cell Signaling Technology (Beverly, MA, USA).
The rabbit anti-mouse Bax monoclonal antibody (cat. no. MS-714) and
rabbit anti-mouse cytochrome c monoclonal antibody (cat. no.
MS-1273) were purchased from Fuzhou Maixin Biotech Co., Ltd.
(Fuzhou, China). TumorTACS in situ Apoptosis Detection kit
was obtained from Roche Palo Alto LLC (Palo Alto, CA, USA). Annexin
V-fluorescein isothiocyanate (FITC) Apoptosis Detection kit was
purchased from BD Biosciences (San Jose, CA, USA). All other
chemicals, unless otherwise stated, were obtained from
Sigma-Aldrich (St. Louis, MO, USA).
Preparation of EE-JXY
JXY is a TCM formula. Each dose of JXY contains: 30
g HDW, 15 g Spica prunellae, 15 g SF and 15 g PC. The four
herbs were purchased from Guo Yi Tang Hospital of Fujian University
of Traditional Chinese Medicine (Fuzhou, China). EE-JXY was
prepared according to the methods outlined in our previous study
(22). EE-JXY was dissolved in
normal saline (NS, 6 mg/ml) for oral administration in mice.
Cell culture
The HepG2 human hepatoma cell line was obtained from
the American Type Culture Collection (Manassas, VA, USA). The cells
were cultured in DMEM supplemented with 10% (v/v) FBS, 100 U/ml
penicillin and 100 μg/ml streptomycin, at 37°C in a
humidified incubator containing 5% CO2. The cells were
subcultured at 80–90% confluence.
Detection of apoptosis by flow
cytometry
Following incubation of the cells with various
concentrations of EE-JXY (0, 0.05, 0.1, 0.2 and 0.4 mg/ml) for 24
h, the rate of apoptosis was determined by flow cytometry, using
fluorescence-activated cell sorting with a FACSCalibur™ (BD
Biosciences). The apoptotic cells were identified using the Annexin
V-FITC/propidium iodide (PI) Apoptosis Detection kit. The ratios of
Annexin V+/PI‒ and Annexin
V+/PI+ cells were calculated using CellQuest™
software (version 3.3; BD Biosciences), which indicated the rates
of early and late stage apoptosis, respectively.
Measurement of mitochondrial membrane
potential (Δψm) by flow cytometry
JC-1 is a cationic dye that exhibits
potential-dependent accumulation in mitochondria, which is
indicated by a fluorescence emission shift from green to red, and
can be used as an indicator of mitochondrial potential. Briefly,
1×106 treated HepG2 cells were resuspended following
trypsinization in 1 ml DMEM. The cells were then incubated with 10
μg/ml JC-1 at 37°C, in an atmosphere containing 5%
CO2, for 30 min. Both red and green fluorescence
emissions were analyzed by flow cytometry after JC-1 staining.
Analysis of caspase activation
The activity of caspase-3 and -9 was determined by
colorimetric assay using caspase-3 and -9 activation kits
respectively, according to the manufacturer’s instructions.
Briefly, following treatment with various concentrations of EE-JXY
for 24 h, the HepG2 cells were lysed with lysis buffer (Beyotime
Inc., Shanghai, China) on ice. The lysed cells were then
centrifuged at 16,000 × g for 10 min. The protein concentration of
the clarified supernatant was determined using the Bradford assay
and 100 μg of each protein sample was incubated with 50
μl of the colorimetric tetrapeptides: Asp-Glu-Val-Asp
(DEAD)-p-nitroaniline (pNA), which is the specific substrate of
caspase-3; or Leu-Glu-His-Asp (LEHD)-pNA, which is the specific
substrate of caspase-9, at 37°C in the dark for 2 h. The absorbance
of the samples was measured at 405 nm in an ELISA plate reader
(EXL800; BioTek Instruments, Inc., Winooski, VT, USA). The data
were normalized to the activity of the caspases in control cells,
and are presented as ‘fold of control’.
Western blot analysis
A total of 2×105 HepG2 cells were seeded
into six-well plates in 2 ml DMEM and treated with various
concentrations of EE-JXY for 24 h. The treated cells were then
lysed with mammalian cell lysis buffer (M-PER; Thermo Fisher
Scientific, Waltham, MA, USA) containing protease (EMD Millipore,
Billerica, MA, USA) and phosphatase inhibitor cocktails
(Sigma-Aldrich). The cell lysates were separated by 12% SDS-PAGE
and electroblotted onto polyvinylidene fluoride (PVDF) membranes
using the iBlot® Western Detection
Stack/iBlot® Dry Blotting system. The PVDF membranes
were then blocked with SuperBlock T20 Tris-Buffered Saline (TBS)
Blocking Buffer (Thermo Fisher Scientific) for 30 min and washed in
TBS containing 0.25% Tween-20 (TBST). The membranes were
subsequently incubated overnight at 4°C with the primary
antibodies. After further washing with TBST, the membranes were
incubated with a rabbit anti-mouse horseradish peroxidase
(HRP)-conjugated secondary antibody for 1 h (1:50). The membranes
were visualized using SuperSignal Pico Substrate (Thermo Fisher
Scientific), and images were captured using a Kodak Image Station
400R (Kodak, Rochester, NY, USA).
Tumor apoptosis assay
A total of 16 male BALB/c nude mice, weighing
between 18 and 22 g were purchased from the School of Basic Medical
Science, Peking University (Beijing, China). The animals were
maintained in a pathogen-free facility (23°C±2°C, 55%±5% humidity,
12-h light/12-h dark cycle). Mice were sacrificed by CO2
inhalation. The animals were injected with a HepG2 cell suspension
(1×106 cells per mouse) in the right flank. After seven
days the mice were randomly divided into two groups: Mice in the
EE-JXY group were orally administered EE-JXY (0.06 g/kg), and mice
in the control group were administered the same volume of saline
containing 0.1% dimethyl sulfoxide. Tumor sections from the mice
were fixed with 4% paraformaldehyde for 48 h. The 5-μm tumor
sections were analyzed by terminal deoxynucleotidyl transferase
(TdT)-mediated dUTP nick end labeling (TUNEL) staining, using a
TumorTACS In Situ Apoptosis Detection kit (Roche Palo Alto
LLC), in order to detect fragmented DNA, according to the
manufacturer’s instructions. Microscopic immunohistochemical images
were captured using an Olympus microscope (Olympus Corporation,
Tokyo, Japan) and Moticam 5000 C camera from Motic Instruments,
Inc. (Richmond, BC, Canada) and analyzed by Motic Med 6.0 software.
The number of positive cells and the total number of cells were
counted in five arbitrarily selected microscopic fields, at x100
magnification (each 7,050 μm2 in size). A dark
brown nucleus represented the positive staining of apoptotic tumor
cells with TUNEL. The apoptotic index (AI) was calculated according
to the following formula: AI=number of positive cells/number of
total cells. All procedures on mice were performed according to the
Animal Care Guidelines issued by the Ministry of Science and
Technology of the People’s Republic of China. The present study was
approved by the Animal Care Committee of Fujian University of
Traditional Chinese Medicine (Fuzhou, China).
Immunohistochemical staining for Bax and
cytochrome c
Tumor samples were fixed in 10% buffered formalin
for 24 h and were conventionally processed into paraffin-embedded
tumor sections. The sections were then subjected to antigen
retrieval and blocking of endogenous peroxidase activity using
commercial kits (Fuzhou Maixin Biotech Co., Ltd.; cat. nos.
MVS-0101 and BLK-0001, respectively). For immunostaining, the
sections were incubated with primary mouse monoclonal Bax antibody
(1:100) or cytochrome c antibody (1:150). The sections were
then incubated with biotinylated appropriate secondary antibody
followed by conjugated HRP-streptavidin (Fuzhou Maixin Biotech Co.,
Ltd.). Subsequently 3,3′-diaminoben-zidine (Sigma-Aldrich) was
added to the sections, which were incubated at room temperature and
counterstained with diluted Harris hematoxylin (Sigma-Aldrich). The
cells were quantified by counting the number of positive cells and
the total number of cells in five arbitrarily selected fields from
each tumor at 100x magnification. Data are presented as the
percentage of positive cells.
Statistical analysis
All data represent the mean of three determinations.
The data were analyzed using SPSS package for Windows version 11.5
(SPSS Inc., Chicago, IL, USA). Statistical analyses of the data
were performed with a Student’s t-test and one-way analysis of
variance. P<0.05 was considered to indicate a statistically
significant difference.
Results
EE-JXY induces apoptosis of HepG2
cells
In our previous study, the results of an MTT assay
demonstrated that treatment with EE-JXY markedly inhibited cell
growth (22). In the present
study, apoptosis of HepG2 cells was analyzed by FACS analysis using
Annexin V/PI staining. Annexin V-positive/PI-negative and Annexin
V-positive/PI-positive populations (labeled as LR or UR in the
fig. 1) identify cells undergoing
early and late apoptosis, respectively. Following treatment of the
cells with 0.1, 0.2 and 0.4 mg/ml EE-JXY for 24 h, the percentage
of apoptotic cells was markedly increased (P<0.01, Fig. 1).
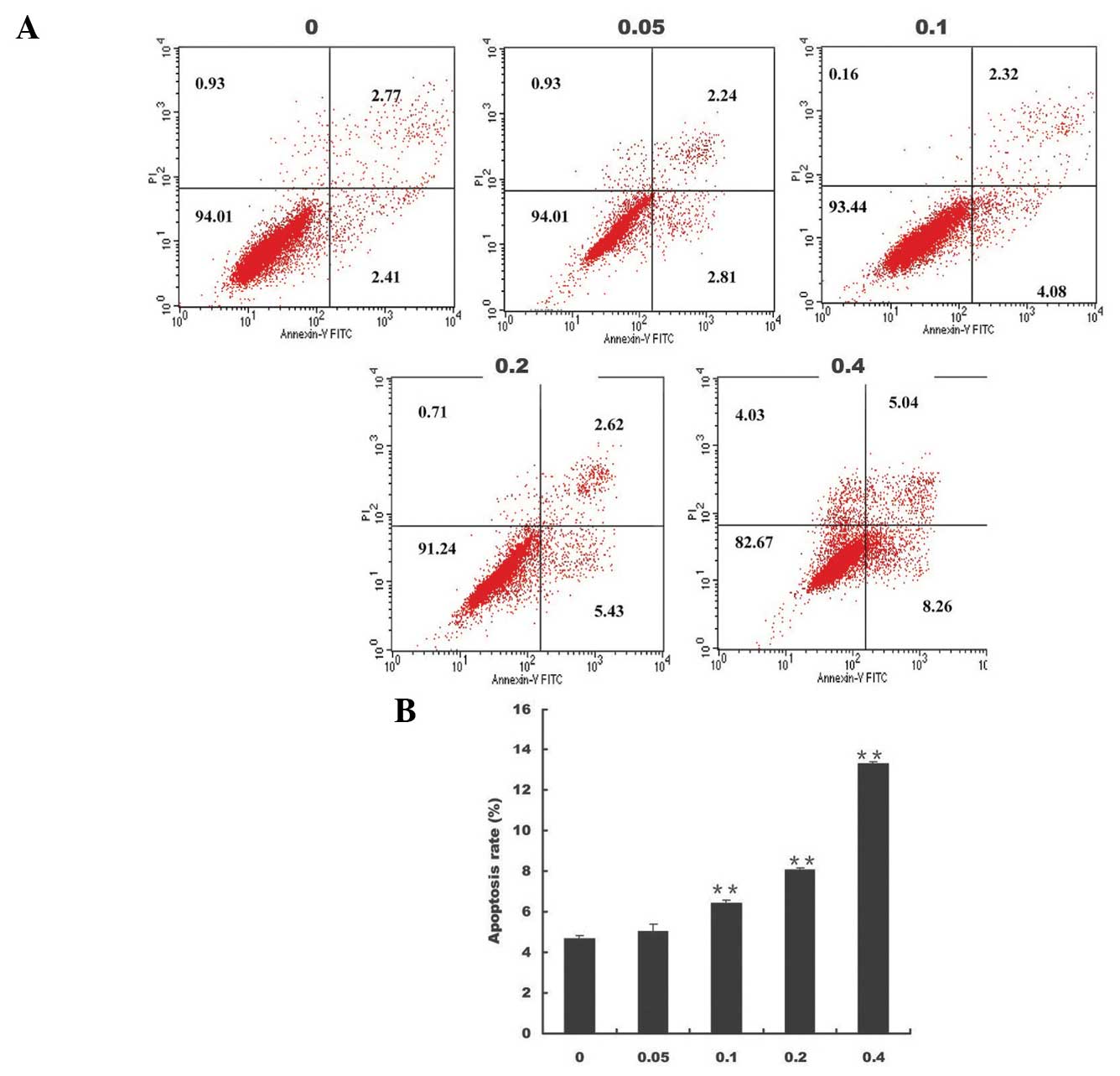 | Figure 1Effects of EE-JXY on apoptosis of
HepG2 human liver carcinoma cells. (A) HepG2 cells were treated
with the indicated concentrations of EE-JXY for 24 h, stained with
Annexin V/PI, and analyzed by FACS. Representative FACS
scatter-grams of Annexin V/PI staining display four different cell
populations: Double-negative staining (LL, lower left) indicating
live cells; Annexin V-positive/PI-negative staining (LR, lower
right) indicating cells in early apoptosis; Annexin V/PI
double-positive staining (UR, upper right) indicating cells in late
apoptosis; Annexin V-negative and PI-positive staining (UL, upper
left) indicating dead cells. Data shown are representative of three
independent experiments. (B) Quantification of FACS analysis. Data
shown are presented as the mean ± standard deviation (UR+LR) from
three independent experiments. **P<0.01, compared
with the control group. EE-JXY, ethyl acetate fraction of Jiedu
Xiaozheng Yin; PI, propidium iodide; FACS, fluorescence-activated
cell sorting; FITC, fluorescein isothiocyanate. |
EE-JXY induces mitochondrial potential
(Δψm) loss
FACS analysis with JC-1 staining was used to
determine the alterations in mitochondrial membrane potential
following treatment with EE-JXY. JC-1 is a lipophilic, cationic dye
that selectively enters mitochondria, and differences in
fluorescence can be detected by FACS using green and red channels.
JC-1 fluorescence shifted from a JC-1-green-bright/JC-1-red-bright
signal in untreated HepG2 cells to a JC-1-green-bright/red-dim
signal in cells treated with EE-JXY. The percentage of cells with a
reduced ratio of JC-1-green-bright/red-dim following treatment with
0.05, 0.1 and 0.2 mg/ml EE-JXY was 0.85 (P<0.05), 0.36
(P<0.01) and 0.08% (P<0.01), respectively, as compared with
the saline-treated control cells (Fig.
2).
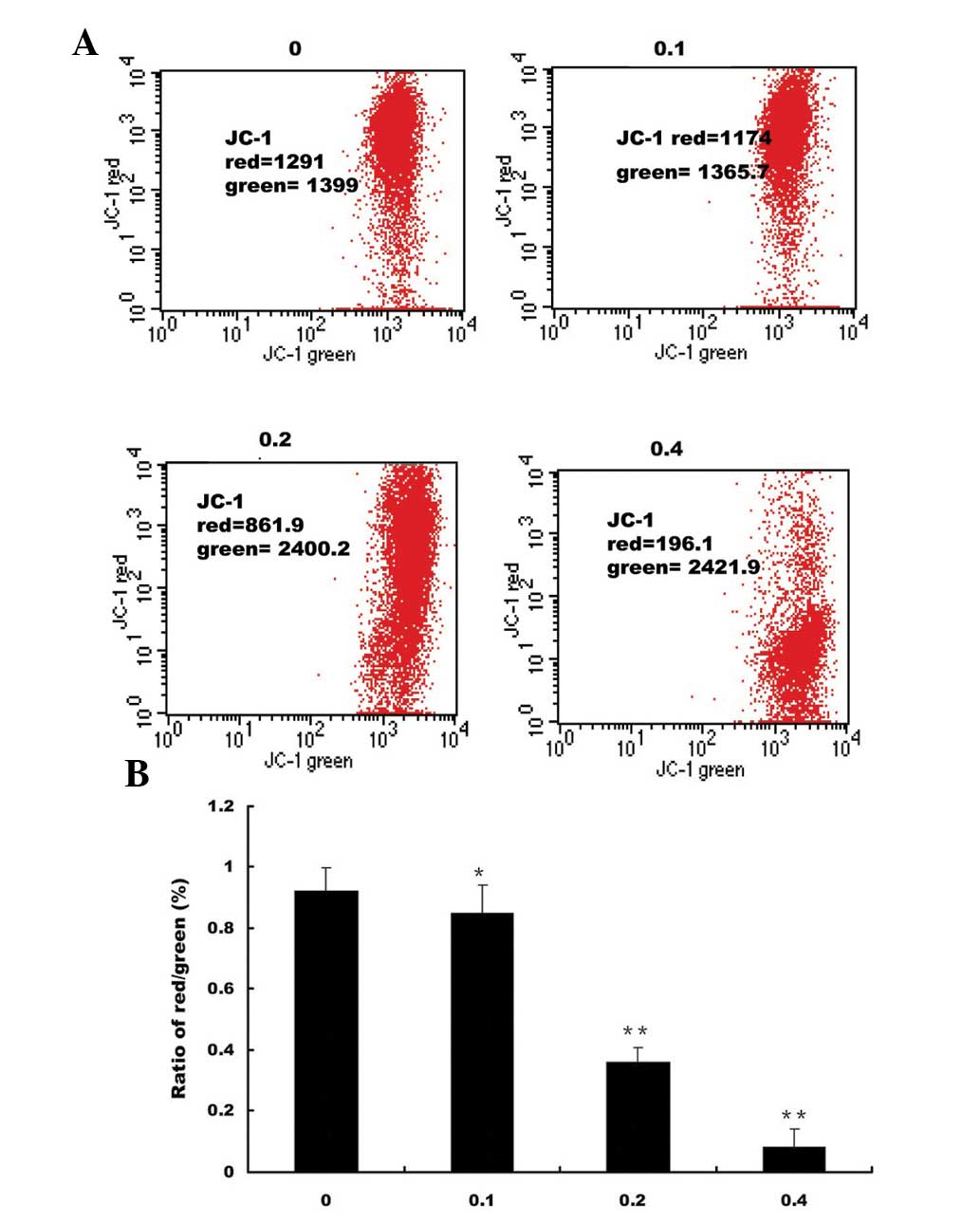 | Figure 2Effects of EE-JXY on mitochondrial
membrane potential in HepG2 human liver carcinoma cells. (A) HepG2
cells were treated with the indicated concentrations of EE-JXY for
24 h and stained with JC-1. The mean JC-1 fluorescence intensity
was detected using FACS. Data shown are representative of three
independent experiments. (B) Quantification of FACS analysis. Data
is presented as the mean ± standard deviation from three
independent experiments. *P<0.05 and
**P<0.01, compared with the control cells. JC-1,
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-benzimidazol-carbocyanine
iodide; EE-JXY, ethyl acetate fraction of Jiedu Xiaozheng Yin; PI,
propidium iodide; FACS, fluorescence-activated cell sorting; FITC,
fluorescein isothiocyanate. |
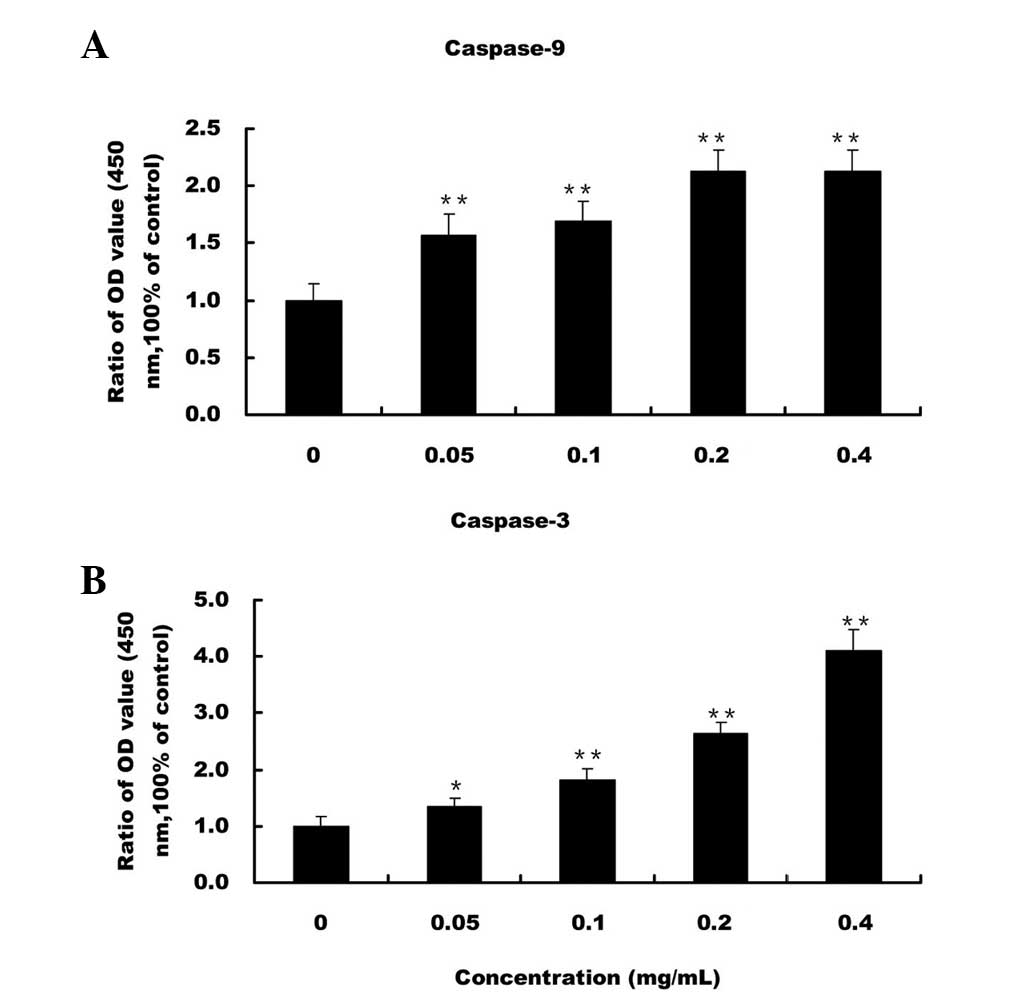
EE-JXY activates caspase-9 and
caspase-3
The present study detected the activation of
caspase-9 and caspase-3 by colorimetric assay using the following
specific chromophores: DEVD-pNA, which is a specific substrate of
caspase-3, and LEHD-pNA, which is a specific substrate of
caspase-9. Treatment with EE-JXY dose-dependently induced the
activation of caspase-9 and caspase-3 in the HepG2 cells (P<0.05
and P<0.01 respectively, as compared with the control cells;
Fig. 3).
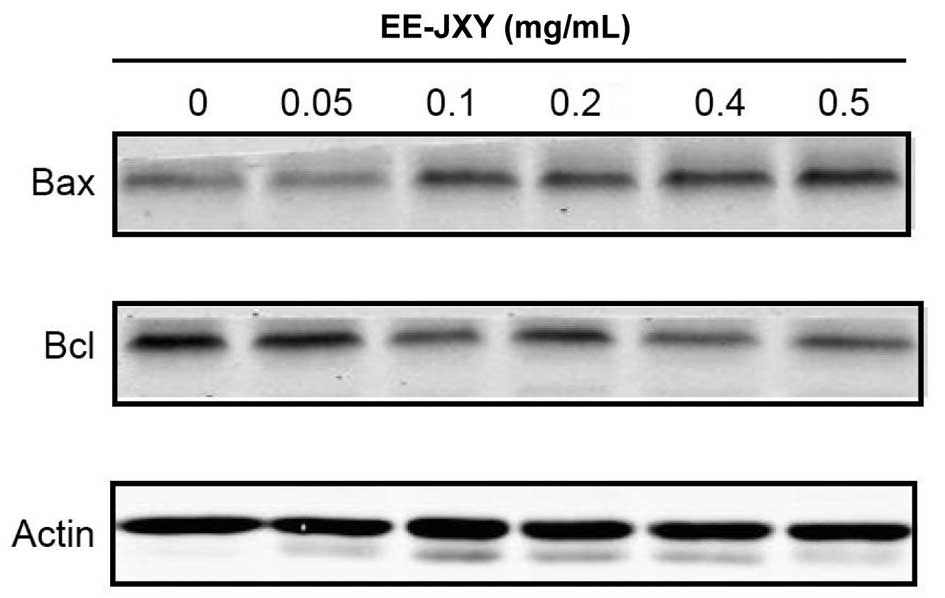
EE-JXY decreases the expression levels of
Bcl-2 and increases the expression levels of Bax
The anti-apoptotic protein Bcl-2 and the
pro-apoptotic protein Bax are able to mediate cell death or
survival, through regulation of mitochondria (24). In the present study a western blot
analysis was conducted to examine the protein expression levels of
Bcl-2 and Bax in the EE-JXY-treated HepG2 cells. Treatment with
EE-JXY markedly increased the protein expression levels of Bax and
reduced the protein expression levels of Bcl-2 in the HepG2 cells,
in a dose-dependent manner (Fig.
4).
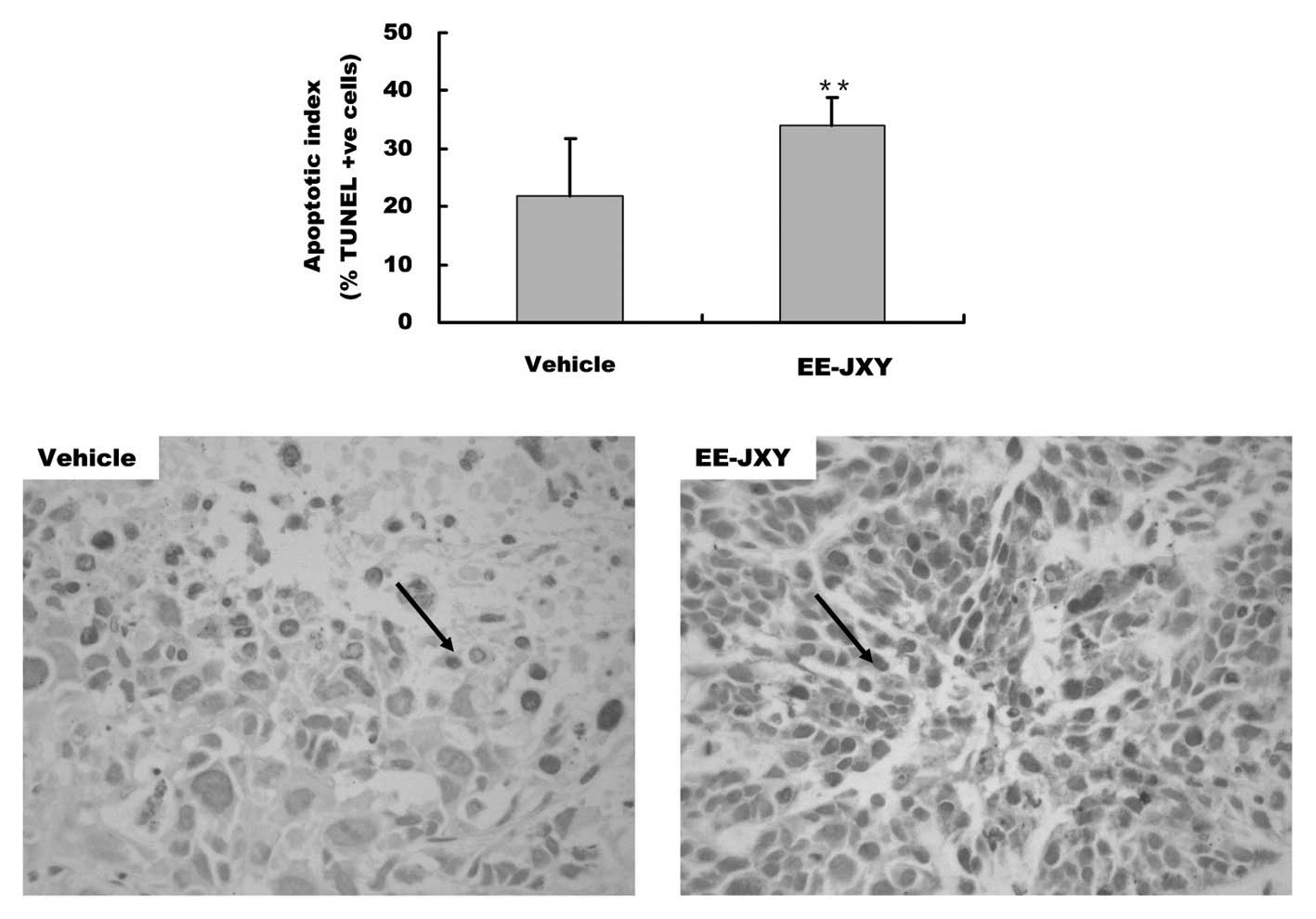
EE-JXY induces tumor apoptosis in
vivo
In our previous study, in vivo results
demonstrated that treatment with EE-JXY reduced tumor volume and
weight by ~39%, as compared with the control group (22). To further confirm the in
vitro pro-apoptotic effects of EE-JXY, the present study
compared the AI of tumors between the two groups. The AI of the
EE-JXY group was 35%, which was significantly higher as compared
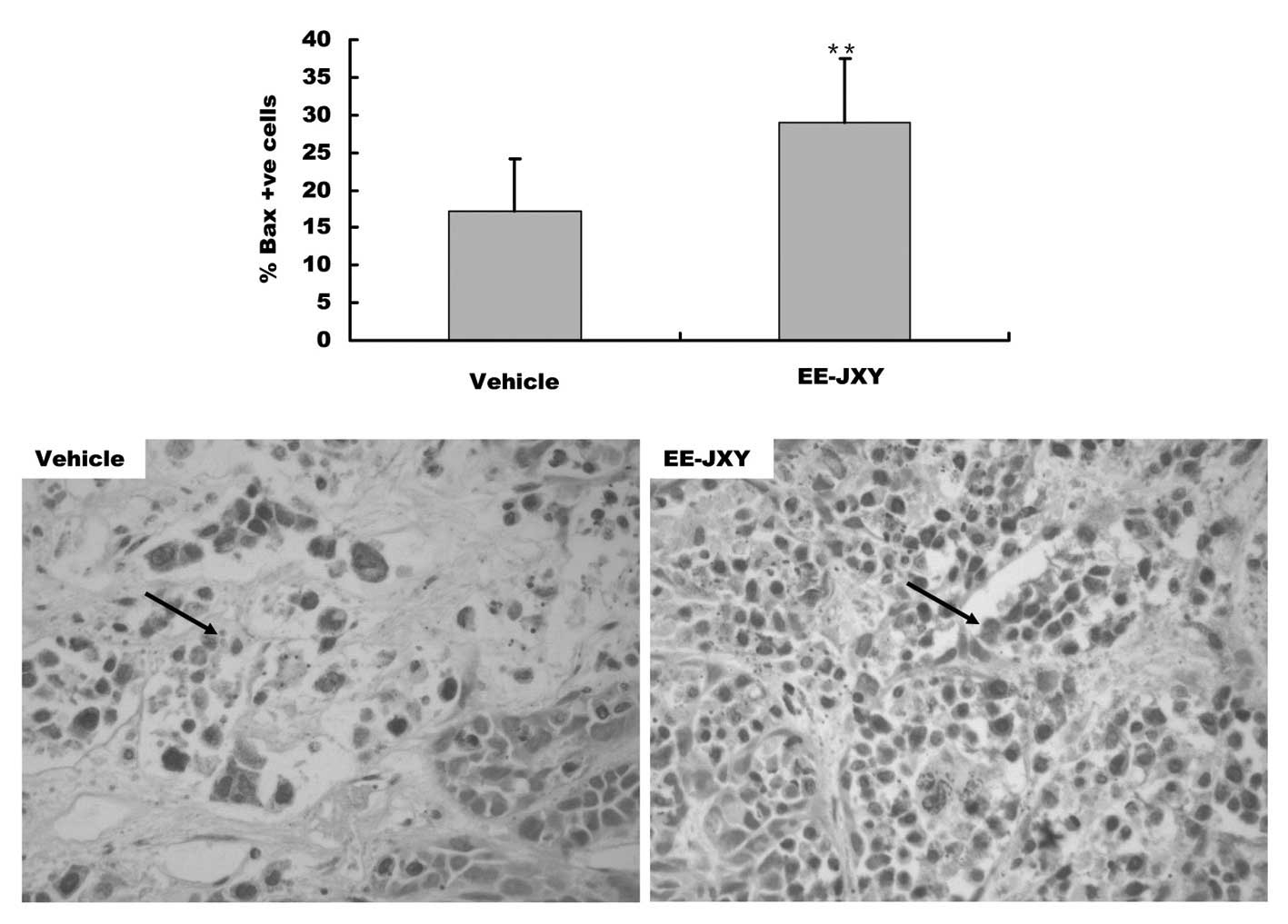
with the control group (P<0.01, Fig. 5). Furthermore, immunohistochemical
staining showed that the percentage of Bax-positive tumor cells in
the EE-JXY group was 32% higher, as compared with in the vehicle
group (P<0.01, Fig. 6). The
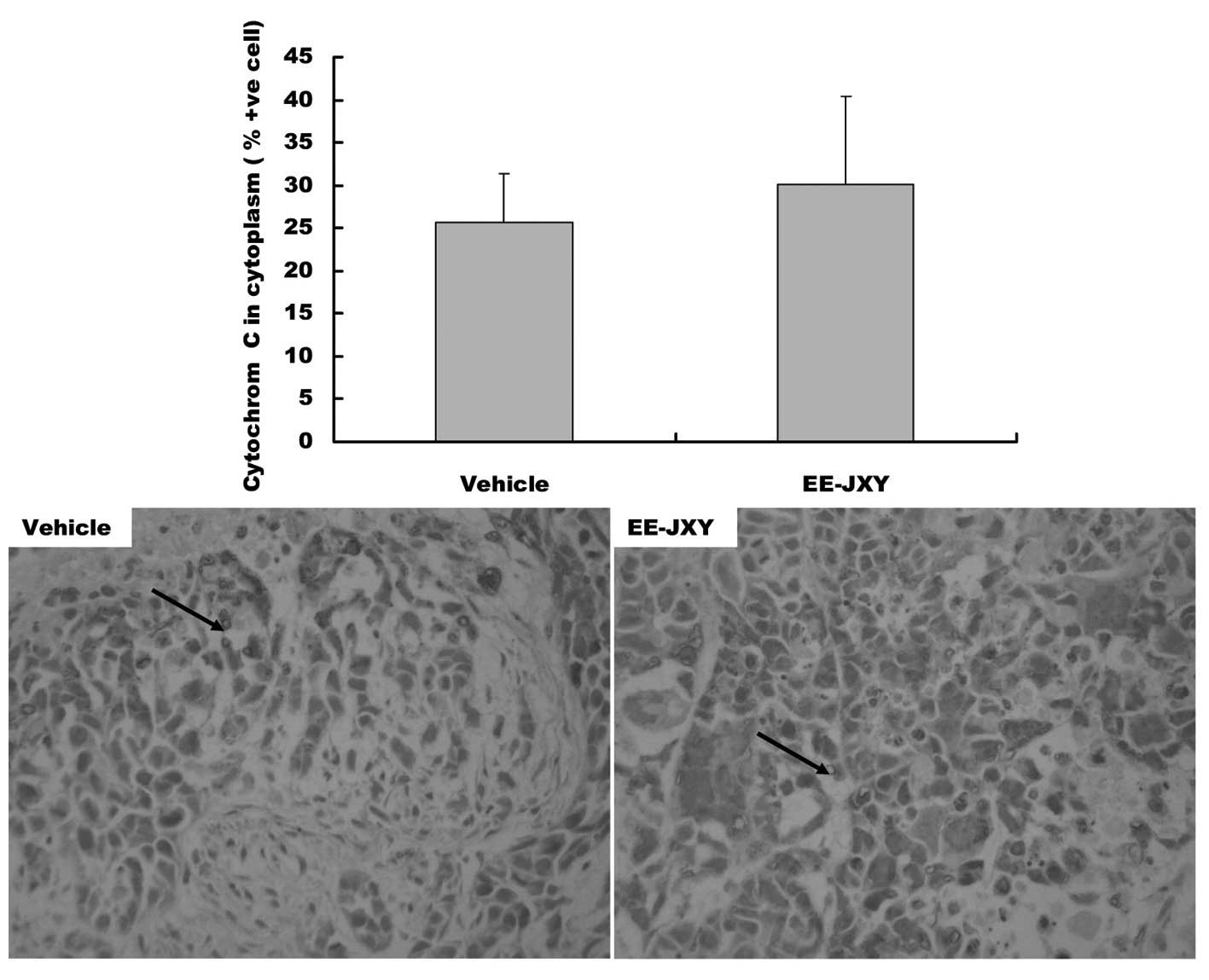
expression levels of cytochrome c were also increased in the
cytoplasm of EE-JXY group (P<0.01, Fig. 7).
Discussion
JXY is a polyherbal formula that was initially
established by Professor Jian Du, according to the principles of
TCM theory. JXY is composed of HDW, Prunella, SF and PC. It
has been used to treat malignancies of the liver, lung and stomach
(25). The present study
demonstrated that treatment with JXY inhibited tumor cell growth in
a dose- and time-dependent manner, and JXY-induced apoptosis was
accompanied by a collapse of the mitochondrial membrane potential
(Δψm) and activation of caspase proteins. Furthermore, JXY
inhibited the growth of xenografted HepG2 tumors in nude mice, and
this was accompanied by no significant side effects. Concordant
with the in vitro findings, treatment with JXY promoted
apoptosis and increased the expression levels of Bax and cytochrome
c in tumor tissue in vivo.
Mitochondria are capable of inducing apoptosis
through the release of numerous caspase activators. Among these
activators, cytochrome c activates caspases by forming a
complex with apoptotoic protease activating factor-1 and
procaspase-9, which triggers the activation of caspase-9 and
subsequently cleaves the effector caspase-3 (26). These results suggest a direct link
between the mitochondria and JXY-induced apoptosis.
The mitochondrial pathway is the main intrinsic
apoptotic pathway. It is regulated by pro-apoptotic proteins, such
as Bax and Bak, and anti-apoptotic proteins, such as Bcl-2 and
Bcl-xl. These proteins control the permeability of the MOM through
homo- and hetero-association. Activation of either of these
pro-apoptotic proteins is sufficient to induce MOM permeabilization
(MOMP) (27,28). MOMP leads to the release of
pro-apoptotic proteins, including cytochrome c and
Diablo/Smac, which subsequently trigger the activation of the
caspase cascade (29). A rapid
collapse of mitochondrial transmembrane electrical potential (Δψm)
has previously been reported in TCM-induced apoptosis of cancer
cells (30).
JXY is composed of four medicinal herbs. According
to the TCM theory, these four herbs may have synergistic effects in
inhibiting tumor growth. Previous pharmacological studies have
demonstrated that each herb exhibits distinct antitumor effects.
Extraction of HDW and Prunella has previously been
demonstrated to induce cell apoptosis, via the
mitochondrion-dependent pathway (17,31–33)
and via regulation of c-Jun N-terminal kinase expression (34). Woo et al (35) reported that
2α,3α-dihydroxyurs-12-ene-28-oic acid from Prunella induced
apoptogenic activity in Jurkat T leukemia cells, which was mediated
by a loss of Δψm, mitochondrial cytochrome c release, and
subsequent activation of caspase-9 and caspase-3, leading to the
activation of caspase-7 and caspase-8. Apoptosis was also shown to
be regulated by Bcl-2 and Bax. A previous study demonstrated that
disruption of the mitochondrial membrane and the release of
cytochrome c was regulated by the ratio of active anti- and
pro-apoptotic Bcl-2 family members (36). Pro-apoptotic factors, including
Bcl-2, prevent mitochondrial membrane disruption, whereas Bax
promotes these events (37). Feng
et al (38) reported that
oleanolic acid from Prunella vulgaris increased the rate of
apoptosis of SPC-A-1 cells, by increasing the ratio of Bax/Bcl-2
(39). Furthermore, SF has been
shown to induce the apoptosis of SGC7901 cells by loss of
mitochondrial membrane potential, reduction in the Bcl-2/Bax ratio,
and significant activation and cleavage of caspase-3 (39). To determine whether the levels of
Bcl-2 family proteins were altered in JXY-treated HepG2 cells, the
present study examined the protein expression levels of Bcl-2 and
Bax. Increased protein expression levels of Bax and decreased
protein expression levels of Bcl-2 were observed following
treatment of the HepG2 cells with various doses of JXY, and these
alterations occurred in a dose-dependent manner. These results
confirm that JXY may induce apoptosis via the mitochondrial
pathway.
Cytochrome c is a pro-apoptotic family
protein, which is usually decreased in tumors (40,41).
Zhang et al (42)
previously reported that matrine and oxymatrine, two major
components in SF, induced apoptosis by causing a collapse in
mitochondrial membrane potential, inducing cytochrome c
release from the mitochondria, reducing the ratio of Bcl-2/Bax, and
increasing activation of caspase-3 (42,43).
In conclusion, the present study provides novel
evidence suggesting that JXY is an effective and safe therapy for
the treatment of cancer. However, the potential for development of
JXY as an adjuvant agent in hepatoma cancer chemotherapy requires
further study.
Acknowledgments
The present study was supported by the CHEN Ke-ji
Integrative Medicine Development Fund (grant no. CKJ2010020), the
International Science Joint Project of the Ministry of Science and
Technology of the People’s of China (grant no. 2008DFA32200) and
the National Natural Science Foundation of China (grant no.
81302954).
References
|
1
|
Kano S: Artemisinin-based combination
therapies and their introduction in Japan. J Infect Chemother.
16:375–382. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim YJ, Chung JW, Lee SJ, Choi KS, Kim JH
and Hahm KB: Progression from chronic atrophic gastritis to gastric
cancer; tangle, toggle, tackle with Korea red ginseng. J Clin
Biochem Nutr. 46:195–204. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cardini F, Lesi G, Lombardo F and Van der
Sluijis C; MSCG-Menopause Survey Collaborative Group: The use of
complementary and alternative medicine by women experiencing
menopausal symptoms in Bologna. BMC Womens Health. 10:72010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Innocenti G, Dall’Acqua S, Scialino G,
Banfi E, Sosa S, Gurung K, Barbera M and Carrara M: Chemical
composition and biological properties of Rhododendron anthopogon
essential oil. Molecules. 15:2326–2338. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Beinfield H and Korngold E: Chinese
medicine and cancer care. Altern Ther Health Med. 9:38–52.
2003.PubMed/NCBI
|
|
6
|
Huang CF, Lin SS, Liao PH, Young SC and
Yang CC: The immunopharmaceutical effects and mechanisms of herb
medicine. Cell Mol Immunol. 5:23–31. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pan B, Cheng T, Nan KJ, Qiu GQ and Sun XC:
Effect of Fuzheng Yiliu decoction combined with chemotherapy on
patients with intermediate and late stage gastrointestinal cancer.
World J Gastroenterol. 11:439–442. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Antonsson B, Conti F, Ciavatta A,
Montessuit S, Lewis S, Martinou I, Bernasconi L, Bernard A, Mermod
JJ, Mazzei G, Maundrell K, Gambale F, Sadoul R and Martinou JC:
Inhibition of Bax channel-forming activity by Bcl-2. Science.
277:370–372. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Antonsson B, Montessuit S, Lauper S, Eskes
R and Martinou JC: Bax oligomerization is required for
channel-forming activity in liposomes and to trigger cytochrome c
release from mitochondria. Biochem J. 345:271–278. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schinzel A, Kaufmann T and Borner C: Bcl-2
family members: integrators of survival and death signals in
physiology and pathology [corrected]. Biochimi Biophys Acta.
1644:95–105. 2004. View Article : Google Scholar
|
|
11
|
Cory S and Adams JM: The Bcl-2 family:
regulators of the cellular life-or-death switch. Nat Rev Cancer.
2:647–656. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Han Y, Zhang S, Wu J, Yu K, Zhang Y, Yin L
and Bi L: Matrine induces apoptosis of human multiple myeloma cells
via activation of the mitochondrial pathway. Leuk Lymphoma.
51:1337–1346. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gao LW, Zhang J, Yang WH, Wang B and Wang
JW: Glaucocalyxin A induces apoptosis in human leukemia HL-60 cells
through mitochondria-mediated death pathway. Toxicol In Vitro.
25:51–63. 2011. View Article : Google Scholar
|
|
14
|
Wang P, Zhang K, Zhang Q, Mei J, Chen CJ,
Feng ZZ and Yu DH: Effects of quercetin on the apoptosis of the
human gastric carcinoma cells. Toxicol In Vitro. 26:221–228. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Benarba B, Meddah B and Aoues A: Bryonia
dioica aqueous extract induces apoptosis through mitochondrial
intrinsic pathway in BL41 Burkitt’s lymphoma cells. J
Ethnopharmacol. 141:510–516. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin J, Chen Y, Wei L, Chen X, Xu W, Hong
Z, Sferra TJ and Peng J: Hedyotis diffusa Willd extract induces
apoptosis via activation of the mitochondrion-dependent pathway in
human colon carcinoma cells. Int J Oncol. 37:1331–1338.
2010.PubMed/NCBI
|
|
17
|
Psotová J, Kolár M, Sousek J, Svagera Z,
Vicar J and Ulrichová J: Biological activities of Prunella vulgaris
extract. Phytother Res. 17:1082–1087. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin HJ, Tseng CP, Lin CF, Liao MH, Chen
CM, Kao ST and Cheng JC: A Chinese herbal decoction, modified Yi
Guan Jian, induces apoptosis in hepatic stellate cells through an
ROS-mediated mitochondrial/caspase pathway. Evid Based Complement
Alternat Med. 2011:4595312011. View Article : Google Scholar
|
|
19
|
Zhang Y, Zeng F, Liu X, Li Y, Zhou J,
Huang Y, Wang Y, Zhou S, Zhu W, Shu E, Zhou G and Chen G:
Chan-Yu-Bao-Yuan-Tang induces apoptosis in NSCLC and SCLC cell
lines via a mitochondria-mediated pathway. Xenobiotica. 41:593–602.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chae HJ, Yang SK, Kim DS, Kim HM, Chae SW,
Keum KS and Kim HR: Ge-Jee-Bok-Ryung-Hwan induces apoptosis in
human cervical carcinoma HeLa cells - an endoplasmic reticulum
stress pathway. Life Sci. 75:2997–3016. 2004.PubMed/NCBI
|
|
21
|
He Y, Zheng X, Sit C, Loo WT, Wang Z, Xie
T, Jia B, Ye Q, Tsui K, Chow LW and Chen J: Using association rules
mining to explore pattern of Chinese medicinal formulae
(prescription) in treating and preventing breast cancer recurrence
and metastasis. J Transl Med. 10(Suppl 1): S122012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cao Z, Lin W, Huang Z, Chen X, Zhao J,
Zheng L, Ye H, Liu Z, Liao L and Du J: Ethyl acetate extraction
from a Chinese herbal formula, Jiedu Xiaozheng Yin, inhibits the
proliferation of hepatocellular carcinoma cells via induction of
G0/G1 phase arrest in vivo and in vitro. Int J Oncol. 42:202–210.
2013.
|
|
23
|
Cao Z, Lin W, Huang Z, Chen X, Zhao J,
Zheng L, Ye H, Liu Z, Liao L and Du J: Jiedu Xiaozheng Yin, a
Chinese herbal formula, inhibits tumor angiogenesis via
downregulation of VEGF-A and VEGFR-2 expression in vivo and in
vitro. Oncol Rep. 29:1083–1086. 2013.
|
|
24
|
Kim PK, Annis MG, Dlugosz PJ, Leber B and
Andrews DW: During apoptosis Bcl-2 changes membrane topology at
both the endoplasmic reticulum and mitochondria. Mol Cell.
14:523–529. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cao Z, Lin W, Huang Z, Chen X, Zhao J,
Zheng L, Ye H, Liu Z, Liao L and Du J: Ethyl acetate extraction
from a Chinese herbal formula, Jiedu Xiaozheng Yin, inhibits the
proliferation of hepatocellular carcinoma cells via induction of
G0/G1 phase arrest in vivo and in vitro. Int J Oncol. 42:202–210.
2013.
|
|
26
|
Elmore S: Apoptosis: a review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gross A, McDonnell JM and Korsmeyer SJ:
BCL-2 family members and the mitochondria in apoptosis. Genes Dev.
13:1899–1911. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wei MC, Lindsten T, Mootha VK, Weiler S,
Gross A, Ashiya M, Thompson CB and Korsmeyer SJ: tBID, a
membrane-targeted death ligand, oligomerizes BAK to release
cytochrome c. Genes Dev. 14:2060–2071. 2000.PubMed/NCBI
|
|
29
|
Wolter KG, Hsu YT, Smith CL, Nechushtan A,
Xi XG and Youle RJ: Movement of Bax from the cytosol to
mitochondria during apoptosis. J Cell Biol. 139:1281–1292. 1997.
View Article : Google Scholar
|
|
30
|
Sen S and D’Incalci M: Apoptosis.
Biochemical events and relevance to cancer chemotherapy. FEBS Lett.
307:122–127. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen XZ, Cao ZY, Chen TS, Zhang YQ, Liu
ZZ, Su YT, Liao LM and Du J: Water extract of Hedyotis diffusa
Willd suppresses proliferation of human HepG2 cells and potentiates
the anticancer efficacy of low-dose 5-fluorouracil by inhibiting
the CDK2-E2F1 pathway. Oncol Rep. 28:742–748. 2012.PubMed/NCBI
|
|
32
|
Lin J, Wei L, Xu W, Hong Z, Liu X and Peng
J: Effect of Hedyotis diffusa Willd extract on tumor angiogenesis.
Mol Med Rep. 4:1283–1288. 2011.PubMed/NCBI
|
|
33
|
Psotova J, Svobodova A, Kolarova H and
Walterova D: Photoprotective properties of Prunella vulgaris and
rosmarinic acid on human keratinocytes. J Photochem Photobiol B.
84:167–174. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu XK, Wang L and Zhang MZ: Involvement
of JNK and caspase-3 in human lymphoma cell apoptosis induced by
Prunella vulgaris. Zhonghua Yi Xue Za Zhi. 90:690–693. 2010.In
Chinese. PubMed/NCBI
|
|
35
|
Woo HJ, Jun do Y, Lee JY, Woo MH, Yang CH
and Kim YH: Apoptogenic activity of 2α,
3α-dihydroxyurs-12-ene-28-oic acid from Prunella vulgaris var.
lilacina is mediated via mitochondria-dependent activation of
caspase cascade regulated by Bcl-2 in human acute leukemia Jurkat T
cells. J Ethnopharmacol. 135:626–635. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cory S and Adams JM: The Bcl2 family:
regulators of the cellular life-or-death switch. Nat Rev Cancer.
2:647–656. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Youle RJ and Strasser A: The BCL-2 protein
family: opposing activities that mediate cell death. Nat Rev Mol
Cell Biol. 9:47–59. 2008. View Article : Google Scholar
|
|
38
|
Feng L, Au-Yeung W, Xu YH, Wang SS, Zhu Q
and Xiang P: Oleanolic acid from Prunella vulgaris L. induces
SPC-A-1 cell line apoptosis via regulation of Bax, Bad and Bcl-2
expression. Asian Pac J Cancer Prev. 12:403–408. 2011.PubMed/NCBI
|
|
39
|
Rasul A, Yu B, Yang LF, Ali M, Khan M, Ma
T and Yang H: Induction of mitochondria-mediated apoptosis in human
gastric adenocarcinoma SGC-7901 cells by kuraridin and
Nor-kurarinone isolated from Sophora flavescens. Asian Pac J Cancer
Prev. 12:2499–2504. 2011.
|
|
40
|
Vinothini G, Murugan RS and Nagini S:
Mitochondria-mediated apoptosis in patients with adenocarcinoma of
the breast: Correlation with histological grade and menopausal
status. Breast. 20:86–92. 2011. View Article : Google Scholar
|
|
41
|
Hryciuk-Umer E, Kupisz K, Bojarska-Junak
A, Andrzejczak A, Trzaskowska E, Kotiuszko K and Klatka J:
Evaluation of cytochrome concentration in peripheral blood
lymphocytes of patients with laryngeal cancer. Otolaryngol Pol.
65:414–416. 2011.In Polish. View Article : Google Scholar
|
|
42
|
Zhang S, Zhang Y, Zhuang Y, Wang J, Ye J,
Zhang S, Wu J, Yu K and Han Y: Matrine induces apoptosis in human
acute myeloid leukemia cells via the mitochondrial pathway and Akt
inactivation. PLoS One. 7:e468532012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ling Q, Xu X, Wei X, Wang W, Zhou B, Wang
B and Zheng S: Oxymatrine induces human pancreatic cancer PANC-1
cells apoptosis via regulating expression of Bcl-2 and IAP
families, and releasing of cytochrome c. J Exp Clin Cancer Res.
30:662011. View Article : Google Scholar : PubMed/NCBI
|