Introduction
Millions of individuals suffer from inherited and
acquired retinal diseases, which have become the leading cause of
blindness in adults (1,2). However, no satisfactory treatment is
currently available for these disorders. As insight into the
molecular mechanisms of the ocular diseases has increased, gene
therapy has been proposed as a promising therapeutic tool for the
treatment of ocular diseases. Successful gene therapy depends on
efficient gene transfer to the targeted cells to provide stable
gene expression with minimal toxicity. How to render foreign gene
expression safe and efficient in target cells is a key issue.
Although the use of a viral vector is able to provide a high
transfer efficiency, the limited payload capacity, difficult large
scale production and safety issues, such as potential
immunogenicity and oncogenesis may hamper their clinical
application (3–5). Nonviral vectors have attracted
interest, as they are simple to prepare, stable, easy to modify and
safe compared with viral vectors. However, they are also
disadvantaged by a low transfection efficiency and transient gene
expression (6,7). These limitations have prompted the
requirement for the development of an effective delivery method,
which has a high level of biosafety and a low level of
cytotoxicity.
Ultrasound (US)-targeted microbubble (MB)
destruction (UTMD)-mediated gene delivery systems hold promise as
effective delivery methods (8–12).
Numerous proof-of-principle studies have indicated that ultrasonic
irradiation itself promotes gene transfection and expression, UTMD
is able to further enhance the gene transfection efficiency in
vitro and in vivo (8–10).
It has become an intense area of investigation as it is a
non-invasive, target-specific type of gene delivery. In the sphere
of ophthalmology, the application of UTMD-mediated gene therapy has
been observed to be efficient in previous studies (11,12).
Polyethylenimine (PEI) is a cationic polymer with a
high charge density, which has been widely examined in numerous
studies as a gene-delivery vector (13). PEI-mediated gene delivery is based
on the electrostatic interaction of the polycation with the
negatively charged phosphate groups of DNA. PEI is able to condense
DNA into compact particles via the electrostatic interaction with
condensing compounds, protecting the DNA from degradation by
nucleases or other enzymes, and the compact particles may be
engulfed by cells via natural processes, such as endocytosis,
pinocytosis and phagocytosis (14). The proton-sponge effect of PEI is
responsible for efficient gene transfer, which is able to evade
lysosomal degradation by rupture of the endosomal vesicle prior to
fusion. To the best of our knowledge, UTMD-mediated PEI/enhanced
green fluorescent protein plasmid (pEGFP) transfection of retinal
pigment epithelial (RPE) cells in vitro and rat retina in
vivo has not been previously reported. The aim of the present
study was to evaluate whether the combination of UTMD and PEI was a
useful and suitable tool for the transfection of RPE cells in cell
culture and in vivo in the rat.
Materials and methods
pEGFP and MBs
An expression vector for the EGFP gene, pEGFP-N1
(4.7 kb), was provided by the Experimental Research Center of
Shanghai Jiao Tong University Affiliated Renji Hospital (Shanghai,
China). The pEGFP-N1 was purified from a culture of Escherichia
coli DH5a using the EndoFree Plasmid Maxi kit (Qiagen Inc.,
Valencia, CA, USA) according to the manufacturer’s instructions.
The purity of the plasmid DNA was determined by measuring
absorption at a 260 nm wavelength (A260) using spectrophotometry
(DU-640; Bechman Coulter, Fullerton, CA, USA). The concentration of
isolated plasmid DNA was resuspended to a final concentration of 1
μg/μl in buffer (Sigma-Aldrich, St. Louis, MO, USA)
and stored at −20°C. The A260:A280 ratio of pEGFP-N1 was between
1.8 and 2.0, indicating that the purified plasmid DNA was free of
contaminants.
Second generation MBs, termed SonoVue, which are
coated with a thin lipid monolayer membrane shell, and consist of a
gas core of SF6 were purchased from Bracco (Milan,
Italy). The average diameter of the MB was between 2.5 and 6.0
μm. They were reconstituted in a saline solution according
to the manufacturer’s instructions and yielded a preparation
containing 2–5×108 MBs/ml. In order to calculate the MB
cell ratio, 2×108 MBs/ml was used as a basis.
In vitro study
Cell culture
The ARPE-19 human RPE cell line was obtained from
the American Type Culture Collection (CRL-2302; American Type
Culture Collection, Rockville, MD, USA) and incubated in Dulbecco’s
modified Eagle’s medium (DMEM; Gibco-BRL, Grand Island, NY, USA)
with 10% fetal bovine serum (Gibco-BRL), and 100 U/ml penicillin
and 100 μg/ml streptomycin, at 37°C in a humidified
environment of 5% CO2. The total cell count was
determined using a cell counting chamber (Huarui Medical Devices
Co. Ltd., Jiangsu, China). The initial cell viability was
determined by means of exclusion with Trypan blue dye
(Sigma-Aldrich, St. Louis, MO, USA). Dead cells were colored pale
blue, whereas living cells remain unstained. Cell viability (%) =
number living cells / total number of living and dead cells x
100.
Preparation of transfection
complex
Branched PEI with an average molecular weight of 25
kDa was purchased from Sigma-Aldrich and diluted 1 mg PEI coupling
medium (Tianrun Medical Devices Co., Ltd., Jiangsu, China) in 1,000
ml deionized water, neutralized with HCl and filtering at 0.2
μm (Millipore, Bedford, MA, USA). The PEI/pEGFP complexes
were developed by mixing PEI and the plasmids at 0.5:5 of PEI
nitrogen/DNA phosphate ratio (N/P ratio). The N/P ratio was based
on the recognition that 1 μl PEI stock solution contained 10
nmol amine nitrogen and 1 μg DNA contained 3 nmol phosphate
(15). Agarose gel electrophoresis
was performed at 80 V for 40 min to determine the N/P ratio at
which PEI was able to condense the DNA efficiently. The PEI/pEGFP
complexes were incubated for 30 min at room temperature prior to
the experiment. Once the cells grew to 70–80% confluence, they were
transfected with the PEI/pEGFP complexes. These cells were infected
with PEI/pEGFP alone or in combination with US or UTMD. The cells
infected with PEI/pEGFP and UTMD served as the experimental
group.
US exposure
A therapeutic US machine (Topteam 161; Chattanooga
Medical Supply, Inc., Chattanooga, TN, USA) was used in the present
experiment. The cells were exposed to US [frequency, 1 MHz;
intensity, 1, 2 and 3 w/cm2; duration, 1,2 and 3 min;
pulse wave with a 20 and 50% duty cycle (DC) and continuous wave;
and pulse recurrent frequency, 100 Hz] with or without MB. The
dosage of SonoVue was selected according to the ratio of MBs to
cells (20:1, 50:1 and 80:1). The probe was placed at the bottom of
the plates with a small quantity of coupling medium between them. A
self-made plastic disc of the same size as the wells was placed
between the probe and the bottom of the plates to ensure the same
thickness of coupling medium between the probe and the plates, and
to avoid ultrasonic radiation affecting the adjacent wells. In the
process of the irradiation, the plate was moved slowly around the
circumference of the plastic dish.
Effect of UTMD on cell viability
The viability of the ARPE-19 cells under the effect
of the US with contrast agent was assessed with a cell counting
kit-8 (Qiagen, Crawley, UK) according to the manufacturer’s
instructions. The ARPE-19 cells were transferred into the 96-well
plates, in every other four wells, at a concentration of
1×104 cells/well and grown in a humidified incubator at
37°C and 5% CO2 for 24 h. When the cells reached 70–80%
confluence, the medium in each well was replaced with 100 μl
fresh DMEM for the control group, or with a 100 μl mixed
solution of MBs and fresh DMEM for the UTMD group. The optimum
parameters of UTMD attained from the experiment investigating cell
viability were used in the following experiment.
Assessment of transfection
efficiency
After the gene transfer treatment, ARPE-19 cells
were incubated for 48 h. EGFP expression was observed and images
were captured using an inverted fluorescence microscope (Axio
Observer Z1; Carl Zeiss, Inc., Oberkochen, Germany). The ratio of
EGFP-positive RPE cells was quantitatively examined using flow
cytometric analysis (Epics XL; Beckman Coulter, Miami, FL,
USA).
In vivo study
Normal adult Sprague-Dawley (SD) rats (female;
weight, 250 g) were used in the present experiment. All animals
were handled humanely in accordance with the policies stated in the
Vision and Ophthalmology Statement for the Use of Animals in
Ophthalmic and Vision Research and with the guidelines approved by
national and local institutions. The rats were anesthetized with an
intraperitoneal injection of 10% chloral hydrate. Pupillary
dilatation was achieved using tropicamide eye drops (Xingqi
Parmaceutical Co., Ltd., Shengyang, China). The eyes were gently
protruded using a plastic circle and subsequently covered with
ofloxacin eye ointment (Xingqi Parmaceutical Co., Ltd.). A 26-gauge
needle was inserted 1 mm posterior to the corneal limbus, causing a
self-sealing wound tunnel, which was observed under a surgical
microscope (SM-2000 J; Eder, Shanghai, China). PEI/pEGFP complexes
(4 μl) alone or in combination with MBs (1 μl) or
normal saline (1 μl) were injected into the eyes of the
rats.
A total of 32 rats were used in the present study as
follows: i) PEI/pEGFP group (16 rats): PEI/pEGFP complexes and
normal saline, without MBs and US exposure; and ii) PEI/pEGFP +
UTMD group (16 rats): PEI/pEGFP complexes and MBs with US
exposure.
Considering the risk of volume overload, the
infusions were administered slowly. Directly after the subretinal
injection, the eyes of the rats in the PEI/pEGFP + UTMD group were
exposed to US. The frequency used was 1 MHz, US intensity was 2
w/cm2 and the pulse repetition frequency was 100 Hz,
with 50% DC. The entire treatment lasted for 5 min.
A total of 12 eyes were enucleated following
sacrifice via an overdose administered on day 5 after subretinal
injection from each group. The fundus oculi were prepared following
enucleation of the globe via removal of the anterior segment with a
blade and carefully transferring the whole fundus oculi to a
microscope slide. The transfected tissue was visualized using an
inverted fluorescence microscope (Zeiss Axiovert S100; Zeiss,
Oberkochen, Germany). The gene-transfer efficiency was observed and
images were captured. If no EGFP-positive cells were observed, the
cells were defined as negative; star-like EGFP-positive cells
observed were defined as a weak positive; and diffuse EGFP-positive
cells observed were defined as a strong positive. Two eyes were
harvested from each group to produce frozen sections on day 5. The
enucleated eyes were embedded in optimal cutting temperature
compound (Sakura; Torrance, CA, USA) and cryosections (10 mm) were
obtained. Two eyes were also harvested from each group for
histology on day 5. The sections were stained with hematoxylin and
eosin to visualize the retinal architecture using microscopy. All
specimens were assessed by two observers who were blinded to the
experimental conditions and received no information regarding the
nature of the specimens.
Statistical analysis
Statistical analyses were performed using the SPSS
13.0 software package (SPSS, Inc., Chicago, IL, USA). All data are
expressed as the mean ± standard deviation. All transfection
conditions were performed in three parallel wells following an
identical procedure and repeated three times. Multiple group
comparisons were performed using a one-way analysis of variance.
χ2 tests were used for count data analysis. P<0.05
was considered to indicate a statistically significant
difference.
Results
Gel retardation analysis of PEI/pEGFP
complex
Agarose gel electrophoresis retardation analysis
revealed that PEI could condense DNA efficiently (Fig. 1). With the increment of the N/P
ratio, the plasmid DNA migrated more slowly. At N/P≥3, the plasmid
DNA migration could not be observed, and PEI could effectively
condense the plasmid DNA. According to the results of agarose gel
electrophoresis, the N/P ratio was selected as 8 in the present
study.
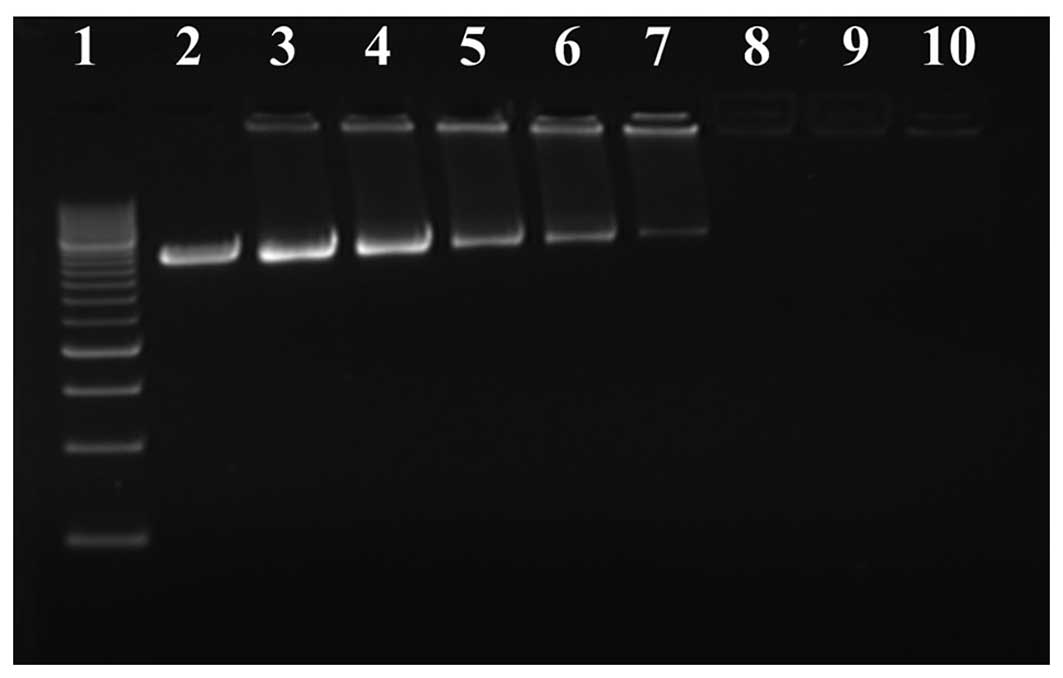 | Figure 1Electrophoretic patterns of plasmid
DNA complexes prepared with PEI at various N/P ratios. Lane 1, DNA
maker; 2, plasmid DNA alone; 3, N/P= 0.5; 4, N/P=1.0; 5, N/P=1.5;
6, N/P=2; 7, N/P=2.5; 8, N/P=3; 9, N/P=4; and 10, N/P=5. When
N/P≥3, the plasmid DNA migration could not be observed, and PEI was
able to effectively condensate the plasmid DNA. PEI,
polyethylenimine; N/P ratio, PEI nitrogen/DNA phosphate ratio. |
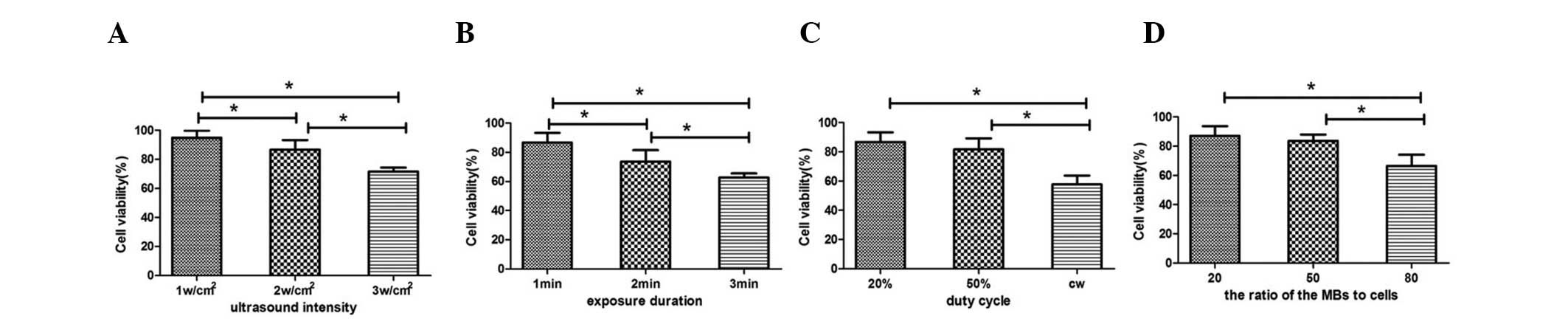
Effect of UTMD on cell viability
The effects of UTMD on the viability of ARPE-19
cells are shown in Fig. 2. The
cell viability of all experimental groups is lower than that of the
control group. When the DC was set at 20% and the ratio of MBs to
cells set at 20:1, the increase in US intensity (between 1 and 3
w/cm2) and duration (between 1 and 3 min) resulted in a
decrease in cell viability (Fig.
2A and B). The cell viability was the lowest in the 3
w/cm2 (71.70±2.77%) and the 3 min groups (62.71±2.74%),
the two exhibiting levels of <80%. The cell viability in the 2
and 3 w/cm2 groups was significantly lower than that in
the 1 w/cm2 group (P=0.031 and 0.000, respectively;
Fig. 2A). The cell viability in
the 2 and 3 min groups was significantly lower than that in the 1
min group (P=0.016 and 0.004, respectively; Fig. 2B). The cell viability in the 20% DC
and 50% DC groups was significantly higher than that in the
continuous wave group (51.25±5.43%), but no significant difference
was identified between the 20 and 50% DC groups (86.67±6.65 vs.
81.59±7.55%; Fig. 2C). Under the
experimental condition of 1-min duration, 20% DC and 2
w/cm2 intensity, the increase in the ratio of MBs to
cells (20:1, 50:1 and 80:1) resulted in an increase in cell
mortality. The viability of the cell was lowest in the 80:1 group
(66.40±7.64%), and no significant difference was identified between
the 20:1 and the 50:1 MB concentration groups (84.72±6.65 vs.
83.56±4.37%; Fig. 2D). Under the
optimal US parameters (frequency, 1 MHz; pulse recurrent frequency,
100 Hz; intensity, ≤2 w/cm2; duration, 1 min; pulse
wave, 20 and 50% DC; and ratio of MBs to cells, 20:1 and 50:1), the
cell viability was >80%, and the cells were equally distributed
with no significant cell damage. The optimal parameters were used
in the following experiments.
Gene transfer by pEGFP alone, pEGFP + US,
PEI/pEGFP and PEI/pEGFP + US
Compared with the group subjected to pEGFP alone
(0.63±0.18%), the pEGFP + US group (1.09±0.34%) exhibited a weak
but non-significant tendency to improve transgene expression.
Compared with the pEGFP alone and pEGFP + US groups, the PEI/pEGFP
(7.31±1.06%) and PEI/pEGFP + US (7.46±1.04%) groups exhibited a
significantly higher expression of the EGFP-positive cells.
However, no significant improvement was observed in the transgene
expression between the PEI/pEGFP + US and PEI/pEGFP group.
UTMD-mediated PEI/pEGFP transfection
To optimize the conditions of the UTMD-mediated
PEI/pEGFP transfection of the ARPE-19 cells, different parameters
were examined.
The pulse waves of 20 and 50% DC were examined for 1
min duration, 1 w/cm2 intensity, and the ratio of the
MBs to the cells was at 50:1. The EGFP-positive ratio was
significantly higher in the 50% DC group (12.48±1.01%) than that in
the 20% DC group (9.13±0.68%) (Fig.
3A). The parameter of 50% DC was selected for the following
experiments.
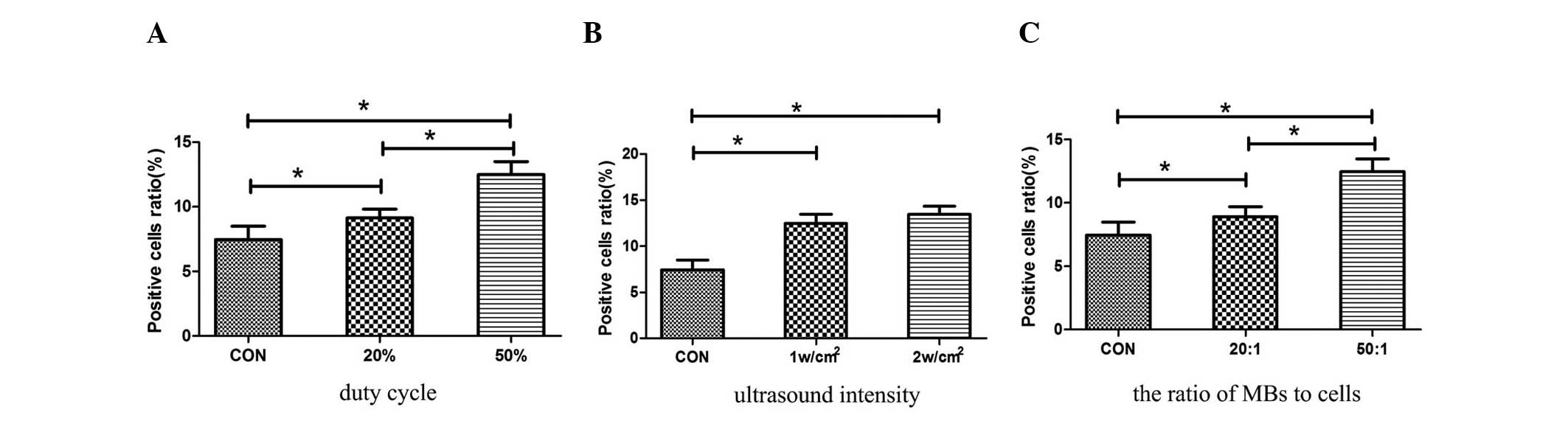 | Figure 3UTMD-mediated PEI/pEGFP transfection
of ARPE-19 cells. (A) Under the conditions of a 50% duty cycle,
UTMD was able to significantly enhance transfection efficiency
compared with that in the control and 20% duty cycle groups
(P=0.0001 and 0.0001, respectively). (B) Under the conditions of a
1 and 2 w/cm2, UTMD was able to significantly enhance
transfection efficiency compared with that in the control group
(P=0.001 and 0.001, respectively). No significant difference was
identified between the 1 and 2 w/cm2 groups. (C) Gene
transfer efficiency in the 50:1 (MBs:cells) group was significantly
higher than that in the 20:1 (MBs:cells) group (P=0.0001), and the
two were significantly higher than that in the control group
(P=0.0001 and 0.001, respectively). pEGFP, enhanced green
fluorescent protein plasmid; PEI, polyethylenimine; US, ultrasound;
UTMD, ultrasound-targeted microbubble destruction; MBs,
microbubbles. |
The US intensities of 1 and 2 w/cm2 were
examined under the condition of 50% DC, 1-min duration and the
ratio of the MBs to the cells 50:1. No significant difference was
identified in the EGFP-positive ratio between the 1 and 2
w/cm2 groups (12.48±1.01% vs. 13.45±0.89%, respectively;
Fig. 3B). In consideration of the
effect of the UTMD on cell viability, 1 w/cm2 was
selected as the optimal parameter.
Under the condition of 50% DC, 1-min duration and 1
w/cm2 intensity, the optimal ratio of the MBs to the
cells (20:1 and 50:1) was selected. The EGFP-positive ratio was the
highest in cells treated by US with the ratio of the MBs to the
cells at 50:1 (Fig. 3C). Thus, a
ratio of the MBs to the cells at 50:1 was determined to be
appropriate.
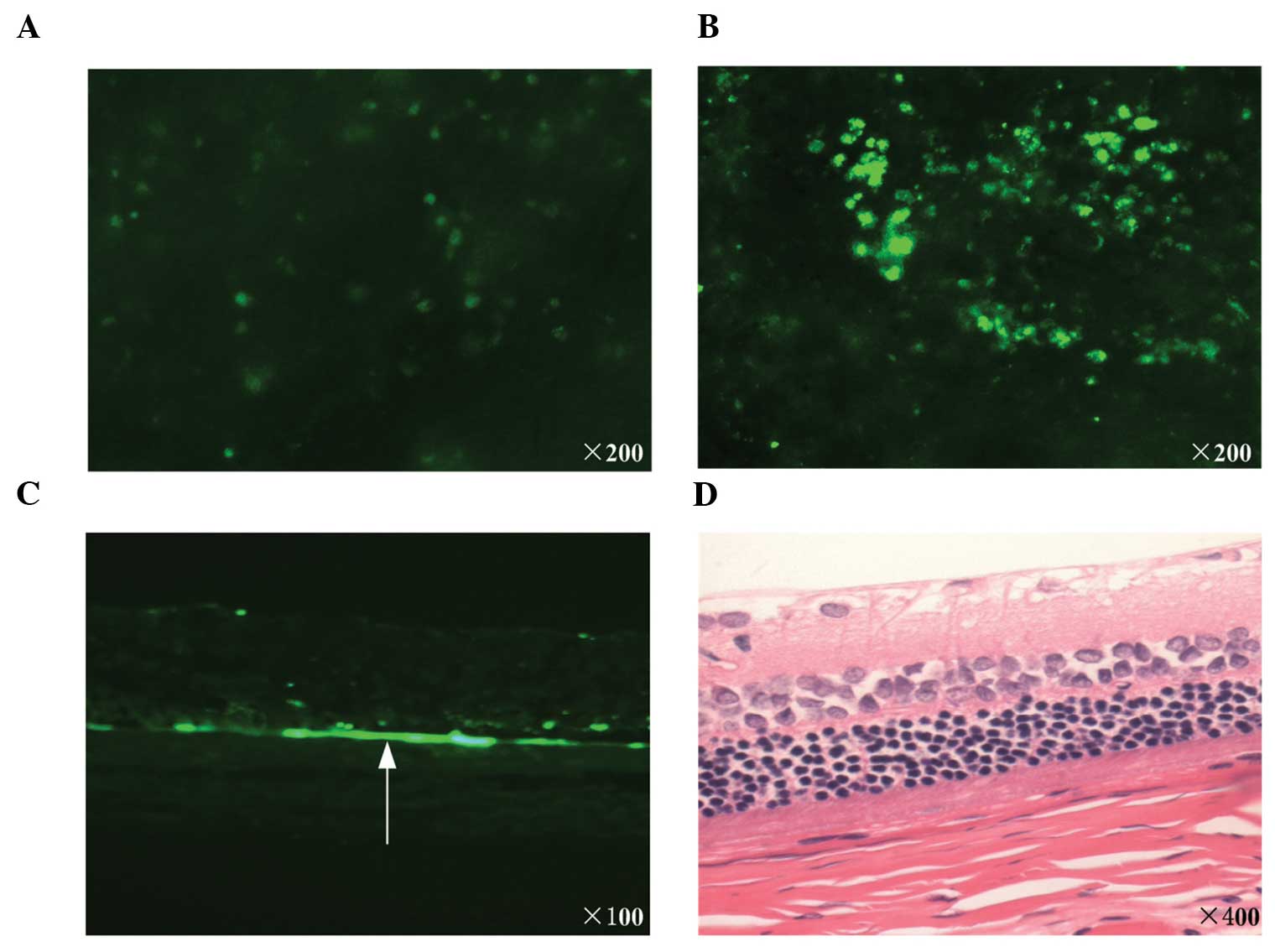
In vivo study
Star-like EGFP-positive cells (weak positive;
Fig. 4A) were observed in the rats
from each groups. Negative expression was observed only in the rats
of the PEI/pEGFP group (Table I).
Diffuse EGFP-positive cells were observed (strong positive;
Fig. 4B) in 7 of the 12 rats in
the group treated with PEI/pEGFP complexes with UTMD (Table I). The difference between the
experimental group and the control group was significant
(P=0.0038). The frozen sections of the optic cups revealed green
fluorescence, predominantly distributed in the retina (Fig. 4C). The histological analysis
revealed that no marked tissue damage was present in the UTMD group
following transfection (Fig.
4D).
 | Table IDistribution of the number of rats in
different EGFP expression groups. |
Table I
Distribution of the number of rats in
different EGFP expression groups.
| Condition | EGFP expression (n)
|
|---|
| Negative | Weak positive | Strong
positive |
|---|
| PEI/pEGFP | 3 | 9 | 0 |
| PEI/pEGFP+UTMD | 0 | 5 | 7 |
Discussion
With advances in the preparation technology of MBs
and the innovations in US imaging, contrast-enhanced US is no
longer confined to the detection of tissue perfusion, but has
gradually extended to specific US molecular imaging and targeted
therapy. MBs as cavitation nuclei are able to volumetrically expand
and contract in response to the compression and rarefaction phases
of US waves (16). When the
acoustic pressure reaches a certain threshold, MBs may collapse
violently and cause a series of biological effects. The physical
responses of MBs are able to mechanically perturb the integrity of
blood vessel walls and cell membranes, thus increasing their
permeability to therapeutic agents (16). In recent years, numerous
proof-of-principle studies have indicated that the combination of
low-intensity US and MBs allows direct DNA transfer into the
cytosol through small pores in the cells caused by cavitation
effects, and enhances gene transfection in vitro and in
vivo (17–19).
Non-viral vectors for gene delivery offer a host of
potential advantages over viruses. However, compared with viral
systems, the majority of non-viral vectors are less efficient with
hard-to-transfect cell types (20). Multiple alternative non-viral gene
delivery approaches have been reported for the in vitro
transfection of RPE cells, but the results were unsatisfactory. A
previous study was designed to evaluate the transfection capacity
of solid lipid nanoparticles in the human RPE established cell
lines. The results demonstrated that the transfection efficacy was
low, and only 2.5% EGFP positive cells were observed at 72 h after
transfection (21). Urtti et
al (22) reported that the
transfection efficiencies of primary RPE cells were <1% for
lipofectin, <5% for degraded dendrimers, and between 1 and 3%
when using DOTAP/DOGS. The low and unsatisfactory transfection
efficiency of RPE cells in vitro indicates a requirement for
more efficient delivery systems to augment the transfection
efficiency. The present study was designed to determine whether the
combination of UTMD with PEI was able to enhance the gene
transfection of the RPE cells in vitro and in
vivo.
To establish the optimal conditions of UTMD-mediated
gene transfer to human RPE cells, various US conditions were
examined. The US intensity, exposure duration, DC and MB
concentration were examined. The present results revealed that the
viability of the cells reduced with an increase in the exposure
intensity, duration, DC and the ratio of the MBs to cells.
Considering cell viability and gene transfer efficiency, the
optimal UTMD condition was as follows: US intensity, 1
w/cm2; exposure duration, 1 min; DC, 50%; and ratio of
the MBs to cells, 50:1.
In the present study, PEI-EGFP complexes with or
without US were transfected into the human RPE cells with a
7.45±1.25 and 7.46±1.04% transfection efficacy, respectively.
Similarly to the present study, Sunshine et al (23) demonstrated that branched 25 kDa PEI
transfected ARPE-19 cells with an 8±1% transfection efficacy. A
number of studies have revealed that ultrasound-mediated
transfection (USMT) is able to enhance gene transfer efficiency,
but the present findings indicated that US alone without MBs was
not able to increase the transfer efficiency. It is possible that a
higher US intensity is required for the transient pore formation in
human RPE cells, but this may decrease the viability of cells.
UTMD may facilitate targeted gene transfection, thus
significantly enhance gene transfection in vitro. Under
optimal conditions, UTMD-mediated gene transfection was
12.48±1.01%, which was significantly greater than other groups
without causing any apparent adverse effects. The transfection
efficiencies of UTMD and PEI were enhanced 19.8-fold compared with
transfection of an EGFP plasmid alone. The results of the present
in vitro study revealed that UTMD was able to significantly
enhance PEI-mediated gene expression in RPE cells.
In the area of ophthalmology, investigations into
UTMD-mediated gene delivery have predominantly focused on the
cornea and retina. Sonoda et al (11) performed a study to estimate the
practical efficacy and safety of US with MB-mediated gene delivery
to the cornea. The results revealed that US alone exerted no
significant enhancement in gene transfer in the in vivo
study. However, US with MBs is able to markedly increase GFP gene
transfer in vivo and in vitro without causing any
apparent side effects, and the transfer efficiency was
significantly higher than that with US or naked plasmid alone. Li
et al (12) reported that
UTMD was able to enhance rAAV2 transfection efficiency in human RPE
cells in vitro and in rat retina in vivo, but USMT
and MBs alone did not affect the transfection efficiency of the
retina in vivo. In the present study, no EGFP-positive cells
(negative) and star-like EGFP-positive cells (weak positive) were
observed in the rats treated with PEI-pEGFP alone. Diffuse
EGFP-positive cells were observed (strong positive) in 7 of the 12
eyes in the UTMD group, and the histological analysis demonstrated
that no marked tissue damage was present in the UTMD group
following transfection.
Multiple administrative routes are currently used to
deliver bio-active materials to the eye. If the retina is the
target, topical, systemic and periocular approaches are limited by
the blood-retinal barrier (BRB) and other ocular barriers. In the
present study, subretinal injection was selected, which is able to
provide direct access to the retinal tissues. Due to the risk of
retinal detachment and hemorrhage, this invasive method is not the
first choice for the treatment of diseases of the eye clinically.
Therefore the development of non-invasive and efficient methods,
which are able to bypass the BRB and enhance the delivery of
therapeutic materials to the retina is important. Park et al
(24) demonstrated that the
appropriate intensity of focused US combined with MBs are able to
induce a temporary and reversible disruption of the BRB in rats,
and the barrier was found to be restored within a few hours after
sonication. In their study, no significant retinal damage was
identified in the histology at the two lower acoustic pressure
amplitudes test. US with MBs may offer a noninvasive, localized and
repeatable means to reversibly disrupt the BRB for the delivery of
ocular substances, which requires further investigation.
As the SonoVue membranes and DNA bear a negative
charge (25), the binding of the
SonoVue MB and the plasmid DNA may be weak and transient. PEI, as
one of the most effective cationic gene vectors, is able to
condense DNA into compact particles via its electrostatic
interaction. The PEI/DNA complexes may be adsorbed to the surface
of MBs through electrostatic interaction. They may form a
DNA/PEI/SonoVue complex. The complex could be released when
targeted with US irradiation, and enhance the transfection
efficiency without off-target effects. In the present study, the
transfection efficiency was enhanced following treatment. With an
increase in the N/P ratio, the viability of the cells may be
reduced (26). In the present
study, a relatively low N/P ratio (N/P=8) was selected, and the
results revealed that this N/P ratio was able to increase the
transfection efficiency effectively in vitro and in
vivo. However, the efficiency of different N/P ratios should be
analyzed in further investigations.
In the present study, it was demonstrated that the
combination of UTMD and PEI was able to significantly enhance the
gene expression of plasmid DNA in human RPE cells in vitro
and in SD rat retina in vivo without any apparent tissue
damage. UTMD provided a new method for the systemic administration
of the non-viral vectors. Although this method was highly dependent
upon the acoustic conditions and the MB concentration, its
simplicity and non-invasiveness could provide a safe and effective
non-viral gene delivery system for the gene transfer of certain
inherited and acquired diseases of the retina.
Acknowledgments
The present study was supported by grants from the
Natural Science Foundation of Shanghai Science Commission (no.
11ZR1421100), the Scientific Effort Project of Shanghai Science and
Technology committee (no. 1441968200) and the Natural Science
Foundation of China (no. 81200700).
References
|
1
|
Surace EM and Auricchio A: Versatility of
AAV vectors for retinal gene transfer. Vision Res. 48:353–359.
2008. View Article : Google Scholar
|
|
2
|
Zhang L, Li X, Zhao M, He P, Yu W, Dong J,
et al: Antisense oligonucleotide targeting c-fos mRNA limits
retinal pigment epithelial cell proliferation; a key step in the
progression of proliferative vitreo-retinopathy. Exp Eye Res.
83:1405–1411. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lehrman S: Virus treatment questioned
after gene therapy death. Nature. 401:517–518. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu Q and Muruve DA: Molecular basis of
the inflammatory response to adenovirus vectors. Gene Ther.
10:935–940. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun JY, Anand-Jawa V, Chatterjee S and
Wong KK: Immune responses to adeno-associated virus and its
recombinant vectors. Gene Ther. 10:964–976. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Glover DJ, Lipps HJ and Jans DA: Towards
safe, non-viral therapeutic gene expression in humans. Nat Rev
Genet. 6:299–310. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park HJ, Yang F and Cho SW: Nonviral
delivery of genetic medicine for therapeutic angiogenesis. Adv Drug
Deliv Rev. 64:40–52. 2012. View Article : Google Scholar
|
|
8
|
Zhigang W, Zhiyu L, Haitao R, et al:
Ultrasound-mediated microbubble destruction enhances VEGF gene
delivery to the infarcted myocardium in rats. Clin Imaging.
28:395–398. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang Q, Wang Z, Ran H, et al: Enhanced
gene delivery into skeletal muscles with ultrasound and microbubble
techniques. Acad Radiol. 13:363–367. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ren JL, Wang ZG, Zhang Y, et al:
Transfection efficiency of TDL compound in HUVEC enhanced by
ultrasound-targeted micro-bubble destruction. Ultrasound Med Biol.
34:1857–1867. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sonoda S, Tachibana K, Uchino E, et al:
Gene transfer to corneal epithelium and keratocytes mediated by
ultrasound with micro-bubbles. Invest Ophthalmol Vis Sci.
47:558–564. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li HL, Zheng XZ, Wang HP, Li F, Wu Y and
Du LF: Ultrasound-targeted microbubble destruction enhances
AAV-mediated gene transfection in human RPE cells in vitro and rat
retina in vivo. Gene Ther. 16:1146–1153. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Neu M, Fischer D and Kissel T: Recent
advances in rational gene transfer vector design based on poly
(ethylene imine) and its derivatives. J Gene Med. 7:992–1009. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Weerasinghe P, Li Y, Guan Y, Zhang R,
Tweardy DJ and Jing N: T40214/PEI complex: a potent therapeutics
for prostate cancer that targets STAT3 signaling. Prostate.
68:1430–1442. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu YZ, Zhu JA, Jiang YG and Hu B:
Ultrasound microbubble contrast agents: application to therapy for
peripheral vascular disease. Adv Ther. 26:425–434. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qin S, Caskey CF and Ferrara KW:
Ultrasound contrast microbubbles in imaging and therapy: physical
principles and engineering. Phys Med Biol. 54:R27–R57. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen YC, Jiang LP, Liu NX, Wang ZH, Hong K
and Zhang QP: P85, Optison microbubbles and ultrasound cooperate in
mediating plasmid DNA transfection in mouse skeletal muscles in
vivo. Ultrason Sonochem. 18:513–519. 2011. View Article : Google Scholar
|
|
18
|
Chen S, Shimoda M, Wang MY, et al:
Regeneration of pancreatic islets in vivo by ultrasound-targeted
gene therapy. Gene Ther. 17:1411–1420. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Koike H, Tomita N, Azuma H, et al: An
efficient gene transfer method mediated by ultrasound and
microbubbles into the kidney. J Gene Med. 7:108–116. 2005.
View Article : Google Scholar
|
|
20
|
Sunshine JC, Bishop CJ and Green JJ:
Advances in polymeric and inorganic vectors for nonviral nucleic
acid delivery. Ther Deliv. 2:493–521. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
del Pozo-Rodríguez A, Delgado D, Solinís
MA, Gascón AR and Pedraz JL: Solid lipid nanoparticles for retinal
gene therapy: transfection and intracellular trafficking in RPE
cells. Int J Pharm. 360:177–183. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Urtti A, Polansky J, Lui GM and Szoka FC:
Gene delivery and expression in human retinal pigment epithelial
cells: effects of synthetic carriers, serum, extracellular matrix
and viral promoters. J Drug Target. 7:413–421. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sunshine JC, Sunshine SB, Bhutto I, Handa
JT and Green JJ: Poly (β-amino ester)-nanoparticle mediated
transfection of retinal pigment epithelial cells in vitro and in
vivo. PLoS One. 7:e375432012. View Article : Google Scholar
|
|
24
|
Park J, Zhang Y, Vykhodtseva N, Akula JD
and McDannold NJ: Targeted and reversible blood-retinal barrier
disruption via focused ultrasound and microbubbles. PLoS One.
7:e427542012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yanagisawa K, Moriyasu F, Miyahara T, Yuki
M and Iijima H: Phagocytosis of ultrasound contrast agent
microbubbles by Kupffer cells. Ultrasound Med Biol. 33:318–325.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kircheis R, Wightman L, Schreiber A, et
al: Polyethylenimine/DNA complexes shielded by transferrin target
gene expression to tumors after systemic application. Gene Ther.
8:28–40. 2001. View Article : Google Scholar : PubMed/NCBI
|