Introduction
Glioma is the most common type of intracranial
neuroepithelial tumor and the most aggressive primary tumor,
exhibiting rapid growth rates (1).
Furthermore, the 2-year survival rate for patients with poorly
differentiated glioma is only 10% (2,3).
Glioma accounts for 44.6% of tumors in the central nervous system,
with high recurrence and mortality rates (4). Survival times are low and mortality
rates are high in patients with glioma, and the disease is
associated with poor prognosis (5). Furthermore, the outcomes of
radiotherapy treatment combined with chemotherapy do not improve
patient prognosis (6).
Expression profiling analysis is an effective method
used to demonstrate abnormalities in miRNA expression patterns
(7). Glioma exhibits a unique
miRNA expression profile, which distinguishes it from the
surrounding healthy brain tissue. Furthermore, miRNA expression
profiles vary between the different stages of glioma (8). Compared with healthy brain tissue, 17
miRNAs, including miR-21, -221, -222, -125b and -10b, are
overexpressed in glial cells, whereas 33 miRNAs, including
miR-181a/b/c, -124, -137, -7 and -128, are downregulated in glial
cells (9). Furthermore, six miRNAs
(miR-16, -107, -185, -425, -451 and -486) are upregulated in
CD133− cells, but downregulated in tumorous glial stem
cells (CD133+). Research has demonstrated that miR-16
expression is markedly decreased in glioma cell lines compared with
healthy cells and that the upregulation of miR-16 may suppress
glioma growth and invasiveness (10,11).
Transcription and expression levels of matrix
metalloproteinase (MMP)-2 and -9 are associated with the degree of
malignancy in glioma (12). MMP-2
and -9 may be used as an indicator of malignant human brain glioma
(13). MMP expression
predominantly modulates the local invasiveness of glial cells.
Therefore, MMP-2 and -9 expression may reflect the degradation of
glima matrix (14). Studies have
demonstrated that U251 multiform glioblastoma expresses MMP-9
during cancer cell invasion (15,16).
In addition, MMP tissue inhibitor treatment is capable of
decreasing percentage cell invasion from 42 to 10% (17).
Paeoniflorin is an active ingredient of the commonly
used herbal medicine derived from Paeonia (18). Pharmacological studies have
demonstrated that paeoniflorin prevents free radical damage,
inhibits intracellular calcium-overload and exhibits anticancer
activities, as well as exhibiting a number of biological effects,
such as inhibiting cancer cell proliferation, improving
microcirculation, an prevents oxidization and convulsion (19). Paeoniflorin treatment induces human
cervical cancer cell apoptosis via the upregulation of the
pro-apoptotic genes, Bax and caspase-3, and the downregulation of
the anti-apoptotic gene, Bcl-2 (20). Paeoniflorin inhibits
H2O2-induced apoptosis in SH-SY5Y cells,
reducing H2O2-induced MMP expression changes.
Paeoniflorin treatment inhibits cluster of differentiation 147
expression in THP-1 cells and reduces MMP-9 secretion. In a
previous study, the levels of transforming growth factor β1 and
thymidylate synthetase were significantly higher in healthy samples
compared with paeoniflorin-treated samples, which corresponded with
an improvement in sample histology. By contrast, MMP-2 and -9
expression levels demonstrated the opposite results (21).
It is hypothesized that paeoniflorin may be useful
for the treatment of glioma. The present study investigated the
molecular mechanisms underlying the effects of paeoniflorin on
glial cells. In order to test this hypothesis, the effects of
different concentrations of paeoniflorin treatment on human glioma
cells were analyzed.
Materials and methods
Primary reagents
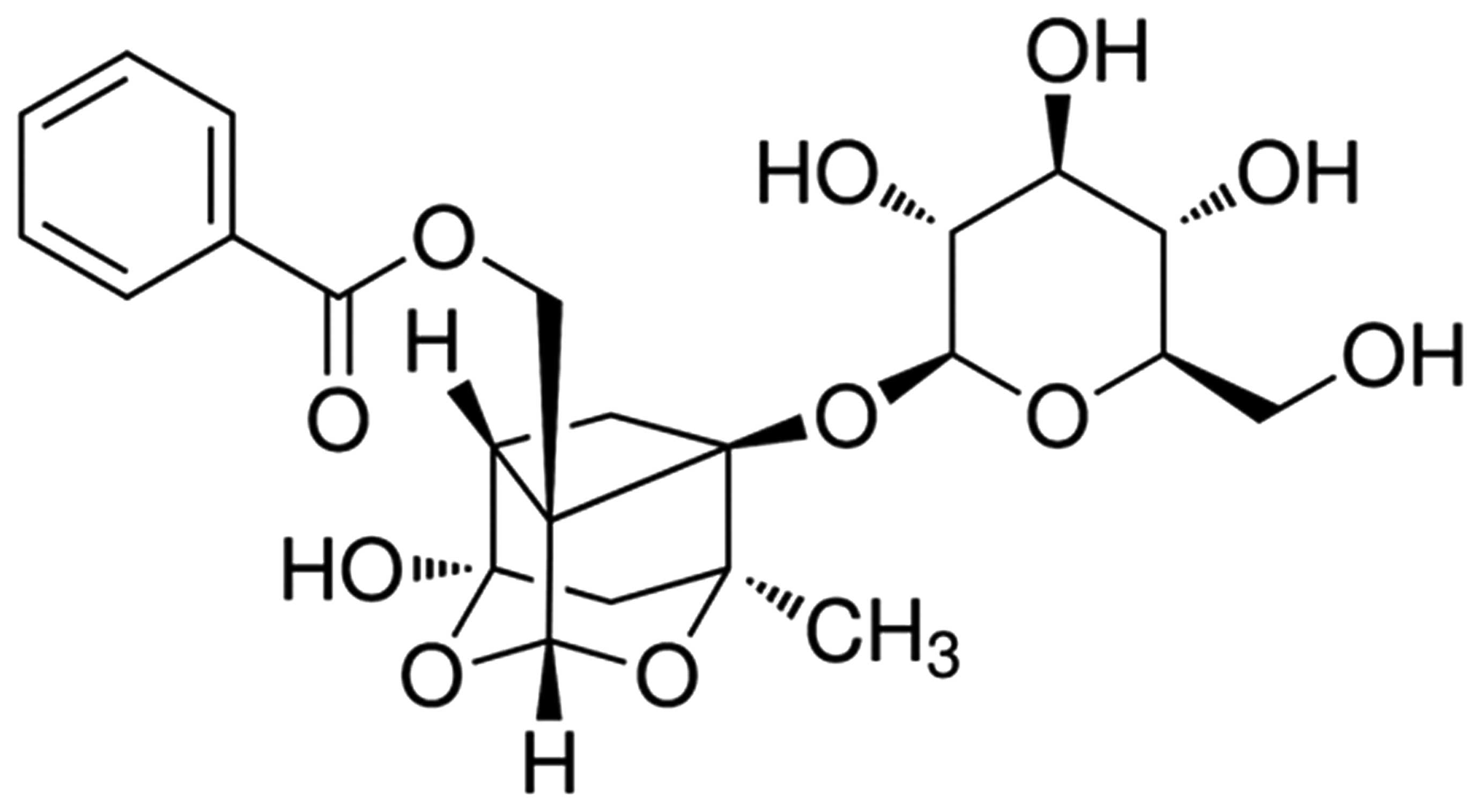
The chemical structure of paeoniflorin is indicated
in Fig. 1. Paeoniflorin (98%;
Sigma-Aldrich, St. Louis, MO, USA) was dissolved in physiological
saline solution. Dulbecco’s modified Eagle’s medium (DMEM), fetal
calf serum and Lipofectamine 2000® were purchased from
Invitrogen Life Technologies (Carlsbad, CA, USA).
3-[4,5-dimethylthiazol-2-thiazolyl]-2,5-diphenyl-tetrazolium
bromide (MTT) was purchased from Beyotime Institute of
Biotechnology (Haimen, China).
Cancer cell lines
The U87 glioma cell line was purchased from the cell
bank of the Chinese academy of sciences (Shanghai, China). U87
cells were cultured in DMEM, supplemented with 10% fetal calf
serum, 100 U/ml penicillin and 100 mg/ml streptomycin at 37°C and
5% CO2.
MTT viability assay
U87 cells (5.0×103 cells/well) were
seeded in 96-well culture clusters and incubated at 37°C and 5%
CO2 in a humidified incubator, for 24 h. Following
treatment with different concentrations of paeoniflorin (0, 5, 10
and 20 μΜ), cell viability was measured using an MTT assay.
MTT (~10-μl; 10 mg/ml) was added into each well and the
wells were incubated at 37°C and 5% CO2, for 4 h.
Subsequently, 200 μl dimethylsulfoxide was added to each
well. The wells were then agitated for 10 min at room temperature.
Viable cells were detected using an enzyme-linked immunosorbent
assay reader (SpectraMax® M5e, BioTek, USA) at 570
nm.
Caspase-3 activity measurement
U87 cells (5.0×103 cells/well) were
seeded in 96-well culture clusters and incubated at 37°C and 5%
CO2 in a humidified incubator for 24 h. Following
treatment with paeoniflorin, A549 cells (Cell Bank of the Chinese
Academy of Sciences, Shanghai, China) were centrifuged at 16,000 ×
g for 15 min at 4°C. Caspase-3 activity of cells was measured using
a colorimetric caspase-3 assay kit (Beyotime Institute of
Biotechnology). Protein extracts (50-μg) were obtained from
U87 cells and were incubated and added to a reaction buffer
(Tianjin Hualida Biotechnology Co., Ltd., Tianjin, China),
containing 85 μl assay buffer and 10 μl caspase-3
substrate (Ac-DEVD-pNA) at 37°C for 4–6 h. The change was
calculated at 405 nm using a microplate spectrophotometer (BioTek
Instruments, Inc., Winooski, VT, USA).
Apoptosis assay
Flow cytometry (BD Biosciences, Franklin Lakes, NJ,
USA) was conducted in order to investigate whether paeoniflorin
treatment induced U87 cell apoptosis. Following treatment with
paeoniflorin, A549 cells were collected and washed twice with
phosphate-buffered saline (PBS). Annexin V-fluorescein
isothiocyanate (FITC; 5 μl; BD Pharmingen, San Diago, CA,
USA) was added to the A549 cells and stained using a binding buffer
for 30 min in the dark according to the manufacturer’s
instructions. Subsequently, 10 μl propidium iodide (PI) was
added to the cells and incubated for 15 min at room temperature in
the dark. Samples were then analyzed using flow cytometry (FACS
Calibur; BD Biosciences).
MMP-9 expression
Gelatin zymography assays were used in order to
investigate whether paeoniflorin inhibits MMP-9 expression in U87
cells. Following treatment with paeoniflorin, U87 cells were
harvested and MMP-9 protein was electrophoresed on a 10% SDS-PAGE,
containing 1% gelatin. Following gel electrophoresis, the gel was
washed in 1.5% Triton X-100 (Shanghai Biological Co., Ltd.,
Shanghai, China) for 0.5–1 h and then washed in water. Gels were
incubated in buffer (pH 8.0) at 37°C for 12 h. Gels were then
stained with 0.2% Coomassie Brilliant Blue R-250 dye (Qingdao Jacob
Chemical Reagent Sales Co., Ltd., Shandong, China) for 1 h. MMP-9
protein expression was then quantified using a MiniBis system (DNR
Bio-Imaging Systems Ltd., Jerusalem, Israel) and prestained
SDS-PAGE standards (Hou-Bio Tech. Ltd., Shandong, China).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) of miR-16 expression
RT-qPCR was used in order to investigate whether
paeoniflorin treatment induced miR-16 expression in U87 cells.
Following treatment with paeoniflorin, total RNA was extracted from
the cells using TRIzol® reagent according to
manufacturer’s instructions (Invitrogen Life Technologies).
SuperScript® III Reverse Transcriptase (Invitrogen Life
Technologies) was used to analyze cDNA. and subsequently,
SYBR® Green PCR Master mix (Life Technologies, Grand
Island, NY, USA) was used to obtain the final cDNA. miR-16 mRNA
expression was quantified using an RT-PCR kit (Invitrogen Life
Technologies) according to the manufacturer’s instructions and an
7900HT Real-time PCR detection system. The following primers were
used: 5′-TAGCAGCACGTAAATATTGGC-3′ for miR-16;
5′-TGGTGTCGTGGAGTCG-3′ for β-actin; U6, forward
5′-CGCTTCGGCACATATACTA-3′ and reverse 5′-CGCTTCACGAATTTGCGTGTCA-3′.
The cycling conditions were as follows: 94°C for 10 min, 35 cycles
of 94°C for 30 sec, 60°C for 30 sec and 72°C for 30 sec, followed
by 73°C for 5 min.
miR-16 expression and anti-miR-16
transfection
miR-16 precursor and anti-miR-16 (Ambion Life
Technologies, Carlsbad, CA, USA) were obtained from Sangon Biotech
Co., Ltd. (Shanghai, China). U87 cells (5×105
cells/well) were cultured in 6 well plates and transfected with
miR-16 precursor/anti-miR-16 (Ambion Life Technologies) using
Lipofectamine 2000 (Invitrogen Life Technologies, Carlsbad, CA,
USA) for 6 h, following treatment with 10 μM peaoniflorin
for 24 h. Subsequently, the transfection medium was replaced with
DMEM containing 10% fetal bovine serum without antibiotic (Beijing
Genetic Company, Beijing, China) in a humidified atmosphere at 37°C
with 5% CO2 for 18 h.
Statistical analysis
Experiments were performed at least three times and
data are provided as the mean ± standard error. Data were analyzed
by Student’s t-test using SPSS 17.0 software (SPSS, Inc,. Chicago,
IL, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
MTT analysis and caspase-3 activity
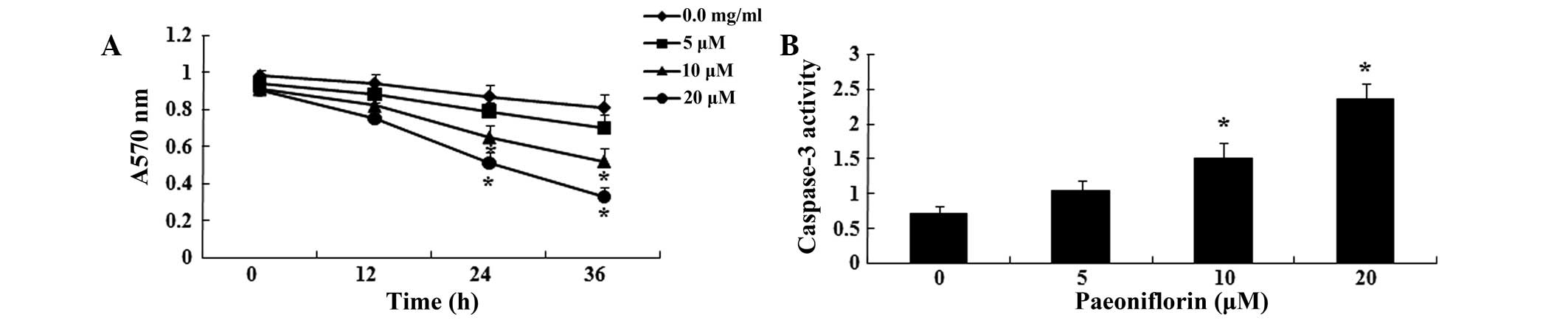
In order to determine the effects of paeoniflorin on
U87 cells, U87 cell viability was analyzed following treatment with
paeoniflorin (0, 5, 10 and 20 μΜ), using MTT assays. As
shown in Fig. 2A, treatment with
10 and 20 μΜ paeoniflorin for 24 or 36 h significantly
reduced U87 cell viability compared with control cells (P<0.05).
Cell viability decreased in a time- and concentration-dependent
manner. Following paeoniflorin treatment (0, 5, 10 and 20
μΜ), caspase-3 activity in U87 cells was analyzed using a
caspase-3 assay kit. As shown in Fig.
2B, treatment with 10 and 20 μΜ paeoniflorin for 24 h
significantly increased caspase-3 activity in U87 cells, compared
with 0 μM treatment (P<0.05). Caspase-3 activity
increased in a concentration-dependent manner in U87 cells.
Flow cytometric analysis and cell
apoptosis
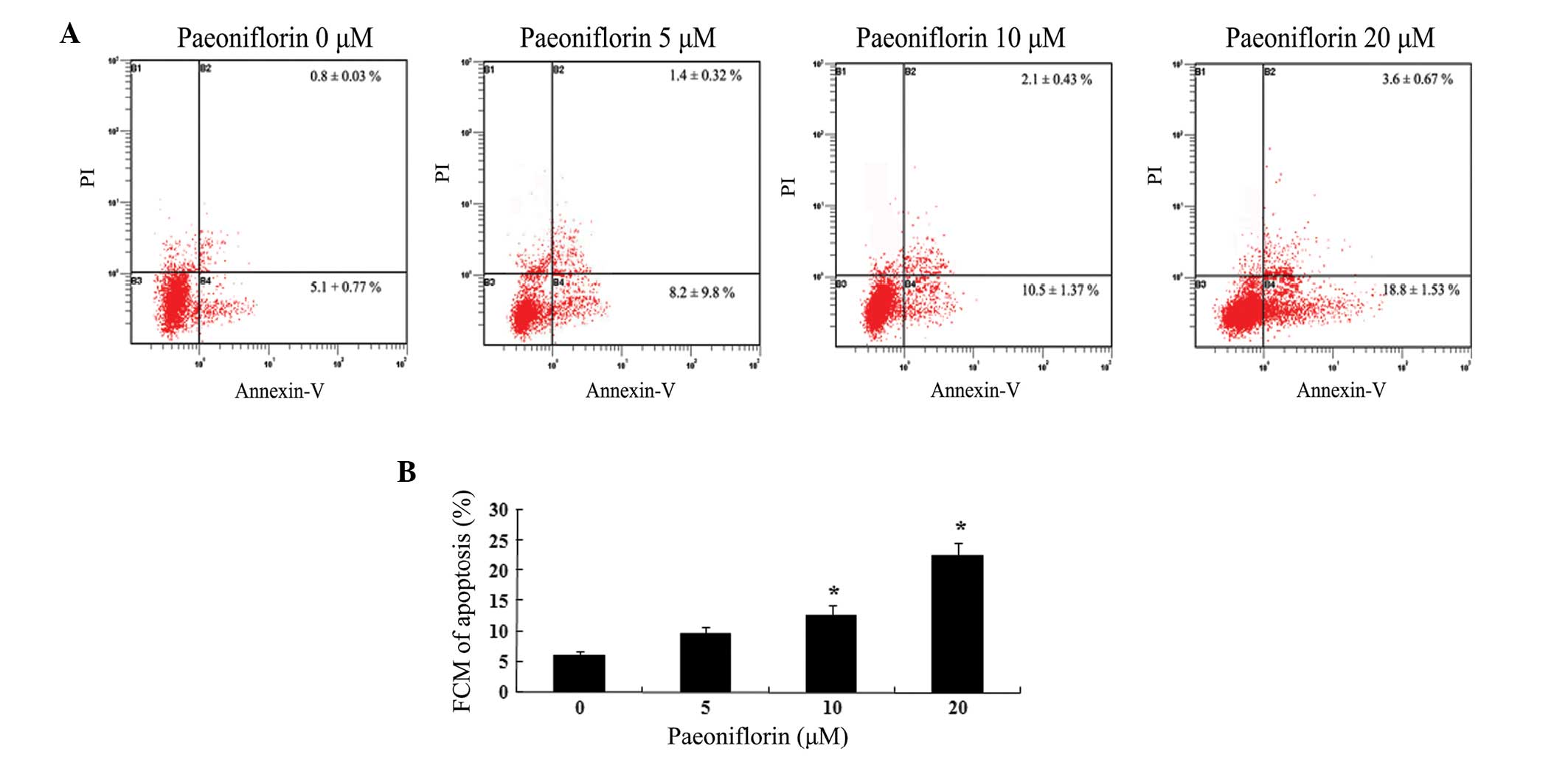
In order to investigate the effect of paeoniflorin
on cell apoptosis, U87 cells were treated with different
concentrations of paeoniflorin for 24 h. Flow cytometry assays
demonstrated that paeoniflorin exerted a dose dependent inhibitory
effect on U87 cell growth (Fig.
3A). As demonstrated in Fig.
3B, treatment with 10 and 20 μΜ paeoniflorin for 24 h
significantly increased the U87 cell apoptosis compared with the 0
μM paeoniflorin-treated group (P<0.05).
Paeoniflorin-induced inhibition of
MMP-9
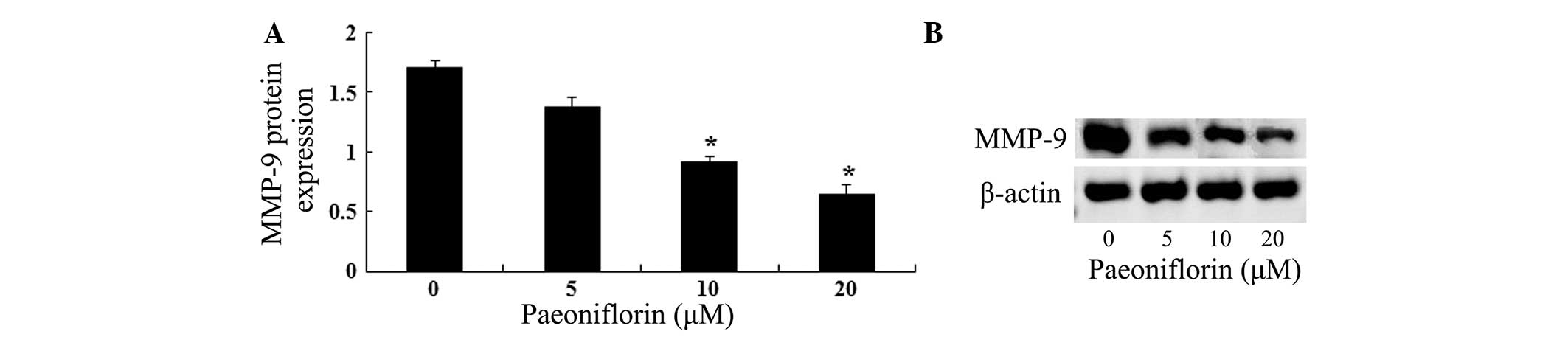
In order to investigate the association between
paeoniflorin-induced U87 cell growth inhibition and MMP-9 protein
expression induction, gelatin zymography assays were conducted. As
shown in Fig. 4A, the results of
gelatin zymography assays suggested that paeoniflorin inhibited
MMP-9 protein expression in a dose-dependent manner. As shown in
Fig. 4B, treatment with 10 and 20
μΜ paeoniflorin for 24 h significantly reduced the MMP-9
protein expression in U87 cells compared with control cells
(P<0.05).
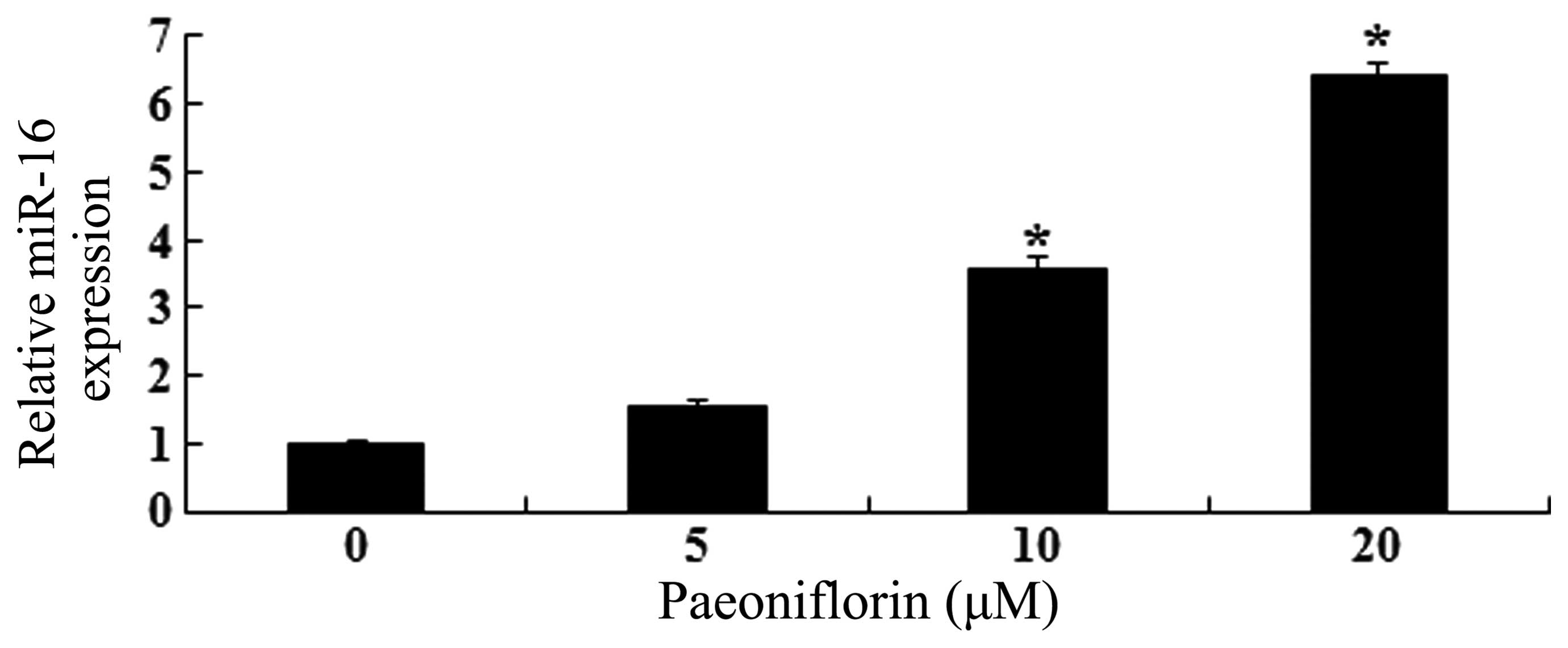
Paeoniflorin induces miR-16
expression
As shown in Fig. 5,
paeoniflorin treatment promoted miR-16 expression levels in a
dose-dependent manner. Treatment with 10 and 20 μΜ
paeoniflorin for 24 h significantly increased miR-16 expression
levels in U87 cells, compared with control cells (P<0.05).
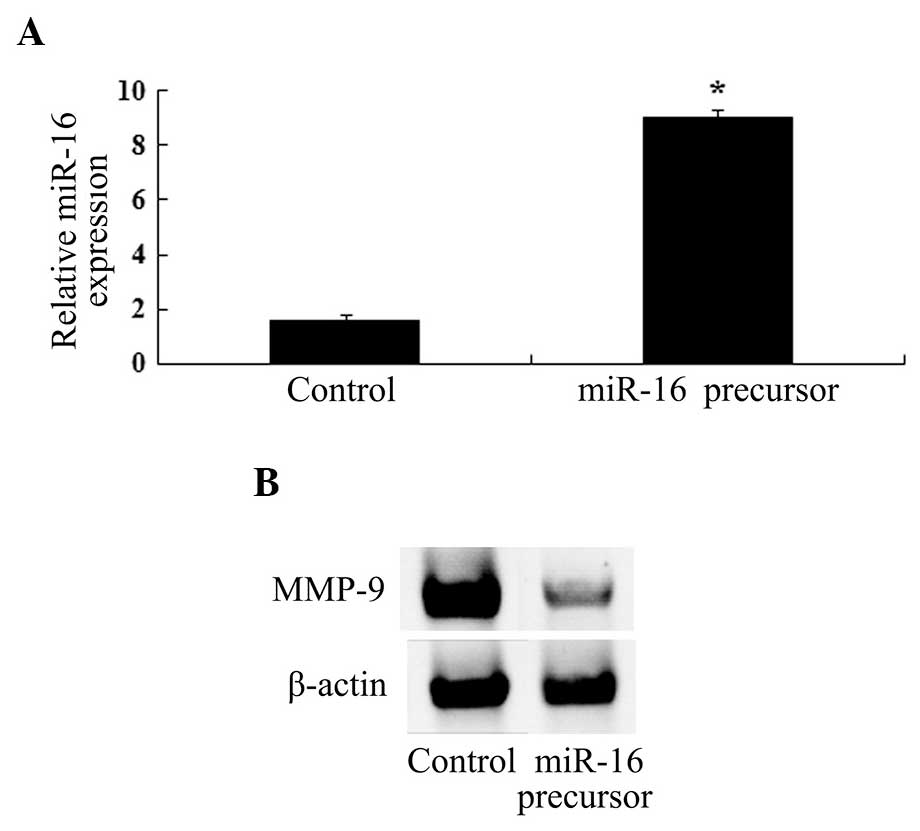
Overexpression of miR-16 and MMP-9
expression levels
In order to investigate the association between
miR-16 expression and paeoniflorin-induced MMP-9 protein
expression, an miR-16 precursor was transfected into U87 cells. As
shown in Fig. 6A and B, miR-16
upregulation led to significant inhibition of MMP-9 protein
expression.
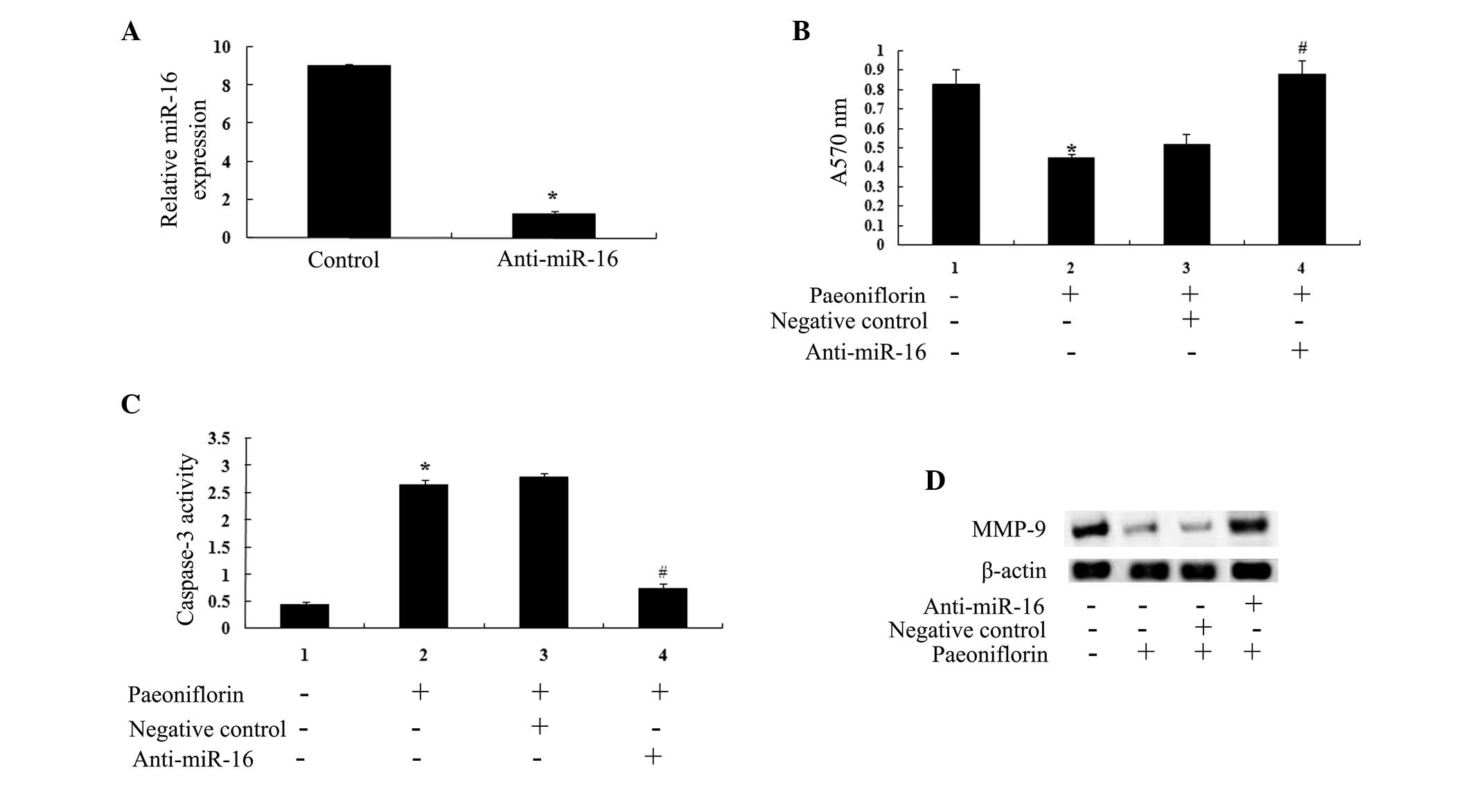
Anti-miR-16 reverses the antitumor
effects of paeoniflorin
An anti-miR-16 antibody was transfected into the U87
cells. The results indicated that miR-16 expression was
significantly lower in anti-miR-16-transfected U87 cells compared
with control cells (Fig. 7A). The
anti-miR-16 antibody significantly reduced the anticancer effects
of paeoniflorin treatment (10 μΜ) on U87 cell proliferation
(Fig. 7B) and U87 cell apoptosis
(Fig. 7C) at 24 h. The results of
the present study suggested that anti-miR-29b may influence the
anticancer effects of paeoniflorin (10 μΜ) via the
downregulation of MMP-9 expression (Fig. 7D).
Discussion
Malignant glioma is the most predominant type of
primary brain tumor in adults with relatively high rates of
recurrences (22). Diffuse glioma
cells are able to infiltrate the surrounding brain tissue, which is
one of the most important characteristics of glioma (23). Therefore, novel approaches for
glioma therapy are required.
Paeoniflorin, an active compound derived from the
medicinal herb Paeonia, has been shown to exhibit a variety
of biological effects (24).
Paeoniflorin treatment may increase superoxide dismutase (SOD)
levels and reduce malondialdehyde (MDA) content in ischemic brain
tissue. It has been suggested that paeoniflorin treatment,
following cerebral ischemia, may inhibit the production of free
radicals, improve SOD activity and decrease MDA content in the
brain. Therefore, paeoniflorin treatment may protect the brain from
secondary neuron injury in patients with cerebral ischemia
(25). A study has reported that
paeoniflorin may modulate multidrug resistance of the human gastric
cancer cell line, via the inhibition of nuclear factor (NF)-κB
activation (26). Paeoniflorin
treatment may decrease MMP-9 expression levels in human liver
carcinoma cells. It inhibits human liver carcinoma cell growth,
metastasis and invasion (27). The
results of the present study suggested that paeoniflorin may be an
effective agent for the inhibition of proliferation and induction
of apoptosis in U87 cells.
The upregulation of MMP-9 expression and the
downregulation of p16 expression in glioma may be associated with
tumor invasiveness. MMP-9 expression was shown to be lower in
non-malignant astrocytoma cells, than in anaplastic astrocytoma and
glioblastoma multiforme cells exhibiting high levels of malignancy
(28). High levels of MMP-9
expression may reflect the degree of malignancy and invasiveness in
brain glioma. High MMP-9 expression and low phosphatase and tensin
homolog expression levels are indicators of increased glioma
invasiveness. The combination of the two indices may be used as an
important reference for diagnosis and prognosis for patients with
glioma (14,29). The results of the present study
demonstrated that paeoniflorin is associated with the expression of
MMP-9 in U87 cells.
miRs are involved in the development of a number of
diseases, including cancer. They are typically underexpressed in
cancer tissues and the inhibition of the expression of certain miRs
may lead to the occurrence of cancer. A small number of miRs are
overexpressed in cancer tissues and are associated with tumor
genes. However, the majority of miRs are underexpressed in tumor
tissues, serving as tumor suppressor genes in cancer (30). A number of experiments have
demonstrated the involvement of miR-16 as a tumor suppressor gene
in glioma growth, via the inhibition of Bcl2 and the NF-κB1/MMP-9
signaling pathway (10).
In the present study, treatment of U87 cells with
paeoniflorin resulted in a significant increase in miR-16
expression levels. The results of the present study suggested that
upregulation of miR-16 promotes MMP-9 expression in U87 cells.
Paeoniflorin treatment exerted anticancer effects against human
glioma cells via upregulating miR-16 and downregulating MMP-9
expression.
In conclusion, paeoniflorin may be useful for the
treatment of human glioma. The results of the present study
demonstrated that paeoniflorin treatment may lead to decreased
proliferation and increased apoptosis of human glioma cells
(31). To the best of our
knowledge, the results of the present study support the hypothesis
that paeoniflorin may be an effective antitumor agent for the
treatment of human glioma (32).
Paeoniflorin inhibited MMP-9 protein expression and promoted miR-16
expression in U87 cells. Upregulating miR-16 inhibited MMP-9
protein expression levels in anti-miR-16-transfected U87 cells.
Therefore, miR-16 is associated with the downregulation of MMP-9
expression in U87 cells. Paeoniflorin treatment appeared to inhibit
proliferation and accelerate apoptosis of human glioma cells via
miR-16 upregulation and MMP-9 expression downregulation. To the
best of our knowledge this is the first study to suggest that
paeoniflorin may inhibit proliferation and accelerate apoptosis of
human glioma cells via miR-16 upregulation and MMP-9
downregulation.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant no. 81202964).
References
|
1
|
Huang Q, Zhang QB, Dong J, et al: Glioma
stem cells are more aggressive in recurrent tumors with malignant
progression than in the primary tumor, and both can be maintained
long-term in vitro. BMC Cancer. 8:3042008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim JK, Jin X, Sohn YW, Jin X, Jeon HY,
Kim EJ, Ham SW, Jeon HM, Chang SY, Oh SY, et al: Tumoral RANKL
activates astrocytes that promote glioma cell invasion through
cytokine signaling. Cancer Lett. 353:194–200. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu B, Gao YQ, Wang XM, Wang YC and Fu LQ:
Germacrone inhibits the proliferation of glioma cells by promoting
apoptosis and inducing cell cycle arrest. Mol Med Rep.
10:1046–1050. 2014.PubMed/NCBI
|
|
4
|
Janinis J, Efstathiou E, Panopoulos C, et
al: Phase II study of temozolomide in patients with relapsing high
grade glioma and poor performance status. Med Oncol. 17:106–110.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nakano I: Therapeutic potential of
targeting glucose metabolism in glioma stem cells. Expert Opin Ther
Targets. 18:1233–1236. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huber RM, Flentje M, Schmidt M, Pöllinger
B, Gosse H, Willner J and Ulm K; Bronchial Carcinoma Therapy Group:
Simultaneous chemoradiotherapy compared with radiotherapy alone
after induction chemotherapy in inoperable stage IIIA or IIIB
non-small-cell lung cancer: study CTRT99/97 by the Bronchial
Carcinoma Therapy Group. J Clin Oncol. 2006 Sep 20;24(27):
4397–404. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Godlewski J, Nowicki MO, Bronisz A,
Williams S, Otsuki A, Nuovo G, Raychaudhury A, Newton HB, Chiocca
EA and Lawler S: Targeting of the Bmi-1 oncogene/stem cell renewal
factor by microRNA-128 inhibits glioma proliferation and
self-renewal. Cancer Res. 68:9125–9130. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Silber J, Lim DA, Petritsch C, Persson AI,
Maunakea AK, Yu M, Vandenberg SR, Ginzinger DG, James CD, Costello
JF, et al: miR-124 and miR-137 inhibit proliferation of
glioblastoma multiforme cells and induce differentiation of brain
tumor stem cells. BMC Med. 6:142008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang TQ, Lu XJ, Wu TF, Ding DD, Zhao ZH,
Chen GL, Xie XS, Li B, Wei YX, Guo LC, et al: MicroRNA-16 inhibits
glioma cell growth and invasion through suppression of BCL2 and the
nuclear factor-κB1/MMP9 signaling pathway. Cancer Sci. 105:265–271.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li X, Ling N, Bai Y, Dong W, Hui GZ, Liu
D, Zhao J and Hu J: MiR-16-1 plays a role in reducing migration and
invasion of glioma cells. Anat Rec (Hoboken). 296:427–432. 2013.
View Article : Google Scholar
|
|
12
|
Gao H, Zhang S, Cao S, Yang Z, Pang Z and
Jiang X: Angiopep-2 and activatable cell-penetrating peptide
dual-functionalized nanoparticles for systemic glioma-targeting
delivery. Mol Pharm. 11:2755–2763. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nakano A, Tani E, Miyazaki K, Yamamoto Y
and Furuyama J: Matrix metalloproteinases and tissue inhibitors of
metalloproteinases in human gliomas. J Neurosurg. 83:298–307. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rao JS, Steck PA, Tofilon P, Boyd D,
Ali-Osman F, Stetler-Stevenson WG, Liotta LA and Sawaya R: Role of
plasminogen activator and of 92-KDa type IV collagenase in
glioblastoma invasion using an in vitro matrigel model. J
Neurooncol. 18:129–138. 1994. View Article : Google Scholar
|
|
15
|
Yan W, Zhang W, Sun L, et al:
Identification of MMP-9 specific microRNA expression profile as
potential targets of anti-invasion therapy in glioblastoma
multiforme. Brain Res. 1411:108–115. 2011.PubMed/NCBI
|
|
16
|
Wong ET, Alsop D, Lee D, et al:
Cerebrospinal fluid matrix metalloproteinase-9 increases during
treatment of recurrent malignant gliomas. Cerebrospinal Fluid Res.
5:12008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ramaswamy P, Aditi Devi N, Hurmath Fathima
K and Dalavaikodihalli Nanjaiah N: Activation of NMDA receptor of
glutamate influences MMP-2 activity and proliferation of glioma
cells. Neurol Sci. 35:823–829. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zheng YQ, Wei W, Zhu L and Liu JX: Effects
and mechanisms of Paeoniflorin, a bioactive glucoside from paeony
root, on adjuvant arthritis in rats. Inflamm Res. 56:182–188. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang H, Zhou H, Wang CX, Li YS, Xie HY,
Luo JD and Zhou Y: Paeoniflorin inhibits growth of human colorectal
carcinoma HT 29 cells in vitro and in vivo. Food Chem Toxicol.
50:1560–1567. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang L and Zhang S: Modulating Bcl-2
family proteins and caspase-3 in induction of apoptosis by
paeoniflorin in human cervical cancer cells. Phytother Res.
25:1551–1557. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiang Z, Chen W, Yan X, Bi L, Guo S and
Zhan Z: Paeoniflorin protects cells from GalN/TNF-α-induced
apoptosis via ER stress and mitochondria-dependent pathways in
human L02 hepatocytes. Acta Biochim Biophys Sin (Shanghai).
46:357–367. 2014. View Article : Google Scholar
|
|
22
|
Gorlia T, Stupp R, Brandes AA, et al: New
prognostic factors and calculators for outcome prediction in
patients with recurrent glioblastoma: a pooled analysis of EORTC
Brain Tumour Group phase I and II clinical trials. Eur J Cancer.
48:1176–1184. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou GS, Song LJ and Yang B:
Isoliquiritigenin inhibits proliferation and induces apoptosis of
U87 human glioma cells in vitro. Mol Med Rep. 7:531–536. 2013.
|
|
24
|
No authors listed. Retraction:
Pharmacokinetic interaction of paeoniflorin and sinomenine:
Pharmacokinetic parameters and tissue distribution characteristics
in rats and protein binding ability in vitro. J Pharmacol Sci.
104:2832007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu J, Zhu X, Qi X, Che J and Cao B:
Paeoniflorin protects human EA. hy926 endothelial cells against
gamma-radiation induced oxidative injury by activating the
NF-E2-related factor 2/heme oxygenase-1 pathway. Toxicol Lett.
218:224–234. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fang S, Zhu W, Zhang Y, Shu Y and Liu P:
Paeoniflorin modulates multidrug resistance of a human gastric
cancer cell line via the inhibition of NF-κB activation. Mol Med
Rep. 5:351–356. 2012.
|
|
27
|
Lu JT, He W, Song SS and Wei W:
Paeoniflorin inhibited the tumor invasion and metastasis in human
hepatocellular carcinoma cells. Bratisl Lek Listy. 115:427–433.
2014.PubMed/NCBI
|
|
28
|
Lee YD, Cui MN, Yoon HH, Kim HY, Oh IH and
Lee JH: Down-modulation of Bis reduces the invasive ability of
glioma cells induced by TPA, through NF-κB mediated activation of
MMP-9. BMB Rep. 47:262–267. 2014. View Article : Google Scholar :
|
|
29
|
Rao JS, Yamamoto M, Mohaman S, Gokaslan
ZL, Fuller GN, Stetler-Stevenson WG, Rao VH, Liotta LA, Nicolson GL
and Sawaya RE: Expression and localization of 92 kDa type IV
collagenase/gelatinase B (MMP-9) in human gliomas. Clin Exp
Metastasis. 14:12–18. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Banno K, Iida M, Yanokura M, et al:
MicroRNA in cervical cancer: OncomiRs and tumor suppressor miRs in
diagnosis and treatment. ScientificWorldJournal. 2014:1780752014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang B, Pan X, Cobb GP and Anderson TA:
microRNAs as oncogenes and tumor suppressors. Dev Biol. 302:1–12.
2007. View Article : Google Scholar
|
|
32
|
Wang F, Sun JY, Zhu YH, Liu NT, Wu YF and
Yu F: MicroRNA-181 inhibits glioma cell proliferation by targeting
cyclin B1. Mol Med Rep. 10:2160–2164. 2014.PubMed/NCBI
|