Introduction
Postoperative cognitive dysfunction (POCD) is
characterized by impaired concentration, memory and learning
following surgery. As POCD can persist for a prolonged period of
time, it is detrimental to the health of millions of elderly
patients worldwide, in addition to presenting a significant
economic burden on society (1). As
a result, the development of effective preventive or therapeutic
targets for POCD is urgently required.
It has been suggested that neuroinflammation
resulting from surgery is involved in the development of POCD
(2,3). It is well-established that, shortly
following surgery, the neuroinflammatory response-associated
signaling pathways are activated, including nuclear factor (NF)-κB.
This can lead to the production and release of pro-inflammatory
cytokines, including tumor necrosis factor (TNF)-α, interleukin
(IL)-1β, IL-4, IL-6 and IL-8, and further neuroinflammatory
responses in the brain (4–7). Therefore, effectively suppressing the
activation of neuroinflammatory response-associated signaling
pathways may offer potential for the prevention and treatment of
POCD.
TNF-α is a multifunctional pro-inflammatory
cytokine, predominantly secreted by macrophages (8). TNF-α is involved in the regulation of
various biological processes, including cell proliferation,
differentiation, apoptosis, lipid metabolism and coagulation,
through binding to its receptors (9). In addition, TNF-α is able to activate
neuroinflammatory-associated signaling pathways, including the
NF-κB and mitogen-activated protein kinase (MAPK) signaling
pathways (4,5,7).
Accordingly, TNF-α may be a potential preventive and therapeutic
target in the treatment of POCD. However, the detailed role of
TNF-α in surgery-induced POCD remains to be elucidated.
In the present study, in order to elucidate the role
of TNF-α in surgery-induced POCD in aged patients, a rat model was
used, in which laparotomy was performed to the aged rats in order
to mimic human abdominal surgery. Subsequently, a Morris water maze
(MWM) assay was performed to evaluate the cognitive functions of
the rats following surgery, with or without the administration of
the R-7050 TNF-α receptor antagonist. In addition, the expression
levels of several key pro-inflammatory cytokines, and the activity
of neuroinflammation-associated NF-κB signaling were examined in
the hippocampal tissues of the aged rats.
Materials and methods
Animals and groups
The present study was approved by the Ethics
Committee of Dezhou People’s Hospital (Dezhou, China). Male
Sprague-Dawley rats (n=60; 24 months old) were purchased from the
Laboratory Animal Centre of Life Science Institute (Shanghai,
China). The rats were housed separately under conditions of
controlled temperature (22±1°C) in a 12 h light/dark cycle, and
were allowed free access to standard rat chow and sterile water.
Each group contained 10 rats.
Surgery
Laparotomy was performed under anesthesia
(Pelltobarbitalum Natricum; 5 mg/100 g; Sigma-Aldrich, Santa Clara,
CA, USA). In the rats subjected to laparotomy, a 3 cm vertical
incision was made at ~0.5 cm below the lower right rib, and the
incision penetrated the peritoneal cavity. The surgeon inserted an
index finger into the opening and vigorously manipulated the
viscera and musculature for 1 min. Subsequently, sterile chromic
gut sutures (Henan Songhe Medicines & Health Products,
Zhengzhou, China) were used to suture the peritoneal lining and
muscle. In the sham-operated rats, the abdominal area was shaved
and cleaned using 70% ethanol (Sigma-Aldrich) and the animals
remained under anesthesia for the same duration as the rats in the
laparotomy group
Intracisternal administration of the
R-7050 TNF-α receptor antagonist
To investigate the role of TNF-α in the development
of POCD following surgery in aged rats, a seperate group of the
rats were administered with R-7050, a TNF-α receptor antagonist
(EMD Biosciences, Inc., San Diego, CA, USA) during surgery under
anesthesia. In brief, the dorsal aspect of the skull was shaved and
cleaned using 70% ethanol. A 27-gauge needle (Sigma-Aldrich),
attached via PE50 tubing (Smiths Medical, Ashford, UK) to a 25
μl Hamilton syringe (Sigma-Aldrich), was inserted into the
cisterna magna. To confirm entry into the cisterna magna, 2
μl clear cerebral spinal fluid was drawn and released,
following which 3 μl R-7050 was administered. In addition, a
separate group of rats were shaved and cleaned, as above, and
administered with 3 μl sterile saline as a vehicle
control.
MWM assay
All the rats were trained in the MWM five times each
day for six consecutive days. Each rat was placed on a platform in
the center of the MWM for 30 sec and was then released into the
water from an assigned release point. The rat was allowed to swim
for 60 sec to reach the platform. If unsuccessful, the rat was
picked up and placed on the platform for another 30 sec. The
swimming distance and the time taken to reach the platform were
recorded using video tracking. The swimming distance and time taken
to reach the platform were used to caculate the speed and then
analyzed using MWM software (XR-XM101; Xinruan Information
Technology, Shanghai, China). On postoperative days 1, 3 and 5, the
MWM assay was repeated three times.
Western blotting
The proteins were extracted using a Nuclear and
Cytoplasmic Protein Extraction kit (Thermo Fisher Scientific,
Waltham, MA, USA). The protein concentrations were determined using
a Bradford DC Protein assay (Bio-Rad Laboratories, Inc., Hercules,
CA, USA). Subsequently, the proteins were separated using 10%
SDS-PAGE (Sigma-Aldrich) and transferred onto a polyvinylidene
difluoride (PVDF; Life Technologies, Carlsbad, CA, USA) membrane,
which was then incubated with phosphate-buffered saline (PBS; Life
Technologies) containing 50 g/l skimmed milk at room temperature
for 4 h. Following this, the PVDF membrane was incubated with the
following antibodies: Rabbit anti-TNF alpha (polyclonal; 1:100;
cat. no. ab6671), rabbit anti-IL-1β (monoclonal; 1:50; cat. no.
ab200478), rabbit anti-IL-4 (poly-clonal; 1:50; cat. no. ab9622),
rabbit anti-IL-6 (polyclonal; 1:50, cat. no. ab6672), mouse
anti-c-JNK (monoclonal; 1:100; cat. no. ab46821), chicken
anti-p-c-JNK (polyclonal; 1:100; cat. no. ab46821), rabbit anti-p38
(polyclonal; 1:50; cat. no. ab7952), rabbit anti-p-p38 (polyclonal;
1:50; cat. no. ab47363), rabbit anti-p65 (polyclonal; 1:50;
ab16502) and mouse anti-GAPDH (monoclonal; 1:100; cat. no. ab8245),
respectively, at 37°C for 1 h. Following washing with PBS three
times, the PVDF membrane was incubated with HRP-conjugated goat
anti mouse IgG (1:10,000; cat. no. ab186694), goat anti-rabbit IgG
(1:5,000; cat. no. ab175773) or goat anti-chicken IgY (1:5,000;
cat. no. ab175754). at room temperature for 1 h. All antibodies
were purchased from Abcam (Cambridge, UK). Chemiluminent detection
was then performed using an Enhanced Chemiluminescence kit (Pierce
Biotechnology, Inc., Rockford, IL, USA).
Statistical analysis
The data are expressed as the mean ± standard
deviation. Differences between two groups were determined using
Student’s t-test with SPSS software, version 17.0 (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Aged rats exhibit defects in cognitive
function following surgery
Prior to surgery, all the aged rats were trained in
the MWM for 6 days. The swimming distances, time taken to reach the
platform and speed in the MWM were used to evaluate the spatial
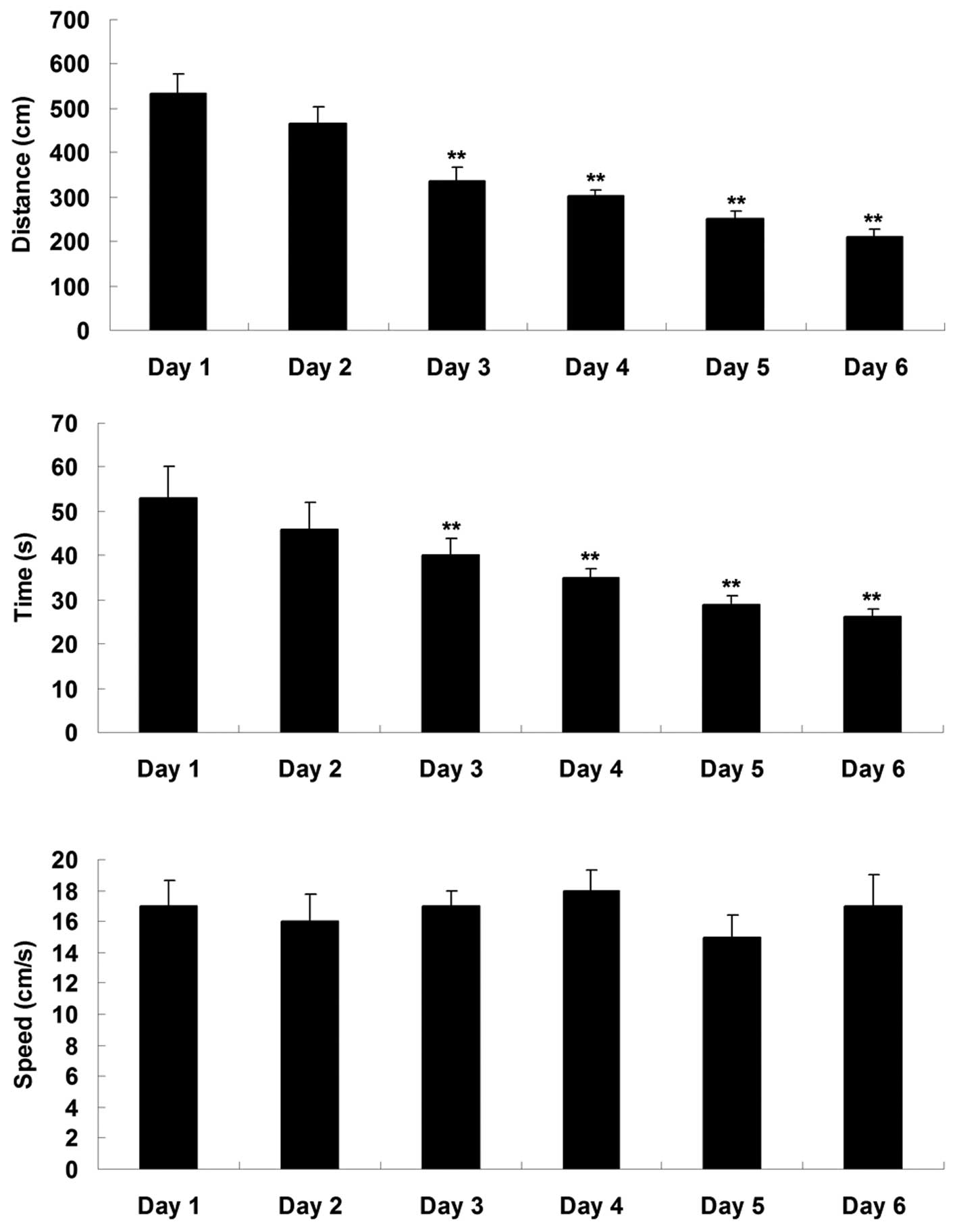
memory function of the rats. As shown in Fig. 1, the swimming distance and time
taken to reach the platform were significantly reduced during the
six training days, indicating that their spatial memory gradually
increased.
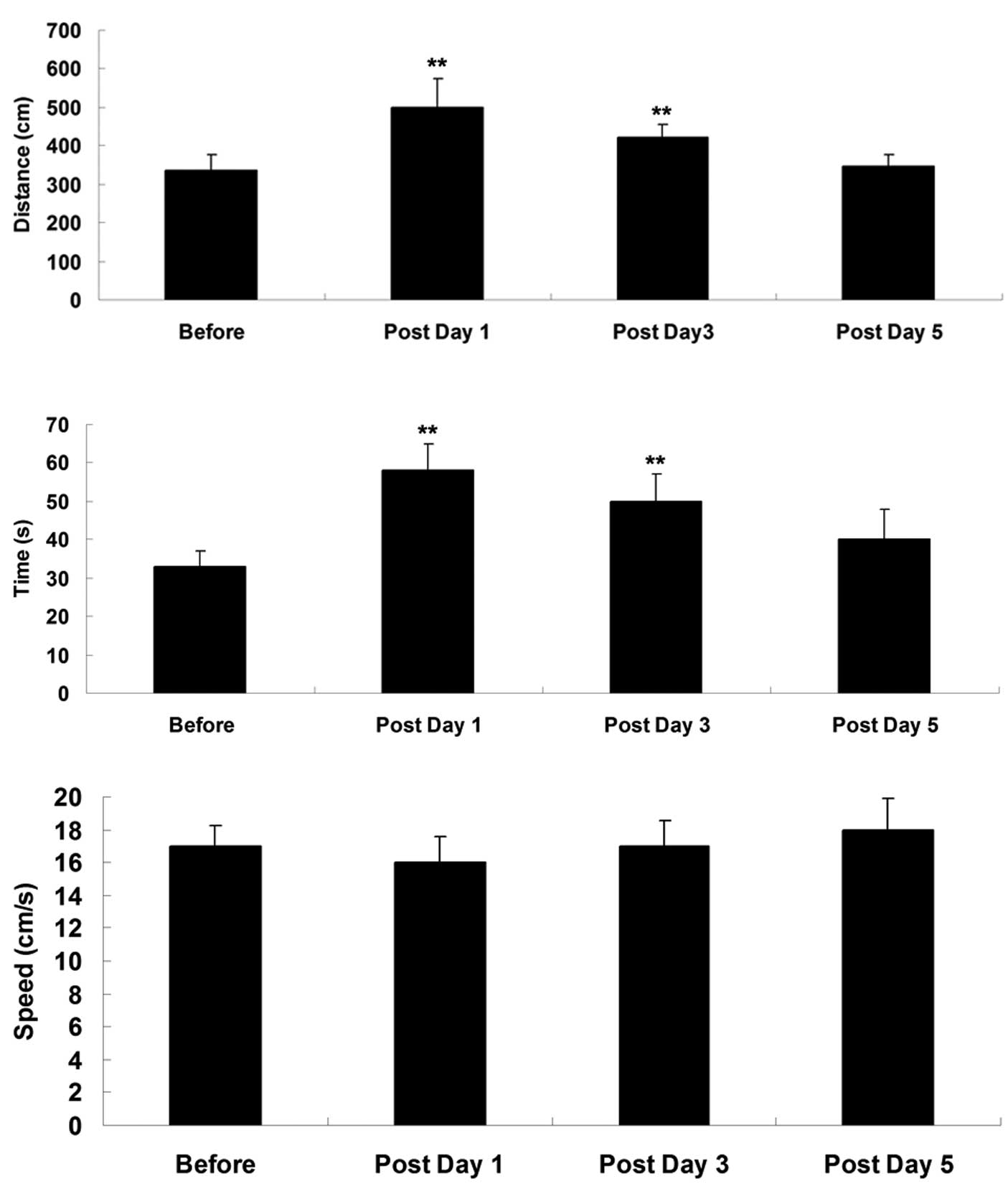
Subsequently, an MWM assay was performed on
postoperative days 1, 3 and 5. As shown in Fig. 2, the swimming distance and time
taken to reach the platform were significantly increased on
postoperative days 1 and 3, compared with the measurements obtained
prior to surgery, suggesting that their spatial memory function was
impaired shortly following surgery. However, on postoperative day
5, the swimming distance and time taken to reach the platform were
not significantly different, compared with those in the
sham-operated control group, suggesting that their cognitive
function had recovered.
Intracisternal administration of TNF-α
receptor antagonist attenuates the defects in cognitive function in
aged rats following surgery
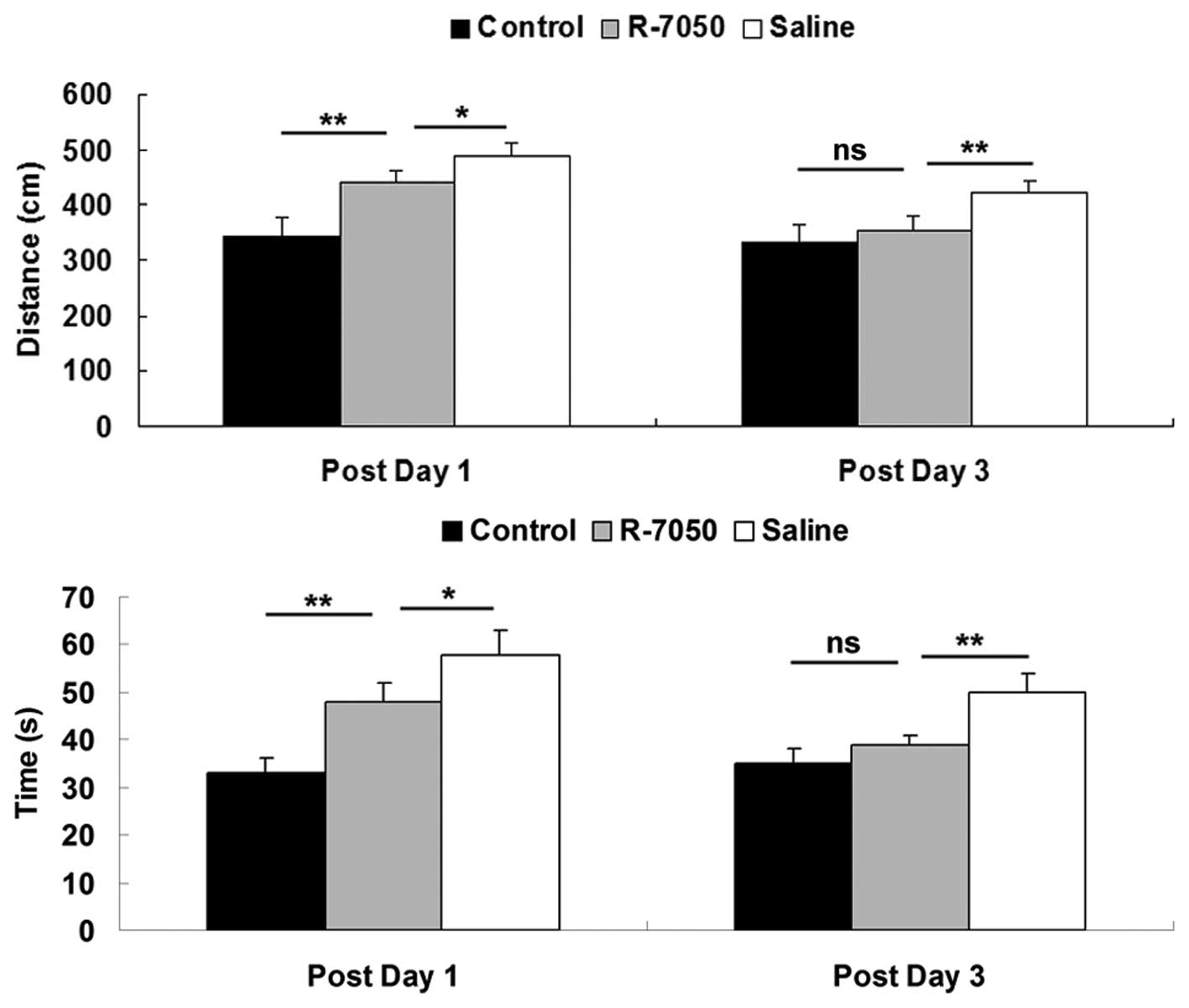
To examine the role of TNF-α in POCD in aged rats,
the rats in the present study received an intracisternal
administration of the R-7050 TNF-α receptor antagonist during
surgery. As shown in Fig. 3,
following administration of the R-7050 TNF-α receptor antagonist,
the swimming distance and time taken for the aged rats to reach the
platform were notably reduced on postoperative days 1 and 3,
compared with the rats in the saline-treated group. However, the
swimming distance and time taken to reach the platform in the aged
rats treated with the R-7050 TNF-α receptor antagonist remained
higher than those in the sham-operated control group on
postoperative day 1. These observations indicated that the
intracisternal administration of the R-7050 TNF-α receptor
antagonist notably attenuated the defects in spatial memory
function observed in the aged rats shortly following surgery.
Intracisternal administration of the
TNF-α receptor antagonist R-7050 inhibits the upregulation of
pro-inflammatory cytokines in aged rats following surgery
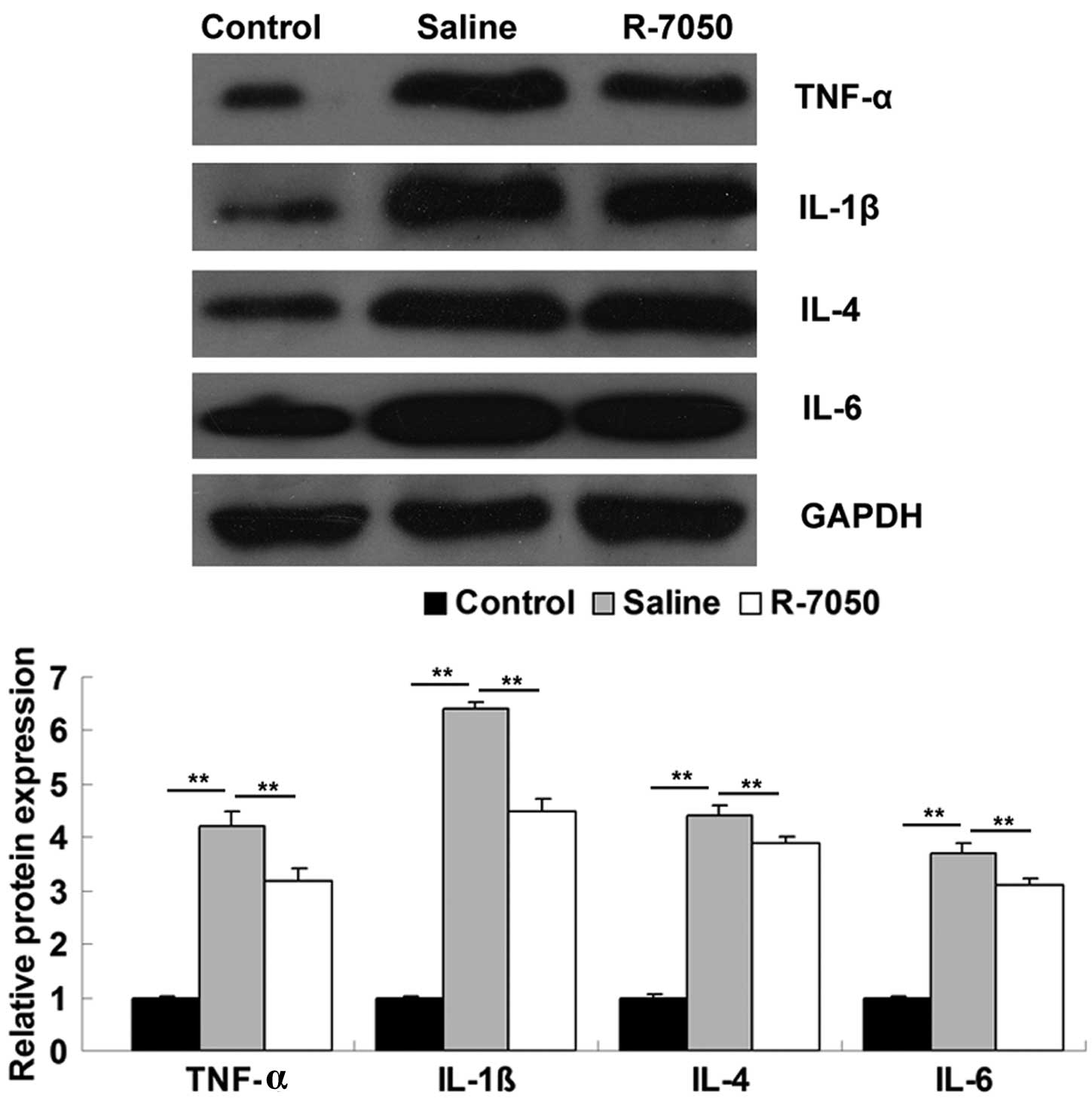
The present study subsequently investigated the
molecular mechanism underlying the effects of TNF-α further.
Western blotting was used to determine the protein expression
levels of pro-inflammatory cytokines, including TNF-α, IL-1β, IL-4
and IL-6, in the hippocampal tissues of aged rats on postoperative
day 1, with or without administration of the R-7050 TNF-α receptor
antagonist. The rats in the shamoperated group were used as the
controls. As shown in Fig. 4, the
protein expression levels of TNF-α, IL-1β, IL-4 and IL-6 were
significantly upregulated on postoperative day 1, compared with the
sham-operated group. However, the intracisternal administration of
the TNF-α receptor antagonist significantly attenuated the
surgery-induced upregulation of these pro-inflammatory cytokines.
These data suggested that inhibiting TNF-α-mediated
pro-inflammatory signaling may effectively inhibit surgery-induced
neuroinflammatory responses in aged rats.
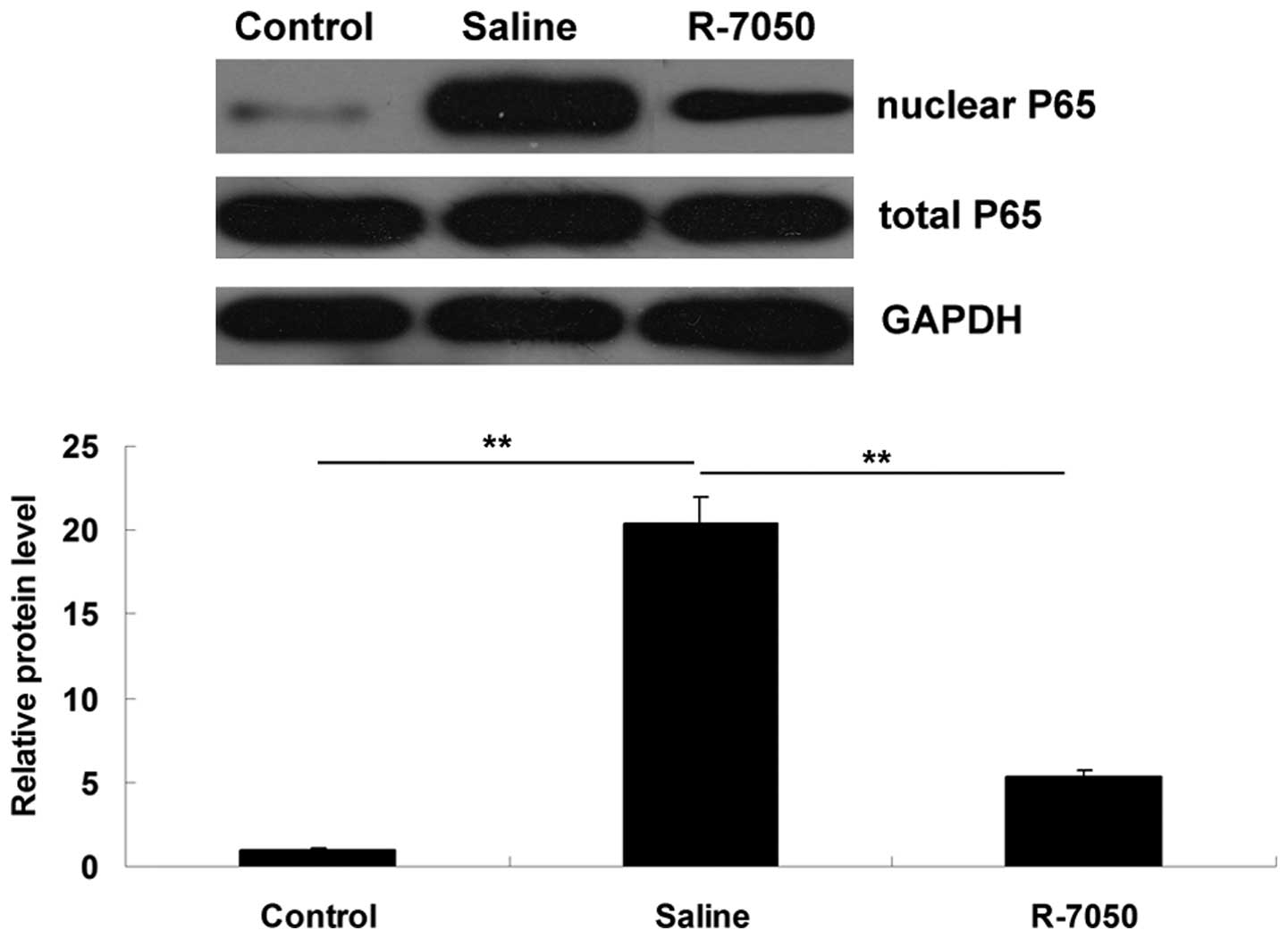
Intracisternal administration of TNF-α
receptor antagonist R-7050 suppresses the activation of the NF-κB
signaling pathway in aged rats following surgery
As NF-κB is a downstream signaling factor of TNF-α
(10), western blotting was
performed to determine the activity of NF-κB signaling in the
hippocampal tissues of the aged rats in each group. As shown in
Fig. 5, compared with the control
group, the protein levels of NF-κB P65 in the nucleus were
significantly upregulated on postoperative day 1, and this
upregulation was inhibited following intracisternal administration
of the R-7050 TNF-α receptor antagonist. These observations
suggested that the R-7050 TNF-α receptor antagonist effectively
inhibited the surgery-induced activation of NF-κB signaling in the
hippo-campal tissues of the aged rats.
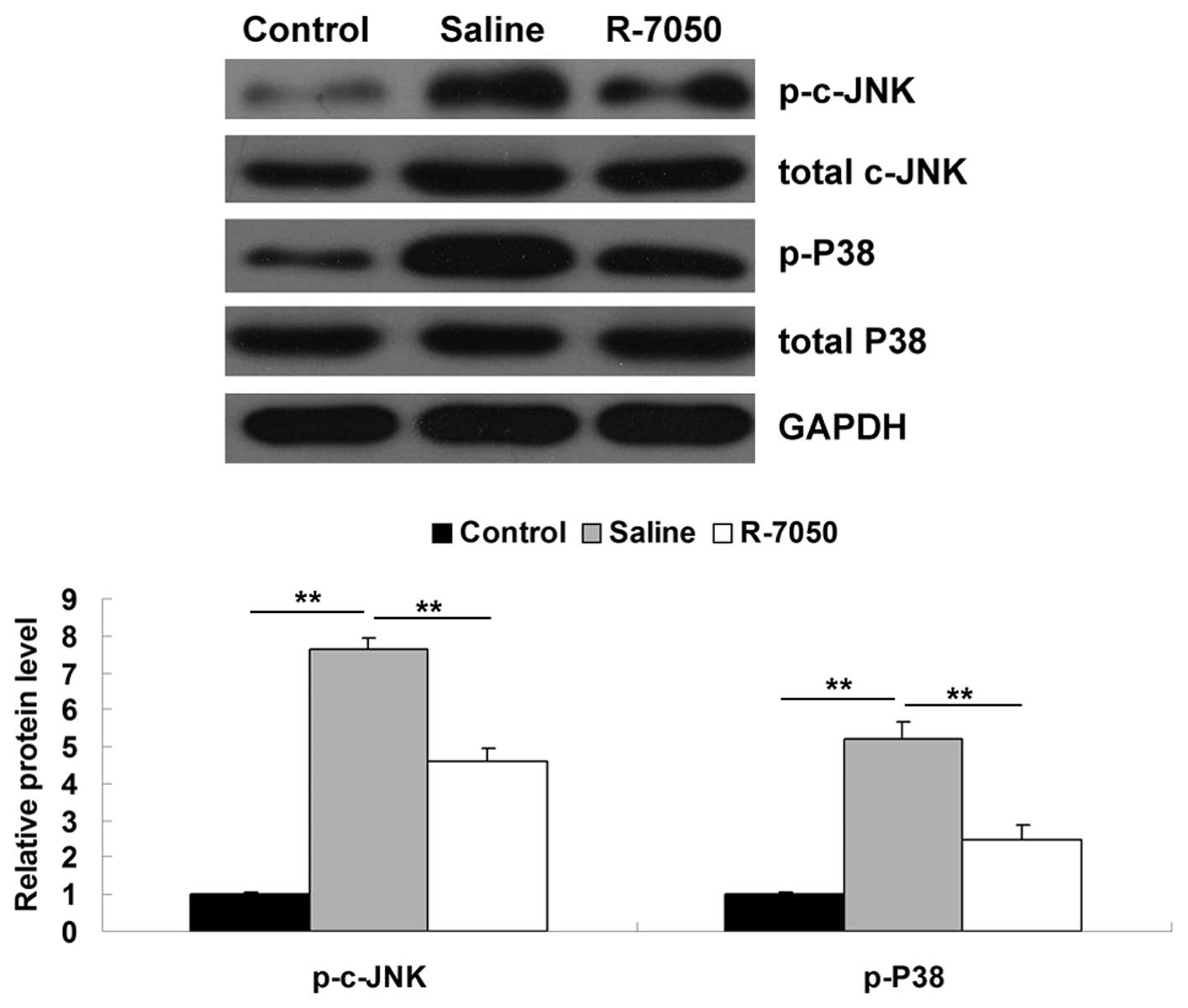
Intracisternal administration of the
TNF-α receptor antagonist R-7050 suppresses the activation of the
MAPK signaling pathway in aged rats following surgery
As MAPK signaling is also involved in TNF-α-mediated
inflammatory responses (11),
western blotting was performed to examine the activity of c-Jun
N-terminal kinases (c-JNK) and p38 MAPK signaling in the
hippocampal tissues of the aged rats in each group. As shown in
Fig. 6, the protein levels of
phosphorylated c-JNK and phosphorylated p38 MAPK were significantly
upregulated on postoperative day 1, compared with the levels in the
control group. However, following intracisternal administration of
the R-7050 TNF-α receptor antagonist, notable attenuation of the
upregulation of these proteins was observed. Therefore, these data
indicated that the inhibition of TNF-α effectively inhibited the
surgery-induced activation of MAPK signaling in the hippocampal
tissues of the aged rats.
Discussion
There is accumulating evidence to suggest that
neuroinflammation may be involved in the development of POCD, as it
is involved in cognitive defects in diseases of the central nervous
system (12,13). However, the detailed molecular
mechanism remains to be elucidated. With the exception of advanced
age as the most significant risk factor, surgical trauma is another
risk factor for the development of POCD (1,14–16).
In the present study, laparotomy was performed to mimic human
abdominal surgery in aged rats. The data obtained demonstrated that
that the laparotomy resulted in impaired cognitive function, while
inhibiting TNF-α, using the R-7050 TNF-α receptor antagonist
significantly attenuated the laparotomy-induced defects in the
cognitive functions of the aged rats. Inhibiting TNF-α inhibited
the activation of NF-κB signaling and stimulated the production of
key pro-inflammatory cytokines, including TNF-α, IL-1β, IL-4 and
IL-6 in the hippocampal tissues of the aged rats.
Previous studies have demonstrated that the
development of POCD is associated with non-infectious
neuroinflammatory responses (3,17).
As peripheral surgery has been observed to induce neuroinflammatory
responses (16,18), laparotomy was performed in the
present study to mimic human abdominal surgery in aged rats.
Peripheral surgery, including laparotomy cause sensitization,
leading to enhanced neuroinflammation (19) and the release of peripheral
pro-inflammatory cytokines, including TNF-α, IL-1β, IL-4 and IL-6
(20). These pro-inflammatory
cytokines are able to enter the brain and further activate
microglial cells, which causes further neuro-inflammatory responses
and brain injury, as described by Kannan et al (21). In the present study, the findings
demonstrated that laparotomy resulted in a significant upregulation
of pro-inflammatory cytokines in the hippocampal tissues of the
aged rats. These observations were consistent with those of
previous studies. Barrientos et al (22) also observed that sple-nectomy led
to hippocampal-dependent memory impairment and upregulation in the
levels of pro-inflammatory cytokines in the hippocampal tissues of
aged rats on postoperative days 1 and 4.
TNF-α has been observed to act on several signaling
pathways through binding to its two receptors, TNF receptor 1
(TNFR1) and TNFR2. TNF-mediated signaling pathways are involved in
NF-κB- and MAPK-mediated activation of inflammation (10,11).
Binding of TNF-α to TNFR1 or TNF receptor-associated factor 2 can
lead to activation of NF-κB signaling, which subsequently leads to
production of pro-inflammatory cytokines, including TNF-α, IL-1β,
IL-4 and IL-6 (10,23,24).
In the present study, it was demonstrated that intracisternal
administration of a TNF-α receptor antagonist suppressed the
activation of the NF-κB signaling pathway, with a resulting
decrease in the production of TNF-α, IL-1β, IL-4 and IL-6 in the
hippocampal tissues of the aged rats following surgery. These
observations suggested that inhibiting TNF-α inhibited the
surgery-induced neuroinflammation in the brain through
downregulation of the NF-κB signaling-induced release of
pro-inflammatory cytokines, and therefore, attenuated the defects
in cognitive function in the aged rats.
MAPK signaling is also involved in TNF-α-mediated
inflammatory responses (11).
c-JNK is a member of the MAPK family (25), and activation of TNF-α-mediated
signaling leads to transcriptional regulation, including c-JNK
phos-phorylation, to stimulate transcriptional activation by
activator protein 1 (AP-1) (26).
In addition, activation of the p38 MAPK signaling pathway also
contributes to AP-1 activation, leading to the transcriptional
activation of several genes involved in inflammation, including
TNF-α, IL-1β, IL-4 and IL-6 (27-29).
Accordingly, the activities of the MAPK signaling pathways,
including c-JNK and p38 MAPK, were investigated in the present
study following the inhibition of TNF-α in the hippo-campal tissue
of aged rats following peripheral surgery. The results of this
investigation suggested that inhibiting TNF-α inhibited the
surgery-induced activation of the c-JNK and p38 MAPK signaling
pathway-induced release of TNF-α, IL-1β, IL-4 and IL-6 in the
hippocampal tissues of the aged rats.
In conclusion, the results of the present study
suggested that, in aged rats, upregulated TNF-α resulting from
peripheral surgery, further activated the downstream NF-κB
signaling pathway, leading to increased release of pro-inflammatory
cytokines, further extensive neuroinflammatory responses and,
ultimately, to defects in cognitive function. Accordingly,
targeting TNF-α may be a promising strategy for the prevention and
treatment of POCD.
References
|
1
|
Colenkova AV, Bondarenko AA, Yu Lubnin A
and Dzyubanova NA: Postoperative cognitive dysfunction in elderly
patients. Anesteziol Reanimatol. 4:13–19. 2012.In Russian.
|
|
2
|
Li M, Yong-Zhe L, Ya-Qun M, Sheng-Suo Z,
Li-Tao Z and Ning-Ling P: Ulinastatin alleviates neuroinflammation
but fails to improve cognitive function in aged rats following
partial hepatectomy. Neurochem Res. 38:1070–1077. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cao XZ, Ma H, Wang JK, et al:
Postoperative cognitive deficits and neuroinflammation in the
hippocampus triggered by surgical trauma are exacerbated in aged
rats. Prog Neuropsychopharmacol Biol Psychiatry. 34:1426–1432.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen J, Buchanan JB, Sparkman NL, Godbout
JP, Freund GG and Johnson RW: Neuroinflammation and disruption in
working memory in aged mice after acute stimulation of the
peripheral innate immune system. Brain Behav Immun. 22:301–311.
2008. View Article : Google Scholar
|
|
5
|
Garcia GE, Xia Y, Chen S, et al:
NF-kappaB-dependent fractalkine induction in rat aortic endothelial
cells stimulated by IL-1beta, TNF-alpha and LPS. J Leukoc Biol.
67:577–584. 2000.PubMed/NCBI
|
|
6
|
Rosczyk HA, Sparkman NL and Johnson RW:
Neuroinflammation and cognitive function in aged mice following
minor surgery. Exp Gerontol. 43:840–846. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Terrando N, Monaco C, Ma D, Foxwell BM,
Feldmann M and Maze M: Tumor necrosis factor-alpha triggers a
cytokine cascade yielding postoperative cognitive decline. Proc
Natl Acad Sci USA. 107:20518–20522. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Riches DW, Chan ED and Winston BW:
TNF-alpha-induced regulation and signalling in macrophages.
Immunobiology. 195:477–490. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang HL, Chang HC, Lin SW, et al: Antrodia
salmonea inhibits TNF-α-induced angiogenesis and atherogenesis in
human endothelial cells through the down-regulation of NF-κB and
up-regulation of Nrf2 signaling pathways. J Ethnopharmacol.
151:394–406. 2014. View Article : Google Scholar
|
|
10
|
Song HY, Regnier CH, Kirschning CJ,
Goeddel DV and Rothe M: Tumor necrosis factor (TNF)-mediated kinase
cascades: Bifurcation of nuclear factor-kappaB and c-jun N-terminal
kinase (JNK/SAPK) pathways at TNF receptor-associated factor 2.
Proc Natl Acad Sci USA. 94:9792–9796. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yuasa T, Ohno S, Kehrl JH and Kyriakis JM:
Tumor necrosis factor signaling to stress-activated protein kinase
(SAPK)/Jun NH2-terminal kinase (JNK) and p38. Germinal center
kinase couples TRAF2 to mitogen-activated protein kinase/ERK kinase
kinase 1 and SAPK while receptor interacting protein associates
with a mitogen-activated protein kinase kinase kinase upstream of
MKK6 and p38. J Biol Chem. 273:22681–22692. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu Z, Ou Y, Duan K and Jiang X:
Inflammation: A bridge between postoperative cognitive dysfunction
and Alzheimer’s disease. Med Hypotheses. 74:722–724. 2010.
View Article : Google Scholar
|
|
13
|
Yu D, Corbett B, Yan Y, et al: Early
cerebrovascular inflammation in a transgenic mouse model of
Alzheimer’s disease. Neurobiol Aging. 33:2942–2947. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Abildstrom H, Rasmussen LS, Rentowl P, et
al: Cognitive dysfunction 1–2 years after non-cardiac surgery in
the elderly. ISPOCD group International study of post-operative
cognitive dysfunction. Acta Anaesthesiol Scand. 44:1246–1251. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Boodhwani M, Rubens FD, Wozny D, et al:
Predictors of early neurocognitive deficits in low-risk patients
undergoing on-pump coronary artery bypass surgery. Circulation.
114(Suppl 1): I461–I466. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Canet J, Raeder J, Rasmussen LS, et al:
Cognitive dysfunction after minor surgery in the elderly. Acta
Anaesthesiol Scand. 47:1204–1210. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Deiner S and Silverstein JH: Postoperative
delirium and cognitive dysfunction. Br J Anaesth. 103(Suppl 1):
i41–i46. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kamer AR, Galoyan SM, Haile M, et al:
Meloxicam improves object recognition memory and modulates glial
activation after splenectomy in mice. Eur J Anaesthesiol.
29:332–337. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hains LE, Loram LC, Taylor FR, et al:
Prior laparotomy or corticosterone potentiates
lipopolysaccharide-induced fever and sickness behaviors. J
Neuroimmunol. 239:53–60. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Didem B, Huseyin B, Osman Y, Yasemin B,
Necati G and Canan T: Early effects of laparotomy and laparoscopy
on bacterial behavior and proinflammatory cytokines on bacterial
peritonitis in rats I: Escherichia coli. J Pediatr Surg.
43:1494–1501. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kannan S, Saadani-Makki F, Muzik O, et al:
Microglial activation in perinatal rabbit brain induced by
intrauterine inflammation: Detection with 11C-(R)-PK11195 and
small-animal PET. J Nucl Med. 48:946–954. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Barrientos RM, Hein AM, Frank MG, Watkins
LR and Maier SF: Intracisternal interleukin-1 receptor antagonist
prevents postoperative cognitive decline and neuroinflammatory
response in aged rats. J Neurosci. 32:14641–14648. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hoesel B and Schmid JA: The complexity of
NF-κB signaling in inflammation and cancer. Mol Cancer. 12:862013.
View Article : Google Scholar
|
|
24
|
Muñoz A and Costa M: Nutritionally
mediated oxidative stress and inflammation. Oxid Med Cell Longev.
2013:6109502013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Arslan F, Lai RC, Smeets MB, et al:
Mesenchymal stem cell-derived exosomes increase ATP levels,
decrease oxidative stress and activate PI3K/Akt pathway to enhance
myocardial viability and prevent adverse remodeling after
myocardial ischemia/reperfusion injury. Stem Cell Res. 10:301–312.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen WL, Sheu JR, Hsiao CJ, Hsiao SH,
Chung CL and Hsiao G: Histone deacetylase inhibitor impairs
plasminogen activator inhibitor-1 expression via inhibiting
TNF-α-activated MAPK/AP-1 signaling cascade. Biomed Res Int.
2014:2310122014. View Article : Google Scholar
|
|
27
|
Lee IT, Lin CC, Cheng SE, Hsiao LD, Hsiao
YC and Yang CM: TNF-alpha induces cytosolic phospholipase A2
expression in human lung epithelial cells via JNK1/2- and p38
MAPK-dependent AP-1 activation. PLoS One. 8:e727832013. View Article : Google Scholar
|
|
28
|
Qiu Q, Xiong W, Yang C, et al:
Lymphocyte-derived micropar-ticles induce apoptosis of airway
epithelial cells through activation of p38 MAPK and production of
arachidonic acid. Apoptosis. 19:1113–1127. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ma JQ, Ding J, Zhang L and Liu CM: Ursolic
acid protects mouse liver against CCl4-induced oxidative stress and
inflammation by the MAPK/NF-κB pathway. Environ Toxicol Pharmacol.
37:975–983. 2014. View Article : Google Scholar : PubMed/NCBI
|