Introduction
Glioblastoma multiforme (GM) is one of the most
aggressive and malignant tumors of the human brain. The treatment
standard of GM is surgical resection with consequent chemotherapy
(1). The survival rate is 12–14
months, and cases of five-year survival are singular (2). The absence of clear borders between
tumor tissue and brain substance, marked infiltration of neoplastic
cells into brain parenchyma and location close to vitally important
brain centers make radical removal of GM impossible (3). The tumor resistance to radio- and
chemotherapy is associated with the cancer stem cells (CSCs)
(4). The ability to restore
damaged DNA, production of ATP-binding cassette transporters,
hypoxic metabolism and the opportunity to actively interact with
endothelial cells that create a barrier for cytotoxic substances
make CSCs almost completely resistant to chemotherapy (5,6).
To date, no efficient drugs which are able to
eliminate GM CSCs have been developed; there is only the
opportunity to impair separate CSC targets. For example, imatinib
inhibits platelet-derived growth factor receptor and stem cell
factor receptor/c-Kit signaling and blocks the mitogen-activated
protein kinase signaling pathway, thus interfering with GM CSC
migration (7). Rapamicin
deactivates phosphatase and tensin homolog (8). Cyclopamine inhibits the Wnt/Sonic
hedgehog signaling pathway in GM CSCs (9). However, these pharmaceuticals are
unable to eliminate inter-phase neoplastic cells disseminated
through the brain substance. To date, attempts to employ
procarbazin (natulan), nimustine hydrochoride (ACNU), fotemustine,
dacarbazine and irinotecan (camptosar) for this purpose have not
been successful (10).
The use of monoclonal antibodies has not been
efficient, either; for instance, treatment of patients of GM with
antibodies to CD44 and CD133 proteins has not had any curative
effects (11). Ipilimumab, an
antibody against cytotoxic T-lymphocyte-associated protein 4, was
not able to penetrate into hypoxic areas of the tumor (12). Bevacizumab (avastin) suppresses
hematopoiesis in bone marrow and increases the risk of bleeding
(13). Only the tyrosine kinase
inhibitors erlotinib and imatinib have produced good outcomes.
However, targeted therapies have not significantly improved the
survival rates of patients with GM, which may be explained by the
absence of universal targets in CSCs. All targets are dynamic and
are associated with specific processes that result from the
functional state of the organism.
An important milestone in the development of novel
approaches in GM therapy was the discovery of targeted migration of
stem cells (SCs) to the neoplasm. A key factor in the process is
stromal cell-derived factor-1 (SDF-1α or CXCL12), a chemokine of
the CXC family encoded by the CXCL12 gene (14). SDF-1 binds with CXCR4 and CXCR7
receptors of the SC membrane and induces migration. The process is
moderated by the stem cell factor (SCF), hepatocyte growth factor,
vascular endothelial growth factor, monocyte chemoattractant
protein-1, high-mobility group protein B1, urokinase-type
plasminogen activator and other ligands. The SCs can overcome the
blood-brain barrier, affect cancer cells that are disseminated in
the brain parenchyma and penetrate into hypoxic areas of GM that
contain CSCs (15). However, the
action of SCs on CSCs demands a specific approach, depending on
their role and complexity.
The CSCs of GM are the product of the evolution of a
neural stem cell (NSC) of the human brain. Common
immunophenotypical clusters of differentiation, integrity of the
main genes and epigenetic mechanisms that regulate key cellular
processes and similarity in proteome and complete transcriptome
profiles (6,16) have confirmed this. NSCs are tools
of local homeostasis and constantly migrate from germinal zones of
the adult brain to interact with neurons and glial cells. Cellular
interaction is the main mechanism of regulation of gene expression;
it is an important factor of coordinated regulation of metabolism
and triggers the programs of determination, differentiation,
adaptation, survival, proliferation and apoptosis. Inductive
interaction of the NSCs and pathologically modified cells retards
their growth and initiates natural mechanisms of cell death
(17).
Appropriately modified SCs can be used in
conventional therapeutic protocols of GM treatment. Development of
systems of somatic and stem cells with specific properties is one
of the top-priority goals of biomedicine globally. At the same
time, it is obvious that it will take a considerate amount of time
and development until the technologies of cell reprogramming, which
are based on the transfer of the somatic cell nucleus into oocyte
cytoplasm, fusion of two pluripotent somatic cells and viral
transfection along with other gene engineering methods, will be
used in practical medicine. In spite of their advantages, possible
genetic consequences and health risks of using such cell systems
rule them out as the strategy of choice in GM therapy. The use of
embryonic SCs in the clinic seems unlikely due to several insoluble
problems: Their control in the body of a patient and serious
ethical considerations as to their sources.
In a previous study by our group from 2013,
comparative proteome mapping and bioinformatics analysis was
performed of the lysates of neural (CD133+) stem cells isolated
from the olfactory sheath of a human, multipotent mesenchymal
stromal cells (CD29+, CD44+, CD73+, CD90+, CD34−) (MMSCs) isolated
from the human bone marrow and CD133+ CSCs of the U87 human
glioblastoma line (18). The study
provided evidence that MMSCs exhibited the largest difference in
their proteome profile. These cells and their progeny continuously
interacted with SCs of other organs, thus completing and modulating
their regulatory functions. It was therefore presumed that
transplantation of this type of the cell may be a tool for
enhancing the efficiency of standard protocols of GM treatment.
The goal of the present study was to assess the
possible enhancing effects of MMSC transplantation in the
chemotherapy of a rat model of glioblastoma.
Materials and methods
Design
A total of 130 adult Wistar rats (2–5 months-old)
weighing 200–220 g at baseline were divided in four groups. The
rats were maintained in cages at room temperature under a normal
diurnal cycle, with free access to food and water. The control
(first) group included C6 glioma models (n=30); the second group
consisted of C6 glioma rat models that received standard therapy
with temozolomide (n=30); the third group were C6 glioma rats that
received MMSC transplantation (n=30), and the fourth group
consisted of C6 glioma rats received temozolomide therapy combined
with MMSCs transplantation. A separate group consisted of
sham-operated rats (n=10).
A combination of surgical, cell biological,
histological and neuroimaging methods was used in the present
study. The animal experiment was performed for 70 days, with
particular attention given to the survival of the animals. Whenever
the state deteriorated, the rats were sacrificed by deep narcosis
[10 mg/kg intraperitonal injection of 200 μl Zoletil 100
(Virbac, Prague, Czech Republic) + Rometar (Bioveta, Ivanovice na
Hane, Czech Republic) in a 1:4 ratio]. The experiment was repeated
three times. Animal care followed good laboratory practice
standards and the Helsinki Declaration on the humane attitude to
animals. All protocols of the present study were approved by the
Ethics Committee of the School of Biomedicine of the Far Eastern
Federal University (Vladivostok, Russia) (protocol no. 12 dated
December 14, 2013).
Culturing of the C6 glioma cell line
The rat C6 glioma line was provided by the
Laboratory of Fundamental and Applied Neurobiology of the Serbsky
State Research Center of Social and Forensic Psychiatry (Moscow,
Russia). The rapidly growing cell line was generated in
Wistar-Furth rats by carcinogenesis induction using
N-nitroso-N-methylurea; morphology, character of
invasive growth and protein spectrum of C6 glioma is closest to
that of human GM (19).
An aliquot of 1×106 cancer cells was
defrosted for 10 minutes at 37°C, washed with Dulbecco’s modified
Eagle’s medium (DMEM; Gibco-BRL, Invitrogen Life Technologies,
Carlsbad, CA, USA) that contained 10% fetal bovine serum (FBS; no.
26140-079; Gibco-BRL) and antibiotic-antimycotic: 10,000 units/ml
penicillin, 10,000 μg/ml streptomycin and 25 μg/ml
fungizone (no. 15240-062; Gibco-BRL). The cells were centrifuged
(120 x g for 4 min at 10°C), fresh medium was added, and cells were
seeded into 50-ml culture flasks (Corning-Costar, Costar, NY, USA)
and cultured until a monolayer developed. To split the cells, they
were detached by enzyme dissociation [0.05% trypsin-EDTA (MP
Biomedicals, Santa Ana, CA, USA) 1:4; 10 min, 37°C), centrifuged
(120 x g, 6 min), the supernatant was removed, fresh medium was
added and the cells were re-suspended.
The tumor was modeled under general anesthesia (200
μl Zoletil/Rometar 1:4, intraperitoneally). The glioma cells
(106 cells in 20 ml) were implanted into the
caudoputamen using a stereotaxic apparatus (Narishige, Tokyo,
Japan) according to the stereotaxic coordinates of the Swanson rat
atlas: Ap-1; L 3.0; V 4.5, TBS-2.4 mm (20). The cells were injected with a
Hamilton syringe at a speed of 5μl/min. Prior to injection,
part of the C6 glioma cells was processed using a
Vybrant® carboxyfluorescein diacetate succinimidyl ester
(CFDASE) Cell Tracer (V12883; Life Technologies, Carlsbad, CA, USA)
according to manufacturer’s instructions.
Multipotent mesenchymal stromal cells
(MMSCs)
Impersonalized samples of bone marrow were provided
by the ZAO Neurovita Clinic of Restorative and Interventional
Neurology and Therapy (Moscow, Russia). The MMSCs were isolated
using a standard procedure (21).
The bone marrow sample was resuspended in RPMI-1640 medium
(Sigma-Aldrich, St Louis, MO, USA) that contained 10% FBS and 1%
penicillin-streptomycin. The cells were cultured in T150 flasks
(TPP, Trasadingen, Switzerland), at 37°C in a 5% CO2
atmosphere. Following four days, the medium with non-adherent cells
was replaced. The adhered cells were cultured to 80% confluence and
passaged at 1:3. The MMSCs were characterized by surface expression
of antigens (CD29+, CD44+, CD73+, CD90+, CD34−), in accordance with
the manufacturer’s instructions, using flow cytometry
(MACSQuant® VYB; Miltenyi Biotec, Cambridge, MA,
USA).
Ten days following implantation of glioma cells,
106 MMSCs (50 μl) were injected in tumor
vicinity. A preliminary experiment showed that during 10 days, the
size of the glioma doubled, leading to a glioma cell/MMSC ratio of
2:1. Prior to injection, all of the cells were marked with
CellTracker™ Red CMTPX (C34552; Life Technologies) according to the
manufacturer’s instructions. Sham-operated rats received injections
of 20 μl DMEM.
Chemotherapy
The standard of GM chemotherapy is the treatment
with temozolomide, a cytostatic anti-tumor alkalyting agent. The
agent rapidly reaches the systemic blood supply and is processed
into an active metabolite whose cytotoxic action is determined by
its ability to disturb the structure and synthesis of DNA, which
most often occurs at the N-7 or O-6 positions of guanine residues
(22). The present study used
temozolomide under the brand name temodal (MSD Shering-Plough Labo,
Heist-op-den-Berg, Belgium). Magnetic resonance imaging (MRI) was
performed on the rats ten days after glioma cell implantation. If
the tumor was clearly imaged, the animals of the appropriate groups
received 50 μg/kg temozolomide orally from days 10–14 of the
experiment.
Neuroimaging
MRI of the brain was performed using a Biospec MR
tomograph (Bruker, Billerica, MA, USA) with a special magnetic coil
for small laboratory animals (2–3 cm inner diameter) under general
anesthesia. MRI was performed at days 10, 20, 30, 40, 50, 60 and 70
of the trial.
Neurological status and body weight
Examination of the neurological status in rats was
accomplished according to the standard algorithm (23). The body weight was measured using a
Sartorius CPA12001S (Sartorius, Göttingen, Germany) laboratory
balance.
Histological study
The 40-μm thick sections were stained with
cresyl violet, toluidine blue, hematoxylin and eosin (all
Sigma-Aldrich) according to standard protocols, and with vanadium
acid fuchsine (Sigma-Aldrich), according to the Victorov method
(24). The brain sections were
studied using a Leica DM 6000 (Leica Microsystems GmbH, Wetzlar,
Germany).
Tumor morphometry
The size of the tumor was defined according to the
formula: V=4\3π abc, where a, b and c are semi-axes of an
ellipsoid. Primarily, the section with the maximal glioma area was
detected, where a large semi-axis (a) and a small semi-axis (b) of
the ellipsoid were defined. Then, the lengths of the frontal
sections from the anterior to the posterior side of a tumor node
were summed, where the anterior-posterior semi-axis was then
defined (c) using a Biospec MR tomograph.
Statistical analysis
Statistical analysis was performed using Excel 2010
(Microsoft Corp., Redmond, WA, USA). Image processing and graphical
analysis was performed with ImageJ 1.43 software (National
Institutes of Health, Bethesda, MD, USA).
Results
Tumor morphology
Stereotaxic implantation of C6 glioma cells to the
rat brain led to tumor formation (Fig.
1A). The T2-Turbo RARE mode scans showed voluminous neoplasms
of irregular shape with signs of compression of brain ventricles
and other structures, as well as foci of hemorrhages and edema in
the brain structure (Fig. 1B).
Histological analysis demonstrated an extensive neoplasm with
unclear borders and edema of the neighboring white matter. The
tumor consisted of cells of different shapes and sizes with
different numbers of nuclei. The tumor spread into the perivasal
and perineural spaces. Acidophilic cells were selectively stained
red with vanadium acid fuchsine and extensive areas of central
necrosis that occasionally transformed in cysts were found.
Fluorescence microscopy (λ=488 nm) showed a heterogeneous
distribution of neoplastic elements in the tumor parenchyma and the
tendency to migrate to the neighboring tissues (Fig. 1C–H).
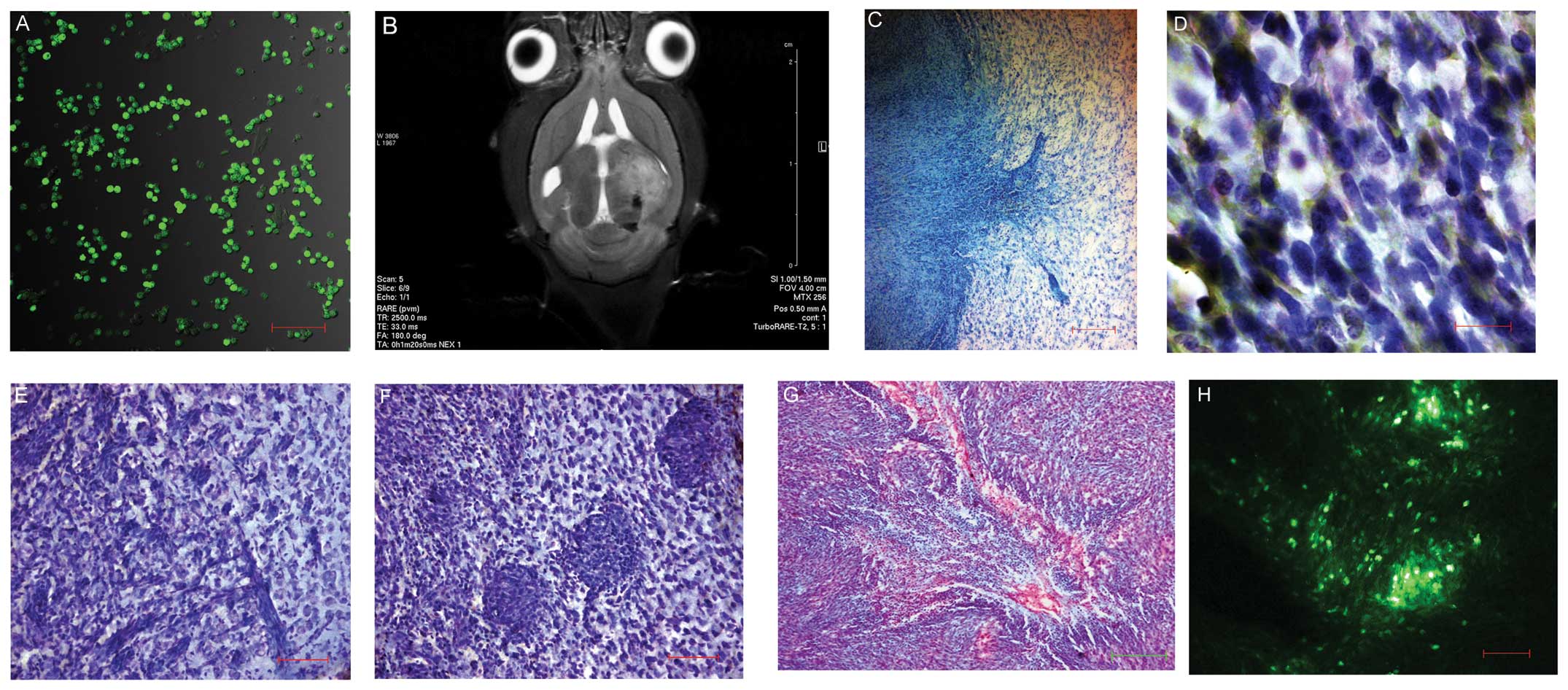 | Figure 1(A) Vybrant® CFDASE
fluorescence of C6 glioma cells in the culture prior to
implantation. LSM T-PMT Carl Zeiss Aim-system, (laser, λ=488 nm;
scale bar, 200 μm). (B) Magnetic resonance thermogram of the
rat brain seven days post C6 glioma cell transplantation.
TurboRARE-T2 mode. Ventricle compression, edema and disposition of
median brain structures are observed. (C) Area of invasive growth
of C6 glioma. Toluidine blue staining (scale bar, 100 μm).
The tumor borders are unclear; invasion area and infiltration of
neoplastic elements into the brain substance are observed. (D)
Polymorphism of C6 glioma cell nuclei. Cresyl violet and toluidine
blue staining (oil immersion; scale bar, 10 μm). (E)
Perineural invasion of C6 glioma. Cresyl violet and toluidine blue
staining (scale bar, 100 μm). (F) Perivasal invasion of C6
glioma. Cresyl violet and toluidine blue staining (scale bar, 100
μm). (G) Central area of necrosis of C6 glioma. Staining
according to the method of I. Viktorov. Acidophilic cells are
selectively stained red (scale bar, 200 μm). (H) C6 glioma
node in the rat brain. Cells were stained using Vybrant®
CFDASE Cell Tracer. LSM T-PMT Carl Zeiss Aim-system 2601876 (laser,
λ=488 nm; scale bar, 100 μm). LSM, laser scanning
microscope; CFDASE, carboxyfluorescein diacetate succinimidyl
ester. |
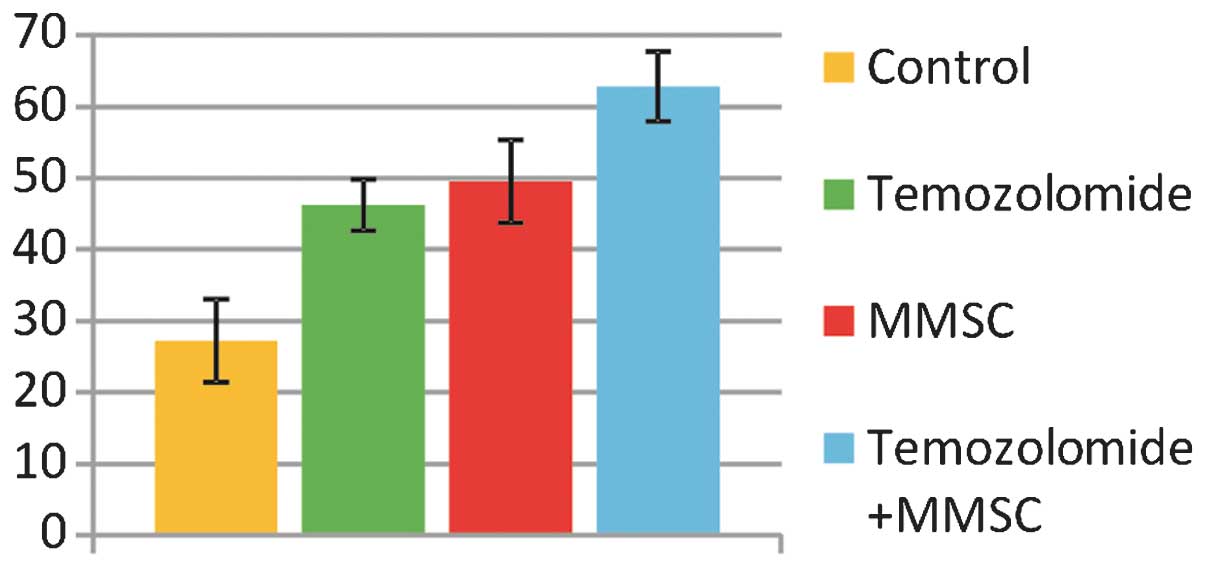
MMSCs increase the survival of a rat
model of GM treated with temozolomide
The average survival of the control group animals
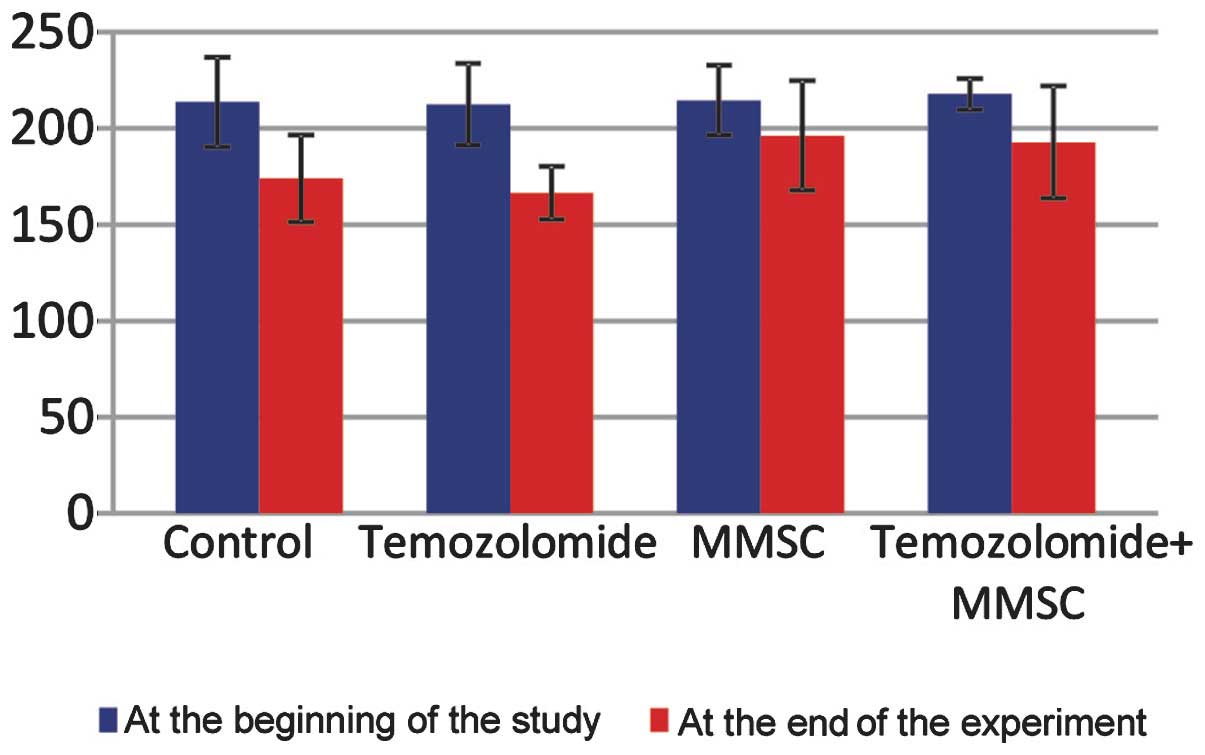
was 27.2±5.2 days from the moment of surgery (Fig. 2). The animals lost body weight
rapidly (Fig. 3), were inert,
disinterested in the events in the cage, refused to feed and were
reluctant to drink. Attempts to pick up an animal by hand or slight
contact with the vibrissae caused reactions of loud squeaking. Mild
neurological symptoms, including ptosis, tremor and paresis of
extremities, rapidly aggravated, resulting in paralysis with
exophthalmos followed by coma and respiratory impairments that
appeared to be a result of brain dislocation. The sham-operated
rats showed no significant changes in body weight and functional
status, and survived until the end of the experiment with no
complications.
The average survival time of the
temozolomide-treated animals was 46.2±3.6 days from the start of
the experiment (Fig. 2). The
animals in this group showed no significant differences in body
weight in comparison with that of the controls (Fig. 3); furthermore, they were reluctant
to eat and were inert. The general condition of the animals was
less severe than that of the control animals; it was more stable
and the development of acute neurological disorders was less
abrupt. Brain symptoms manifested as bilateral hemoptosis and mild
spastic paresis of extremities in combination with spasms of the
paws. Inhibition was replaced by the episodes of excitation that
manifested in circular movements within the cage associated with
the damage of one of the hemispheres. Morphological changes in the
brains of the animals excluded from the experiment due to illness
showed no differences from the morphological changes in the control
group.
The average survival time of the animals with C6
glioma tumors that received transplantation of MMSCs was 49.56±1.94
days from the start of the experiment, which was significantly
different from that of the controls, but not different from that in
the temozolomide-treated group. Neurological examination showed
minor neurological symptoms: Reduced corneal reflex, ptosis and
exophthalmos on the tumor side, reduced flexor and grasp reflexes,
increased reaction to light and sound stimuli, and retardation in
the head shaking test (22). The
animals remained more active for a longer time as compared with the
controls, did not refuse to eat and did not lose weight
significantly. Histological analysis showed an extensive tumor
formation with unclear borders and areas of invasion to the brain
substance (Fig. 1C).
MMSCs were observed at the site of injection and at
a certain distance from it. The cells were ball-shaped and no
branching was present. It appeared that during glioma growth, the
MMSCs migrated following neoplastic cells and possibly interacted
with them. Overlaying of red and green fluorescence showed that the
MMSCs had accumulated along the tumor borders and were also present
in the parenchyma of the neoplasm (Fig. 4A–C). MMSCs were not visible by
fluorescence microscopy at day 30 of the trials, which may have
been due to their death, differentiation or involvement in the
tumor biological process.
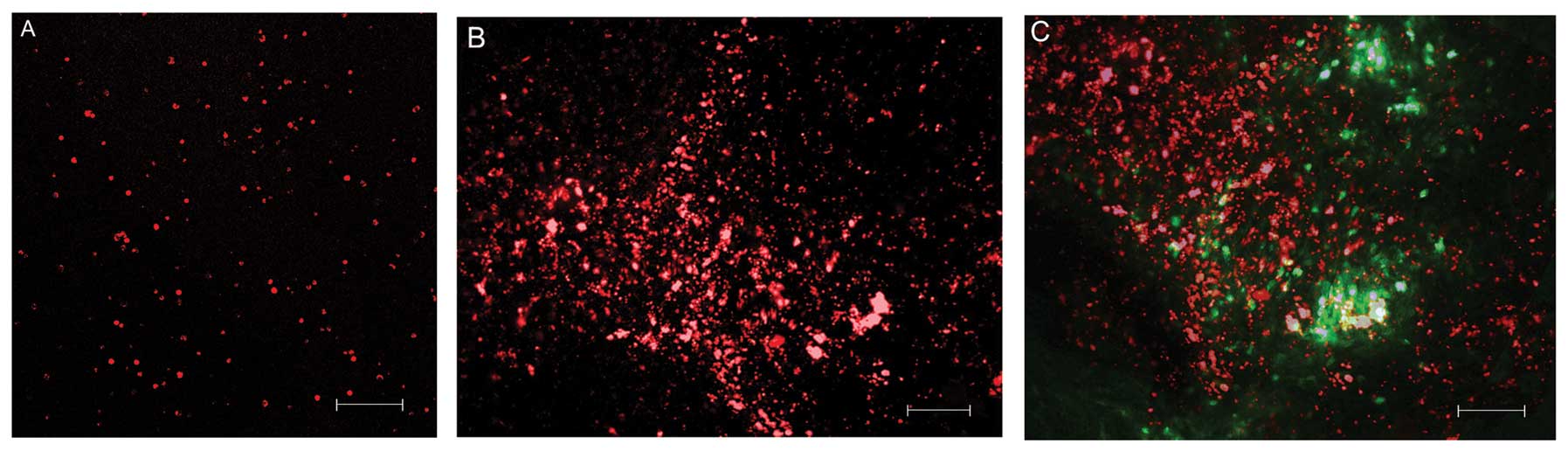 | Figure 4(A) CMTPX-positive MMSCs (red) prior
to transplantation. LSM T-PMT Carl Zeiss Aim-system 2601876 (laser,
λ=650 nm; scale bar, 100 μm). (B) Migration of
CMTPX-positive MMSCs (red) at day 10 in the brain substance
adjacent to the neoplasm (scale bar, 100 μm). (C)
CMTPX-positive MMSCs (laser fluorescence, λ=650 nm, red) in the
tumor parenchyma and the tissue adjacent to the tumor nodes formed
by CFDASE-positive tumor cells (laser fluorescence, λ=488 nm,
green). Overlay of red and green fluorescence (scale bar, 100
μm). MMSCs, multipotent mesenchymal stromal cells; LSM,
laser scanning microscopy; CFDASE, carboxyfluorescein diacetate
succinimidyl ester. |
The survival time of the animals that received
chemotherapy along with MMSC transplantation was 62.8±4.85 days,
which is significantly longer than the survival times in the
control and temozolomide groups. The animals showed no significant
weight loss, were active and did not refuse to eat. Neurological
examination showed mild neurological symptoms with further abrupt
coma and decreased viability.
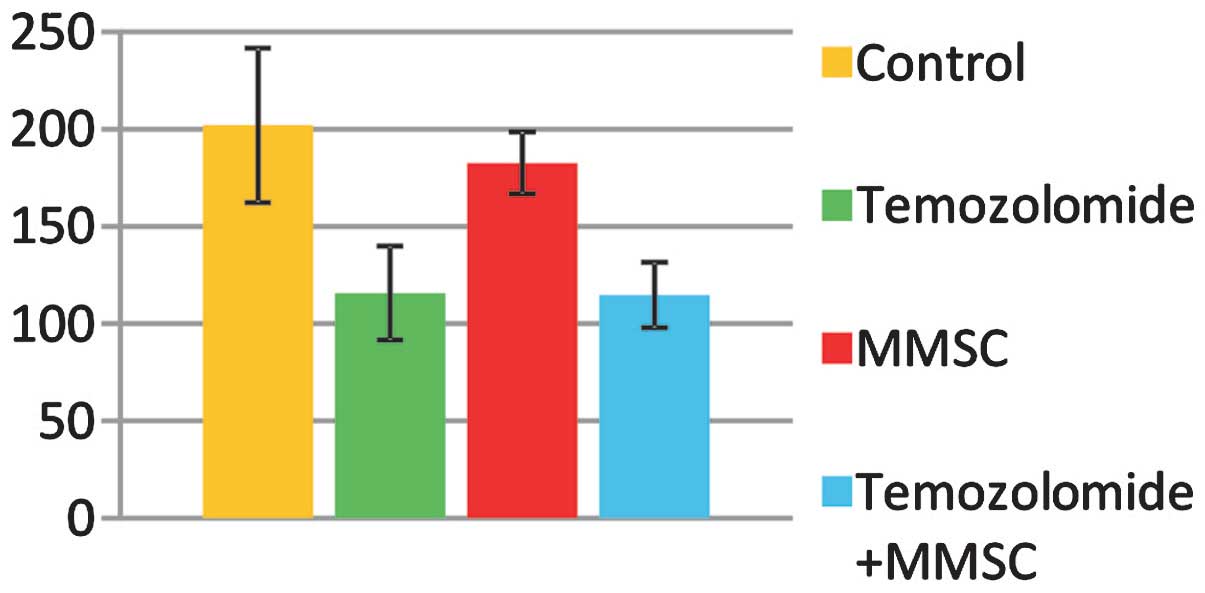
The tumor size varied considerably among the groups
(Fig. 5). The smallest size was
found in the temozolomide-treated group (115.76±16.25
mm3) and in the temozolomide + MMSCs group (114.74±5.54
mm3), which were significantly smaller than the size of
neoplastic nodes in the control group (202.09±39.72 mm3)
and in the MMSCs group (182.72±15.96 mm3)
(P<0.05).
Discussion
From an evolutionary perspective and with regard to
the antagonism between CSCs and healthy NSCs, tumor development can
be understood as the result of local functional dominance of the
neoplastic SCs. Among the CSCs of the C6 glioma cel line, 87.24%
are CD133+ (25), making it
advantageous for the use in models of GM as compared with other
glioma cell lines (26).
Transplantation of C6 glioma cells leads to the collapse of
autoregulation mechanisms of tissue homeostasis in the rat brain,
and the quantitative advantage of CD133+ CSCs permits the C6 cell
line to rapidly develop vascular, lymphatic and neural networks, to
optimize metabolism and to trigger invasive processes at a high
speed, which explains the severity of GM and the high mortality
rates in the control group of the present study.
Temozolomide significantly reduces the size of
glioma, which was proven by the present and previous studies
(27). The cytotoxic activity of
the agent reduces the size of the tumor nodes, but considerably
enhances hypoxia (28,29), which appears to promote the
production of chemokines that induce processes of targeted
migration of SCs to the tumor focus. Factors including healthy SCs
that migrated to the tumor abruptly shifted this balance,
explaining for the increased survival as the main indicator of
partial restoration of the system.
The transplanted MMSCs were able to slow down tumor
cell proliferation, which additionally made the latter susceptible
to the regulatory signals of apoptosis and autophagy (30). Activation of tumor necrosis factor
(TNF), TNF-related apoptosis-inducing ligand, nerve-, insulin-like
and endothelial growth factor receptors, CAR1, CD95 receptors and
DR3, DR4, DR5 trans-membrane proteins in the SCs induces apoptosis
in tumor cells. In co-culture with MMSCs, the cyclins E and D2 as
well as p27KIPl accumulate in the medium, thus blocking
proliferation of tumor cells in G0/G1 phase (31). Death of C6 glioma cells upon
interaction with SCs can be caused by disorders in the
intracellular homeostasis of calcium ions, and a specific signal is
transferred through cell junctions by means of bone morphogenetic
protein 4 and interleukin-1 (32).
Neuroplastic action of SCs and the production of biologically
active substances by SCs in the pathological tissue also can be
regarded as mechanisms of their anti-cancer action (33).
It should be noted that the fate of
xenotransplantation in the present study was initially defined. A
certain degree of immune suppression caused by hormones or
cytostatics can delay the death of the transplanted cell systems.
Thus, in the context of the present study, MMSCs can be viewed as
cell systems which induced apoptosis. A previous study by our group
reported the possibility of apoptosis induction in cells of glial
tumors during interaction with NSCs and hematopoiesis precursors.
When these cell systems were co-cultured with C6 glioma and U87
glioblastoma cells, they induced apoptosis in them and slowed down
the neoplastic process (34). It
is possible that this mechanism reduced the size of tumor nodes and
ameliorated the general condition of the rats.
However, GM is able to recruit normal somatic cells
and SCs. The tumor uses their potential and involves them in
carcinogenesis. Liu et al (35) reported that SCs that had been
co-cultured with tumor cells underwent malignant transformations.
It cannot be ruled out that the death of tumor cells activates
survival mechanisms in CSCs and functions as a factor that selects
the most aggressive and resistant cells in the tumor
microenvironment.
The results of the present study lead to an
important conclusion: The rats with C6 glioma that received
chemotherapy in combination with MMSCs survived significantly
longer than the animals that received temozolomide only
(P<0.05). Therefore, the survival, which is the main criterion
of therapeutic efficiency in oncology, significantly improved when
stem cell transplantation was used. Identification of the
mechanisms of this phenomenon and development of more sensitive and
accurate methods to control cell induction is among priority tasks
of studies to be performed in the near future. Due to the evidence
provided, the MMSC preparations can be viewed as promising tools of
regulation and management of neoplastic processes in the glial
tumor and open opportunities for the development of novel
therapeutic strategies against neurooncological diseases.
Acknowledgments
The present study was funded by the Russian Science
Fund (project no. 14-15-00084).
References
|
1
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hottinger AF, Stupp R and Homicsko K:
Standards of care and novel approaches in the management of
glioblastoma multiforme. Chin J Cancer. 33:32–39. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goffart N, Kroonen J and Rogister B:
Glioblastoma-initiating cells: Relationship with neural stem cells
and the micro-environment. Cancers (Basel). 5:1049–1071. 2013.
View Article : Google Scholar
|
|
4
|
Pointer KB, Clark PA, Zorniak M, Alrfaei
BM and Kuo JS: Glioblastoma cancer stem cells: Biomarker and
therapeutic advances. Neurochem Int. 71:1–7. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ramirez YP, Weatherbee JL, Wheelhouse RT
and Ross AH: Glioblastoma Multiforme therapy and mechanisms of
resistance. Pharmaceuticals (Basel). 6:1475–1506. 2013. View Article : Google Scholar
|
|
6
|
Bryukhovetskyi IS, Bryukhovetskyi AS,
Kumeiko VV, Mishenko PV and Khotimchenko YS: Stem cells in
carcinogenesis of glioblastoma multiforme. Genes Cells. 8:13–19.
2013.In Russian.
|
|
7
|
Dong Y, Han Q, Zou Y, Deng Z, Lu X, Wang
X, Zhang W, Jin H, Su J, Jiang T, et al: Long-term exposure to
imatinib reduced cancer stem cell ability through induction of cell
differentiation via activation of MAPK signaling in glioblastoma
cells. Mol Cell Biochem. 370:89–102. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mendiburu-Eliçabe M, Gil-Ranedo J and
Izquierdo M: Efficacy of rapamycin against glioblastoma cancer stem
cells. Clin Transl Oncol. 16:495–502. 2014. View Article : Google Scholar
|
|
9
|
Eimer S, Dugay F, Airiau K, Avril T,
Quillien V, Belaud-Rotureau MA and Belloc F: Cyclopamine cooperates
with EGFR inhibition to deplete stem-like cancer cells in
glioblastoma-derived spheroid cultures. Neurooncol. 14:1441–1451.
2012.
|
|
10
|
Parney IF and Chang SM: Current
chemotherapy for glioblastoma. Cancer J. 9:149–156. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tamura K, Aoyagi M, Ando N, Ogishima T,
Wakimoto H, Yamamoto M and Ohno K: Expansion of CD133-positive
glioma cells in recurrent de novo glioblastomas after radiotherapy
and chemotherapy. J Neurosurg. 119:1145–1155. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brower V: Early-stage progress on glioma
vaccines. J Natl Cancer Inst. 103:1361–1362. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rovere RK: Bevacizumab as secondline
treatment of glioblastoma - worth the effort? Klin Onkol.
27:219–220. 2014.
|
|
14
|
Nagasawa T: CXC chemokine ligand 12
(CXCL12) and its receptor CXCR4. J Mol Med (Berl). 92:433–439.
2014. View Article : Google Scholar
|
|
15
|
Bryukhovetskyi IS, Bryukhovetskyi AS,
Mischenco PV and Khotimchenko YS: The role of systemic migration
and homing mechanisms of stem cells in the development of malignant
tumors of the central nervous system and the development of new
cancer therapies. Russian Biotherapeutic J. 4:3–12. 2013.In
Russian.
|
|
16
|
Rispoli R, Conti C, Celli P, Caroli E and
Carletti S: Neural stem cells and glioblastoma. Neuroradiol J.
27:169–174. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Paltsev MA, Ivanov AA and Severin SE:
Intercellular interactions. Medicine (Moscow). 2003, In
Russian.
|
|
18
|
Bryukhovetskiy AS, Shevchenko VE,
Chekhonin VP, Bryukhovetskiy IS, Kovalev SV, Baklaushev VP and
Davydov MI: Comparative proteome mapping of tumor stem cells
isolated from U87 glioblastoma, neural stem and multi-potent
mesenchymal stromal cells of human: From cataloguing of cell
proteins to novel paradigm of proteome-based cell therapy of
tumors. Genes Cells. 8:85–92. 2013.
|
|
19
|
Grobben B, De Deyn PP and Slegers H: Rat
C6 glioma as experimental model system for the study of
glioblastoma growth and invasion. Cell Tissue Res. 310:257–270.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Swanson LW: Brain Maps: Structure of the
Rat Brain. 2nd edition. Elsevier; Amsterdam: 1998
|
|
21
|
Pittenger MF: Mesenchymal stem cells from
adult bone marrow. Methods Mol Biol. 449:27–44. 2008.PubMed/NCBI
|
|
22
|
Shen W, Hu JA and Zheng JS: Mechanism of
temozolomide-induced antitumour effects on glioma cells. J Int Med
Res. 42:164–172. 2014. View Article : Google Scholar
|
|
23
|
Tupper DE and Wallace RB: Utility of the
neurological examination in rats. Acta Neurobiol Exp (Wars).
40:999–1003. 1980.
|
|
24
|
Victorov IV, Prass K and Dirnagl U:
Improved selective, simple, and contrast staining of acidophilic
neurons with vanadium acid fuchsin. Brain Res Brain Res Protoc.
5:135–139. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shen G, Shen F, Shi Z, Liu W, Hu W, Zheng
X, Wen L and Yang X: Identification of cancer stem-like cells in
the C6 glioma cell line and the limitation of current
identification methods. In Vitro Cell Dev Biol Anim. 44:280–289.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Barth RF and Kaur B: Rat brain tumor
models in experimental neurooncology: The C6, 9L, T9, RG2, F98,
BT4C, RT-2 and CNS-1 gliomas. J Neurooncol. 94:299–312. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hirst TC, Vesterinen HM, Sena ES, Egan KJ,
Macleod MR and Whittle IR: Systematic review and meta-analysis of
temozolomide in animal models of glioma: Was clinical efficacy
predicted? Br J Cancer. 108:64–71. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Steinbach JP, Wolburg H, Klumpp A, Probst
H and Weller M: Hypoxia-induced cell death in human malignant
glioma cells: Energy deprivation promotes decoupling of
mitochondrial cytochrome c release from caspase processing and
necrotic cell death. Cell Death Differ. 10:823–832. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang L, Lin C, Wang L, Guo H and Wang X:
Hypoxia and hypocia-inducible factors in glioblastoma multiforme
progression and therapeutic implications. Exp Cell Res.
318:2417–2426. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang MH, Hu YD, Xu Y, Xiao Y, Luo Y, Song
AC and Zhou J: Human mesenchymal stem cells enhance autophagy of
lung carcinoma cells against apoptosis during serum deprivation.
Int J Oncol. 42:1390–1398. 2013.PubMed/NCBI
|
|
31
|
Hou L, Wang X, Zhou Y, Ma H, Wang Z, He J,
Hu H, Guan W and Ma Y: Inhibitory effect and mechanism of
mesenchymal stem cells on liver cancer cells. Tumour Biol.
35:1239–1250. 2014. View Article : Google Scholar
|
|
32
|
Li Q, Wijesekera O, Salas SJ, Wang JY, Zhu
M, Aprhys C, Chaichana KL, Chesler DA, Zhang H, Smith CL, et al:
Mesenchymal stem cells from human fat engineered to secrete BMP4
are nononcogenic, suppress brain cancer, and prolong survival. Clin
Cancer Res. 20:2375–2387. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Baraniak PR and McDevitt TC: Stem cell
paracrine actions and tissue regeneration. Regen Med. 5:121–143.
2010. View Article : Google Scholar :
|
|
34
|
Bryukhovetskiy A, Bryukhovetskiy I,
Chekhonin V and Baklaushev V: Experimental cytoregulatory therapy
of brain glial tumors with cell system of hematopoietic precursors
epigenetically reprogrammed of apoptosis induction: Victory in
vitro and failure in vivo. J Neurol. 257(Suppl 1): S152–S153.
2010.
|
|
35
|
Liu J, Zhang Y, Bai L, Cui X and Zhu J:
Rat bone marrow mesenchymal stem cells undergo malignant
transformation via indirect co-cultured with tumour cells. Cell
Biochem Funct. 30:650–656. 2012. View
Article : Google Scholar : PubMed/NCBI
|