Introduction
CD83 is a type I transmembrane glycoprotein with a
highly glycosylated N-terminal ectodomain and a short C-terminal
intracellular domain. Being absent from the majority of resting
cells, CD83 is predominantly induced on the surface of mature
dendritic cells (mDCs) and activated T and B lymphocytes (1–3).
Increasing evidence has demonstrated the significant regulatory
roles of CD83 in the central and peripheral immune system (4–14).
CD83 was demonstrated to be essential for the lineage commitment of
CD4+ T cells in the thymus (4). A previous study has demonstrated that
membrane CD83 (mCD83) promotes the expression of major
histocompatability complex (MHC) class II and CD86 on mDCs by
inhibiting membrane-associated RING-CH1 (MARCH1)-dependent
ubiquitination and degradation of the two target molecules
(5). The co-stimulatory effects of
mCD83 on mDCs per se for CD4+ T cells have remained
controversial (4,6–8).
However, soluble CD83 (sCD83), which is produced predominantly by
ectodomain shedding, has clear suppressive effects in vitro
and in vivo (9–14). A previous study by our group
demonstrated that sCD83 suppresses T-cell proliferation and the
secretion of interleukin (IL)-2 and interferon (IFN)-γ through
prostaglandin E2 (PGE2) produced by monocytes (15).
A previous study demonstrated that native or forced
expression of CD83 confers an immunosuppressive function to
CD4+ T cells (16).
However, a previous study using short hairpin (sh)RNA-mediated gene
silencing of CD83 on CD4+ T cells revealed a reduced
proliferation and lower production of IL-2 and IL-17 by the
CD4+ T cells, indicating that CD83 serves as a positive
co-stimulator for CD4+ T cells (17). It was noted that genetic
manipulation may cause unintended effects to the target cells. On
the other hand, modified expression of CD83 is likely paralleled by
concurrent changes of co-stimulatory molecules on CD4+ T
cells, since CD83 is an important regulator of MHC class II and the
expression of CD86 (5). Therefore,
the biological and functional definition of the expression of CD83
on CD4+ T cells remains to be elucidated.
In the present study, the expression of CD83 on
CD4+ T cells was assessed. The effects of stimulation
with a (TGF)-β on the expression of CD83 on CD4+ T cells
as well as on their differentiation into
CD4+CD25+ forkhead box (Fox)
P3+-induced regulatory T (iTreg) cells were
investigated.
Materials and methods
Lymphocyte purification and cell
culture
Usin Ficoll-Hypaque density gradient centrifugation
at 900 × mononuclear cells were isolated from the blood of healthy
donors who provided written informed consent. This study was
approved by the Ethics Committee of the Second Hispital of Anhui
Medical University (Hefei, China). The mononuclear cell suspension
(8 ml) was added into a T-25 culture flask and incubated at 37°C
with 5% CO2 for 2 h. The cells were gently agitated, the
non-adherent cells were aspirated, and adherent monocytes and B
cells were discarded. Alternatively, untouched CD4+ T
cells were purified from mononuclear cells using a CD4+
T-cell isolation kit (cat. no. 130-096-533) and magnetic columns
(cat. no. 130-042-306) (both from Miltenyi Biotech, Bergisch
Gladbach, Germany). This procedure routinely provided >95% pure
CD4+ T cells. Non-adherent lymphocytes or purified
CD4+ cells were cultured in RPMI-1640 containing 10%
fetal bovine serum, 10 mM
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid and 1%
penicillin-streptomycin (all purchased from Invitrogen Life
Technologies, Carlsbad, CA, USA) at 1×106 cells/ml in
24-well plates, in the presence of pre-coated agonistic murine anti
human CD3 monoclonal antibody (mAb; clone UCHT-1; cat. no. 555329;
0.5 µg/ml) and soluble agonistic murine anti human CD28 mAb
(clone ANC28.1/5D10; cat. no. 177–820; 1.0 µg/ml), purchased
from BD Biosciences, Franklin Lakes, NJ, USA and Ancell, Bayport,
MN, USA, respectively. To assess the importance of TGF-β regulation
on the expression of CD83 on CD4+ T cells, a final
concentration of 2 ng/ml exogenous TGF-β (Sigma-Aldrich, St. Louis,
MO, USA) was added to the cultures at day 0. All cells were
harvested 1, 2 or 3 days following incubation for flow cytometry
and confocal immunofluorescence microscopy analysis.
In vitro cell proliferation assay and
cytokine detection
As mentioned above, purified CD4+ T cells
were collected and stimulated with agonistic anti-CD3/CD28 at
105/ml in 96-well plates. Determination of cell
proliferation was performed 1, 2 and 3 days later using a Cell
Counting kit-8 (Dojindo, Kumamoto, Japan), according to the
manufacturer's instructions. Cell viability was determined by
measuring the absorbance at 450 nm using a multiwell scanning
spectrophotometer (KHB ST-360; Kehua Bio-Engineering, Shanghai,
China). The experiments were repeated in triplicate wells. The
levels of IL-2 and IFN-γ in CD4+ T-cell culture
supernatants were measured in duplicate for each of the serial
aliquots using a Human IL-2 Quantikine ELISA kit (cat. no. D2050)
and Human IFN-γ Quantikine ELISA kit (cat. no. DIF50), purchased
from R&D Systems (Minneapolis, MN, USA), according to the
manufacturer's instructions.
Flow cytometric analysis
Non-adherent lymphocytes or purified CD4+
T cells were collected at 0, 1, 2 and 3 days following stimulation,
and the cells were stained with phycoerythrin (PE)-conjugated
anti-CD83, fluorescein isothiocyanate (FITC)-conjugated anti-CD4 or
FITC-conjugated anti-CD25 (All from BioLegend, San Diego, CA, USA)
and incubated on ice for 30 min in the dark. The controls were
prepared using FITC- or PE-conjugated isotype controls. The
intracellular staining of TGF-β-treated CD4+ T cells
with anti-Foxp3 mAb was performed using an anti-human Foxp3
staining set (eBioscience, San Diego, CA, USA) according to the
manufacturer's instructions. The cells were subsequently analyzed
using a flow cytometer (Beckman Coulter Epics XL; Beckman Coulter,
Miami, FL, USA) by using a two-color acquisition method and the
data were analyzed by using FlowJo 7.6.1 software (Treestar, Inc.,
Ashland, OR, USA).
Confocal immunofluorescence
microscopy
Following stimulation with anti-CD3/CD28 for 2 days,
purified CD4+ T cells were washed twice with ice-cold
phosphate-buffered saline (PBS) and fixed in 4% (w/v)
paraformaldehyde (Sigma-Aldrich) in PBS for 15 min at room
temperature (20°C). The cells were subsequently stained with
FITC-conjugated anti-CD25 mAb and PE-conjugated anti-CD83 mAb, and
incubated on ice for 30 min in the dark. Fluorescently-labeled
cells were observed and recorded by an examiner, in a blinded
manner, using a Zeiss LSM 410 inverted laser scan microscope (Carl
Zeiss, Oberkochen, Germany) equipped with Ar488, Kr568 and HeNe633
lasers. Non-specific fluorescence was assessed by incubating cells
with FITC- or PE-conjugated isotype controls.
Statistical analysis
Values presented in the figures are representative
of at least three independent experiments and are expressed as the
mean± standard derivation. Comparisons between the expression
levels of CD83 at days 1, 2 and 3 were determined by Student's
t-test with SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Time-dependent expression of CD83 on
CD4+ T cells is stimulated by anti-CD3/CD28
To assess the time-dependent kinetics of the
expression of CD83 on CD4+ T cells, non-adherent
lymphocytes isolated from mononuclear cells were stimulated with
agonistic anti-CD3/CD28 and the expression of CD83 on
CD4+ T cells was determined by flow cytometry 1, 2 and 3
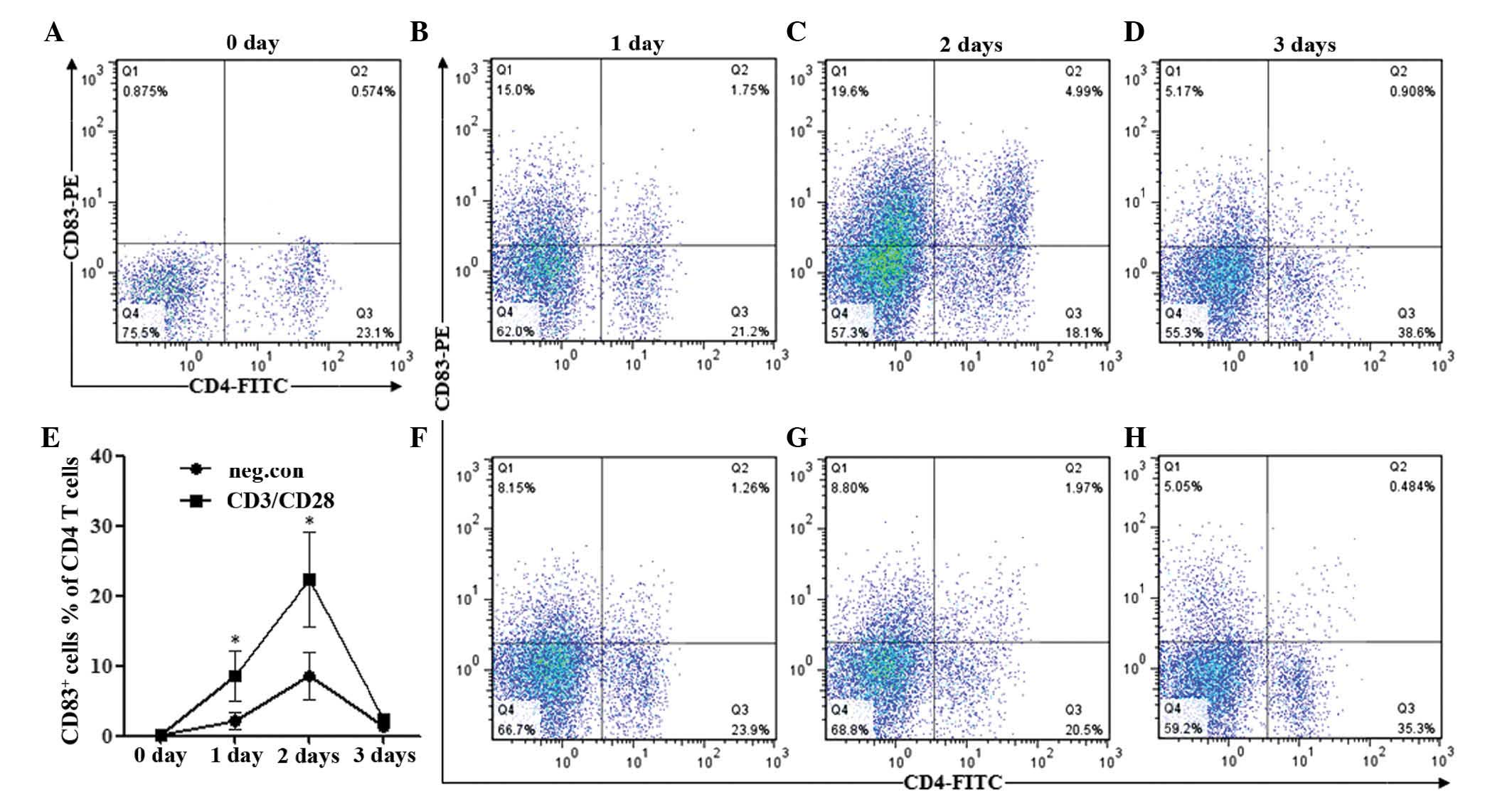
days following stimulation. As shown in Fig. 1A, CD83 was rarely expressed on
resting CD4+ T cells (0 days), whereas the percentages
of CD83-positive cells in the total CD4+ T cells were
significantly upregulated at day 1 (7.69%) and markedly increased
to 21.61% by day 2 (Fig. 1B and
C). However, the activation-induced expression of CD83 on
CD4+ T cells decreased significantly to 2.30% at day 3
(Fig. 1D). By contrast,
unstimulated CD4+ T cells demonstrated less severe
increases in the expression of CD83 compared with those in the
stimulated group on days 1 and 2, and on day 3, CD83 were similar
to those in the stimulated group (Fig.
1E–H). The differences in the percentage of CD83+
cells (with regard to the total CD4+ T-cell population)
between the anti-CD3/CD28-stimulated and the unstimulated group
were statistically significant (P<0.05) at days 1 and 2
(Fig. 1E).
Purified CD4+ T cells were used to
confirm the time-dependent surface expression of CD83. As shown in
Fig. 2A–D, anti-CD3/CD28
stimulation of CD4+ T cells led to an upregulation of
CD83 on days 1 and 2 and substantially low levels of CD83 on
CD4+ T cells on day 3. The time-dependent kinetics were
consistent with those observed in non-adherent lymphocytes. These
data suggested that CD83 was significantly upregulated; however, it
was transiently presented on CD4+ T cells activated by
the canonical CD3/CD28 signal.
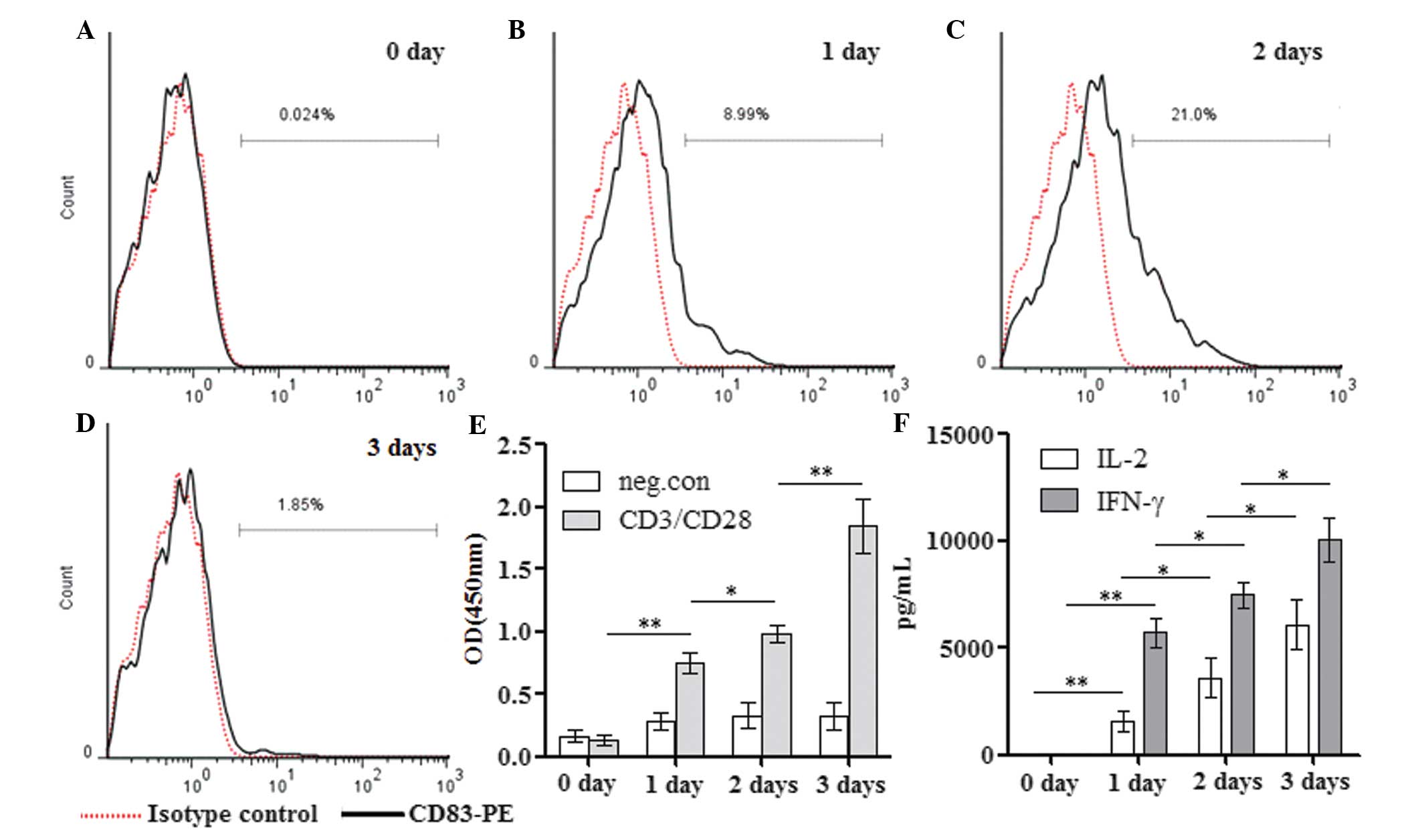 | Figure 2Time-dependent expression of CD83,
proliferation and the production of IL-2 and IFN-γ in
CD4+ T cells. Following purity identification, the
purified CD4+ T cells were either (A) directly stained
with PE-labeled CD83 or (B–D) stimulated with anti-CD3/CD28 for 1–3
days, followed by staining with PE-labeled CD83. All samples were
analyzed by flow cytometry and the typical flow cytometric
histograms indicating the percentages of CD83-positive cells are
shown. (E) Additionally, cell proliferation at days 1, 2 and 3 were
determined using Cell Counting Kit 8. (F) The levels of IL-2 and
IFN-γ in the supernatants from 1-, 2- and 3-day cultures of
activated CD4+ T cells were analyzed by ELISA. Values
are expressed as the mean ± standard deviation of three independent
experiments (**P<0.01; *P<0.05). PE,
phycoerythrin; CD, cluster of differentiation; IFN, interferon; IL,
interleukin; neg.con, negative control. |
Decreased expression of CD83 is not
caused by a reduced proliferation or activation of CD4+
T cells
The present study next determined whether the
decreased expression of CD83 on day 3 was a result of reduced
proliferation or activation of target CD4+ T cells.
Purified CD4+ T cells were stimulated with anti-CD3/CD28
for 1, 2 and 3 days, and cell proliferation as well as the levels
of IL-2 and IFN-γ in culture supernatants were analyzed. This
experiment paralleled the detection of the expression of CD83 on
CD4+ T cells as mentioned above. By contrast to
fluctuated surface expression of CD83, the proliferation of
CD4+ T cells stimulated by anti-CD3/CD28 was
continuously increased from days 1 to 3 (Fig. 2E). Similarly, progressively
increased secretion of IL-2 and IFN-γ was observed in
CD4+ T cells (Fig. 2F).
Therefore, the notable decrease in the surface expression of CD83
at day 3 was not a result of reduced proliferation or the
activation of CD4+ T cells.
Co-localization of CD83 and CD25 on
activated CD4+ T cells
Previous studies have reported that the mRNA
expression of CD83 was predominantly in the naturally occurring
CD4+CD25+ T (nTreg) cells, which rapidly
expressed large quantities of surface CD83 upon activation
(16). It was therefore of
interest to determine whether CD83 is co-localized with CD25 on
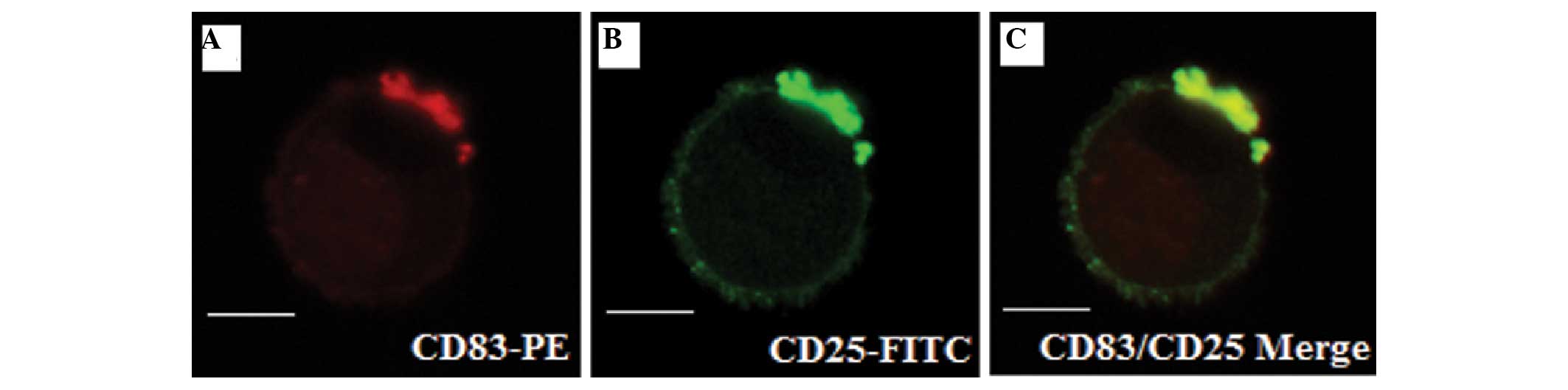
activated CD4+ T cells. Purified CD4+ T cells
were stimulated with anti-CD3/CD28 for 2 days, double-labeled with
CD25-FITC/CD83-PE and observed by immunofluorescence microscopy.
The results demonstrated that CD83 and CD25 were accumulated on the
surface of activated CD4+ T cells (Fig. 3A and B). Furthermore, merging of
the two images revealed a high degree of co-localization between
CD83 and CD25 on the surface of the activated CD4+ T
cells (Fig. 3C). These results
suggested that there was a significant correlation between the
expression levels of CD83 and CD25 on anti-CD3/CD28-stimulated
CD4+ T cells.
TGF-β restores the expression of CD83 on
CD4+ T cells
Induced
CD4+CD25+FoxP3+ T cells represent
an important sub-type of CD4+ T cells, termed iTreg
cells, which are instrumental in the maintenance of immunological
tolerance (18). The highly
co-localized expression of CD83 and CD25 on CD4+ T cells
suggests that the expression of CD83 is closely associated with the
differentiation of CD4+ T cells into iTreg cells upon
activation. The present study aimed to determine whether the sudden
decrease in the expression of CD83 on day 3 was due to the
differentiation of CD4+ T cells, since the canonical
CD3/CD28 signal rarely drives the generation of iTreg cells. With
this suggestive evidence, purified CD4+ T cells were
stimulated with anti-CD3/CD28 in the presence or absence of TGF-β,
an immunosuppressive cytokine, which controls the balance between
iTreg cells and pathogenic effector T cells (19). The expression of CD83 on
CD4+ T cells was detected 3 days later using flow
cytometry. In addition, the differentiation of CD4+ T
cells triggered by TGF-β signaling was identified by co-expression
analysis of FoxP3 and CD25. The experiments demonstrated that TGF-β
signaling increased the percentage of CD83-postive cells in total
CD4+ T cells to 12.1% on day 3, while stimulation
without TGF-β only resulted in 1.78% CD38-positive cells (Fig. 4A–C). The difference was
statistically significant according to Student's t-test (P=0.0006;
Fig. 4D). Furthermore, it was
observed that, in contrast to anti-CD3/CD28 stimulation, the
combination with TGF-β induced a significant increase in the
co-expression of FoxP3 and CD25 on CD4+ T cells on day 3
(Fig. 4E–G). In conclusion, these
findings demonstrated that the decreased surface expression of CD83
on activated CD4+ T cells can be restored by TGF-β,
which simultaneously drives the differentiation of CD4+
T cells into iTreg cells.
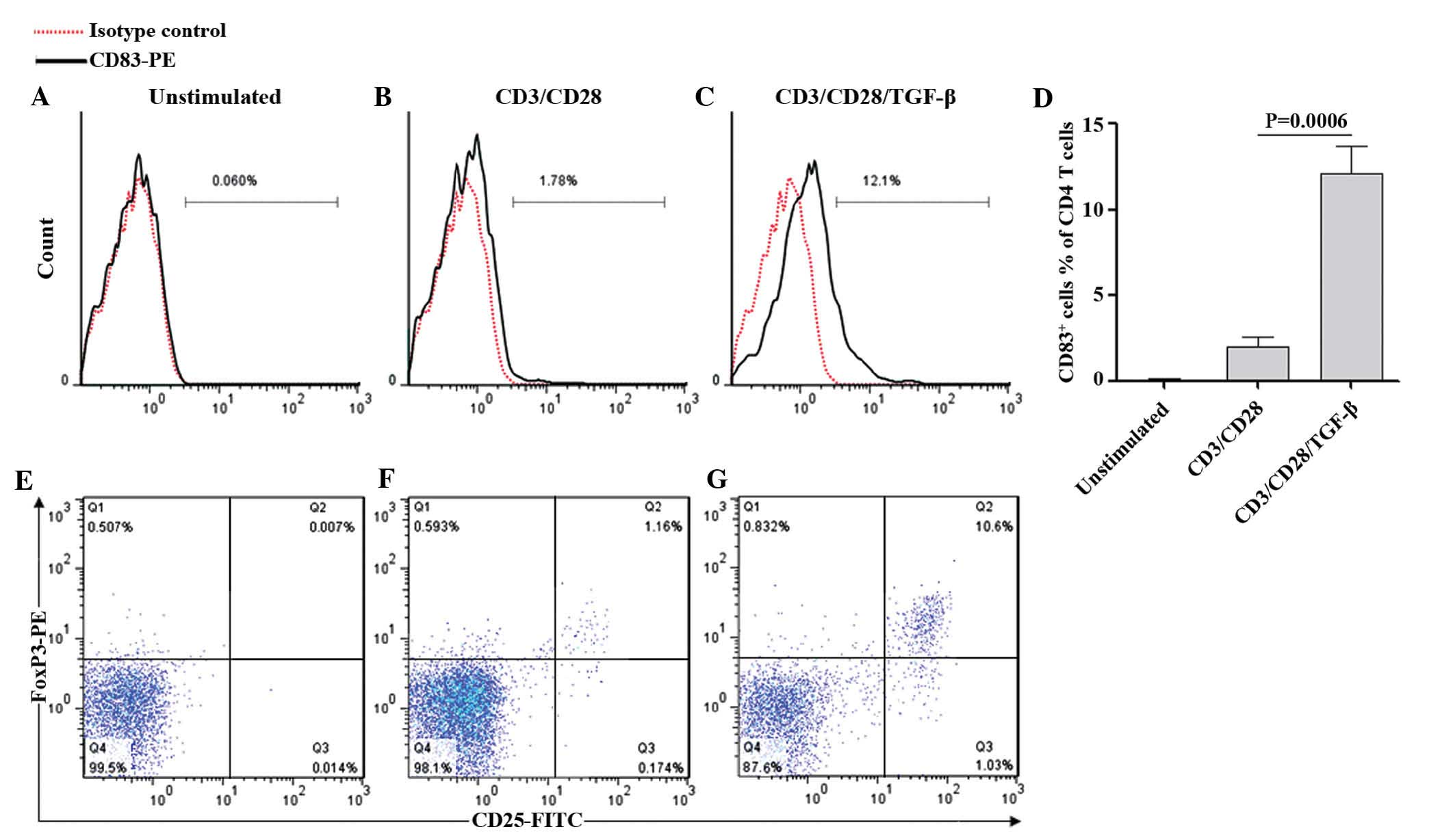 | Figure 4TGF-β restored the expression of CD83
in CD4+ T cells. Purified human CD4+ T cells
were (A) left unstimulated or (B and C) stimulated with
anti-CD3/CD28 in the presence or absence of TGF-β. Following 3 days
of incubation, all samples were collected, stained with PE-labeled
anti-CD83 and analyzed by flow cytometry. The typical flow
cytometric histograms indicating the percentages of CD83-positive
cells are shown in A, B and C. (D) Quantification of the number of
CD83-positive cells in the total CD4+ T cell population.
Values are expressed as the mean ± standard deviation of four
independent experiments and P-values were calculated by Student's
t-test. Additionally, the (E) unstimulated, (F) anti-CD3/CD28- and
(G) anti-CD3/CD28/TGF-β-treated CD4+ T cells were
stained with FITC-labeled anti-CD25, followed by intracellular
FoxP3-PE staining and analyzed by flow cytometry. The data
demonstrated representative data for the donors in A–C and E–G.
FITC, fluorescein isothiocyanate; PE, phycoerythrin; CD, cluster of
differentiation, TGF, transforming growth factor; Fox, forkhead
box. |
Discussion
A growing body of in vitro and in vivo
evidence has demonstrated an immunosuppressive role of sCD83 in T
cell-mediated immunity (9–14). However, the effect of mCD83
expressed on antigen-presenting cells (APCs), including dendritic
cells and B lymphocytes, remains a matter of controversy (4,6–8).
Similarly, CD83 expressed on the surface of CD4+ T cells
poses a novel challenge to elucidate the biological and functional
behavior of mCD83. The present study demonstrated that CD83 was
expressed in a time-dependent manner on activated human
CD4+ T cells, which reached the maximum at day 2 and
decreased significantly on day 3. These time-dependent kinetics of
the expression of CD83 are consistent with observations using
CD4+ T cells isolated from BALB/c mice and stimulated
with anti-CD3 and IL-2 in the presence of irradiated CD4-depleted
splenocytes as APCs (16). The
decreased expression of CD83 at day 3 was not a result of reduced
proliferation or activation of CD4+ T cells, in that
IL-2 and IFN-γ production was sustained until day 3. Therefore,
these findings demonstrated the fine-tuning of activation-induced
expression of CD83 on CD4+ T cells. Of note, non-CD4
cells in non-adherent lymphocytes expressed considerable quantities
of surface CD83. It was suggested that this synchronous
presentation of CD83 originates from CD8+ T cells and
remaining B cells upon activation (20,21).
On the other hand, unstimulated CD4+ T cells also
expressed certain quantities of surface CD83, which may be
explained by the spontaneous activation of undepleted monocytes in
non-adherent lymphocytes, which in turn stimulate and activate
CD4+ T cells.
Upon T-cell receptor (TCR)-mediated cell activation,
naïve CD4+ T cells differentiate into at least four
major lineages (Th1, Th2, Th17 and iTreg cells), which can be
distinguished by their specialized expression profiles, unique
cytokine production and effector functions (22). The present study suggested that
differentiation of CD4+ T cells, which follows cell
activation, may be responsible for the downregulation of CD83 on
CD4+ T cells stimulated by the canonical CD3/CD28
signal. By using a CD83 reporter mouse expressing enhanced green
fluorescent protein under the control of the CD83 promoter, a
previous study has demonstrated that CD83 was predominantly
identified in CD4+CD25+ and in CD4 memory
cells (21). Similarly, an in
vitro study revealed that the mRNA expression levels of CD83
were differentially expressed in nTreg cells, which rapidly
expressed large quantities of surface CD83 upon activation
(16). In this context, the
present study examined the spatial positions of CD83 and CD25 on
activated CD4+ T cells by confocal microscopy. Of note,
co-localization of CD83 and CD25 on the surface of activated
CD4+ T cells was observed. CD25 is the α-chain of the
IL-2 receptor, and an IL-2 signal is essential for the
differentiation, expansion and function of Tregs (23,24).
The function of highly co-localized presentation of CD83 and CD25
remains to be elucidated; however, it may be associated with the
stabilization of surface CD25 on target cells by CD83. A previous
study has demonstrated that the transmembrane domain of CD83
inhibited the activity of membrane-associated ring finger (C3HC4)
1, E3 ubiquitin protein ligase (MARCH1), a member of the MARCH
family of membrane-bound E3 ubiquitin ligases, which ubiquitinate
and downregulate cell surface molecules (5).
Numerous studies have demonstrated that the presence
of TGF-β at the onset of cell cultures can drive naïve
CD4+ T cells to differentiate into iTreg cells (19,25).
The present study therefore investigated the effects of TGF-β
regulation on the expression of CD83 on CD4+ T cells.
The findings support the evidence that the addition of TGF-β can
restore the surface expression of CD83 on purified CD4+
T cells stimulated with anti-CD3/CD28 for 3 days. In addition, the
combined TGF-β stimulation drove CD4+ T cells towards a
phenotype of CD4+CD25+FoxP3+ iTreg
cells. As mentioned above, the close association between CD83 and
iTreg cells was also demonstrated to be present in nTreg cells,
which develop in the thymus and constitutively express CD25
(16,21). These observations may have
important implications for CD83 regulation on Treg cells.
Therefore, it is conceivable that the sustained presentation of
CD83 on CD4+ Treg sub-sets is required for Treg
induction, differentiation, survival or functional maintenance.
Defining the factors which regulate the expression of CD83 and
understanding the mechanisms of CD83 regulation on regulatory T
cells may facilitate the development of Treg cells and novel
therapeutic strategies in immune intervention.
In conclusion, the results of the present study
suggested that CD83 is presented in a time-dependent manner on
CD4+ T cells stimulated by the canonical CD3/CD28
signal. The maximum expression of CD83 expression was observed at
day 2 and was followed by a marked decrease at day 3. Of note, the
addition of TGF-β at the onset of stimulation, which drove the
differentiation of CD4+CD25+FoxP3+
Tregs, restored the expression of CD83 on day 3. Furthermore, CD83
was highly co-localized with CD25, as observed by fluorescence
microscopy. Therefore, the present study suggested that the
continuous expression of CD83 on activated human CD4+ T
cells was correlated with their differentiation into iTreg cells.
Establishing the functional connection between the expression of
CD83 and iTregs may facilitate the further elucidation of iTregs
and their clinical application.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (no. 81171662), the Natural Science
Foundation of Anhui Province (no. 1408085MH169) and the Scientific
Research Foundation for the Doctoral Program of the Second Hospital
of Anhui Medical University (no. 2012BKJ011).
References
|
1
|
Zhou LJ, Schwarting R, Smith HM and Tedder
TF: A novel cell-surface molecule expressed by human
interdigitating reticulum cells, Langerhans cells and activated
lymphocytes is a new member of the Ig superfamily. J Immunol.
149:735–742. 1992.PubMed/NCBI
|
|
2
|
Zhou LJ and Tedder TF: CD14+
blood monocytes can differentiate into functionally mature
CD83+ dendritic cells. Proc Natl Acad Sci USA.
93:2588–2592. 1996. View Article : Google Scholar
|
|
3
|
Stein MF, Lang S, Winkler TH, et al:
Multiple interferon regulatory factor and NF-κB sites cooperate in
mediating cell-type- and maturation-specific activation of the
human CD83 promoter in dendritic cells. Mol Cell Biol.
33:1331–1344. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fujimoto Y, Tu L, Miller AS, Bock C,
Fujimoto M, Doyle C, Steeber DA and Tedder TF: CD83 expression
influences CD4+ T cell development in the thymus. Cell.
108:755–767. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tze LE, Horikawa K, Domaschenz H, et al:
CD83 increases MHC II and CD86 on dendritic cells by opposing
IL-10-driven MARCH1-mediated ubiquitination and degradation. J Exp
Med. 208:149–165. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Prechtel AT, Turza NM, Theodoridis AA and
Steinkasserer A: CD83 knockdown in monocyte-derived dendritic cells
by small interfering RNA leads to a diminished T cell stimulation.
J Immunol. 178:5454–5464. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kretschmer B, Lüthje K, Ehrlich S,
Osterloh A, Piedavent M, Fleischer B and Breloer M: CD83 on murine
APC does not function as a costimulatory receptor for T cells.
Immunol Lett. 120:87–95. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pinho MP, Migliori IK, Flatow EA and
Barbuto JA: Dendritic cell membrane CD83 enhances immune responses
by boosting intracellular calcium release in T lymphocytes. J
Leukoc Biol. 95:755–762. 2014. View Article : Google Scholar
|
|
9
|
Dudziak D, Nimmerjahn F, Bornkamm GW and
Laux G: Alternative splicing generates putative soluble CD83
proteins that inhibit T cell proliferation. J Immunol.
174:6672–6676. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bock F, Rössner S, Onderka J, et al:
Topical application of soluble CD83 induces IDO-mediated immune
modulation, increases Foxp3+ T cells and prolongs allogeneic
corneal graft survival. J Immunol. 191:1965–1975. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Starke C, Steinkasserer A, Voll RE and
Zinser E: Soluble human CD83 ameliorates lupus in NZB/W F1 mice.
Immunobiology. 218:1411–1415. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yuan Y, Wan L, Chen Y, et al: Production
and characterization of human soluble CD83 fused with the fragment
crystallizable region of human IgG1 in Pichia pastoris. Appl
Microbiol Biotechnol. 97:9409–9417. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eckhardt J, Kreiser S, Döbbeler M, et al:
Soluble CD83 ameliorates experimental colitis in mice. Mucosal
Immunol. 7:1006–1018. 2014.PubMed/NCBI
|
|
14
|
Guo Y, Li R, Song X, et al: The Expression
and characterization of functionally active soluble CD83 by pichia
Pastoris using high-density fermentation. PLoS One. 9:e892642014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen L, Zhu Y, Zhang G, Gao C, Zhong W and
Zhang X: CD83-stimulated monocytes suppress T-cell immune responses
through production of prostaglandin E2. Proc Natl Acad Sci USA.
108:18778–18783. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Reinwald S, Wiethe C, Westendorf AM,
Breloer M, Probst-Kepper M, Fleischer B, Steinkasserer A, Buer J
and Hansen W: CD83 expression in CD4+ T cells modulates
inflammation and autoimmunity. J Immunol. 180:5890–5897. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Su LL, Iwai H, Lin JT and Fathman CG: The
transmembrane E3 ligase GRAIL ubiquitinates and degrades CD83 on
CD4+ T cells. J Immunol. 183:438–444. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kumar S, Naqvi RA, Ali R, Rani R, Khanna N
and Rao DN: CD4+CD25+ T regs with acetylated
FoxP3 are associated with immune suppression in human leprosy. Mol
Immunol. 56:513–520. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen W, Jin W, Hardegen N, Lei KJ, Li L,
Marinos N, McGrady G and Wahl SM: Conversion of peripheral
CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta
induction of transcription factor Foxp3. J Exp Med. 198:1875–1886.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kretschmer B, Kühl S, Fleischer B and
Breloer M: Activated T cells induce rapid CD83 expression on B
cells by engagement of CD40. Immunol Lett. 136:221–227. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lechmann M, Shuman N, Wakeham A and Mak
TW: The CD83 reporter mouse elucidates the activity of the CD83
promoter in B, T and dendritic cell populations in vivo. Proc Natl
Acad Sci USA. 105:11887–11892. 2008. View Article : Google Scholar
|
|
22
|
Yamane H and Paul WE: Early signaling
events that underlie fate decisions of naive CD4(+) T
cells toward distinct T-helper cell subsets. Immunol Rev.
252:12–23. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu A and Malek TR: Selective availability
of IL-2 is a major determinant controlling the production of
CD4+CD25+Foxp3+ T regulatory
cells. J Immunol. 177:5115–5121. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Barron L, Dooms H, Hoyer KK, Kuswanto W,
Hofmann J, O'Gorman WE and Abbas AK: Cutting edge: mechanisms of
IL-2-dependent maintenance of functional regulatory T cells. J
Immunol. 185:6426–6430. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rao PE, Petrone AL and Ponath PD:
Differentiation and expansion of T cells with regulatory function
from human peripheral lymphocytes by stimulation in the presence of
TGF-β. J Immunol. 174:1446–1455. 2005. View Article : Google Scholar : PubMed/NCBI
|