Introduction
Multiple myeloma is a plasma cell neoplasm,
characterized by the overgrowth of mature antibody-producing plasma
cells in the bone marrow. Multiple myeloma ranks as the second most
common type of hematologic malignancy, following non-Hodgkin's
lymphoma, with >20,000 new cases diagnosed annually in the USA.
Despite advances in the treatment of multiple myeloma, the disease
remains incurable, with a median survival rate of between 3 and 5
years (1). Patients with multiple
myeloma eventually succumb to recurrent or refractory disease;
therefore, there is an urgent requirement for the identification of
novel effective agents for the treatment of multiple myeloma.
Apoptosis, also termed programmed cell death, is
morphologically distinct from necrotic cell death. Apoptotic cells
typically exhibit chromatin condensation, the formation of
apoptotic bodies and an intact cell membrane (2). There are two predominant apoptotic
pathways: The extrinsic, or death receptor, pathway and the
intrinsic, or mitochondrial, pathway (3). These two pathways converge on the
activation of caspases, which are responsible for the execution of
apoptosis through the cleavage of structural and regulatory
cellular proteins and nuclear DNA. The mitochondrial pathway is
primarily regulated by members of the B-cell lymphoma 2 (Bcl-2)
family, which comprises pro-apoptotic proteins, including
Bcl-2-associated X protein (Bax), and anti-apoptotic proteins,
including Bcl-2 and Bcl-extra large (Bcl-XL) (4). Resistance to apoptosis is important
in the pathogenesis of multiple myeloma, which involves the
activation of multiple survival pathways, including nuclear
factor-κB (NF-κB), Janus kinase 2/signal transducers and activators
of transcription (3), and
phosphoinositide 3-kinase/Akt (5).
Ginseng, the root of Panax ginseng C.A.
Meyer, is a herbal plant, which has received increasing attention
due to its various pharmacological properties, including antitumor
(6) and antioxidant properties
(7). Ginsenoside Rg3 is one of the
predominant constituents isolated from Panax ginseng, which
exhibits cytotoxic effects against a wide range of cancer cells
(8,9). Keum et al (10) reported that the antitumor activity
of ginsenoside Rg3 in HL-60 human pro-myelocytic leukemia cells is
partially mediated through the downregulation of NF-κB. In colon
(8) and prostate (9) cancer cells, ginsenoside Rg3 is also
capable of suppressing the activation of NF-κB, consequently
enhancing the susceptibility of cancer cells to chemotherapeutic
drugs (10). Despite evidence for
the anticancer activity of ginsenoside Rg3, its actions in multiple
myeloma remain to be fully elucidated.
The present study aimed to determine the effects of
ginsenoside Rg3 treatment on human multiple myeloma cells, and
investigate the possible underlying molecular mechanisms.
Materials and methods
Reagents
Ginsenoside Rg3, with a purity of >98%, was
purchased from Jilin Yatai Pharmaceutical Company (Changchun,
China). Dimethyl sulfoxide (DMSO) and 4′,6-diamidino-2-phenylindole
(DAPI) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Fetal bovine serum (FBS) was purchased from GE Healthcare Life
Sciences (Logan, UT, USA), RPMI 1640 medium was purchased from
Invitrogen Life Technologies (Carlsbad, CA, USA), the Annexin V/PE
Apoptosis Assay kit was obtained from Shenzhen Genmed Biological
Company (Shenzhen, China) and the Caspase-3 Activity Assay kit was
purchased from EMD Millipore (Billerica, MA, USA). Mouse monoclonal
antibodies targeting Bax (mouse; cat no. Ab122; 1:800) and GAPDH
were purchased from Beyotime Institute of Biotechnology (Shanghai,
China). Horseradish peroxidase-conjugated goat anti-mouse
immu-noglobulin G (cat. no. ZDR-5307; 1:1,000) was obtained from
Beijing Zhongshan-Golden Bridge Biological Company (Beijing,
China).
Cells and culture
The U266 and RPMI8226 human multiple myeloma cells
were obtained from the Cell Bank of Chinese Academy of Sciences
(Shanghai, China) and cultured in RPMI 1640 medium, supplemented
with 10% heat-inactivated FBS, 1% penicillin/streptomycin (Thermo
Fisher Scientific, Waltham, MA, USA). The cells were maintained in
a humidified incubator in 5% CO2 and 95% air at 37°C,
and were passaged every 3–4 days.
Small interfering (si)RNA
transfection
The U266 cells were seeded into six-well plates at a
confluence of 40%. The cells were subsequently transfected with
siRNA using Lipofectamine 2000 (Invitrogen Life Technologies),
according to the manufacturer's instructions.
Cell proliferation assay
The effects of ginsenoside Rg3 on cell proliferation
were determined using an MTT assay (Beyotime Institute of
Biotechnology, Shanghai, China). Briefly, the U266 cells or
RPMI8226 cells were seeded into 96-well plates at a density of
1×104 cells/well (100 µl) and were exposed to
ginsenoside Rg3 at different concentrations (20, 40 or 80
µM) in RPMI 1640 medium supplemented with 10% FBS. Control
cells were treated with the medium only. Following treatment for 48
h at 37°C, MTT solution at a final concentration of 0.5 mg/ml was
added to the cells in each well. After 2 h incubation at 37°C, 150
µl DMSO was added to each well, in order to dissolve the
blue formazan crystals. The optical densities (OD) of the cells
were measured at a wavelength of 545 nm, with a reference
wavelength of 650 nm. The cell proliferation inhibitory rate was
calculated using the following formula: Inhibitory rate (%)= (1 -
mean OD of experiment group / mean OD of control group) × 100.
Analysis of apoptosis using
annexin-V-fluorescein isothio-cyanate (FITC)/propidium iodide (PI)
with flow cytometry
The U266 cells, exposed to various concentrations of
ginsen-oside Rg3 for 48 h, were harvested by trypsinization and
washed twice with cold PBS. The cells were then centrifuged at 200
x g for 5 min and the cell pellet was resuspended in 1X binding
buffer, containing 10 mM HEPES, 140 mM NaCl and 2.5 mM
CaCl2, at a density of 1×106 cells/ml. The
sample solution (100 µl) was transferred to a 5 ml culture
tube and incubated with FITC-conjugated annexin V and PI for 15 min
at room temperature in the dark. The 1X binding buffer (400
µl) was added to each sample tube, and the samples were
analyzed on a FACScan flow cytometer (BD Biosciences, San Jose, CA,
USA) using Cell Quest Research software version 3.3 (BD
Biosciences).
Western blot analysis
Following treatment with ginsenoside Rg3, the cells
were lysed in lysis buffer, containing50 mmol/l tris (pH 7.4), 150
mmol/l NaCl; 1% NP-40 and 0.1% SDS (Sangon Biotech, Inc.),
supplemented with 1X protease and phosphatase inhibitor cocktail
(Roche Diagnostics, Indianapolis, IN, USA). The protein samples
were then separated on polyacrylamide gels containing 0.1% SDS and
then transferred to a nitrocellulose membrane (GE Healthcare Life
Sciences). The membrane was then blocked for 4 h at 37°C in
tris-buffered saline (TBS) containing 5% fat-free dried milk and
0.5% Tween-20, following which the membrane was incubated with
individual antibodies targeting Bax overnight at 4°C. The membrane
was then washed three times with TBS and incubated for 1 h with
secondary antibodies at room temperature. The signals were
visualized using enhanced chemiluminescence and developed on X-ray
film (Carestream Health, Inc., Rochester, NY, USA). The band
density was measured using the GEL DOC 2000 system equipped with
Quantity One software (Bio-Rad Laboratories, Inc., Hercules, CA,
USA) and normalized against the density of β-actin.
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
The total RNA was extracted using TRIzol Reagent
(Invitrogen Life Technologies), according to the manufacturer's
instructions. The cDNA was synthesized from 1 µg total RNA
using an iScript™ cDNA Synthesis kit (Bio-Rad, Richmond, CA, USA).
Relative gene expression levels were determined using FastStart
Universal SYBR Green Master mix (Roche Diagnostics), with the mRNA
expression levels of β-actin used as an endogenous control. The
expression levels of the target genes were calculated using the
2−ΔΔCt method.
Caspase-3 activity assay
The activity of caspase-3 was determined using a
colorimetric assay kit. The U266 cells at 60% confluence, treated
with various concentrations of ginsen-oside Rg3, were washed with
PBS, and lysed in caspase-3 sample lysis buffer. The cell lysates
were then centrifuged at 12,000 x g for 20 min at 4°C, and the
resulting superna-tants were assessed for protein concentration and
caspase-3 activity. The protein concentration was determined using
a Bicinchoninic Acid Protein Assay kit (Sigma-Aldrich). The cell
lysates were incubated in reaction buffer containing a caspase-3
specific inhibitor (z-DEVD-FMK-pNA; 20 mmol/l) for 4 h at 37°C. The
absorbance was measured at a wavelength of 405 nm. Each sample was
assessed in triplicate.
Statistical analysis
All data are expressed as the mean ± standard
deviation. Statistical significance was determined using Student's
t-test on SPSS 19.0 software (IBM SPSS, Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of ginsenoside Rg3 on the
viability of human multiple myeloma cells
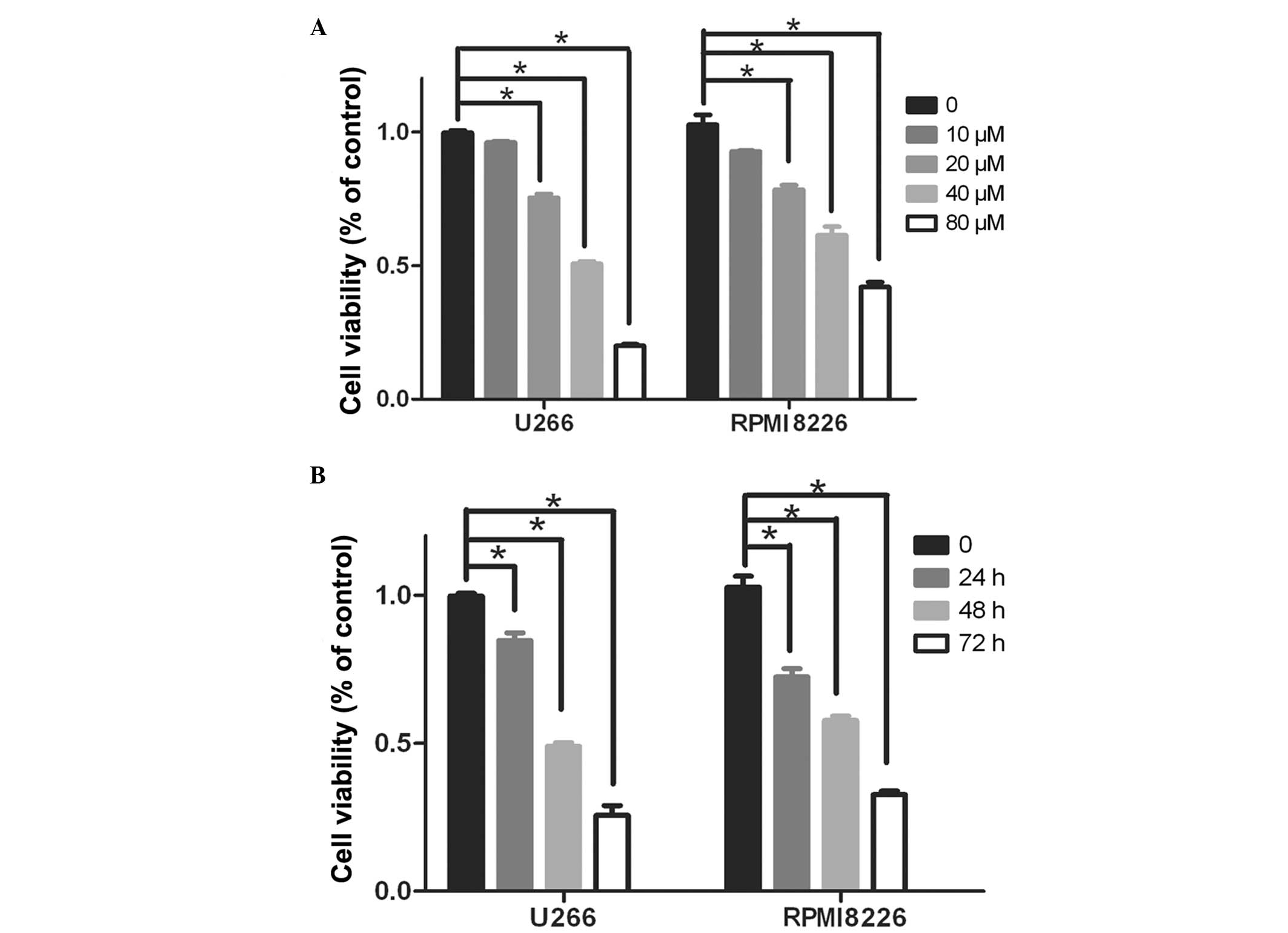
To determine whether ginsenoside Rg3 affected cancer
cell growth in culture, an MTT assay was performed on U266 and
RPMI8226 cells treated with increasing concentrations of Rg3 (0,
10, 20, 40 and 80 µM) for 48 h. Treatment with ginsenoside
Rg3 resulted in a dose-dependent inhibition of cell proliferation
in the two cell lines, compared with the untreated control cells
(Fig. 1A). The half maximal
inhibitory concentration of ginsenoside Rg3 was ~40 µM in
the U266 cells and 70 µM in the RPMI8226 cells. The highest
inhibitory rate was observed at a concentration of 80 µM for
48 h in the two cell lines. When the cell lines were treated with
40 µM Rg3 for 0, 24, 48 and 72 h, the cell viability
declined sig nificantly after 24 h (P<0.005, vs. control;
Fig. 1B).
Ginsenoside Rg3 induces apoptosis in U266
cells
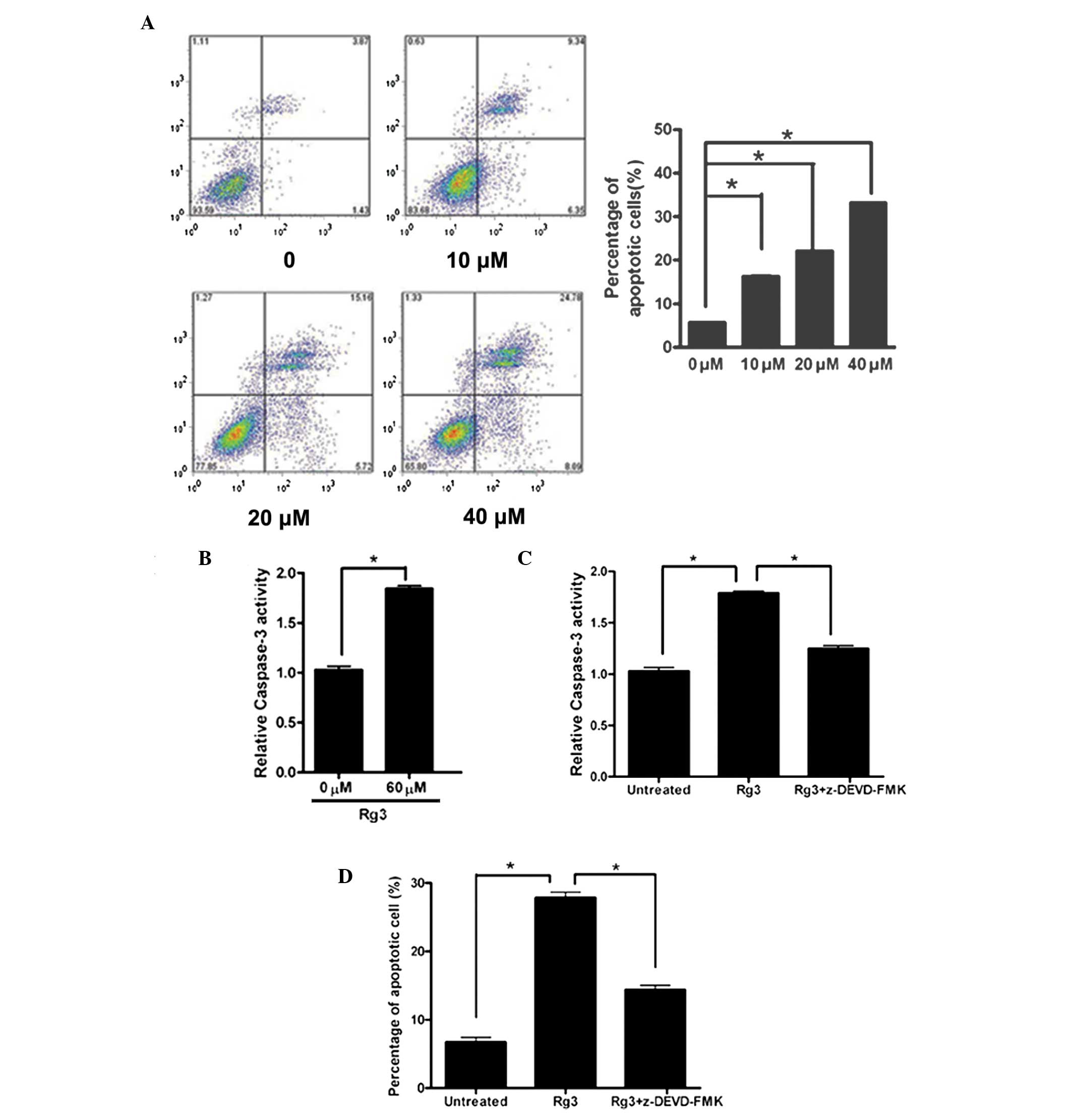
To determine whether the antiproliferative effects
of Rg3 were associated with the induction of apoptosis, the U266
cells were treated with 0–40 µM Rg3. The rate of Rg3-induced
apop-tosis was determined in the U266 cells using annexin-V/pe
staining. As shown in Fig. 2A,
treatment with Rg3 at 10, 20 and 40 µM caused a significant
increase in apoptosis, (16.2±0.46, 22.1±0.83 and 33.2±0.18%,
respectively) compared with the untreated control cells
(5.70±0.10%; P<0.05). Caspase-3 is universally activated during
apoptotic cell death by the extrinsic (death ligand) and intrinsic
(mitochondrial) pathways. To determine whether the caspase cascade
mediated Rg3-induced apoptosis, caspase-3 activity was examined
using pNA. The activity of caspase-3 was significantly higher in
the Rg3 (60 µM)-treated cells, compared with the untreated
control cells (Fig. 2B).
Furthermore, caspase-3 activity was significantly decreased when
the U266 cells were pretreated with z-DEVD-FMK, a caspase-3
inhibitor (Fig. 2C). Pre-treatment
with z-DEVD-FMK attenuated the increased rate of apoptosis in the
Rg3-treated cells, suggesting that Rg3 induced apoptosis in the
U266 cells via the caspase-3-dependent apoptotic pathway (Fig. 2D).
Ginsenoside Rg3 enhances the expression
levels of Bax
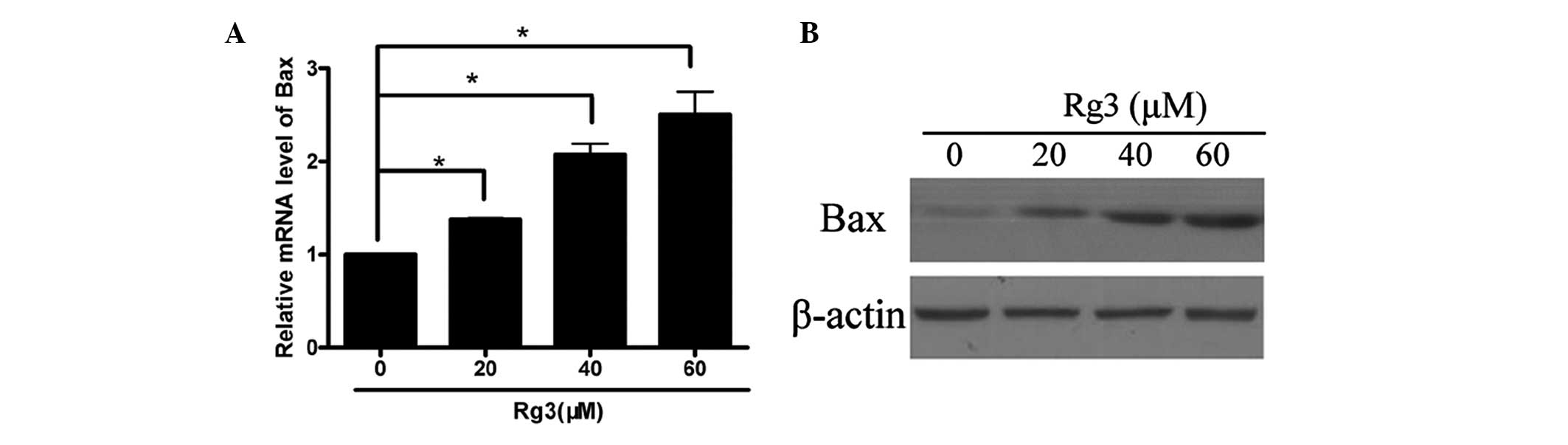
The Bcl-2 family is important in apoptosis in
leukemogenesis. To investigate the molecular mechanism underlying
Rg3-induced apoptosis, the Bcl-2 family proteins were examined. The
U266 cells were treated with different concentrations of
ginsen-oside Rg3. Reverse transcription-quantitative polymerase
chain reaction (RT-qPCR) demonstrated that treatment with 20–80
µM ginsenoside Rg3 resulted in upregulated mRNA expression
levels of Bax (Fig. 3A). Western
blot analysis revealed that exposure to various concentrations of
ginsen-oside Rg3 resulted in a marked reduction in the expression
levels of Bax (Fig. 3B). These
results suggested that treatment with ginsenoside Rg3 enhanced the
expression of Bax at the transcriptional level.
Bax mediates the pro-apoptotic effects of
ginsenoside Rg3
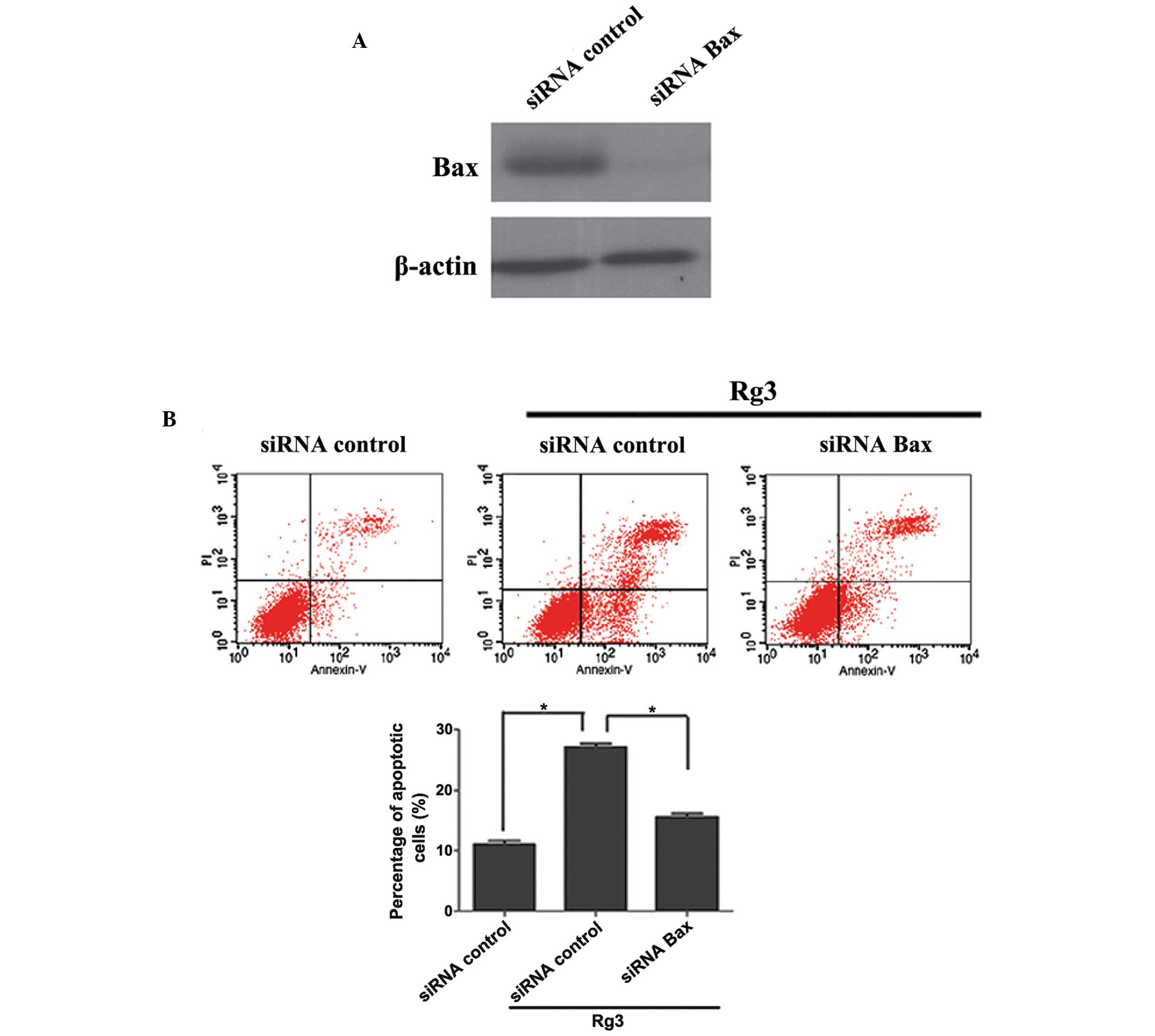
To determine whether Bax was essential for caspase-3
activation, the U266 cells were transfected with small interfering
(si)RNA targeting Bax prior to Rg3 treatment. Transfection of the
cells with Bax-siRNA efficiently knocked down the expression of Bax
in the U266 cells (Fig. 4A).
Furthermore, BAX knockdown also protected the U266 cells from
Rg3-induced apoptosis, indicating that Rg3-induced apop-tosis was
partially due to upregulation of Bax (Fig. 4B). These data suggested that Rg3
induced apoptosis in human multiple myeloma cells, at least in
part, via the Bax-dependent activation of caspase-3.
Discussion
The results of the present study demonstrated that
exposure to ginsenoside Rg3 resulted in the dose-dependent
inhibition of cell proliferation in the U266 and RPMI8226 cell
lines. The anti-proliferative effect of ginsenoside Rg3 has been
documented in other types of cancer cell, including HCC and colon
cancer cells (11,12). These findings indicate that
ginsenoside Rg3 exhibited growth-suppressive activity in solid and
hemato-logic malignancies. The induction of apoptosis is an
important mechanism of antitumor agents. As apoptosis proceeds
without disruption of plasma membrane integrity, it prevents the
onset of an inflammatory response, which favors tumor progression
(13). Therefore, inducing
apoptosis, rather than necrosis, has been regarded as a preferred
and superior strategy for clearing tumor cells. Notably, the
present study demonstrated, using DAPI staining, that treatment
with ginsenoside Rg3 led to apoptotic morphological changes,
including nuclear condensation and fragmentation, and the formation
of apoptotic bodies in the U266 cells. The results of the
annexin-V/PI staining further confirmed the apoptosis-promoting
role of ginsenoside Rg3. These results suggested that the
antiproliferative activity of ginsenoside Rg3 in multiple myeloma
cells is associated with the induction of apoptosis. Ginsenoside
Rg3 has also been reported to suppress tumor growth by inducing
tumor cell apoptosis in HCC (14).
Caspases are a family of proteases, which regulate
apop-tosis. The caspase family includes upstream initia tor
caspases, including caspase-8 and 10, and downstream executor
caspases, including caspase-3. It is widely accepted that
activation of caspase-3 is essential for triggering apoptosis in
several types of cell (2).
Brazilin, which is isolated from Caesalpinia sappan, has
been reported to promote apoptosis in U266 multiple myeloma cells
through the activation of caspase-3 (15). To further investigate the possible
mechanism underlying Rg3-induced apoptosis in human multiple
myeloma, the present study examined the activity of caspase-3 in
Rg3-treated cells. The data revealed that ginsenoside Rg3-induced
apoptosis involved the activation of caspase-3 in the U266 cells,
in a dose-dependent manner. In order to confirm the importance of
caspase-3 for Rg3-induced apoptosis, the U266 cells were pretreated
with an irreversible caspase-3 inhibitor. Rg3-induced apoptosis was
decreased following the inhibition of caspase-3 activation,
suggesting that Rg3-induced apoptosis was caspase-3 dependent.
Bcl-2 family members exhibit either pro- or
anti-apoptotic activities, and regulate the mitochondrial pathway
of apoptosis by controlling the permeabilization of the outer
mitochondrial membrane (16).
Activated Bax is involved in the formation of pores in the outer
mitochondrial membrane, which allow the release of cytochrome
c from the mitochondria, consequently leading to activation
of caspases (16). The present
study demonstrated that exposure to ginsenoside Rg3 resulted in a
marked enhancement of the mRNA and protein expression levels of
Bax. Notably, silencing the expression of Bax reduced the
activation of caspase-3 and the levels of apoptosis in the
Rg3-treated U266 cells. These findings collectively suggested that
ginsenoside Rg3 induced apoptosis in human multiple myeloma cells
by modulating the expression of Bax, which triggered the
caspase-3-dependent pathway. Activation of the mitochondrial
pathway of apoptosis by ginsenoside Rg3 has also been documented in
HCC (14–17) and colon cancer cells (12).
In conclusion, the present study demonstrated that
ginsen-oside Rg3 inhibited the proliferation and induced the
apoptosis of human multiple myeloma cells. Furthermore, Rg3-induced
apoptosis was partially due to upregulation of Bax. However, there
were certain limitations to the present study. The detailed
signaling pathways involved in ginsenoside Rg3-induced apoptosis in
multiple myeloma cells require further elucidation. In addition,
whether these findings can be translated into the clinical setting
remains to be elucidated. Therefore, further investigation is
required to assess the possible therapeutic application of
ginsenoside Rg3 in human multiple myeloma therapy.
References
|
1
|
Mahindra A, Laubach J, Raje N, Munshi N,
Richardson PG and Anderson K: Latest advances and current
challenges in the treatment of multiple myeloma. Nat Rev Clin
Oncol. 9:135–143. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT,
Liu B and Bao JK: Programmed cell death pathways in cancer: A
review of apoptosis, autophagy and programmed necrosis. Cell
Prolif. 45:487–498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Danial NN and Korsmeyer SJ: Cell death:
Critical control points. Cell. 116:205–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kale J, Liu Q, Leber B and Andrews DW:
Shedding light on apoptosis at subcellular membranes. Cell.
151:1179–1184. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oancea M, Mani A, Hussein MA and Almasan
A: Apoptosis of multiple myeloma. Int J Hematol. 80:224–231. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kang KA, Kim HS, Kim DH and Hyun JW: The
role of a ginseng saponin metabolite as a DNA methyltransferase
inhibitor in colorectal cancer cells. Int J Oncol. 43:228–236.
2013.PubMed/NCBI
|
|
7
|
Ma SW, Benzie IF, Chu TT, Fok BS,
Tomlinson B and Critchley LA: Effect of Panax ginseng
supplementation on biomarkers of glucose tolerance, antioxidant
status and oxidative stress in type 2 diabetic subjects: Results of
a placebo-controlled human intervention trial. Diabetes Obes Metab.
10:1125–1127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim SM, Lee SY, Yuk DY, Moon DC, Choi SS,
Kim Y, Han SB, Oh KW and Hong JT: Inhibition of NF-kappaB by
ginsenoside Rg3 enhances the susceptibility of colon cancer cells
to docetaxel. Arch Pharm Res. 32:755–765. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim SM, Lee SY, Cho JS, Son SM, Choi SS,
Yun YP, Yoo HS, Yoon do Y, Oh KW, Han SB and Hong JT: Combination
of ginsenoside Rg3 with docetaxel enhances the susceptibility of
prostate cancer cells via inhibition of NF-kappaB. Eur J Pharmacol.
63:1–9. 2010. View Article : Google Scholar
|
|
10
|
Keum YS, Han SS, Chun KS, Park KK, Park
JH, Lee SK and Surh YJ: Inhibitory effects of the ginsenoside Rg3
on phorbol ester-induced cyclooxygenase-2 expression, NF-kappaB
activation and tumor promotion. Mutat Res. 523–524:75–85. 2003.
View Article : Google Scholar
|
|
11
|
Yu Y, Zhang C, Liu L and Li X: Hepatic
arterial administration of ginsenoside Rg3 and transcatheter
arterial embolization for the treatment of VX2 liver carcinomas.
Exp Ther Med. 5:761–766. 2013.PubMed/NCBI
|
|
12
|
Yuan HD, Quan HY, Zhang Y, Kim SH and
Chung SH: 20(S)-Ginsenoside Rg3-induced apoptosis in HT-29 colon
cancer cells is associated with AMPK signaling pathway. Mol Med
Rep. 3:825–831. 2010.
|
|
13
|
Sethi G, Shanmugam MK, Ramachandran L,
Kumar AP and Tergaonkar V: Multifaceted link between cancer and
inflammation. Biosci Rep. 32:1–15. 2012. View Article : Google Scholar
|
|
14
|
Jiang JW, Chen XM, Chen XH and Zheng SS:
Ginsenoside Rg3 inhibit hepatocellular carcinoma growth via
intrinsic apoptotic pathway. World J Gastroenterol. 17:3605–3613.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim B and Kim SH, Jeong SJ, Sohn EJ, Jung
JH, Lee MH and Kim SH: Brazilin induces apoptosis and G2/M arrest
via inactivation of histone deacetylase in multiple myeloma U266
cells. J Agric Food Chem. 60:9882–9889. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brunelle JK and Letai A: Control of
mitochondrial apoptosis by the Bcl-2 family. J Cell Sci.
122:437–441. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park HM, Kim SJ, Kim JS and Kang HS:
Reactive oxygen species mediated ginsenoside Rg3- and Rh2-induced
apoptosis in hepatoma cells through mitochondrial signaling
pathways. Food Chem Toxicol. 50:2736–2741. 2012. View Article : Google Scholar : PubMed/NCBI
|