Introduction
Cruciferous vegetables are plants exerting antitumor
activity. 3,3′ Diindolylmethane (DIM), which extracted from
cruciferous vegetables, induces antiproliferative and proapoptotic
effects in a variety of tumor cell types, including nasopharyngeal
carcinoma (NPC) cells (1,2). Several underlying mechanisms of the
anti tumor effects of DIM have been reported (3–6),
however, the DIM regulation of telomerase activity, which is
important in NPC, and the associated mechanisms remain to be
elucidated. Telomeres are located on the ends of eukaryotic
chromosomes, are comprised of G- and C-rich hexanucleotide repeats
and protect chromosome ends from recombination, fusion and
degradation (7). Cell division is
accompanied by a gradual reduction in telomere length. A short
telomere triggers the apoptotic program, which, however, can be
avoided by the activation of telomerase (7,8).
Telomerase is composed of human telomerase RNA (hTR),
telomerase-associated protein 1 (TP1) and human telomerase reverse
transcriptase (hTERT). As the rate-limiting component of
telomerase, hTERT induces the immortalization of a number of cell
types in culture (8).
However, whether the effects of DIM may be
associated with telomerase activity has remained to be elucidated.
Previous studies have investigated the underlying mechanisms of the
anti-proliferative and pro-apoptotic effects of DIM and have
revealed that cell cycle arrest (9), cell signaling inhibition (10–12)
and downregulation of the androgen receptor (13) are involved. The inhibition of
telomerase was reported to induce anti-proliferative effects
(14,15). The present study hypothesized that
telomerase may be important in the anti-tumor mechanism of DIM.
Therefore, the effects of DIM on the proliferation and apoptotic
rate of NPC cells were assessed. Furthermore, telomerase activity,
levels of hTERT and hTR, as well as telomere length were assessed
in nasopharyngeal cells treated with DIM.
Materials and methods
Cells and culture
Human nasopharyngeal carcinoma (NPC) 5-8F cells were
purchased from the Cell Bank of the Chinese Academy of Science
(Shanghai, China). The cell line was cultured in the medium
RPMI-1640 (HyClone Corp., Logan, UT, USA) supplemented with 10%
fetal bovine serum (Gibco Life Technologies, Carlsbad, CA, USA) and
cultured in a humidified incubator at 37°C with 5%
CO2.
DIM
DIM (Sigma-Aldrich, St. Louis, MO, USA) was
dissolved in dimethylsulfoxide (Sigma-Aldrich) and was diluted to
the concentrations of 0, 25, 50, 75 and 100 µM in complete
medium (Genom Biotechnology, Hangzhou, China).
Cell proliferation assay
Cells in the logarithmic growth phase were obtained
and were seeded into 96-well plates at 2,500 cells/well. Following
culturing, cell proliferation was assessed using a cell counting
kit-8 (CCK-8; cat. no. C0038; Beyotime Institute of Biotechnology,
Shanghai, China) according to manufacturer's instructions. Briefly,
10 µl CCK8 solution was added to the culture medium followed
by incubation for 1 h. The absorbance was measured at a wavelength
of 450 nm with a reference wavelength of 630 nm.
Flow cytometry
Induction of apoptosis was assessed using flow
cytometric analysis. An Annexin V/propidium iodide (PI) apoptosis
kit (cat. no. LK-AP101-100; Lianke Biotech Co., Ltd., Hangzhou,
China) was used for the detection. The cells were seeded into
six-well plates and the next day, the medium was changed to medium
containing 0, 25, 50, 75 and 100 µm DIM for up to 24 h,
prior to digestion and collection. The samples were stained with
Annexin V-fluorescein isothiocyanate (FITC) and PI for 15 min. The
cells were analyzed immediately by flow cytometry (FACSCanto II; BD
Biosciences, San Jose, CA, USA) using the fluorescence channels FL1
(emission at 525 nm) and FL3 (emission at 670 nm).
Telomeric repeat amplification protocol
(TRAP) assay
For the detection of telomerase activity, a
TRAP-polymerase chain reaction (PCR) assay was used (Millipore,
Billerica, MA, USA). The primers were as follows: TS,
5′-AATCCGTCGAGCAGAGTT-3′ and CX primer,
5′-CCCTTACCCTTACCCTTACCCTAA-3′. Cells (~1×106) were
washed once with ice-cold wash buffer, re-suspended and centrifuged
at 3,000 × g for 5 min at 4°C. The precipitate was homogenized with
40 µl cold lysis buffer for 30 min on ice, followed by
centrifugation at 13,000 × g for 30 min at 4°C. The supernatants
were used for subsequent analyses. The extension reaction was as
follows: 5 µl 10X TRAP buffer, 1 µl
deoxyribonucleoside triphosphates, 1 µl Taq-DNA polymerase,
1 µl telomere strand primer, 2 µl telomerase
extraction, 39 µl diethylpyrocarbonate-treated
H2O and 1 µl CX primer. Prior to PCR, a solution
without CX primer was pre-incubated at 23°C for 30 min. The PCR for
the TRAP assay was performed as follows: 94°C for 5 min; 35 cycles
of 94°C for 30 sec, 50°C for 30 sec and 72°C for 90 sec; followed
by 72°C for 10 min. The PCR products were electrophoresed using 10%
SDS-PAGE. The gels were stained with ethidium bromide for 15 min,
subsequently scanned and images were captured (Geliamce 200 Gel
Imaging system; Perkin Elmer, Waltham, MA, USA).
Reverse transcription (RT)PCR
The total RNA was isolated from ~1×106
cells and a 25 µl reaction mixture, containing 8.5 µl
5X RT buffer, 2 µl RT enzyme mix, 2 µl primer mix and
12.5 µl nuclease-free water, which were contained in the
RT-PCR kit (Takara, Dalian, China), were incubated at 65°C for 5
min, followed by 42°C for 1 h. The cDNA was amplified using the
following specific primers: hTERT forward,
5′-CGGAAGAGTGTCTGGAGCAA-3′ and reverse, 5′-GGATGAAGCGGAGTCGGA-3′;
hTR forward, 5′-TCTAACCCTAACTGAGAAGGGCGTAG-3′ and reverse,
5′-GTTTGCTCTAGAATGAACGGGGAAG-3′; Actin forward,
5′-CGTACCACTGGCATCGTGAT-3′ and reverse,
5′-GTGTTGGCGTACAGGTCTTTG-3′; GAPDH forward,
5′-GAAGGTGAAGGTCGGAGTCA-3′ and reverse, 5′-GGCAGAGATGATGACCCTTT-3′.
All primers were designed and synthesized by Sangon Biotechnology,
Shanghai, China. The PCR assay was performed as follows: 94°C for 5
min; 35 cycles at 94°C for 30 sec, 60°C for 30 sec and 72°C for 90
sec; followed by 72°C for 10 min. The products were subsequently
electrophoresed on a 1.5% agarose gels. The gels were stained with
ethidium bromide for 15 min, scanned and images were captured
(Geliamce 200 Gel Imaging system; Perkin Elmer).
Western blot analysis
The cells were harvested and lysed with lysate
buffer (Guge Biotechnology, Wuhan, China), mixed with protease
inhibitor cocktail (Roche, Basel, Switzerland) and phosphorylase
inhibitor (Roche) on ice for 15 min, and was subsequently
quantified using a BCA kit (Thermo Fisher Scientific, Grand Island,
NY, USA). The total protein extracts from each group of cells were
resolved by 10% SDS-PAGE (Guge Biotechnology) and transferred on
polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA,
USA). Following blocking with 5% sealing liquid (Guge
Biotechnology) for 2 h at room temperature, the PVDF membranes were
washed three times for 15 min with Tris-buffered saline containing
Tween-20 (TBST; Guge Biotechnology) at room temperature and
incubated with primary antibody (rabbit anti-hTERT; 1:500; cat. no.
sc-7212; Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight
at 4°C. Following extensive washing with TBST three times for 15
min each, the membranes were incubated with the secondary antibody,
donkey anti-rabbit immunoglobulin G (cat. no. 926-32213; LI-COR,
Lincoln, NE, USA) for 1 h. Following washing three times for 15 min
with TBST at room temperature, the membranes were scanned with the
Odyssey CLx Infrared Imaging system (LI-COR).
Telomere length detection
A telomere peptide nucleic acid (PNA) fluorescence
in situ hybridization (FISH) kit FITC (cat. no. K5325; Dako
Denmark A/S, Glostrup, Denmark) was used for the detection of
telomere length. The nuclei were isolated by re-suspending
3×105 cells in 2% Triton X-100 and 0.1 M citric acid
buffer (Guge Biotechnology), vortexed and incubated for 10 min at
room temperature. The samples were washed once with
phosphate-buffered saline (PBS) and directly subjected to
denaturation⁄hybridization. Denaturation was performed on a thermo
block at 80°C for 10 min and the samples were allowed to hybridize
at room temperature overnight. The samples were washed with PBS,
incubated on a heat block at 40°C for 10 min and subsequently
centrifuged at 700 x g for 5 min. The samples were re-suspended in
200 ml DNA staining solution. For DNA counterstaining, the cells
were re-suspended in 500 µl staining solution for 2 h prior
to acquisition on a FACScan flow cytometer (Becton Dickinson,
Franklin Lakes, NJ, USA). The mean telomere fluorescence of the
cells was analyzed using Cell Quest software (Becton
Dickinson).
Statistical analysis
All statistical analyses were performed using SPSS
version 20.0 software (IBM SPSS, Chicago, IL, USA). The values are
expressed as the mean ± standard deviation. A t-test was used to
determine the significance. P<0.05 was considered to indicate a
statistically significant difference.
Results
NPC cell growth is repressed and
apoptosis is increased following treatment with DIM
To identify the effects of DIM on NPC cells, the
proliferation ability and the apoptotic rate of NPC cells were
determined following treatment with DIM. A CCK-8 assay was used to
detect the proliferative rate of the NPC cells. The NPC cells were
treated with DIM at various concentrations: 0, 25, 50, 75 and 100
µm, for five durations: 0, 24, 48, 72 and 96 h. As the
duration increased, the absorbance was increased. In addition, at
each time-point, the absorbance decreased as the concentration of
DIM increased. Of note, the absorbance at each time-point revealed
no change at a concentration of 100 µM, indicating that
cells failed to grow substantially (Fig. 1A).
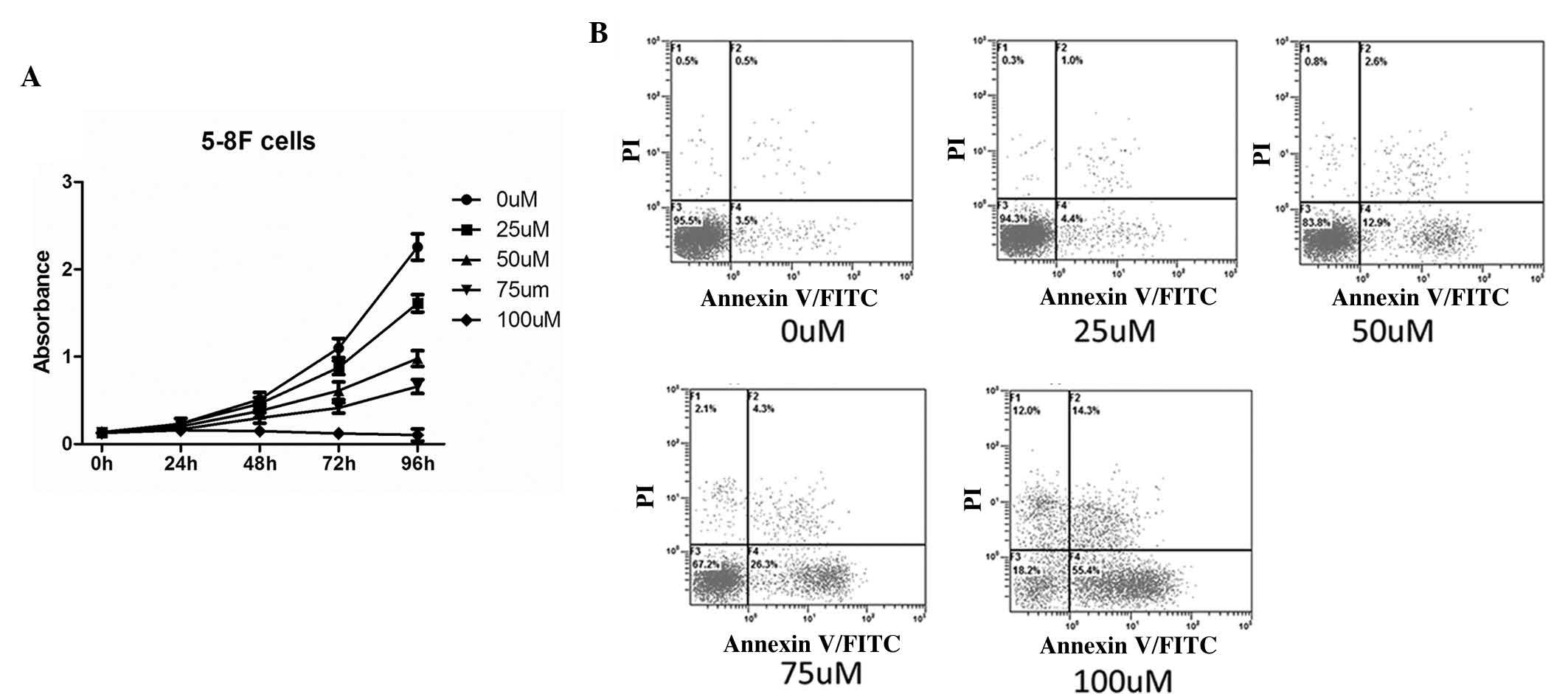 | Figure 1Proapoptotic and antiproliferative
effects of DIM in the 5-8F NPC cell line. (A) The proliferation
rate of the NPC cells treated with DIM at various concentrations
was determined. At the time points of 0, 24, 48, 72 and 96 h, the
proliferation ability was detected by CCK-8 assay. As the
concentration of DIM increased, the proliferation ability of the
5-8F cells was reduced (P<0.05). (B) The apoptotic response of
the NPC cells treated with DIM at various concentrations was
determined. Following 24 h treatment with DIM, the apoptosis rate
of the 5-8F cells was detected by flow cytometry. The apoptotic
rates were 4.0±0.4, 5.4±0.6, 15.5±0.5, 30.6±0.5 and 69.7±0.6%,
respectively. As the concentration of DIM increased, there was an
increasing trend in the apoptotic response of the NPC cells
(P<0.05). DIM, 3,3′-diindolylmethane; NPC, nasopharyngeal
carcinoma; CCK, cell counting kit; PI, propidium iodide; FITC,
fluorescein isothiocyanate. |
The apoptotic response of NPC cells was detected by
flow cytometry. As the concentration of DIM increased, there was an
increasing trend in the apoptotic rate of the NPC cells (Fig. 1B).
Telomerase deactivation is involved in
the anti-cancer effect of DIM
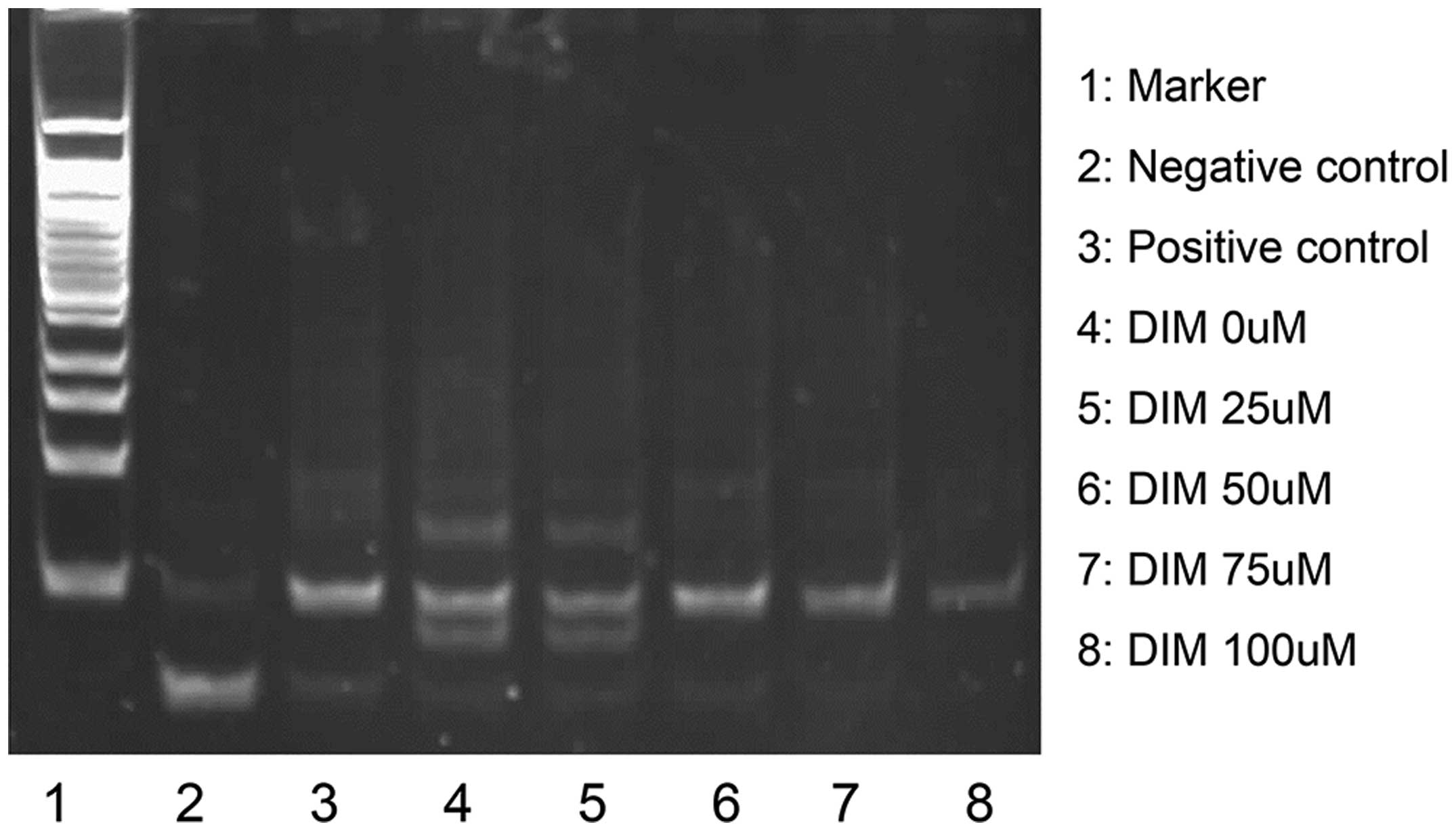
To identify whether telomerase activity was changed
following treatment with DIM, telomerase activity was detected. A
TRAP assay was performed to detect the telomerase activity in 5-8F
cells and 5-8F cells treated with DIM at a range of concentrations
as mentioned above. The results demonstrated that the telomerase
activity was reduced in the DIM-treated cells compared with that in
the control 5-8F cells, and the decreasing effect occurred in a
concentration-dependent manner (Fig.
2).
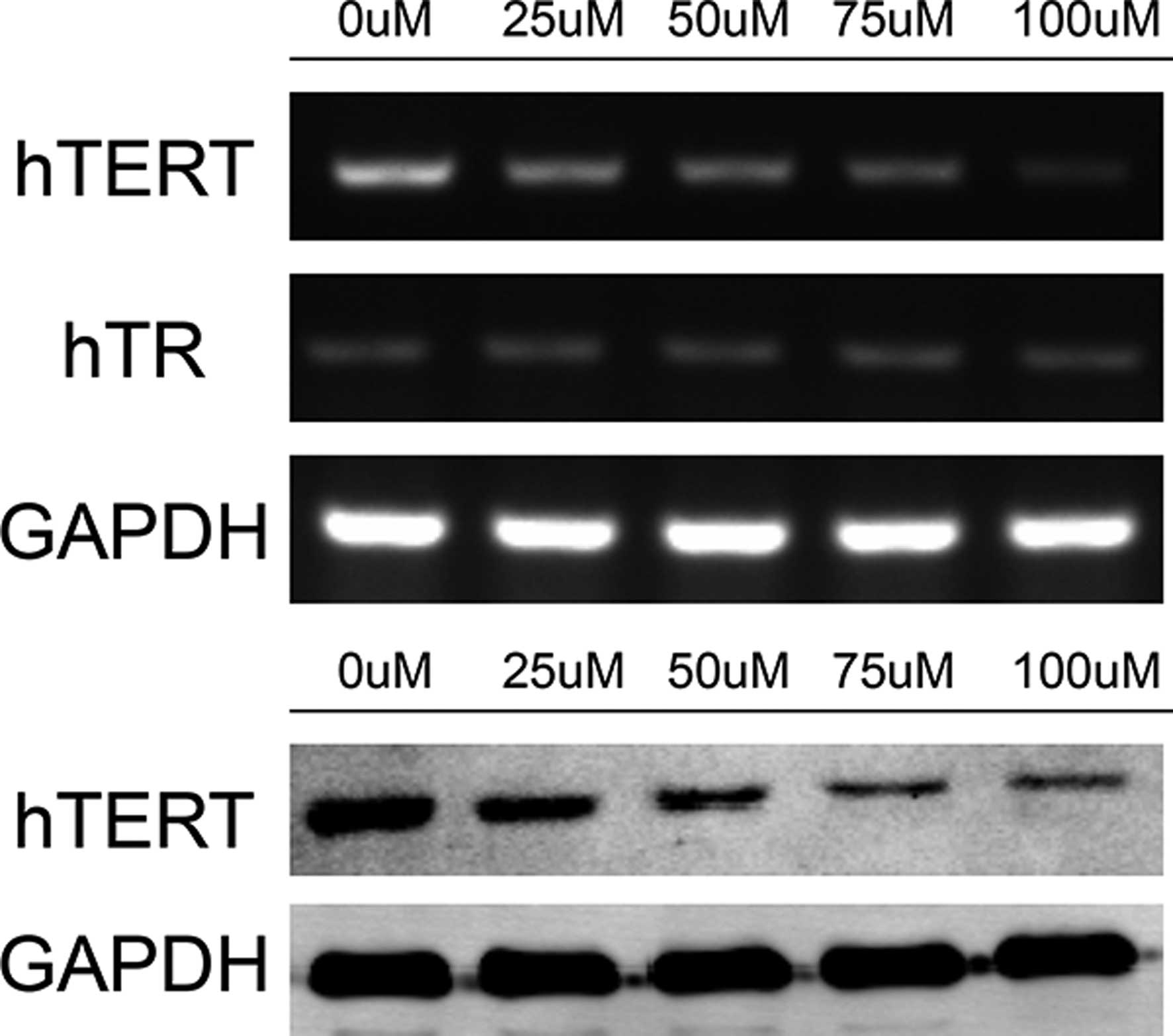
Telomerase subunit hTERT is the possible
target of DIM
To assess which part of telomerase was the target of
DIM, the expression levels of the two components of telomerase,
hTERT and hTR, were detected. The mRNA expression levels of hTERT
and hTR were detected by RT-PCR, and the protein expression of
hTERT was detected by western blot analysis. The results
demonstrated that the mRNA and protein expression levels of hTERT
were downregulated in the 5-8F cells treated with DIM compared with
those in the control 5-8F cells (Fig.
3). The mRNA expression of hTR remained unchanged (Fig. 3), indicating that hTERT was the
possible target of DIM in the regulation of the proliferation and
apoptosis of NPC cells.
Telomeres are shortened in the NPC cells
treated with DIM
To further confirm the functions of DIM with hTERT,
the length of telomeres was detected. A telomere length detection
kit was used for the detection and the results demonstrated that
the telomeres in the 5-8F cells treated with DIM were shortened
compared with those in the control 5-8F cells in a
concentration-dependent manner (Fig.
4). Combined with the finding of apoptosis being induced by DIM
in NPC cells, the present study hypothesized that telomere
shortening leads to cell death, as indicated by the increased
apoptotic rate.
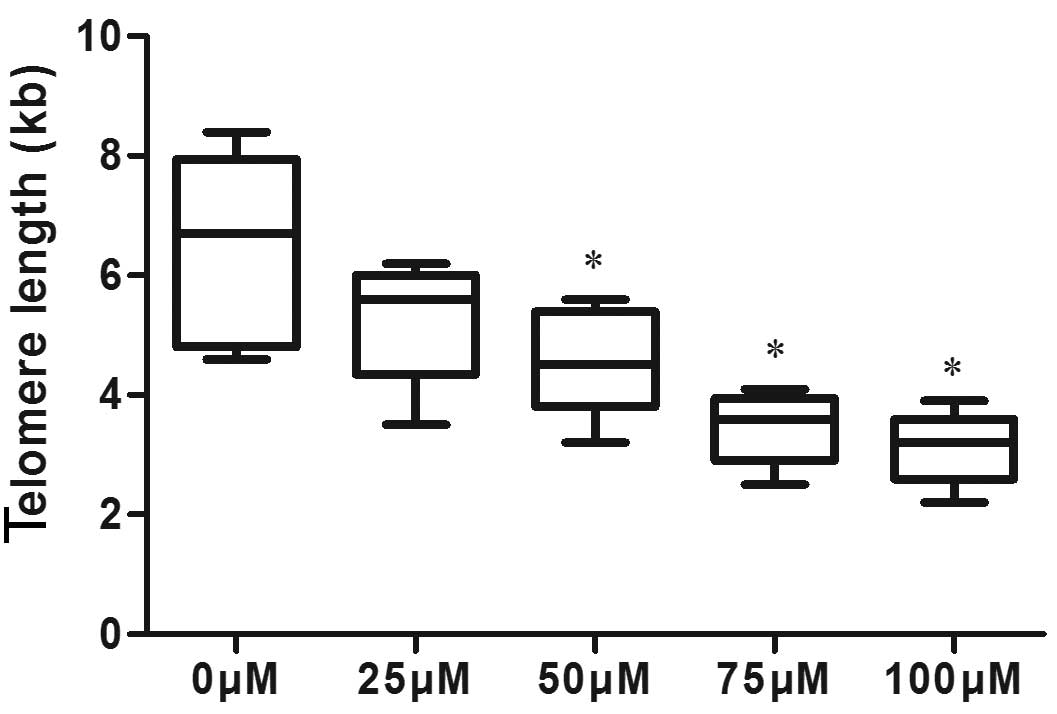 | Figure 4Telomere length in 5-8F cells and 5-8F
cells treated with DIM. The cells were treated with 0, 25, 50, 75
and 100 µM DIM for 24 h, and a telomere length detection kit
was used to detect telomere length. The telomere length was graphed
as boxes and error bars indicate the mean ± standard deviation of
three replicates. The results were 6.44±1.6, 5.26±1.0. 4.58±0.92,
3.46±0.61 and 3.12±0.61, respectively, and these results indicated
that telomeres in cells treated with DIM were shortened compared
with those in untreated 5-8F cells. *P<0.05, compared
with the 0 µM DIM group. DIM, 3,3′-diindolyl-methane. |
Discussion
DIM is a tumor-preventive agent and is a natural
product present in cruciferous plants. The anti-tumor effect of DIM
has been vastly investigated (2–6,16,17).
The underlying mechanisms of in the anti-tumor effects were
reported in several previous studies which demonstrated that DIM
induces apoptosis of tumor cells (1–4),
inhibits proliferation (2,5,6),
regulates several signaling pathways (2–4),
including phosphoinositide 3-kinase, mitogen-activated protein
kinase, Akt and nuclear factor-κB, regulates the cell cycle
(5), inhibits tumor angiogenesis
(16,17), downregulates the androgen receptor
(7) and exerts a preventive and
curative role in the development and progression of certain types
of tumor (2,5).
Proliferation and apoptosis are important hallmark
properties of cancer cells (18).
Based on this, the present study aimed to investigate the effects
of DIM on the proliferation and apoptosis of NPC cells, to identify
its effects on telomerase and elucidate the possible underlying
mechanism. The proliferation ability of cells with and without DIM
treatment was detected using a CCK-8 assay, and the apoptosis of
the cells was detected by flow cytometry. The results demonstrated
that DIM inhibited proliferation and induced apoptosis of the NPC
cells.
Telomerase was first characterized by its ability to
elongate and maintain telomere length; however, previous studies on
mice and cells have revealed that it also functions in other
processes (19–22). The novel functions of telomerase
were assessed following the elimination of telomerase activity and
several functions were reported, including induction of apoptosis,
participation in DNA repair, association with DNA replication
protein primase, regulation of DNA damage responses, modulation of
several signaling pathways and regulation of gene expression.
Telomerase activity was hypothesized to affect
changes in proliferation and apoptosis of NPC cells (23); therefore, the present study used
the TRAP-PCR assay for the detection of telomerase activity. The
results demonstrated that telomerase activity was inhibited
following treatment with DIM. Based on these results, the present
study aimed to determine which sub-unit of telomerase is a target
of DIM. RT-PCR and western blot analyses were performed to detect
the mRNA and protein expression levels of hTERT, respectively, and
the mRNA expression of hTR. The results demonstrated that the mRNA
and protein expression levels of hTERT were downregulated in the
DIM-treated cells; however, the mRNA expression of hTR remained
unchanged. This suggested that DIM decreased telomerase activity by
downregulating the mRNA and protein expression of hTERT.
The expression of hTERT was downregulated in the
cells treated with DIM, which confirmed that hTERT was the target
of DIM. Inhibition of hTERT by DIM resulted in the inhibition of
the telomerase activity. The present study next aimed to determine
whether the function of telomerase in this process was associated
with telomere length. A FISH assay was used to assess whether the
telomere length was affected by DIM, and the results revealed that
telomere length was shortened in the DIM-treated cells, suggesting
that telomerase inhibition led to decreases in telomere length,
inhibited proliferation and directly induced the apoptosis of NPC
cells.
Several previous studies have reported that
telomerase has a role in the effect of the anti-proliferative and
anti-tumor effects of drugs (24–26).
Zhao et al (24) revealed
that telomerase was inhibited by harmine and the proliferation of
MCF-7 cells was decreased. Long-term effects of hTERT inhibition
were demonstrated by Qian et al (25), who revealed that SGC-7901 cell
proliferation was inhibited by the inhibition of telomerase
activity. The present study demonstrated that telomerase activity
was inhibited by DIM through the inhibition of hTERT.
Telomerase was identified to be associated with the
sensitivity of NPC cells to radiotherapy and chemotherapy (23,27,28).
It was hypothesized that DIM may also be involved in the
sensitivity to radiotherapy and chemotherapy, and several previous
studies confirmed this. Ahmad et al (29) reported that DIM increased the
chemotherapeutic sensitivity of prostatic cancer cells, which were
multidrug-resistant. Fan et al (30) reported that physiological
sub-micromolar concentrations of DIM protected cultured cells
against radiation. DIM was thoroughly studied for its functions in
cancer therapy and prevention, and indole-3-carbinol, the precursor
of DIM, was already approved for clinical use in the treatment of
respiratory papillomatosis in the USA (31). It is no exaggeration to suggest
that there is a wide prospect for DIM in clinical application;
however, further study is required.
In conclusion, the present study reported that the
proliferation of NPC cells was inhibited and apoptosis was induced
by DIM. NPC cells treated with DIM showed obvious decreases in
telomerase activity, and it was speculated that the changes in cell
proliferation and apoptosis of NPC cells treated by DIM were
associated with telomerase. The mRNA and protein expression of
hTERT were downregulated following treatment with DIM, while the
expression of hTR mRNA was unaltered, indicating that hTERT was the
target of DIM. Furthermore, telomeres were shortened in DIM-treated
NPCs, which confirmed that pro-apoptotic and anti-proliferative
effects of DIM were mediated through the regulation of
telomerase.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (no. 81372880), the
Independent Research Project of Wuhan University (nos.
2042014kf0184 and 2042014kf0119), the doctoral program of the
Higher Education Research Fund (nos. 20130141120093 and
20110141110062) and the Natural Science Foundation of Hubei
province (no. 2012FFA045).
References
|
1
|
Wang Q, Tiffen J, Bailey CG, et al:
Targeting amino acid transport in metastatic castration-resistant
prostate cancer: effects on cell cycle, cell growth and tumor
development. J Natl Cancer Inst. 105:1463–1473. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen C, Chen SM, Xu B, et al: In vivo and
in vitro study on the role of 3,3′-diindolylmethane in treatment
and prevention of nasopharyngeal carcinoma. Carcinogenesis.
34:1815–1821. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xu Y, Zhang J and Dong WG:
Indole-3-carbinol (I3C)-induced apoptosis in nasopharyngeal cancer
cells through Fas/FasL and MAPK pathway. Med Oncol. 28:1343–1348.
2011. View Article : Google Scholar
|
|
4
|
Banerjee S, Kong D, Wang Z, Bao B, Hillman
GG and Sarkar FH: Attenuation of multi-targeted
proliferation-linked signaling by 3,3′-diindolylmethane (DIM): from
bench to clinic. Mutat Res. 728:47–66. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen Z, Tao ZZ, Chen SM, Chen C, Li F and
Xiao BK: Indole-3-carbinol inhibits nasopharyngeal carcinoma growth
through cell cycle arrest in vivo and in vitro. PloS one.
8:e822882013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim SJ, Lee JS and Kim SM:
3,3′-Diindolylmethane suppresses growth of human esophageal
squamous cancer cells by G1 cell cycle arrest. Oncol Rep.
27:1669–1673. 2012.PubMed/NCBI
|
|
7
|
Blackburn EH: Switching and signaling at
the telomere. Cell. 106:661–673. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shay JW and Wright WE: Senescence and
immortalization: role of telomeres and telomerase. Carcinogenesis.
26:867–874. 2005. View Article : Google Scholar
|
|
9
|
Tadi K, Chang Y, Ashok BT, et al:
3,3′-Diindolylmethane, a cruciferous vegetable derived synthetic
anti-proliferative compound in thyroid disease. Biochem Biophys Res
Commun. 337:1019–1025. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nicastro HL, Firestone GL and Bjeldanes
LF: 3,3′-Diindolylmethane rapidly and selectively inhibits
hepatocyte growth factor/c-Met signaling in breast cancer cells. J
Nutr Biochem. 24:1882–1888. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu J, Li Y, Guan C and Chen Z:
Anti-proliferative and pro-apoptotic effects of
3,3′-diindolylmethane in human cervical cancer cells. Oncol Rep.
28:1063–1068. 2012.PubMed/NCBI
|
|
12
|
Li Y, Wang Z, Kong D, et al: Regulation of
FOXO3a/beta-catenin/GSK-3beta signaling by 3,3′-diindolyl-methane
contributes to inhibition of cell proliferation and induction of
apoptosis in prostate cancer cells. J Biol Chem. 282:21542–21550.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bhuiyan MM, Li Y, Banerjee S, et al:
Down-regulation of androgen receptor by 3,3′-diindolylmethane
contributes to inhibition of cell proliferation and induction of
apoptosis in both hormone-sensitive LNCaP and insensitive C4-2B
prostate cancer cells. Cancer Res. 66:10064–10072. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lagah S, Tan IL, Radhakrishnan P, et al:
RHPS4 G-quadruplex ligand induces anti-proliferative effects in
brain tumor cells. PloS one. 9:e861872014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo WQ, Li LZ, He ZY, et al:
Anti-proliferative effects of Atractylis lancea (Thunb) DC via
down-regulation of the c-myc/hTERT/telomerase pathway in Hep-G2
cells. Asian Pac J Cancer Prev. 14:6363–6367. 2013. View Article : Google Scholar
|
|
16
|
Meng Q, Qi M, Chen DZ, et al: Suppression
of breast cancer invasion and migration by indole-3-carbinol:
associated with up-regulation of BRCA1 and E-cadherin/catenin
complexes. J Mol Med (Berl). 78:155–165. 2000. View Article : Google Scholar
|
|
17
|
Kong D, Li Y, Wang Z, Banerjee S and
Sarkar FH: Inhibition of angiogenesis and invasion by
3,3′-diindolylmethane is mediated by the nuclear factor-kappaB
downstream target genes MMP-9 and uPA that regulated
bioavailability of vascular endothelial growth factor in prostate
cancer. Cancer Res. 67:3310–3319. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mukherjee S, Firpo EJ, Wang Y and Roberts
JM: Separation of telomerase functions by reverse genetics. Proc
Natl Acad Sci USA. 108:E1363–E1371. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Martinez P and Blasco MA: Telomeric and
extratelomeric roles for telomerase and the telomere-binding
proteins. Nat Rev Cancer. 11:161–176. 2011. View Article : Google Scholar
|
|
21
|
Park JI, Venteicher AS, Hong JY, et al:
Telomerase modulates Wnt signalling by association with target gene
chromatin. Nature. 460:66–72. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cong Y and Shay JW: Actions of human
telomerase beyond telomeres. Cell Res. 18:725–732. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fujiwara T, Kagawa S and Tazawa H:
Synergistic interaction of telomerase-specific oncolytic
virotherapy and chemotherapeutic agents for human cancer. Curr
Pharm Biotechnol. 13:1809–1816. 2012. View Article : Google Scholar
|
|
24
|
Zhao L and Wink M: The beta-carboline
alkaloid harmine inhibits telomerase activity of MCF-7 cells by
down-regulating hTERT mRNA expression accompanied by an accelerated
senescent phenotype. Peer J. 1:e1742013. View Article : Google Scholar
|
|
25
|
Qian X, Cheng J, Chen A, et al: Long-term
effects of short hairpin RNA-targeted human telomerase reverse
transcriptase on suppression of SGC-7901 cell proliferation by
inhibition of telomerase activity. Oncol Rep. 19:575–581.
2008.PubMed/NCBI
|
|
26
|
Zou L, Zhang P, Luo C and Tu Z:
shRNA-targeted hTERT suppress cell proliferation of bladder cancer
by inhibiting telomerase activity. Cancer Chemother Pharmacol.
57:328–334. 2006. View Article : Google Scholar
|
|
27
|
Pang LY and Argyle D: Cancer stem cells
and telomerase as potential biomarkers in veterinary oncology. Vet
J. 185:15–22. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
McCaul JA, Gordon KE, Minty F, Fleming J
and Parkinson EK: Telomere dysfunction is related to the intrinsic
radio-resistance of human oral cancer cells. Oral Oncol.
44:261–269. 2008. View Article : Google Scholar
|
|
29
|
Ahmad A, Sakr WA and Rahman KW: Mechanisms
and therapeutic implications of cell death induction by indole
compounds. Cancers. 3:2955–2974. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fan S, Meng Q, Xu J, et al: DIM
(3,3′-diindolylmethane) confers protection against ionizing
radiation by a unique mechanism. Proc Natl Acad Sci USA.
110:18650–18655. 2013. View Article : Google Scholar
|
|
31
|
Wiatrak BJ: Overview of recurrent
respiratory papillomatosis. Curr Opin Otolaryngol Head Neck Surg.
11:433–441. 2003. View Article : Google Scholar : PubMed/NCBI
|