Introduction
Non-alcoholic fatty liver disease (NAFLD) is one of
the major causes of chronic liver injury, which affects 10–24% of
the general population worldwide (1). The prevalence of NAFLD has markedly
increased, which is associated with rates of severe obesity,
insulin resistance and other metabolic syndromes (2). The underlying pathogenesis of NAFLD
remains to be fully elucidated, however, the pathological changes
may be explained by the 'two-hit hypothesis' (3). The 'first hit” consists of the
accumulation of hepatic lipids, which is predominantly associated
with insulin resistance, while the 'second hit' consists of
oxidative stress, mitochondrial dysfunction and inflammation, which
may aggravate the existing steatosis and lead to non-alcoholic
steatosis hepatitis (NASH) (1,4). At
present, there remains no satisfactory therapeutic treatment for
NAFLD.
Mesenchymal stem cells (MSCs) possess the ability to
differentiate into multiple cells, including adipocytes,
osteocytes, hepatocytes, chondrocytes and neurons (5,6),
which regulate the immune response and are important in tissue
repair, thus attracting attention for potential as a novel
therapeutic approach (7,8). Compared with other sources of MSCs,
adipose tissue-derived stem cells (ADSCs) have the advantages of
being abundant, readily obtainable from liposuction or excised fat
and accumulated rapidly for autologous transplantation with minimal
immunogenicity and improved immunoregulatory ability (9). These characteristics make ADSCs more
attractive for clinical usage in cell-based therapy. Previous
studies have demonstrated the immunoregulatory roles of ADSCs in
tissue and organ repair (10–12),
particularly on the protective effects of ADSCs on acute or chronic
liver failure and liver fibrosis (13,14).
However, the liver injury model used in these investigations was
induced by chemical drug treatments, including tetrachloromethane,
d-galactosamine and thioacetamide. Therefore, the underlying
mechanisms and the pathological changes observed were different to
those observed in the most common liver diseases, including
viral-induced (hepatitis B or C) chronic inflammation and fatty
liver disease (FLD) (15,16). As one of the most common forms of
FLD, NAFLD refers to a series of pathological changes, including
hepatic steatosis, NASH, liver fibrosis and cirrhosis, which are
not easily mimicked by chemical drug treatment. Administration of a
high-fat-diet (HFD) is usually used to establish NAFLD animal
models (17), which more closely
resembles the clinical pathological progression of NAFLD
disease.
In the present study, in order to investigate
whether transplantation with ADSCs attenuates disease progression
at an early stage of NAFLD, intrahepatic transplantation of ADSCs
was performed on an HFD-induced NAFLD rat model, and the liver
function and histopathological characteristics of the liver
following transplantation were carefully evaluated.
Materials and methods
Animal experiments
A total of 40 adult male Sprague-Dawley rats
(weight, 180–200 g) were obtained from the Center for Animal
Experiment of Fujian Medical University (Fuzhou, China; license no.
SCXKmin 2012–0002) and were housed at constant temperature (22±2°C)
and 60% relative humidity, with a 12:12 h light-dark cycle. Rats
had ad libitum access to food and autoclaved water. All
animal procedures were approved by the Animal Ethics Committee of
Fuzhou General Hospital of the Fujian province of China (Fuzhou,
China).
Isolation and culture of rat ADSCs
Male Sprague-Dawley rats were anesthetized with 2%
pentobarbital sodium (40 mg/kg; wt; i.p.; Merck Chemicals,
Shanghai, China), and the adipose tissues (~3×1.5×0.5 cm) were
collected by excision from the subcutaneous inguinal region of male
Sprague-Dawley rats, and washed thoroughly with phosphate-buffered
saline (PBS; GE Healthcare Life Sciences, Logan, UT, USA).
Subsequently, the tissues were cut into small sections
(~0.1mm3) and digested with 0.1% type I collagenase
(Sigma-Aldrich, St. Louis, MO, USA) at 37°C with gentle agitation
for 60 min. Subsequently, the type I collagenase activity was
neutralized by adding an equal volume (10 ml per rat) of α-modified
Eagle's minimum essential medium (α-MEM; GE Healthcare Life
Sciences) containing 20% fetal bovine serum (FBS; Gibco Life
Technologies, Carlsbad, CA, USA), followed by filtering through an
100 µm cell strainer and centrifugation at 400 × g for 10 min at
room temperature. The supernatant containing the lipid droplets was
discarded and the cell pellet was washed twice with PBS. The cell
pellets containing the erythrocytes were then lysed with osmotic
lysates (Biyuntian Biotech Co., Ltd., Shanghai, China). The
remaining cells were suspended in the complete medium, consisting
of α-MEM, with 20% FBS, supplemented with 1%
penicillin/streptomycin (Gibco Life Technologies). Subsequently,
the resuspended cells were immediately transferred into 25
cm2 cell culture flasks (Corning Incorporated, Corning,
NY, USA) at a density of 1×106 cells/ml, and incubated
at 37°C with 5% CO2. The medium was replaced after 24 h
and the adherent cells were further expanded with complete medium,
which was replaced every 2 days. Once the cells had reached ~80%
confluence, they were detached using 0.25% trypsin-0.02% EDTA
(Gibco Life Technologies) and passaged at a dilution of 1:3.
Cultured cells at the third passage were used for the analysis of
cell surface markers and for transplantation into the livers of the
NAFLD rats.
Flow cytometry analysis
The surface biomarkers of the ADSCs were
characterized using flow cytometry to ensure cell quality. The
cultured cells were detached using 0.25% trypsin/0.02% EDTA and
were suspended in PBS containing 5% bovine serum albumin
(Sigma-Aldrich). Subsequently, the cells were incubated with the
following primary antibodies for 60 min at room temperature:
PE-conjugated anti-mouse/rat CD29 (monoclonal; 1:200; eBioscience,
San Diego, CA, USA; cat. no. 12-0291-82), mouse anti-rat/human CD31
(monoclonal; 1:100; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA; cat. no. SC-80913), mouse anti-rat/human CD34 (monoclonal;
1:100; Santa Cruz Biotechnology, Inc.; cat. no. SC-7324),
PE-conjugated anti-mouse/rat CD44H (monoclonal; 1:200; eBioscience;
cat. no. 12-0444-82), rabbit anti-rat/human/mouse CD45 (polyclonal;
1:100; Santa Cruz Biotechnology, Inc.; cat. no. SC-25590) and
rabbit anti-rat/human/mouse CD90 (polyclonal; 1:100; Santa Cruz
Biotechnology, Inc.; cat. no. SC-9163). The cells were then washed
twice with PBS and further incubated with fluorescent-conjugated
secondary antibodies (donkey anti-mouse IgG-Alexa Fluor®
488 (polyclonal, 1:1,000) or donkey anti-rabbit IgG-Alexa
Fluor® 647 (polyclonal, 1:1,000), obtained from
Invitrogen Life Technologies (Carlsbad, CA, USA; cat. no. A-21202
or A31573) for 30 min at room temperature. Finally, the cells were
washed twice with PBS and characterized using a
fluorescence-activated cell sorter (FACS; BD Biosciences, Franklin
Lakes, NJ, USA). The raw data was further analyzed using FlowJo 7.6
software (FlowJo, LLC, Ashland, OR, USA).
Transplantation of ADSCs into the livers
of NAFLD rats
The NAFLD rat model was induced using a previously
described procedure (17) with
modification. Briefly, the rats were fed either with normal chow
(control group) or with a HFD, which contained 88% normal chow, 2%
cholesterol and 10% lard, for 6 weeks. Subsequent to the
development of hepatic steatosis, the NAFLD rats (n=20) were
randomly divided into two groups: The NAFLD group (n=10), treated
with PBS (1 ml/rat), as a mock-treatment group; and the ADSCs
therapy group (n=10), which received intrahepatic transplantation
of ADSCs (2×106 cells/rat). The transplantation was
performed as follows: The rats were temporarily anesthetized with
diethyl ether (Sinopharm Chemical Reagent Co. Ltd., Shanghai,
China), following which the abdominal cavity was opened under
aseptic conditions, and the portal vein was exposed using moistened
swabs. Subsequently, the suspension of ADSCs in PBS was injected
into the portal vein using a 24-gauge needle. All NAFLD rats were
continuously fed with HFD and were sacrificed with 2% pentobarbital
sodium (100 mg/kg; wt; i.p.) (Merck Chemicals) 2 or 4 weeks after
transplantation. The liver tissues (~400mg per rat) and sera (~3 ml
per rat) were simultaneously collected for further
investigation.
Biochemical assays of liver function
The collected sera was separated by centrifugation
at 1,000 × g at 4°C for 10 min, then was stored at −80°C for
further use. The serum levels of total cholesterol (TC),
triglycerides (TG), fatty acids (FA), alanine aminotransferase
(ALT), aspartate aminotransferase (AST) and total bilirubin (TBIL)
were measured using TC, TG, FA, ALT, AST and TBIL ELISA kits,
respectively, according to the manufacturer's instructions (Nanjing
Jiancheng Bioengineering Institute, Nanjing, China).
To further investigate whether ADSC transplantation
improved hepatic lipid peroxidation, each liver sample was
homogenized in stroke-physiological saline solution (Fuzhou
Neptunus Futao Pharmaceuticals Co., Ltd, Fuzhou, China), and was
then centrifuged at 5,000 × g for 10 min at 4°C. The supernatants
were collected and the activity of liver superoxide dismutase
(SOD), and the content of malondialdehyde (MDA) were measured using
SOD and MDA assay kits (Nanjing Jiancheng Bioengineering
Institute), according to the manufacturer's instructions. The
activity of SOD and the MDA content were normalized to the level of
total protein, which was measured using a bicinchoninic acid assay
kit (Beijing TransGen Biotech Co., Ltd., Beijing, China).
Histopathological assessment
The obtained liver tissues were fixed in 4%
paraformaldehyde for 24 h, then were gradually dehydrated with
ethanol, embedded in paraffin and stained with hematoxylin and
eosin (H&E) staining kit (Jiancheng Bioengineering Institute,
Nanjing, China). The presence of hepatic steatosis and inflammation
evaluated by two pathologists in a double-blinded manner. To
further visualize the accumulation of fat in the liver, oil red O
staining was performed using a commercial kit (Nanjing Jiancheng
Bioengineering Institute), according to the manufacturer's
instructions. The histopathological examination was performed using
an Olympus microscope (BX51; Tokyo, Japan).
Assessment of pro-inflammatory
cytokines
The secretion levels of tumor necrosis factor
(TNF)-α, interleukin (IL)-6, cyclooxygenase (COX)-2, IL-1β,
monocyte chemoattractant protein (MCP)-1 and interferon (IFN)-γ in
the serum were evaluated using ab ELISA (Shanghai BlueGene Biotech
Co., Ltd., Shanghai, China), according to the manufacturer's
instructions. Briefly, the standards and samples were added to an
antibody-coated 96-well plate, incubated at 37°C for 1 h and,
followed with double-distilled water 3 times, the substrate was
then added to each well at 37°C for 10 min. Absorbance values were
measured at 450 nm using a SpectraMax M5e microplate reader
(Molecular Devices, LLC, Sunnyvale, CA, USA).
Statistical analyses
GraphPad Prism, version 6.00 (GraphPad Software,
Inc., La Jolla, CA, USA) for Windows was used for statistics
analysis. All quantitative data are expressed as the mean ±
standard deviation. Statistical analysis among different groups was
performed using Student's t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Characterization of rat ADSCs
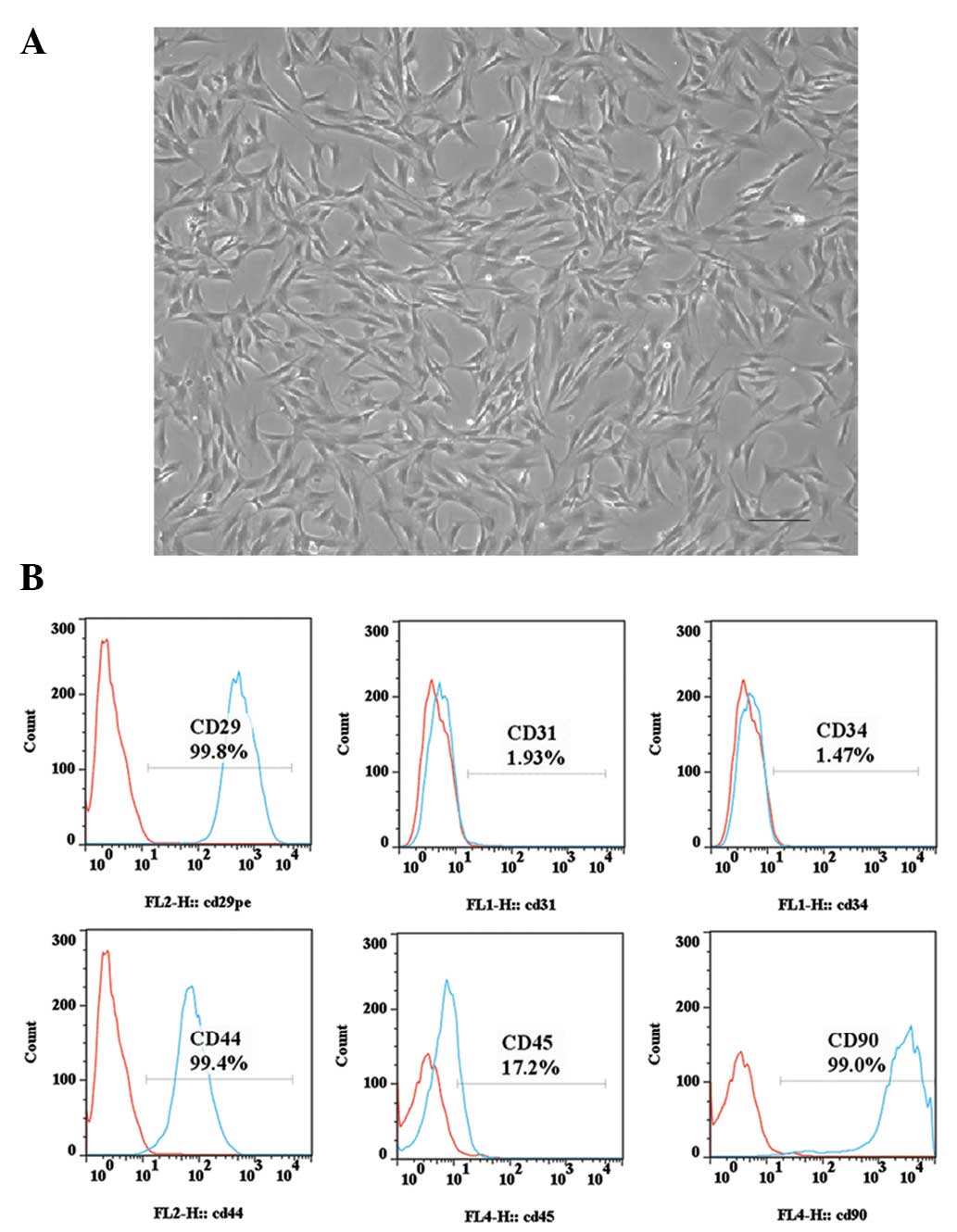
The derived ADSCs were first characterized by their
fibroblast-like morphology, which is consistent with the
morphological characteristics of ADSCs from other species (Fig. 1A) (13). Subsequently, the specific surface
biomarkers of ADSCs were characterized using fluorescence-activated
cell sorting, in which CD29 (99.8%), CD44 (99.4%) and CD90 (99.0%)
were positively expressed, CD45 (17.2%) was moderately expressed,
and CD31 (1.93%) and CD34 (1.47%) were negatively expressed
(Fig. 1B). This expression pattern
of the surface biomarkers was consistent with that reported for
characterization of ADSCs (14).
Therefore, the derived cells were deemed suitable for further
transplantation experiments.
ADSC transplantation improves the hepatic
functions of NAFLD model rats
To confirm whether transplantation of ADSCs was able
to attenuate hepatic injuries, serum levels of ALT, AST and TBIL,
which are associated with liver function, were measured 2 or 4
weeks following ADSC therapy. As shown in Fig. 2, the serum levels of ALT and TBIL
in the NAFLD rats were significantly higher following 2 weeks of
further feeding with an HFD (8 weeks HFD in total), compared with
the normal rats, however, the AST level was not significantly
different. On further feeding of NAFLD rats with HFD for 4 weeks
(10 weeks HFD in total), the serum levels of ALT, TBIL and AST were
significantly higher, compared with the normal rats, indicating
severe damage to the liver function of NAFLD rats. At 2 weeks
post-ADSC transplantation into the NAFLD rats, the serum levels of
ALT, TBIL and AST were all significantly reduced, almost to the
levels observed in the normal rats, which demonstrated that
transplantation with ADSCs protected the liver function against
damage. However, only ALT remained significantly lower 4 weeks
after ADSC transplantation, compared with the NAFLD rats. The
levels of TBIL and AST were no longer significantly different
compared with the NAFLD rats, possibly due to the continuous HFD
feeding during the treatment of all rats.
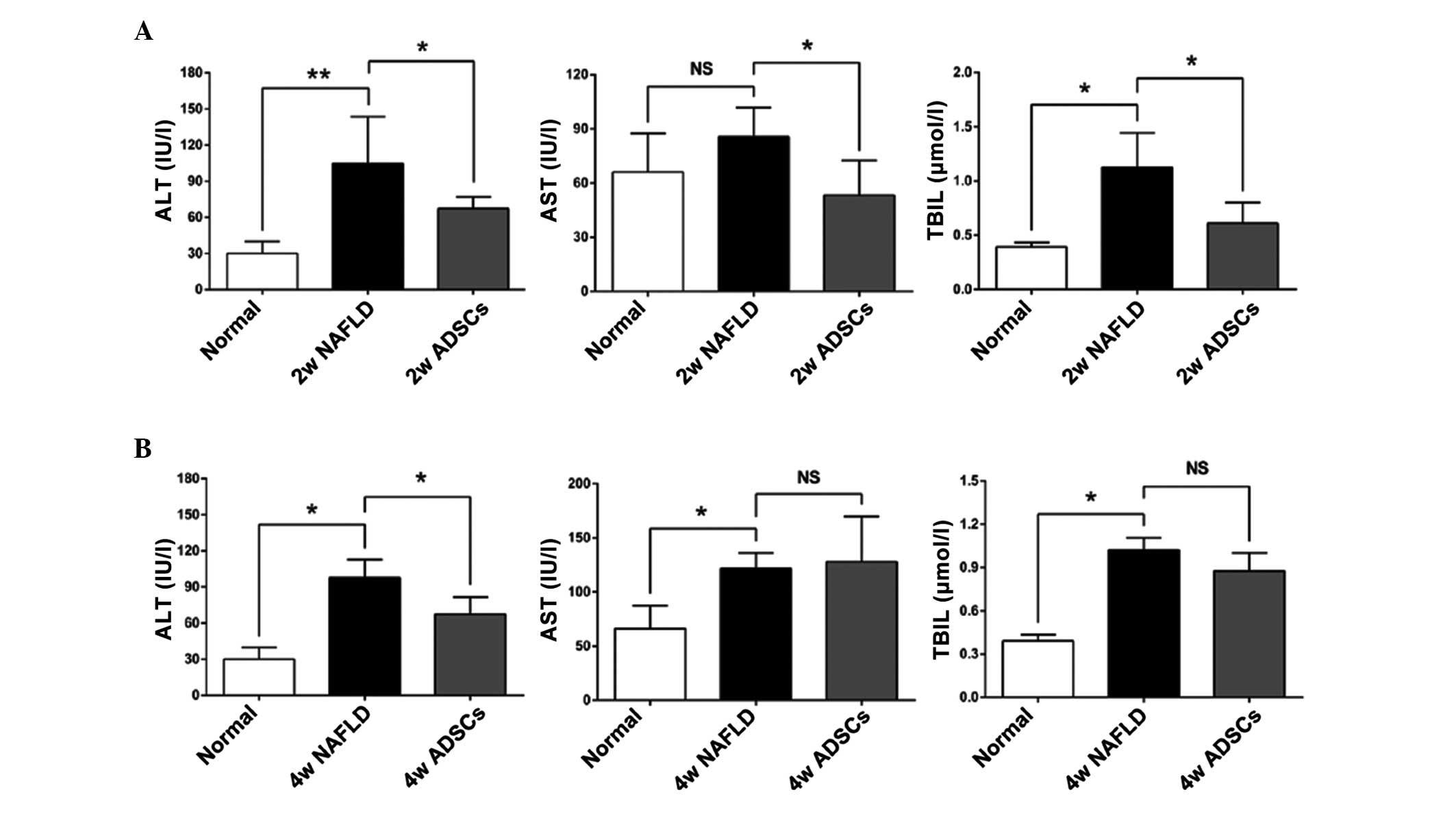 | Figure 2Serum levels of ALT, AST and TBIL
following ADSC transplantation. At (A) 2 or (B) 4 weeks post-ADSC
transplantation, the serum levels of ALT, AST and TBIL were
measured by ELISA. Compared with the NAFLD group, the ADSC groups
exhibited significantly reduced serum levels of ALT, AST and TBIL 2
weeks after transplantation, however, only ALT was reduced 4 weeks
after transplantation. For all groups, n=5. *P<0.05;
**P<0.01. Normal, untreated rats; 2w/4w NAFLD, NAFLD
rats fed a high fat diet (HFD) for a further 2/4 weeks; 2w/4w
ADSCs, 2/4 weeks after intrahepatic transplantation of ADSCs into
NAFLD rats.. ALT, alanine aminotransferase; AST, aspartate
aminotransferase; TBIL, total bilirubin; ADSCs, adipose
tissue-derived stem cells; NAFLD, non-alcoholic fatty liver
disease; HFD, high-fat-diet; NS, not significant. |
Transplantation with ADSCs promotes lipid
metabolism in NAFLD rats
Disorder of lipid metabolism is the predominant
biochemical change in the development of NAFLD (1). To determine whether ADSC
transplantation promoted the lipid metabolism in the livers of
NAFLD rats, the serum levels of TC, TG and FA were measured 2 or 4
weeks after transplantation with the ADSCs. As shown in Fig. 3, higher levels of TC, TG and FA
were observed in the NAFLD rats, compared with the normal rats,
which indicated that the NAFLD rats had imbalanced lipid
metabolism. At 2 weeks post-ADSC transplantation in the NAFLD rats,
the serum levels of TC and TG were significantly reduced, while the
serum level of FA was not significantly reduced, compared with the
NAFLD rats. However, at 4 weeks post-ADSC transplantation, the
serum levels of TC, TG and FA were all significantly lower than
those in the NAFLD rats, suggesting that the transplanted ADSCs
promoted lipid metabolism.
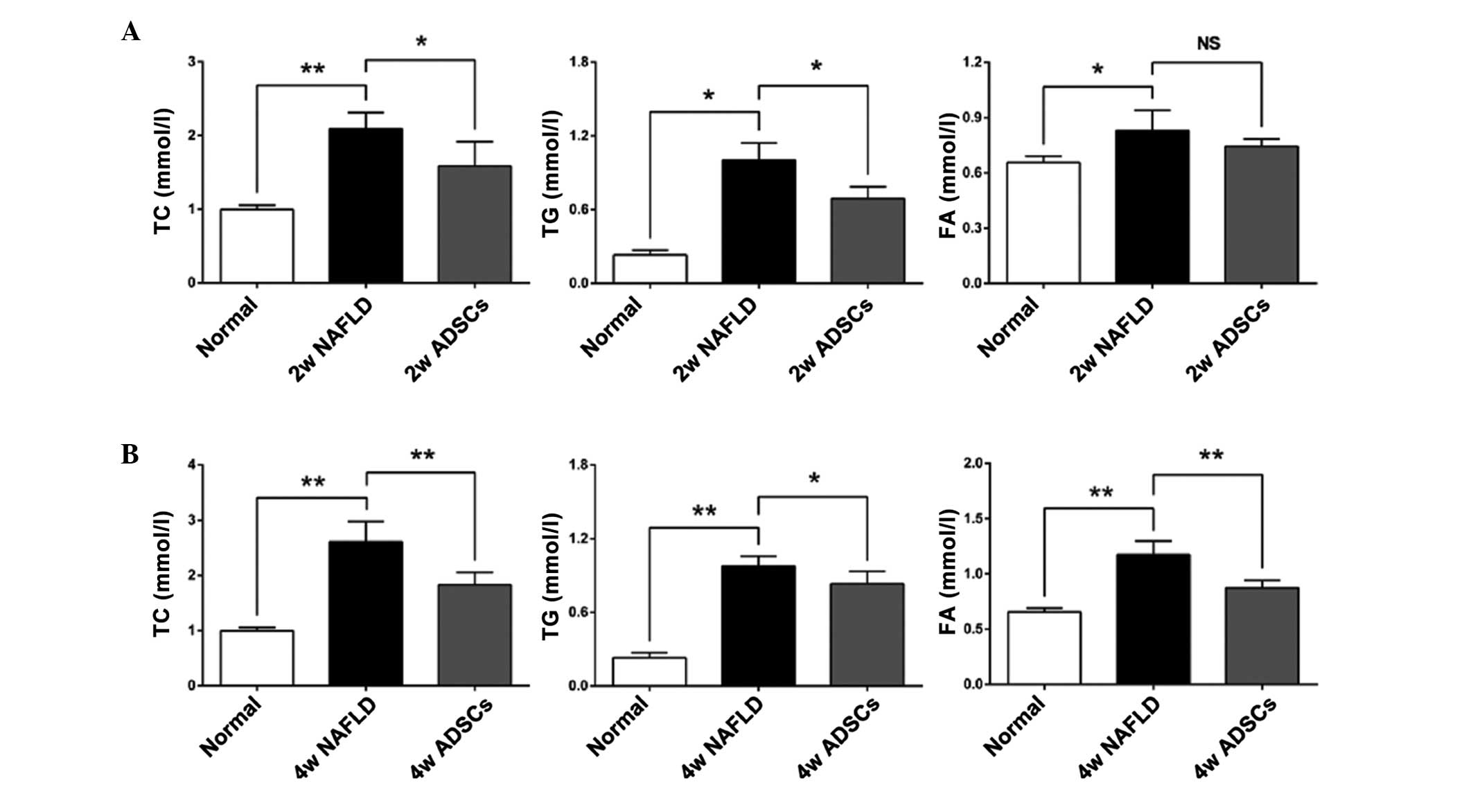 | Figure 3Serum levels of FA, TG and TC
following transplantation of ADSCs. The serum levels of TC, TG and
FA were measured (A) 2 weeks or (B) 4 weeks after ADSCs
transplantation. Compared with the NAFLD groups, transplantation
with ADSCs significantly reduced the serum levels of TC and TG at 2
and 4 weeks post-transplantation. The serum level of FA was only
reduced 4 weeks after transplantation. For all groups, n=5. Data
are expressed as the mean ± standard
deviation..*P<0.05; **P<0.01. FA, fatty
acids; TG, triglycerides; TC, total cholesterol; ADSCs, adipose
tissue-derived stem cells; NAFLD, non-alcoholic fatty liver
disease; NS, not significant; Normal, untreated rats; 2w/4w NAFLD,
NAFLD rats fed high fat diet for a further 2/4 weeks; 2w/4w ADSCs,
2/4 weeks after intrahepatic transplantation of ADSCs into NAFLD
rats. |
Transplantation with ADSCs reduces
oxidative stress in the liver tissues
To further examine the effect of ADSC
transplantation on oxidative stress in the livers of NAFLD rats,
the SOD activity and MDA content in the liver homogenates were
evaluated. As shown in Fig. 4, the
activity of SOD and MDA content in the NAFLD rats, which received
another 2 weeks HFD feeding (8 weeks HFD in total) were not
significantly different, compared with the normal rats. However,
the SOD activity was significantly reduced and the MDA content was
significantly increased in the NAFLD rats, which received another 4
weeks HFD feeding (10 weeks HFD in total), compared with the normal
rats, indicating that oxidative stress was induced in the NAFLD
rats. At 2 weeks post-ADSC transplantation in the NAFLD rats, the
SOD activity and MDA content were not significantly altered.
However, the SOD activity was significantly higher and the MDA
content was significantly lower, compared with the NAFLD rats, at 4
weeks post-ADSC transplantation. These results suggested that ADSC
transplantation reduces oxidative stress in the liver.
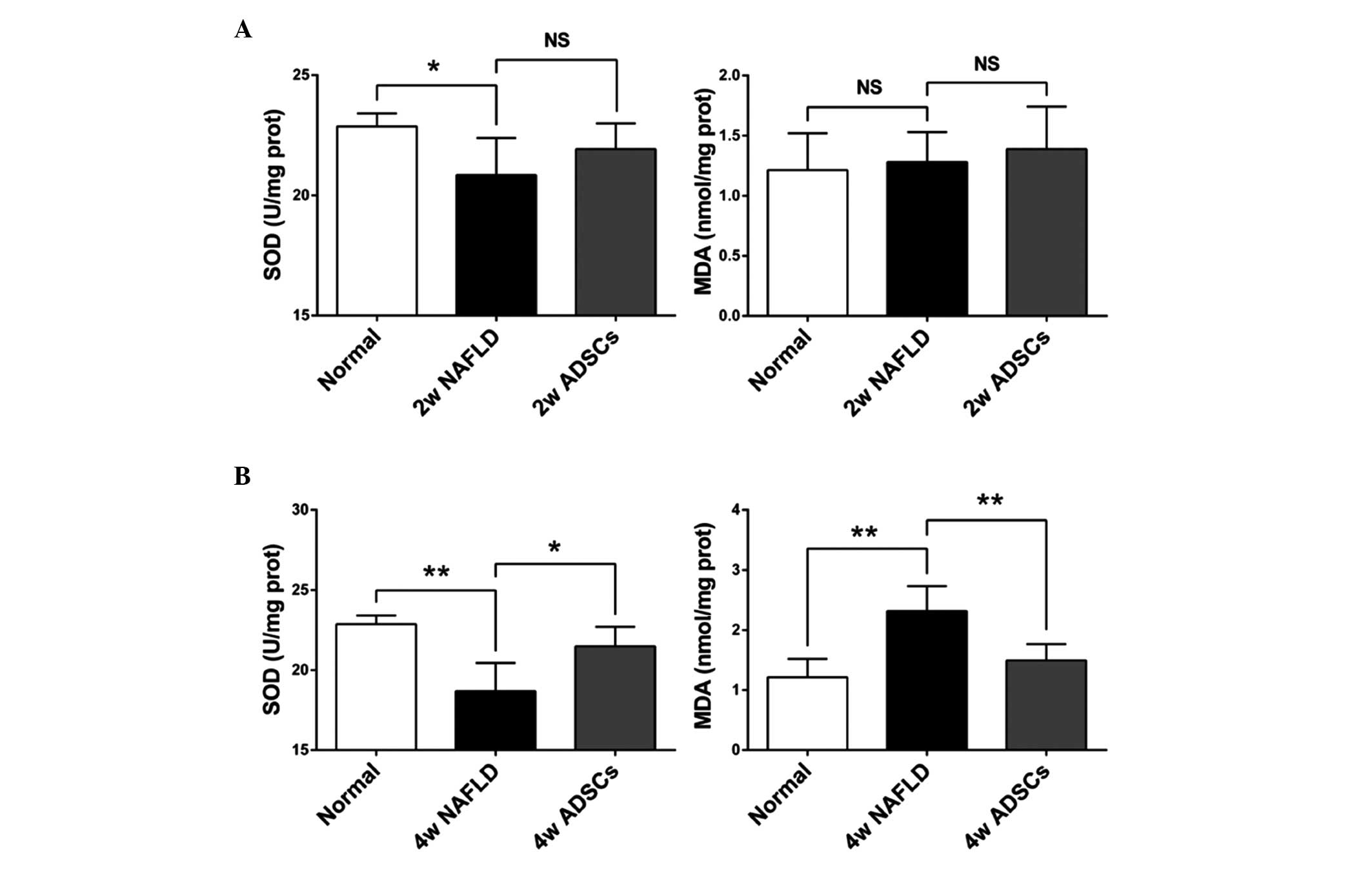 | Figure 4SOD activity and MDA content in liver
tissues following transplantation of ADSCs. The liver homogenate
levels of MDA and SOD activity, which are closely associated with
oxidative stress, were measured following (A) 2 or (B) 4 weeks of
ADSCs transplantation. Compared with the NAFLD groups, ADSCs were
able to significantly reduce the MDA contents and increase the SOD
activity following 4 weeks of transplantation. For all groups, n=5.
Data are expressed as the mean ± standard deviation.
*P<0.05; **P<0.01. SOD, superoxide
dismutase; MDA, malondialdehyde; ADSCs, adipose tissue-derived stem
cells; NAFLD, non-alcoholic fatty liver disease; NS, not
significant; Normal, untreated rats; 2w/4w NAFLD, NAFLD rats fed a
high fat diet for a further 2/4 weeks; 2w/4w ADSCs, 2/4 weeks after
intrahepatic transplantation of ADSCs into NAFLD rats. |
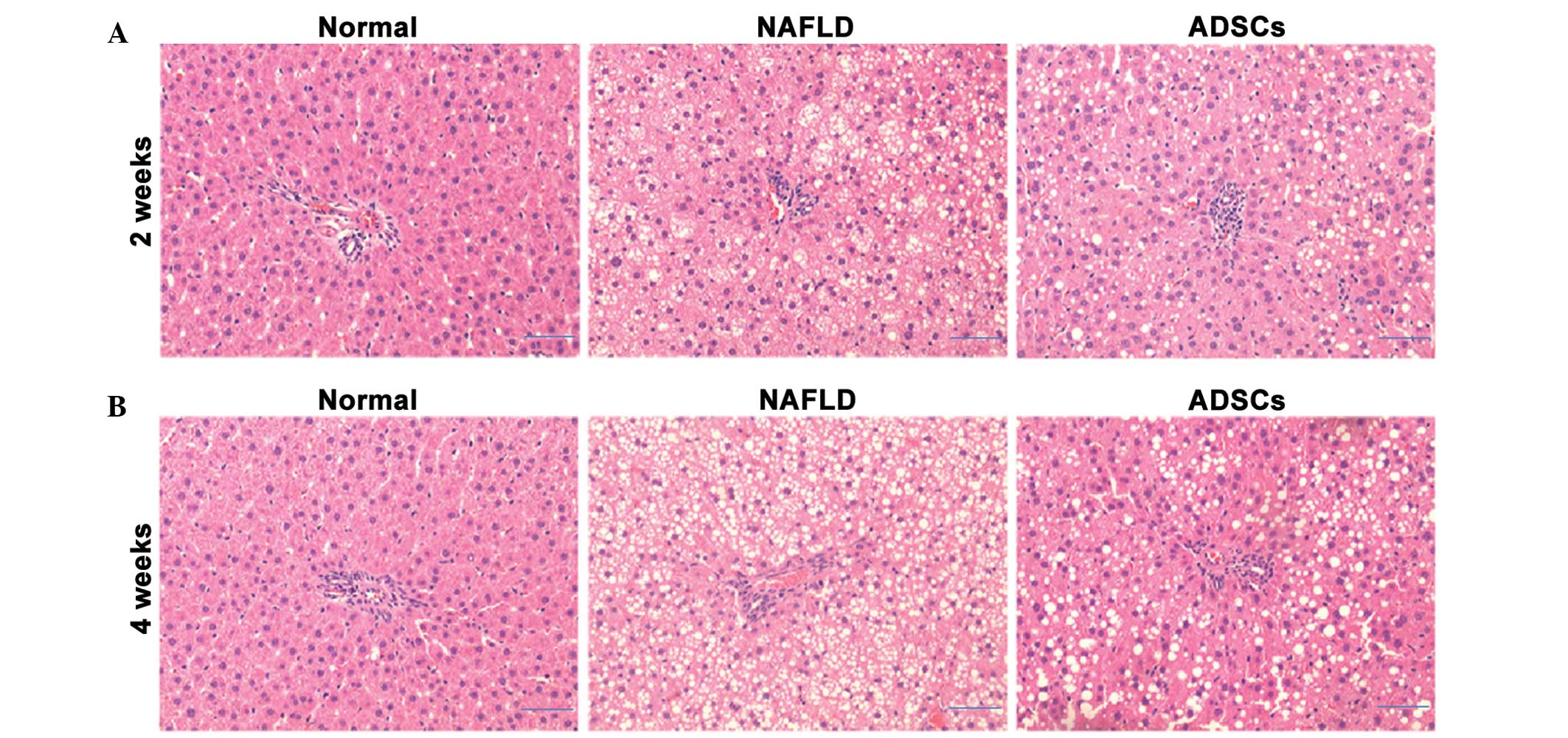
ADSCs transplantation decelerates hepatic
pathological changes in NAFLD rats
Hepatic histopathological assessment is the gold
standard for NAFLD diagnosis (18). In order to determine whether
transplantation with ADSCs has a protective role by slowing
pathological progression, the liver tissues were evaluated
following H&E staining. As shown in Fig. 5, no evidence of steatosis or
inflammation was identified in the liver tissues of the normal
group. Following HFD feeding, the NAFLD group exhibited typical
steatosis, indicating that the NAFLD model was successfully
established. However, evidence of steatosis was limited 2 weeks
post-ADSC transplantation, and low gradesteatosis was observed 4
weeks post-ADSC transplantation, which indicated that the ADSCs
attenuated the development of NAFLD.
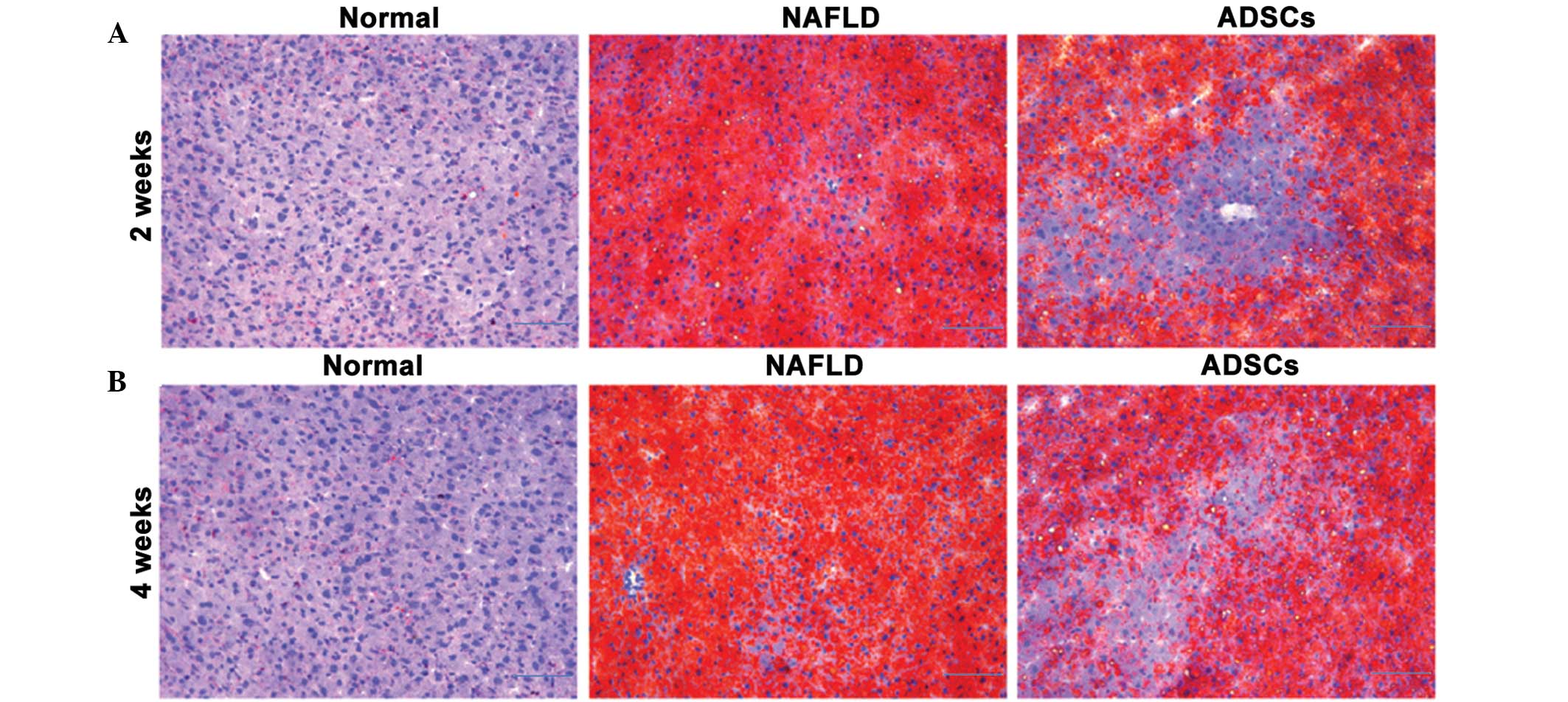
The excessive accumulation of hepatic lipids is the
major pathological feature in the progression of NAFLD (19). To further confirm whether
transplantation with ADSCs reduced lipid accumulation in the liver
tissues, the hepatic lipid contents were evaluated using oil red O
staining. As shown in Fig. 6, no
lipid accumulation was observed in the normal rats, however,
numerous lipids were observed in the liver tissues of the NAFLD
rats. The ADSC group exhibited lower lipid accumulation, compared
with the NAFLD rats, suggesting that the ADSCs reduced lipid
accumulation in the livers of NAFLD rats, thus decelerating disease
progression.
ADSC transplantation selectively reduces
the serum levels of TNF-α and IL-6 in NAFLD rats
Inflammation is important in the pathogenesis of
hepatic steatosis to NASH. To further assess whether
transplantation with ADSCs had an immunoregulatory role by reducing
the secretion of pro-inflammatory cytokines, the serum levels of
TNF-α, IL-6, COX-2, IL-1β, MCP-1 and IFN-γ were detected by ELISA.
As shown in Fig. 7, the serum
levels of IL-6 and IFN-γ were significantly increased in the NAFLD
rats, which received another 2 weeks HFD feeding (8 weeks HFD in
total), while no significant differences were observed in the other
cytokines between this group and the normal rats. This indicated
that the rat livers were not undergoing inflammation. However,
following 4 weeks HFD feeding of the NAFLD rats (10 weeks HFD in
total), the serum levels of TNF-α, IL-6, IL-1β, MCP-1 and IFN-γ
were all significantly increased, with no significant change in
COX-2 levels, compared with the normal rats. These data suggested
that the rat livers were undergoing inflammation. At 2 weeks
post-ADSC transplantation, none of the measured cytokines were
significantly different, compared with the NAFLD rats, due to the
fact that inflammation was not present in the rat livers. Notably,
the serum levels of IL-6 and TNF-α, which are predominantly
secreted by the kuffer cells in the liver and actively contribute
to the acute inflammatory responses (20,21),
were significantly reduced at 4 weeks post-ADSC transplantation,
compared with the NAFLD rats, although no significant changes were
observed in the other cytokines measured,. Taken together, these
results demonstrated that ADSC transplantation selectively reduced
the secretion of pro-inflammatory cytokines, including TNF-α and
IL-6, attenuating the progression of NAFLD.
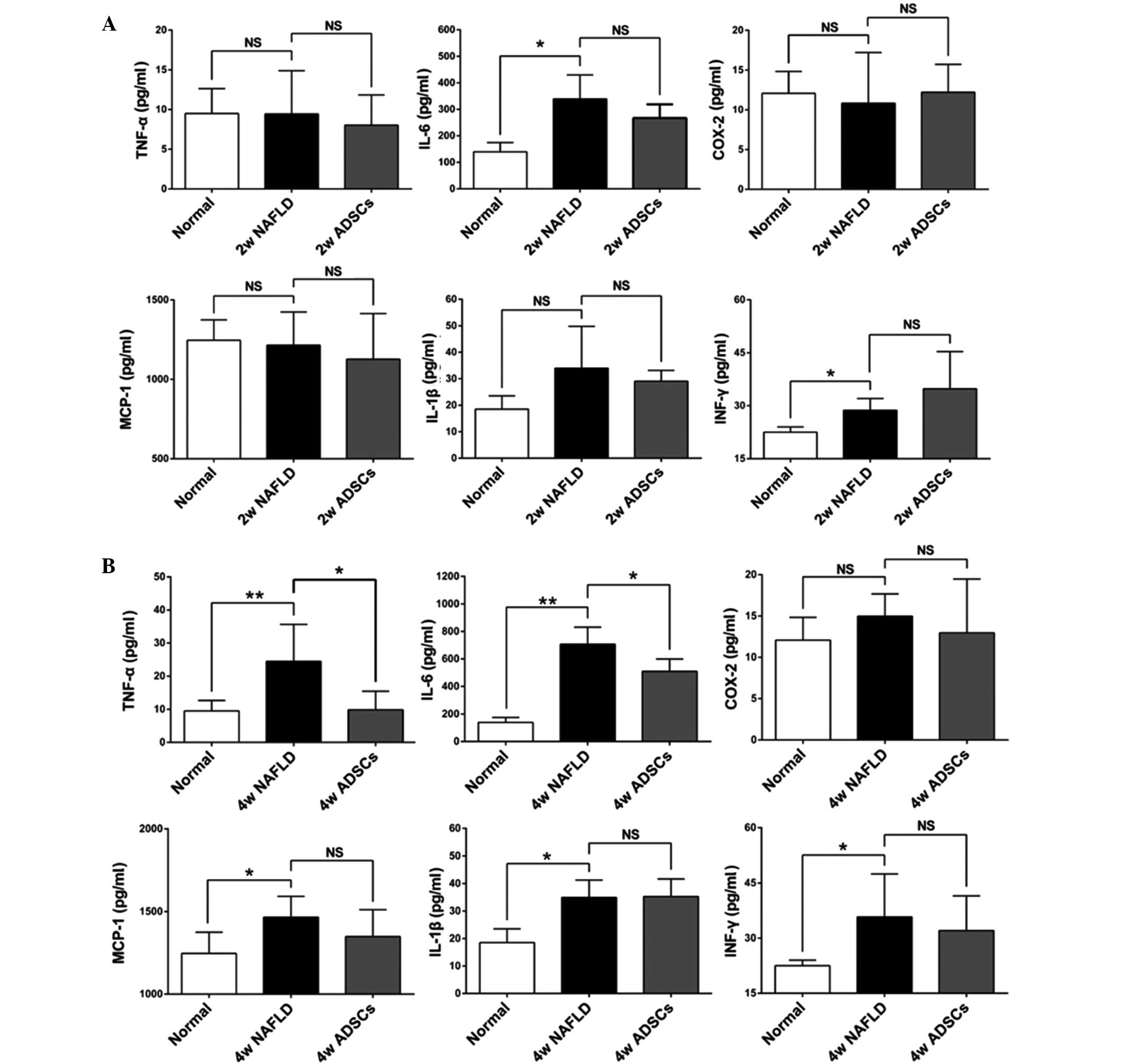 | Figure 7Serum levels of pro-inflammatory
cytokines following ADSC transplantation. The serum levels of
TNF-α, IL-6, COX-2, IL-1β, MCP-1 and IFN-γ were measured by ELISA
(A) 2 or (B) 4 weeks after ADSC transplantation. Compared with the
NAFLD rats, significantly reduced the serum levels of TNF-α and
IL-6 were observed 4 weeks after transplantation. For all groups,
n=5. Data are expressed as the mean ± standard deviation.
*P<0.05; **P<0.01. ADSCs, adipose
tissue-derived stem cells; TNF-α, tumor necrosis factor-α; IL-6,
interleukin 6; MCP-1, monocyte chemoattractant protein 1; IFN-γ,
interferon-γ; NAFLD, non-alcoholic fatty liver disease; NS, not
significant; Normal, untreated rats; 2w/4w NAFLD, NAFLD rats fed a
high fat diet for a further 2/4 weeks; 2w/4w ADSCs, 2/4 weeks after
intrahepatic transplantation of ADSCs into NAFLD rats. |
Discussion
MSC transplantation is being investigated as an
alternative therapeutic approach for liver transplantation in
chronic liver diseases (22).
Previous studies have demonstrated that multi-potent stromal cells
and the hepatocyte-like cells from bone marrow-derived stem cells
may offer a potential solution to prevent the progression of NAFLD
in animal models (23,24). However, whether ADSCs have a
therapeutic effect on NAFLD remains to be fully elucidated. In the
present study, ADSCs were able to efficiently protect the NAFLD
rats from disease progression, which suggested that ADSC
transplantation may be an effective therapeutic approach for
NAFLD.
According to the 'two-hit' hypothesis, the 'first
hit' is marked by the accumulation of hepatic lipids, but not due
to the alcohol abuse (1,4,25),
following which liver function is damaged. In the present study,
transplantation with ADSCs improved liver function through
reductions in ALT activity and total TBIL (Fig. 2), in addition to promoting lipid
metabolism (Fig. 3). These
observations were consistent with the hepatic pathological
morphological changes in the H&E (Fig. 5) and oil red O staining (Fig. 6), confirming that ADSCs attenuated
the disease progression of NAFLD.
Under pathological conditions, excessive lipid
accumulations can cause damage via peroxidation, resulting in the
occurrence of marked oxidative stress (26–28).
In the present study, transplantation with ADSCs reduced oxidative
stress by increasing the activity of SOD and reducing the content
of MDA (Fig. 4), which may result
from the suppression of lipid peroxidation by ADSC
transplantation.
It has been demonstrated that inflammation is
important in the progression of NAFLD (26,29,30).
In the present study, high levels of pro-inflammatory cytokines,
including IL-6, TNF-α, IL-1β, MCP-1 and IFN-γ were observed in the
NAFLD rats, which suggested that inflammation was induced in the
model. However, the secretions of IL-6 and TNF-α, which are
considered to be major inflammatory mediators of steatosis, insulin
resistance and associated inflammatory disorders (31,32),
were significantly inhibited following ADSC transplantation. These
results suggested that ADSC transplantation selectively inhibited
inflammation, mediated by IL-6 and TNF-α. Notably, the level of
COX-2 was not increased in the NAFLD rats, compared with the normal
rats, which may be due to the possibility that the animal model had
not progressed to NASH, as significant increases in COX-2 are
reported to appear only in the NASH stage (33,34).
Certain key parameters, including serum TBIL and AST
were only reduced 2 weeks after transplantation with ADSCs,
however, these changes were not observed 4 weeks after
transplantation. This may be due to continued HFD feeding
attenuating the therapeutic effects of ADSC transplantation. To
more effectively prevent NAFLD progression, intrahepatic
transplantation may be combined with multiple supplementary
injections of ADSCs via the tail or penis vein, to further maintain
the therapeutic effects.
ADSCs may be more suitable for clinical use due to
the advantages of being abundant, readily maintained, highly
proliferative and exhibiting improved immunoregulatory activities
(9,35), however, further investigation into
the possible tumorigenic risk are to be performed prior to clinical
application. In the present study, the application of ADSC
transplantation reduced the rate of disease progression in the
NAFLD rat model. The results demonstrated that ADSC transplantation
significantly improved liver function, reduced lipid accumulation
and attenuated the inflammatory state of the liver, therefore
decelerating the progression of NAFLD in the rat model. In
conclusion, ADSC transplantation may offer a promising therapeutic
approach for the treatment of NAFLD in the future.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant no. 31201008), the Scientific
Foundation of Fuzhou Health Department (grant no. 2013-S-wq15), the
Research Development Foundation of Fujian Medical University (grant
no. FZS13001Y), the Key Medical Foundation of Nanjing Military
Command of China (grant no. 14ZX22), the National Natural Science
Foundation of Fujian Province of China (grant no. 2012J01407), the
Research Development Foundation of Fuzhou General Hospital of
Fujian Province of China (grant no. 2014J08) and the University
Natural Science Foundation of Jiangsu Province (grant no.
12KJB180013).
References
|
1
|
Ibrahim MA, Kelleni M and Geddawy A:
Nonalcoholic fatty liver disease: Current and potential therapies.
Life Sci. 92:114–118. 2013. View Article : Google Scholar
|
|
2
|
Henao-Mejia J, Elinav E, Jin C, Hao L,
Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ,
et al: Inflammasome-mediated dysbiosis regulates progression of
NAFLD and obesity. Nature. 482:179–185. 2012.PubMed/NCBI
|
|
3
|
Rezazadeh A, Yazdanparast R and Molaei M:
Amelioration of diet-induced nonalcoholic steatohepatitis in rats
by Mnsalen complexes via reduction of oxidative stress. J Biomed
Sci. 19:262012. View Article : Google Scholar
|
|
4
|
Shaker M, Tabbaa A, Albeldawi M and
Alkhouri N: Liver transplantation for nonalcoholic fatty liver
disease: New challenges and new opportunities. World J
Gastroenterol. 20:5320–5330. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nagaishi K, Ataka K, Echizen E, Arimura Y
and Fujimiya M: Mesenchymal stem cell therapy ameliorates diabetic
hepatocyte damage in mice by inhibiting infiltration of bone
marrow-derived cells. Hepatology. 59:1816–1829. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Banas A, Teratani T, Yamamoto Y, Tokuhara
M, Takeshita F, Quinn G, Okochi H and Ochiya T: Adipose
tissue-derived mesenchymal stem cells as a source of human
hepatocytes. Hepatology. 46:219–228. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kubo N, Narumi S, Kijima H, Mizukami H,
Yagihashi S, Hakamada K and Nakane A: Efficacy of adipose
tissue-derived mesenchymal stem cells for fulminant hepatitis in
mice induced by concanavalin A. J Gastroenterol Hepatol.
27:165–172. 2012. View Article : Google Scholar
|
|
8
|
Ghannam S, Bouffi C, Djouad F, Jorgensen C
and Noël D: Immunosuppression by mesenchymal stem cells: Mechanisms
and clinical applications. Stem Cell Res Ther. 1:22010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun CK, Chang CL, Lin YC, Kao YH, Chang
LT, Yen CH, Shao PL, Chen CH, Leu S and Yip HK: Systemic
administration of autologous adipose-derived mesenchymal stem cells
alleviates hepatic ischemia-reperfusion injury in rats. Crit Care
Med. 40:1279–1290. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Meyerrose TE, De Ugarte DA, Hofling AA,
Herrbrich PE, Cordonnier TD, Shultz LD, Eagon JC, Wirthlin L, Sands
MS, Hedrick MA, et al: In vivo distribution of human
adipose-derived mesenchymal stem cells in novel xenotransplantation
models. Stem Cells. 25:220–227. 2007. View Article : Google Scholar
|
|
11
|
Philippe B, Luc S, Valérie PB, Jérôme R,
Alessandra BR and Louis C: Culture and use of mesenchymal stromal
cells in phase I and II clinical trials. Stem Cells Int.
2010:5035932010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Y, Chen XM and Sun DL: Effects of
coencapsulation of hepatocytes with adipose-derived stem cells in
the treatment of rats with acute-on-chronic liver failure. Int J
Artif Organs. 37:133–141. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saito Y, Shimada M, Utsunomiya T, Ikemoto
T, Yamada S, Morine Y, Imura S, Mori H, Sugimoto K, Iwahashi S, et
al: The protective effect of adipose-derived stem cells against
liver injury by trophic molecules. J Surg Res. 180:162–168. 2013.
View Article : Google Scholar
|
|
14
|
Harn HJ, Lin SZ, Hung SH, Subeq YM, Li YS,
Syu WS, Ding DC, Lee RP, Hsieh DK, Lin PC, et al: Adipose-derived
stem cells can abrogate chemical-induced liver fibrosis and
facilitate recovery of liver function. Cell Transplant.
21:2753–2764. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liedtke C, Luedde T, Sauerbruch T,
Scholten D, Streetz K, Tacke F, Tolba R, Trautwein C, Trebicka J
and Weiskirchen R: Experimental liver fibrosis research: Update on
animal models, legal issues and translational aspects. Fibrogenesis
Tissue Repair. 6:192013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nanji AA and French SW: Animal models of
alcoholic liver disease - focus on the intragastric feeding model.
Alcohol Res Health. 27:325–330. 2003.
|
|
17
|
Seki A, Sakai Y, Komura T, Nasti A,
Yoshida K, Higashimoto M, Honda M, Usui S, Takamura M, Takamura T,
et al: Adipose tissue-derived stem cells as a regenerative therapy
for a mouse steatohepatitis-induced cirrhosis model. Hepatology.
58:1133–1142. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vernon G, Baranova A and Younossi ZM:
Systematic review: The epidemiology and natural history of
non-alcoholic fatty liver disease and non-alcoholic steatohepatitis
in adults. Aliment Pharmacol Ther. 34:274–285. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Milagro FI, Campión J and Martínez JA:
Weight gain induced by high-fat feeding involves increased liver
oxidative stress. Obesity (Silver Spring). 14:1118–1123. 2006.
View Article : Google Scholar
|
|
20
|
Stauffer JK, Scarzello AJ, Jiang Q and
Wiltrout RH: Chronic inflammation, immune escape, and oncogenesis
in the liver: A unique neighborhood for novel intersections.
Hepatology. 56:1567–1574. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Budhu A and Wang XW: The role of cytokines
in hepatocellular carcinoma. J Leukoc Biol. 80:1197–1213. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Y, Lian F, Li J, Fan W, Xu H, Yang X,
Liang L, Chen W and Yang J: Adipose derived mesenchymal stem cells
transplantation via portal vein improves microcirculation and
ameliorates liver fibrosis induced by CCl4 in rats. J Transl Med.
10:1332012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ezquer M, Ezquer F, Ricca M, Allers C and
Conget P: Intravenous administration of multipotent stromal cells
prevents the onset of non-alcoholic steatohepatitis in obese mice
with metabolic syndrome. J Hepatol. 55:1112–1120. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Winkler S, Borkham-Kamphorst E, Stock P,
Brückner S, Dollinger M, Weiskirchen R and Christ B: Human
mesenchymal stem cells towards non-alcoholic steatohepatitis in an
immunodeficient mouse model. Exp Cell Res. 326:230–239. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gaggini M, Morelli M, Buzzigoli E,
DeFronzo RA, Bugianesi E and Gastaldelli A: Non-alcoholic fatty
liver disease (NAFLD) and its connection with insulin resistance,
dyslipidemia, atherosclerosis and coronary heart disease.
Nutrients. 5:1544–1560. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Takaki A, Kawai D and Yamamoto K:
Molecular mechanisms and new treatment strategies for non-alcoholic
steatohepatitis (NASH). Int J Mol Sci. 15:7352–7379. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mollica MP, Lionetti L, Moreno M, Lombardi
A, De Lange P, Antonelli A, Lanni A, Cavaliere G, Barletta A and
Goglia F: 3,5-diiodo-l-thyronine, by modulating mitochondrial
functions, reverses hepatic fat accumulation in rats fed a high-fat
diet. J Hepatol. 51:363–370. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zou Y, Li J, Lu C, Wang J, Ge J, Huang Y,
Zhang L and Wang Y: High-fat emulsion-induced rat model of
nonalcoholic steatohepatitis. Life Sci. 79:1100–1107. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Asrih M and Jornayvaz FR: Inflammation as
a potential link between nonalcoholic fatty liver disease and
insulin resistance. J Endocrinol. 218:R25–R36. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Del Ben M, Polimeni L, Carnevale R,
Bartimoccia S, Nocella C, Baratta F, Loffredo L, Pignatelli P,
Violi F and Angelico F: NOX2-generated oxidative stress is
associated with severity of ultrasound liver steatosis in patients
with non-alcoholic fatty liver disease. BMC Gastroenterol.
14:812014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Meli R, MattaceRaso G and Calignano A:
Role of Innate Immune Response in Non-Alcoholic Fatty Liver
Disease: Metabolic Complications and TherapeuticTools. Front
Immunol. 5:1772014. View Article : Google Scholar
|
|
32
|
Jung UJ and Choi MS: Obesity and its
metabolic complications: The role of adipokines and the
relationship between obesity, inflammation, insulin resistance,
dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci.
15:6184–6223. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen J, Liu D, Bai Q, Song J, Guan J, Gao
J, Liu B, Ma X and Du Y: Celecoxib attenuates liver steatosis and
inflammation in non-alcoholic steatohepatitis induced by high-fat
diet in rats. Mol Med Rep. 4:811–816. 2011.PubMed/NCBI
|
|
34
|
Giannitrapani L, Ingrao S, Soresi M,
Florena AM, La Spada E, Sandonato L, D'Alessandro N, Cervello M and
Montalto G: Cyclooxygenase-2 expression in chronic liver diseases
and hepatocellular carcinoma: An immunohistochemical study. Ann N Y
Acad Sci. 1155:293–299. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Seki T, Yokoyama Y, Nagasaki H, Kokuryo T
and Nagino M: Adipose tissue-derived mesenchymal stem cell
transplantation promotes hepatic regeneration after hepatic
ischemia-reperfusion and subsequent hepatectomy in rats. J Surg
Res. 178:63–70. 2012. View Article : Google Scholar : PubMed/NCBI
|