Introduction
Hepatic fibrosis results from chronic injuries to
the liver caused by chronic hepatitis, alcohol abuse, toxic agents,
metabolic diseases involving an overload of iron or copper,
autoimmune diseases, or congenital abnormalities (1,2).
Hepatic fibrosis, which may ultimately lead to cirrhosis, is the
pathological basis of all chronic hepatic diseases, and is
characterized by an imbalance between the excessive synthesis and
decreased degradation of extracellular matrix (ECM) components,
specifically type I and type III collagen (3,4). The
pathophysiology of ECM formation during fibrosis is multifaceted
and complex, and is associated with alterations in the expression
levels of both ECM proteases, such as matrix metalloproteinases
(MMPs), and ECM protease inhibitors, such as tissue inhibitors of
metalloproteinases (TIMPs) (5).
ECM formation also requires an increase in the synthesis of
fibronectin and collagens (5). The
process of ECM formation is maintained by growth factors, such as
transforming growth factor-β1 (TGF-β1), and connective tissue
growth factor, as well as pro-inflammatory cytokines, such as tumor
necrosis factor α (TNF-α), interleukin (IL)-1β, and IL-6 (6,7).
Activated hepatic stellate cells (HSCs) are known to participate in
ECM remodeling by producing various types of collagen, MMPs, TIMPs,
and TGF-β1, thus deeply influencing fibrotic progression and
regression (8,9). Numerous studies have indicated that
the activation of HSCs is the cytological basis and the main
initiator of hepatic fibrosis (10–13).
Consequently, the inhibition of HSC proliferation has become an
important antifibrotic therapeutic strategy.
Krüppel-like factors (KLF) are a subclass of the
zinc-finger family of transcription factors. They are characterized
by a DNA-binding domain that contains a conserved sequence,
CX2CX3FX5LX2HX3H (14). The KLF
family consists of ≤16 members, which are in turn separated into
structurally associated subgroups (15). KLFs regulate gene expression and
are responsible for cell proliferation, apoptosis, differentiation,
embryonic development, and somatic cell reprogramming (16,17).
KLFs are also important regulators in the pathogenesis of various
diseases, including ECM remodeling (18–21).
Human KLF4 was initially identified in 1998 from a human umbilical
vein endothelial cell cDNA library, using a DNA probe containing
the zinc-finger region of human erythroid KLF (22). KLF4 has many important functions,
including the regulation, proliferation, and differentiation of
various epithelial and endothelial tissues (23,24).
Recent studies have shown that KLF4 regulates the expression of
certain genes, including MMP-1, MMP-13, TIMP-1, and TGF-β1, and is
responsible for ECM remodeling in Sprague-Dawley rat and mouse
aortic vascular smooth muscle cell lines (25,26).
However, the effects of KLF4 on HSCs and hepatic fibrosis remain
unknown. In the present study, KLF4 expression was inhibited by
transfecting chemically synthesized KLF4-specific small interfering
(si)RNA into human LX2 HSCs, with the aim of ascertaining the
effect of KLF4 on the ECM and its associated genes.
Materials and methods
Materials
The LX2 human HSC line was donated by Professor D.X.
Sun (Division of Liver Diseases, Bethune International Peace
Hospital, Shijiazhuang, China) and was originally sourced from the
Institute of Tumor Research of the Chinese Academy of Medical
Sciences (Beijing, China). Lipofectamine® 2000 and
TRIzol® reagent were obtained from Invitrogen Life
Technologies (Carlsbad, CA, USA). Fetal bovine serum (FBS),
Dulbecco's modified Eagle medium (DMEM), and Opti-MEM were
purchased from GE Healthcare Life Sciences (Logan, UT, USA). Rabbit
anti-human KLF4 monoclonal antibody (cat. no. ab151733), rabbit
anti-human collagen type I monoclonal antibody (cat. no. ab138492),
rabbit anti-human collagen type III monoclonal antibody (cat. no.
ab7778), and rabbit anti-human TIMP-1 monoclonal antibody (cat. no.
ab109125) were purchased from Epitomics, Inc. (Burlingame, CA,
USA). Rabbit anti-human MMP-1 polyclonal antibody (cat. no.
10371-2-AP) was purchased from ProteinTech Group, Inc. (Chicago,
IL, USA). IRDye800-conjugated monoclonal goat IgG secondary
antibody (cat. no. 611-132-002) was purchased from Rockland
Research Corp. (Rockland, MA, USA). The reverse transcription (RT)
reagents were purchased from Thermo Fisher Scientific, Inc.
(Waltham, MA, USA). The RT-quantitative polymerase chain reaction
(RT-qPCR) assay kit was purchased from BioTeke Corporation
(Beijing, China). The TGF-β1, TNF-α, and IL-1β enzyme-linked
immunosorbent assay (ELISA) kits were purchased from Shanghai
ExCell Biological Products Co., Ltd. (Shanghai, China).
Design of siRNAs
Using the Homo sapiens KLF4 mRNA nucleotide
sequence from GenBank (GI: 194248076; http://www.ncbi.nlm.nih.gov/nuccore/NM_004235.4), and
referring to the standard design strategy for siRNAs (27), three pairs of 21 bp reverse repeat
sequences targeting KLF4 mRNA were designed and synthesized by
Shanghai GenePharma Co., Ltd. (Shanghai, China): siRNA1 sense,
5′-UCC AUU ACC AAG AGC UCA UTT-3′, antisense, 5′-AUG AGC UCU UGG
UAA UGG ATT-3′; siRNA2 sense, 5′-GGU CAU CAG CGU CAG CAA ATT-3′,
antisense 5′-UUU GCU GAC GCU GAU GAC CTT-3′; and siRNA3 sense,
5′-GGA CUU UAU UCU CUC CAA UTT-3′; and antisense, 5′-AUU GGA GAG
AAU AAA GUC CTT-3′. An unrelated sequence was used as a control,
sense, 5′-UUC UCC GAA CGU GUC ACG UTT-3′ and antisense, 5′-ACG UGA
CAC GUU CGG AGA ATT-3′.
Cell culture
The LX2 cells were cultured in DMEM supplemented
with 12% FBS, 100 U penicillin, and 100 µg streptomycin
(Sangon Biotech Co., Ltd., Shanghai, China), at 37°C in a
humidified atmosphere containing 5% CO2. The medium was
changed every 2 days. The cells were subsequently digested with
0.25% trypsin (Gibco Life Technologies, Carlsbad, CA, USA) once the
cells had reached 80–90% confluence.
siRNA transfection
The LX2 cells were digested and dispersed with 0.25%
trypsin, prior to being seeded in 6-well plates. Once the cells had
reached 70–80% confluence, they were separated into four groups and
transfected with various siRNAs as follows: Control group; siRNA1
group; siRNA2 group; and siRNA3 group. Triplicate wells were
established for each group. The cells were transfected with siRNA
using Lipofectamine® 2000, according to the
manufacturer's instructions. Briefly, the LX2 cells were seeded
into 6-well plates at a density of 1.8×105 cells/well,
and cultured for 24 h until they reached ~80% confluence. The 5
µl siRNAs (20 µM) were subsequently mixed with 5
µl Lipofectamine® 2000 in 250 µl Opti-MEM
medium for 20 min at room temperature to allow complex formation.
The transfection mixture was then added to each well with 2 ml
FBS-free DMEM. Following a 6 h incubation, 200 µl FBS was
added to the mixture and incubated for an additional 24 h or 48 h,
prior to RNA harvesting and protein isolation.
RNA purification and RT-qPCR
Following an additional 24 h incubation period, the
total RNA was isolated using TRIzol® reagent, and
reverse transcribed into cDNA according to the manufacturer's
instructions. The cDNA (10 ng) was then used as the template for
qPCR. Using the primer design software Primer Premier 5.0 (Premier
Biosoft, Palo Alto, CA, USA), the specific primers for each gene
were synthesized by BGI (Beijing, China) as follows: KLF4 forward,
5′-ATC TTT CTC CAC GTT CGCGT-3′, reverse, 5′-GGA AGT CGC TTC ATG
TGGGA-3′; type I collagen forward, 5′-CCC AGC CAC AAA GAG TCT
ACAT-3′, reverse, 5′-TCA TGG TAC CTG AGG CCGTT-3′; type III
collagen forward, 5′-CGC CCT CCT AAT GGT CAAGG-3′, reverse 5′-TTC
TGA GGA CCA GTA GGGCA-3′; MMP-1 forward, 5′-CAT GCT TTT CAA CCA
GGCCC-3′, reverse 5′-GGG TAC ATC AAA GCC CCGAT-3′; and TIMP-1
forward, 5′-ACT TCC ACA GGT CCC ACAAC-3′, and reverse, 5′- GCA TTC
CTC ACA GCC AACA-3′. GAPDH was used as an internal control and had
the following primer sequence: Forward, 5′-TGG TAT CGT GGA AGG
ACTCA-3′, and reverse 5′-CCA GTA GAG GCA GGG ATGAT-3′. RT-qPCR was
performed in a Corbett rotor real-time cycler (Qiagen China Co.,
Ltd., Shanghai, China). The PCR consisted of an initial
denaturation step of 5 min at 95°C, 40 cycles of 10 sec at 95°C, 15
sec at 55°C and 15 sec at 72°C, followed by a heating step that
involved the passage from 70°C to 99°C at a rate of 0.1°C/sec,
allowing the acquisition of sufficient data to produce the
denaturing curve of the amplified products. The comparative
threshold method was used to calculate the relative levels of mRNA
in the treated samples, as compared with the amount in the control
group samples (28,29). Each treatment was performed in
triplicate, and the results were presented as the mean ± standard
deviation.
Protein extraction and western
blotting
After 48 h, the cells were washed with ice-cold
phosphate-buffered saline (PBS). The total protein was subsequently
extracted using 50 µl protein lysis buffer (Sangon Biotech
Co., Ltd.) per 5×106 cells, prior to centrifugation of
the cells at 12,000 x g for 20 min at 4°C. The supernatant was
harvested, and the protein concentration was determined using a
bicinchoninic acid assay (Sangon Biotech Co., Ltd.) and then stored
at −80°C. The protein samples (60 µg) were then subjected to
8 or 12% SDS-PAGE, prior to being electrically transferred to
polyvinylidene difluoride membranes (Merck Millipore, Darmstadt,
Germany). The membranes were then blocked with 5% non-fat milk in
PBS containing 0.1% Tween® 20 for 1 h at room
temperature, and were incubated with either anti-KLF4 (1:1,000),
anti-collagen I (1:200), anti-collagen III (1:250), anti-MMP1
(1:300), or anti-TIMP1 (1:200) antibodies in tris-buffered saline
containing 0.05% Tween® 20 at 4°C overnight. The washed
membranes were then incubated with IRDye800-conjugated secondary
antibody (1:20,000) for 1 h at 37°C, prior to being scanned with
the Odyssey Infrared Imaging system (Li-COR Biosciences, Lincoln,
NE, USA). The integrated intensity for each detected band was
determined using ImageJ 1.46 software (National Institutes of
Health, Bethesda, MA, USA). β-actin was used as the control (cat.
no. ab119716; polyclonal rabbit lgG; 1:4,000; Abcam, Cambridge,
UK).
ELISA of TGF-β1, TNF-α, and IL-1β
At 48 h post-transfection the supernatant of the
cultured cells was collected and the concentrations of TGF-β1,
TNF-α and IL-1β were measured using ELISA kits according to the
manufacturer's instructions. The absorbance was measured using a
microplate spectrophotometer (XMark; Bio-Rad Laboratories, Inc.) at
450 nm. TGF-β1, TNF-α and IL-1β levels were calculated based on a
standard curve.
MTT cell viability assay
The cells in the exponential growth phase were
seeded into 96-well plates at a density of 5×104⁄ml with
200 µl added to each well. Six repeated wells and negative
control wells were used for each group. The HSCs were subsequently
transfected with siRNA3 and cultured in DMEM supplemented with 12%
FBS in 96-well plates for 12, 24, and 48 h, and 20 µl MTT
solution (5 mg/ml; Sangon Biotech Co., Ltd.) was added to each
well. The cells were then cultured for a further 4 h, and the
solution was replaced with 150 µl dimethyl sulfoxide (Sangon
Biotech Co., Ltd.). The absorbance value (A), was measured at 492
nm using an enzyme-labeling instrument (XMark; Bio-Rad
Laboratories, Inc., Hercules, CA, USA), and subsequently used to
calculate viability rate. Viability
rate=(Aexperimental−Acontrol) × 100%.
Statistical analysis
The data are expressed as the mean ± standard
deviation, and were analyzed using SPSS 13.0 software (SPSS Inc.,
Chicago, IL, USA). Independent t-tests, one way analysis of
variance, and least significant difference tests were subsequently
carried out. P<0.05 was considered to indicate a statistically
significant difference.
Results
Silencing of KLF4 expression with
synthetic siRNAs
Using Lipofectamine® 2000 as the
transfection reagent, siRNA1, siRNA2, and siRNA3 were transfected
into the LX2 cells. The results of the RT-qPCR indicated that the
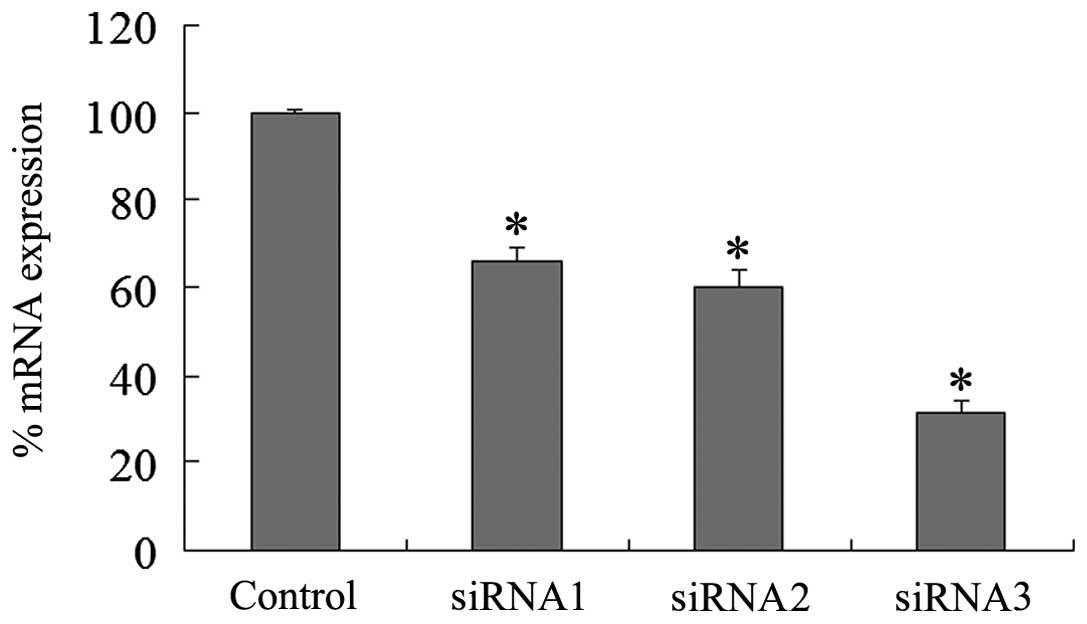
mRNA expression levels of KLF4 were decreased by ~34, 40, and 69%
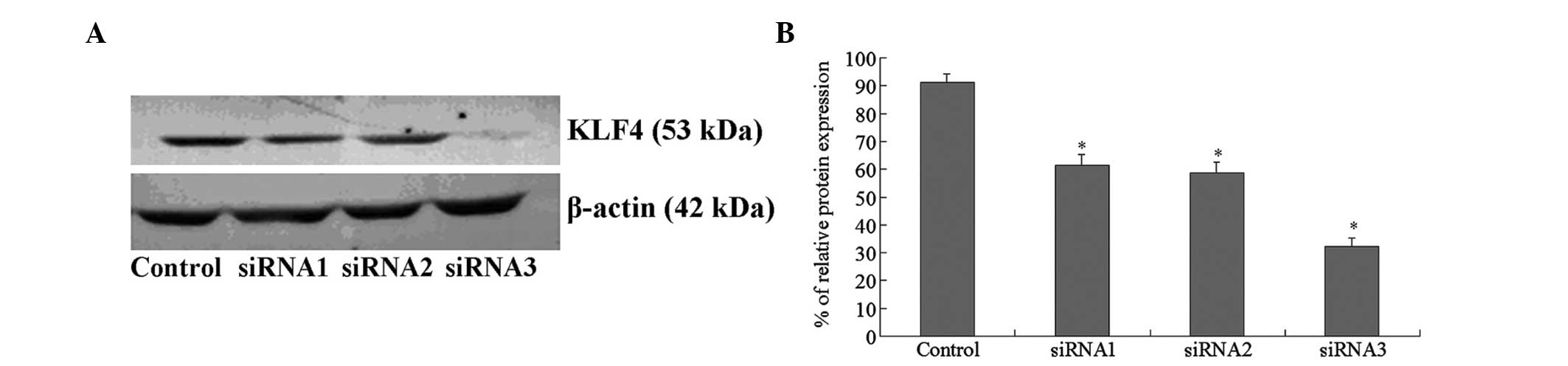
in the siRNA1, siRNA2, and siRNA3 groups, respectively (Fig. 1). Western blot analyses showed that
the protein expression levels of KLF4 were also inhibited to
various extents in the three siRNA groups. siRNA3 exhibited the
strongest inhibitory effect (Fig.
2), and was therefore selected to determine the effects of KLF4
gene silencing.
Effects of KLF4 siRNA on collagen
synthesis in LX2 cells
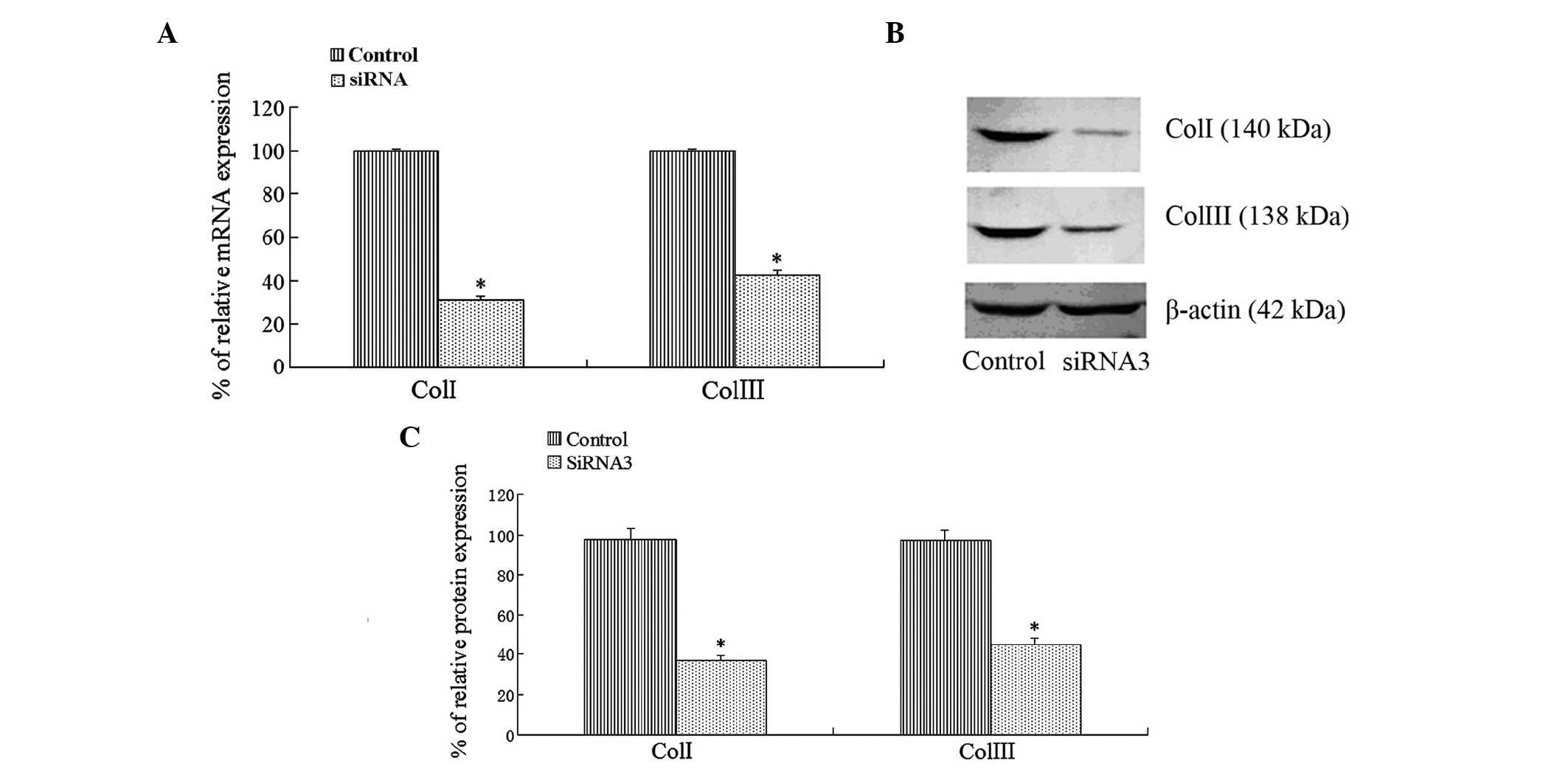
The mRNA and protein expression levels of type I and
type III collagen were determined by RT-qPCR and western blotting,
respectively. The results indicated that the mRNA and protein
expression levels of type I and type III collagen were
significantly decreased in the siRNA3 group, as compared with the
control group, following the transfection of KLF4 siRNA into the
LX2 cells (Fig. 3).
Effects of KLF4 siRNA on collagen
degradation in LX2 cells
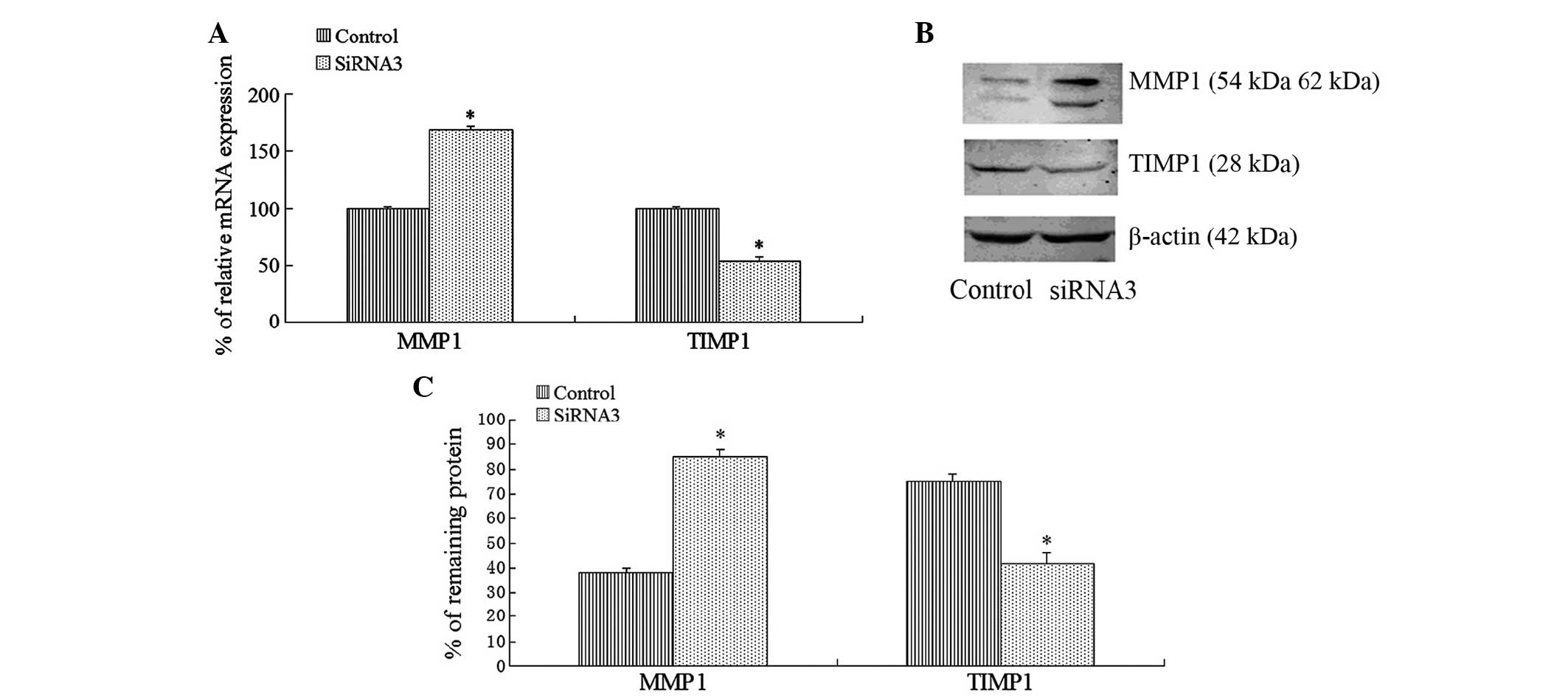
Following the transfection of KLF4-specific siRNA
into the LX2 cells, the results of the RT-qPCR and western blot
analyses indicated that the mRNA expression levels of MMP-1 were
significantly upregulated after 24 h in the siRNA3 group, and that
the protein expression levels of MMP-1 were also significantly
upregulated after 48 h, as compared with the control group.
Furthermore, knockdown of KLF4 expression by KLF4 siRNA
significantly reduced the mRNA and protein expression levels of
TIMP-1 (Fig. 4).
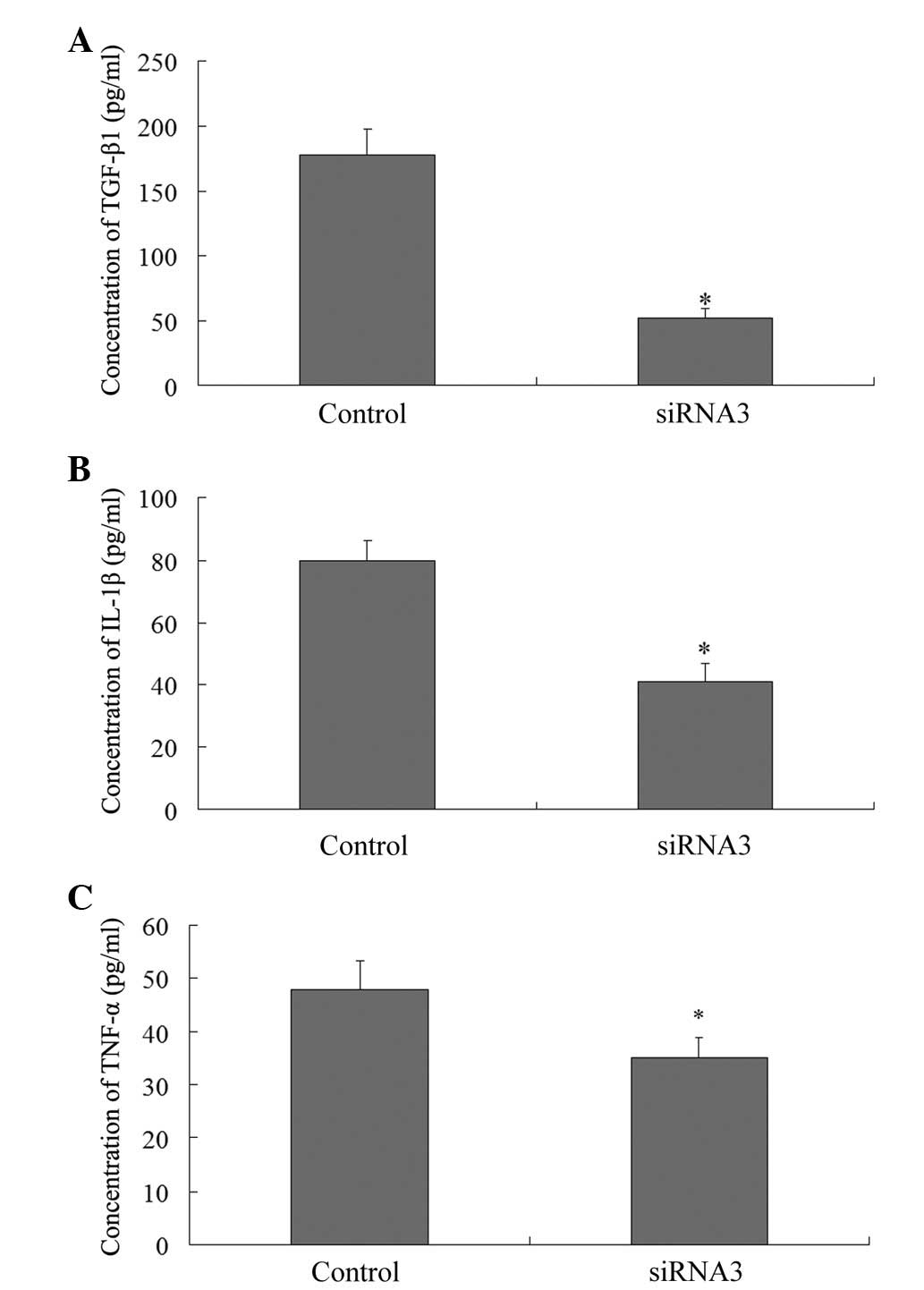
Effects of KLF4 siRNA on TGF-β1, TNF-α,
and IL-1β
The expression levels of TGF-β1, TNF-α, and IL-1β
were measured using an ELISA 48 h post-transfection. The expression
levels of the four cytokines were markedly decreased, as compared
with the control group (178±20.3 pg/ml TGF-β1, vs. 52±8.1 pg/ml
TGF-β1; 48±5.4 pg/ml TNF-α, vs. 35±4.1 pg/ml TNF-α; and 80±6.2
pg/ml IL-1β, vs. 41±5.7 pg/ml IL-1β; P<0.05) (Fig. 5).
KLF4 siRNA inhibits the viability of LX2
cells
KLF4 siRNA3 significantly inhibited the growth of
LX2 cells, as compared with the control group. The viability rates
of LX2 cells were determined to be 75.56% at 12 h, 52.35% at 24 h,
and 50.14% at 48 h post-transfection (Table 1).
 | Table IEffects of KLF4 on LX2 hepatic
stellate cell viability, as determined by an MTT assay. |
Table I
Effects of KLF4 on LX2 hepatic
stellate cell viability, as determined by an MTT assay.
| Group | Cell viability (%)
|
|---|
| 12 h | 24 h | 48 h |
|---|
| Control | 101.25±1.35 | 100.69±1.22 | 101.56±2.31 |
| KLF4 | 75.56±3.56a | 52.35±2.34a | 50.14±2.41a |
Discussion
RNA interference is an advanced gene blocking
technique that permits the efficient, specific, and continuous
inhibition of intracellular gene targets. The selection of a potent
siRNA sequence targeting a specific gene is one of the most
important steps in order to allow sufficient inhibition of gene
expression. Numerous studies on the silencing effects of siRNA have
revealed that the binding of the target mRNA secondary structure
region to the siRNA antisense strand has a strong influence on the
level of siRNA activity (30,31).
If the mRNA secondary structure is complex, the siRNA will combine
with less efficiency to the region. In the present study, three
synthetic siRNAs were designed to target the coding regions of KLF4
mRNA, located between 909–1702 bp. Using Lipofectamine®
2000, the siRNAs were subsequently transfected into LX2 cells. The
results indicated that all three KLF4 siRNAs were able to
effectively inhibit the mRNA and protein expression of KLF4, and
siRNA3 exhibited the maximum inhibitory effect. The variations in
the inhibitory effects of the siRNAs may be due to differences in
the local secondary structures of the KLF4 mRNA, and to variations
in the accessibility of the 1682–1702 bp region.
A substantial alteration in liver fibrosis or
cirrhosis is due to the deposition of ECM, which is predominantly
composed of type I and type III collagen, which accounts for
~80–90% of increased total collagen (32). The increases in type I and type III
collagen is an important indicator of liver fibrosis (33). Although numerous hepatic cell types
are able to synthesize ECM proteins, HSCs are unequivocally the
primary cells involved in the production of excessive ECM detected
in liver fibrosis (34).
Therefore, in the present study, KLF4 siRNAs were transfected into
HSCs in order to investigate the mRNA and protein expression levels
of type I and type III collagen. The results indicated that the
expression levels of type I and type III collagen were
significantly decreased in the siRNA3 group, as compared with the
control group. Furthermore, the results of an MTT cell viability
assay indicated that KLF4 siRNA significantly inhibited the growth
of HSCs. These data demonstrated that KLF4 siRNA was able to
effectively suppress the synthesis of collagen by HSCs, and that
KLF4 gene silencing may be a promising target for novel
antifibrotic therapies.
The present study also assessed the role of KLF4 in
the regulation of ECM metabolism in HSCs. MMPs are a class of
calcium-dependent enzymes that have a major role in ECM degradation
(35). There are currently eight
subcategories of MMPs that have been identified in the liver.
Current evidence indicates that, except for the granulocyte MMP-8,
MMP-1 is the only collagenase with specificity for native
interstitial type I and type III collagens produced in the liver
(36). Previous studies have
demonstrated that a negative correlation exists between MMP-1 and
the degree of liver fibrosis, and that MMP-1 expression is
inhibited in liver fibrosis (37–39).
MMP-1 activity is regulated by TIMP-1, interacting at a 1:1
stoichiometry ratio (40). During
fibrosis, the mRNA and protein expression levels of TIMP-1 are
markedly increased. Therefore, the imbalance between MMP-1 and
TIMP-1 is a principal feature of hepatic fibrosis (41,42).
The present study showed that the silencing of KLF4 expression was
able to increase MMP-1 expression, and reduce TIMP-1 expression in
HSCs. Thus KLF4 gene silencing may promote ECM degradation.
Numerous cytokines are involved in the activation,
proliferation, and secretion of HSCs. Among these complex
cytokines, TGF-β1 is widely accepted as the strongest activating
factor for HSCs (43). Based on
data from HSCs and liver damage in animal models, a conclusive
statement regarding liver fibrosis may be drawn: TGF-β1 is required
for liver fibrosis and the reduction of TGF-β1 signaling reduces
fibrogenesis (44–46). A recent study revealed that
elevated KLF4 binds to the TGF-β1 promoter region and activates
TGF-β1 transcription, which leads to ECM synthesis in
myofibroblasts (26). The results
of the present study were consistent with these previous findings,
and demonstrated that the downregulation of KLF4 by siRNA decreased
the expression levels of TGF-β1 in HSCs. In addition, the process
of liver fibrosis development is accompanied by inflammation, and a
close association between pro-inflammatory cytokines such as TNF-α,
and liver fibrosis and cirrhosis has been reported (47). The results of the present study
showed that KLF4 gene silencing significantly decreased the
expression levels of TNF-α, suggesting that KLF4 gene silencing
also decreases liver inflammation. IL-1β is another important
pro-inflammatory cytokine known to promote local inflammatory
responses, and consequently promote chronic liver fibrosis
(48). In addition, IL-1β protects
TGF-β1 and TNF-α from proteolysis by modulating its bioactivity and
bioavailability. In this microenvironment, the cytokines may have a
key role in the onset of fibrosis, and in the continued
inflammatory response (49). The
results of the present study also indicated that there was a
significant decrease in the amount of secreted IL-1β following KLF4
gene silencing.
In conclusion, knockdown of KLF4 expression
significantly inhibited ECM synthesis and proliferation of HSCs,
most likely through MMP-1 and TIMP-1 activity, as well as cytokine
modulation. The inhibition of KLF4 by siRNA may be an efficient and
specific approach for the development of novel therapeutic methods
in the treatment of liver fibrosis. Further studies are required in
order to clarify the underlying mechanism of action of KLF4 on the
production of cytokines and proteolytic enzymes in HSCs, as well as
in other cell types.
Acknowledgments
The present study was supported by the Key Project
of Medical Science Research of the Hebei Provincial Bureau of
Health (grant no. 20110558).
References
|
1
|
Friedman SL: Molecular regulation of
hepatic fibrosis, an integrated cellular response to tissue injury.
J Biol Chem. 275:2247–2250. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pinzani M, Romanelli RG and Magli S:
Progression of fibrosis in chronic liver diseases: Time to tally
the score. J Hepatol. 34:764–767. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brenner DA, Waterboer T, Choi SK, et al:
New aspects of hepatic fibrosis. J Hepatol. 32(Suppl 1): 32–38.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schuppan D, Ruehl M, Somasundaram R and
Hahn EG: Matrix as a modulator of hepatic fibrogenesis. Semin Liver
Dis. 21:351–372. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Desmet VJ and Roskams T: Cirrhosis
reversal: A duel between dogma and myth. J Hepatol. 40:860–867.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gordillo-Bastidas D, Oceguera-Contreras E,
Salazar-Montes A, González-Cuevas J, Hernández-Ortega LD and
Armendáriz-Borunda J: Nrf2 and Snail-1 in the prevention of
experimental liver fibrosis by caffeine. World J Gastroenterol.
l9:9020–9033. 2013. View Article : Google Scholar
|
|
7
|
Hayden MS and Ghosh S: Signaling to
NF-kappaB. Genes Dev. 18:2195–2224. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cisneros L, Londoño MC, Blasco C, et al:
Hepatic stellate cell activation in liver transplant patients with
hepatitis C recurrence and in non-transplanted patients with
chronic hepatitis C. Liver Transpl. 13:1017–1027. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Enami Y, Bandi S, Kapoor S, Krohn N,
Joseph B and Gupta S: Hepatic stellate cells promote hepatocyte
engraftment in rat liver after prostaglandin-endoperoxide synthase
inhibition. Gastroenterology. 136:2356–2364. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wells RG: Cellular sources of
extracellular matrix in hepatic fibrosis. Clin Liver Dis.
12:759–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pellicoro A, Ramachandran P and Iredale
JP: Reversibility of liver fibrosis. Fibrogenesis Tissue Repair 5
(Suppl 1 Proceedings of Fibroproliferative disorders: From
biochemical analysis to targeted therapies Petro E Petrides and
David Brenner). S262012.
|
|
12
|
Kim JB, Ann YH, Park SY, et al: Side
population in LX2 cells decreased by transforming growth factor-β.
Hepatol Res. 44:229–237. 2014. View Article : Google Scholar
|
|
13
|
Brenner DA: Molecular pathogenesis of
liver fibrosis. Trans Am Clin Climatol Assoc. 120:361–368.
2009.PubMed/NCBI
|
|
14
|
Bieker JJ: Krüppel-like factors: Three
fingers in many pies. J Biol Chem. 276:34355–34358. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Turner J and Crossley M: Mammalian
Krüppel-like transcription factors: More than just a pretty finger.
Trends Biochem Sci. 24:236–240. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ghaleb AM, Nandan MO, Chanchevalap S,
Dalton WB, Hisamuddin IM and Yang VW: Krüppel-like factors 4 and 5:
The yin and yang regulators of cellular proliferation. Cell Res.
15:92–96. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu Y, Zhao J, Liu J, Zhang H, Liu M and
Xiao X: Upregulation of the constitutively expressed HSC70 by KLF4.
Cell Stress Chaperones. 13:337–345. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fisch S, Gray S, Heymans S, et al:
Kruppel-like factor 15 is a regulator of cardiomyocyte hypertrophy.
Proc Natl Acad Sci USA. 104:7074–7079. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang B, Haldar SM, Lu Y, et al: The
Kruppel-like factor KLF15 inhibits connective tissue growth factor
(CTGF) expression in cardiac fibroblasts. J Mol Cell Cardiol.
45:193–197. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Haldar SM, Ibrahim OA and Jain MK:
Kruppel-like Factors (KLFs) in muscle biology. J Mol Cell Cardiol.
43:1–10. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shindo T, Manabe I, Fukushima Y, et al:
Krüppel-like zinc-finger transcription factor KLF5/BTEB2 is a
target for angiotensin II signaling and an essential regulator of
cardiovascular remodeling. Nat Med. 8:856–863. 2002.PubMed/NCBI
|
|
22
|
Yet SF, McA'Nulty MM, Folta SC, et al:
Human EZF, a Krüppel-like zinc finger protein, is expressed in
vascular endothelial cells and contains transcriptional activation
and repression domains. J Biol Chem. 273:1026–1031. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen X, Johns DC, Geiman DE, et al:
Krüppel-like factor 4 (gut-enriched Krüppel-like factor) inhibits
cell proliferation by blocking G1/S progression of the cell cycle.
J Biol Chem. 276:30423–30428. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen X, Whitney EM, Gao SY and Yang VW:
Transcriptional profiling of Krüppel-like factor 4 reveals a
function in cell cycle regulation and epithelial differentiation. J
Mol Biol. 326:665–677. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang C, Han M, Zhao XM and Wen JK:
Kruppel-like factor 4 is required for the expression of vascular
smooth muscle cell differentiation marker genes induced by all
trans-retinoic acid. J Biochem. 144:313–321. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Y, Wang Y, Liu Y, Wang N, Qi Y and
Du J: Krüppel-like factor 4 transcriptionally regulates TGF-β1 and
contributes to cardiac myofibroblast differentiation. PLoS One.
8:e634242013. View Article : Google Scholar
|
|
27
|
Levenkova N, Gu Q and Rux JJ: Gene
specific siRNA selector. Bioinformatics. 20:430–432. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
29
|
Bustin SA: Absolute quantification of mRNA
using real-time reverse transcription polymerase chain reaction
assays. J Mol Endocrinol. 25:169–193. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yoshinari K, Miyagishi M and Taira K:
Effects on RNAi of the tight structure, sequence and position of
the targeted region. Nucleic Acids Res. 32:691–699. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sciabola S, Cao Q, Orozco M, Faustino I
and Stanton RV: Improved nucleic acid descriptors for siRNA
efficacy prediction. Nucleic Acids Res. 41:1383–1394. 2013.
View Article : Google Scholar :
|
|
32
|
Wang JY, Guo JS and Yang CQ: Expression of
exogenous rat collagenase in vitro and in a rat model of liver
fibrosis. World J Gastroenterol. 8:901–907. 2002.PubMed/NCBI
|
|
33
|
Dun ZN, Zhang XL, An JY, Zheng LB, Barrett
R and Xie SR: Specific shRNA targeting of FAK influenced collagen
metabolism in rat hepatic stellate cells. World J Gastroenterol.
16:4100–4106. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Moreira RK: Hepatic stellate cells and
liver fibrosis. Arch Pathol Lab Med. 131:1728–1734. 2007.PubMed/NCBI
|
|
35
|
Consolo M, Amoroso A, Spandidos DA and
Mazzarino MC: Matrix metalloproteinases and their inhibitors as
markers of inflammation and fibrosis in chronic liver disease
(Review). Int J Mol Med. 24:143–52. 2009.PubMed/NCBI
|
|
36
|
Milani S, Herbst H, Schuppan D, et al:
Differential expression of matrix-metalloproteinase-1 and -2 genes
in normal and fibrotic human liver. Am J Pathol. 144:528–537.
1994.PubMed/NCBI
|
|
37
|
Murawaki Y, Ikuta Y, Idobe Y and Kawasaki
H: Serum matrix metalloproteinase-1 in patients with chronic viral
hepatitis. J Gastroenterol Hepatol. 14:138–145. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pardo A and Selman M: MMP-1: The elder of
the family. Int J Biochem Cell Biol. 37:283–288. 2005. View Article : Google Scholar
|
|
39
|
Iimuro Y, Nishio T, Morimoto T, et al:
Delivery of matrix metalloproteinase-1 attenuates established liver
fibrosis in the rat. Gastroenterology. 124:445–458. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Birkedal-Hansen H, Moore WG, Bodden MK, et
al: Matrix metalloproteinases: A review. Crit Rev Oral Biol Med.
4:197–250. 1993.PubMed/NCBI
|
|
41
|
Xu GF, Li PT, Wang XY, et al: Dynamic
changes in the expression of matrix metalloproteinases and their
inhibitors, TIMPs, during hepatic fibrosis induced by alcohol in
rats. World J Gastroenterol. 10:3621–3627. 2004.PubMed/NCBI
|
|
42
|
Wang CH, Lee TH, Lu CN, et al:
Electroporative alpha-MSH gene transfer attenuates
thioacetamide-induced murine hepatic fibrosis by MMP and TIMP
modulation. Gene Ther. 13:1000–1009. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Inagaki Y and Okazaki I: Emerging insights
into Transfo rming growth factor beta Smad signal in hepatic
fibrogenesis. Gut. 56:284–292. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhou J, Zhong DW, Wang QW, Miao XY and Xu
XD: Paclitaxel ameliorates fibrosis in hepatic stellate cells via
inhibition of TGF-beta-Smad activity. World J Gastroenterol.
16:3330–3334. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kondou H, Mushiake S, Etani Y, Miyoshi Y,
Michigami T and Ozono K: A Blocking peptide for transforming growth
factor-beta 1 activation prevents hepatic fibrosis in vivo. J
Hepatol. 39:742–748. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Okuno M, Akita K, Moriwaki H, et al:
Prevention of rat hepatic fibrosis by the protease inhibitor,
camostat mesilate, via reduced generation of active TGF-beta.
Gastroenterology. 120:1784–1800. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Marchetti L, Klein M, Schlett K,
Pfizenmaier K and Eisel UL: Tumor necrosis factor (TNF)-mediated
neuroprotection against glutamate-induced excitotoxicity is
enhanced by N-methyl D-aspartate receptor activation. Essential
role of a TNF receptor 2-mediated phosphatidylinositol
3-kinase-dependent NF-kappa B pathway. J Biol Chem.
279:32869–32881. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bortolami M, Kotsafti A, Cardin R and
Farinati F: Fas/FasL system, IL-1beta expression and apoptosis in
chronic HBV and HCV liver disease. J Viral Hepat. 15:515–522. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Saile B and Ramadori G: Inflammation,
damage repair and liver fibrosis – role of cytokines and different
cell types. Z Gastroenterol. 45:77–86. 2007. View Article : Google Scholar : PubMed/NCBI
|