Introduction
Diabetic peripheral neuropathy (DPN) affects up to
50% of patients with diabetes mellitus (1) and is considered to be one of the most
prevalent and life threatening microvascular diabetic
complications. There are currently no efficacious therapeutic
strategies available for the treatment of DPN (2,3). DPN
is a complex disorder and its exact pathogenesis remains to be
fully elucidated; however, various hypotheses have been suggested
(4,5). The four predominant pathways
associated with DPN are the advanced glycation end products (AGEs)
pathway, polyol pathway, protein kinase C (PKC) pathway and
hexosamine pathway (6). Based on
previous evidence, oxidative stress, which is induced by the
excessive production of reactive oxygen species (ROS), is a key
factor in all the above-mentioned pathways (7). Therefore, inhibition of the
production of ROS, or alleviation of its detrimental effects, may
offer potential for the treatment of DPN.
Molecular hydrogen (H2) is the smallest
natural gas molecule (8) and it
has been suggested that H2 may be promising for
extensive used in medical applications, without side effects
(9), as a potential oxidation
inhibitor. Compared with traditional antioxidants such as vitamin
E, H2 possesses two unique properties. Firstly, it has
the ability to readily penetrate cell membranes and rapidly
translocate to the nucleus and mitochondria (10). Secondly, it is able to selectively
neutralize the more cytotoxic hydroxyl (OH−) and
peroxynitrite (ONOO−), without reacting with less potent
ROS (11). It is widely accepted
that H2 inhalation or the administration of
hydrogen-rich saline (HS) may protect against numerous diseases,
including type 2 diabetes (12),
neurodegenerative disease (13),
multiple organ dysfunction syndrome (14), sepsis (15) and atherosclerosis (16). However, whether H2
benefits patients with DPN remains to be elucidated.
There is increasing evidence suggesting that ongoing
hyperglycemia, which invokes oxidative stress events and impairs
neurons and peripheral nerve Schwann cells (SCs) (17), is a risk factor in the progression
of DPN (18). Excessive
ROS-induced DNA breakage can rapidly activate poly(ADP-ribose)
polymerase-1 (PARP-1), an enzyme associated with DNA repair,
resulting in energy depletion and cell apoptosis (3,7).
Notably, overactivation of PARP-1 can cause the substantial release
of PAR fragments from the nucleus into the cytoplasm, triggering
the caspase-independent apoptotic pathway via translocation of
apoptosis-inducing factor (AIF) from the mitochondria to the
nucleus (19). However, excessive
expression of PARP-1 can also active caspase-3, initiating the
caspase-dependent apoptotic pathway (20). PARP-1 itself is degraded by
caspase-3 from 116 kDa, into two cleavage products (89 kDa and 24
kDa), thus partially losing its activity (21).
In the present study, SCs were treated with high
glucose (HG), in order to produce a cellular research model of DPN.
The aim of the present study was to clarify the role of HG in the
process of SC apoptosis, and to examine whether treatment with
hydrogen-rich medium (HM) alleviates HG-induced injury in
vitro.
Materials and methods
Preparation of HM
HM was prepared, according to a method previously
described by Ohsawa et al (11), with minor modifications. Briefly,
H2 (1 l/min) mixed with air (1 l/min) was dissolved in
low glucose Dulbecco's modified Eagle's medium (DMEM; Gibco Life
Technologies, Carlsbad, CA, USA) for 4 h to reach supersaturation
(~0.6 mM), under 0.4 MPa pressure. The saturated HM was then stored
in a sealed aluminum bag under atmospheric conditions at 4°C. HM
was freshly prepared every week and was sterilized using
γ-radiation (Co-60γ irradiation facility; Tianjin Institute of
Technical Physics, Tianjin, China), in order to maintain a
continuous concentration.
Cell culture and treatment
Primary rat SCs (cat. no. R1700; ScienCell Research
Laboratories, Carlsbad, CA, USA) were cultured in low glucose DMEM
(5.6 mM), supplemented with 5% fetal bovine serum, 1% SC growth
supplement and 1% penicillin/streptomycin solution (ScienCell
Research Laboratories) in a humidified atmosphere containing 5%
CO2 at 37°C. Once the cells had reached ~80% confluence,
0.05% trypsin-EDTA was added. The second and third passages were
selected for the subsequent experiments.
The SCs were randomly divided into five groups,
according to the methods of Sun et al (22) with minor modifications. The groups
were as follows: Control group (SCs treated with 5.6 mM primary
DMEM); H2 group (SCs treated with 0.6 mM HM); HG group
(SCs treated with 44.4 mM glucose + 50 mM glucose in complete
medium); HG + H2 group (SCs treated with HG + 0.6 mM
HM). In order to eliminate osmotic interference, a further group of
SCs were treated with 44.4 mM mannitol, which has almost the same
osmotic pressure as 44.4 mM glucose. This group was termed the high
osmotic control group (mannitol). The cells in each groups were
treated for 48 h at 37°C.
Cell viability assay
Cell viability was detected using a Cell Counting
kit-8 assay (CCK-8; Dojindo Molecular Technologies, Inc.,
Kuamamoto, Japan). Briefly, the cells were seeded at a density of
2×103 per well in 96-well plates in the different
treatment conditions for 48 h, with five parallel wells for each
treatment group. Subsequently, 10 µl CCK-8 reagent was added
to each well and incubated at 37°C for another 3 h. Cell density
was determined by measuring the absorbance at 450 nm using a
microplate reader (VERSAMax; Molecular Devices, LLC, Sunnyvale, CA,
USA), with the cell viability expressed as the percentage of
cytoprotection, vs. control group set at 100%.
Cellular apoptosis assay
Apoptosis was determined using an Annexin
V-Fluorescein Isothiocyanate (FITC)/Propidium Iodide (PI) kit
(Nanjing Keygen Biotech. Co., Ltd., Nanjing, China), according to
the manufacturer's instructions. After 48 h treatment, the SCs in
each group were washed twice with pre-cooled phosphate-buffered
saline (PBS) and were resuspended at 2×106/ml in binding
buffer (Nanjing Keygen Biotech. Co., Ltd.). A total of 5 µl
annexin V-FITC and 10 µl PI was then added to each well, and
the cells were incubated in the dark at room temperature for 15
min. Flow cytometry (FACSCalibur; BD Biosciences, San Diego, CA,
USA) was performed to analyze the cells immediately following
incubation. CellQuest Pro software v.5.2.1 (BD Biosciences) was
used to analyze the data.
Caspase-3 activity assay
An EnzChek Caspase-3 Assay kit (Molecular Probes
Life Technologies, Carlsbad, CA, USA) with Z-DEVD-AMC substrate was
used to measure caspase-3 activity, according to the manufacturer's
instructions. Briefly, after 48 h treatment of the cells
(2×105 cells/well) in the different treatment
conditions, the cells were collected, lysed with 1X cell lysis
buffer (Molecular Probes Life Technologies) and assayed in a
standard black 96-well plate. Once the reactions had been performed
with 2X reaction buffer (Molecular Probes Life Technologies), a
fluorescence microplate reader (Fluoroskan Ascent; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) was used to measure the
fluorescence at 360 nm excitation and 460 nm emission
wavelengths.
DCFH-DA assay
Intracellular OH− levels were detected
using a DCFH-DA assay (Beyotime Institute of Biotechnology, Haimen,
China). Briefly, following treatment for 48 h, the cells in each
group were harvested. The cells were seeded into a 6-well plate at
2×106/ml and labeled with DCFH-DA (10 µM) in a
humidified atmosphere containing 5% CO2 in the dark for
20 min at 37°C. Following incubation, the cells were washed with
PBS and the labeled cells were collected. A flow cytometer (BD
Biosciences) was used to detect the fluorescence intensity.
Enzyme-linked immunosorbent assay
(ELISA)
The concentrations of 8-hydroxy-deoxyguanosine
(8-OHdG) and ONOO− were measured using a highly
sensitive 8-OhdG Check ELISA kit (Japanese Institute for the
Control of Ageing, Shizouka, Japan) and ONOO− ELISA kit
(Yueyan Bio, Shanghai, China), respectively. The cellular
supernatants were obtained following centrifugation at 3,000 × g
for 15 min at 4°C, and the levels of 8-OHdG and ONOO− in
the supernatants were determined, according to the manufacturer's
instructions, with minor modifications, such as to the
centrifugation speed. The absorbance was read using the VERSAMax
microplate reader.
Western blot analysis
Western blotting was used to detect the relative
expression levels of the target proteins. Following treatment of
the cells in each group, the cells were lysed with
radioimmunoprecipitation buffer (Beijing Solarbio Science &
Technology, Co., Ltd., Beijing, China). The lysates (50
µg/lane) were separated by 10% SDS-PAGE and were transferred
onto polyvinylidene fluoride membranes (EMD Millipore, Billerica,
MA, USA). The membranes were blocked with 5% fat-free milk for 2 h
and were then incubated with the following antibodies: Rabbit
polyclonal anti-AIF (1:1,000; cat. no. ab1998; Abcam, Cambridge,
MA, USA), rabbit polyclonal anti-PAR (1:1,000; cat. no.
4336-BPC-100; Trevigen Inc., Gaithersburg, MD, USA), rabbit
polyclonal anti-cleaved PARP-1/PARP-1 (1:100; cat. no. sc-25780;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA), rabbit polyclonal
anti-B-cell lymphoma 2 (Bcl-2; 1:100; cat. no. sc-492: Santa Cruz
Biotechnology, Inc.), rabbit polyclonal anti-Bcl-2-associated X
protein (Bax; 1:100; sc-493; Santa Cruz Biotechnology, Inc.) and
anti-β-actin (1:2,000; cat. no. A5060; Sigma-Aldrich, Shanghai,
China), at 4°C overnight with mildly consistent agitation. The
immunoblots were washed three times with Tris-buffered saline
containing 0.05% Tween (10 min/wash), followed by incubation with
goat anti-rabbit immuno-globulin G (1:5,000; cat. no. 05557;
Sigma-Aldrich) for 2 h at room temperature. The blots were washed
again, as mentioned above, and were treated with a prepared
chemiluminescent horseradish peroxidase substrate (EMD Millipore).
The blots were visualized using Quantity One software, version
4.5.2 (Bio-Rad Laboratories, Inc., Hercules, CA, USA), and the
integrated optical density was analyzed using a Gel-Pro analyzer
(Media Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
The data are presented as the mean ± standard
deviation. One-way analysis of variance with least significant
difference comparison were used to analyze differences between the
groups. SPSS 21.0 software (IBM SPSS, Armonk, NY, USA) was used to
perform the statistical analyses. P<0.05 was considered to
indicate a statistically significant difference.
Results
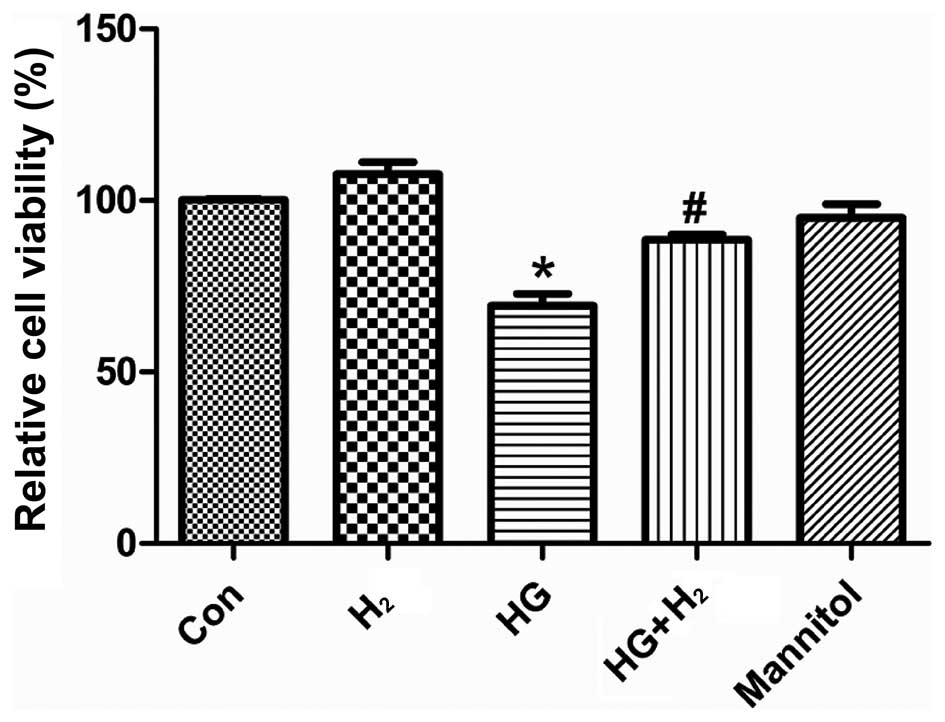
HM increases the viability of SCs exposed
to HG
In order to investigate the effects of HM on
cellular viability following treatment with HG, the viability of
the SCs was examined using a CCK-8 assay (Fig 1). The viability of the SCs exposed
to HG was significantly reduced, compared with the control group
(P<0.05), following treatment for 48 h. Furthermore, treatment
with HM improved cell viability under HG conditions (P<0.05).
However, s no significant difference were observed between the
mannitol and control groups (P>0.05).
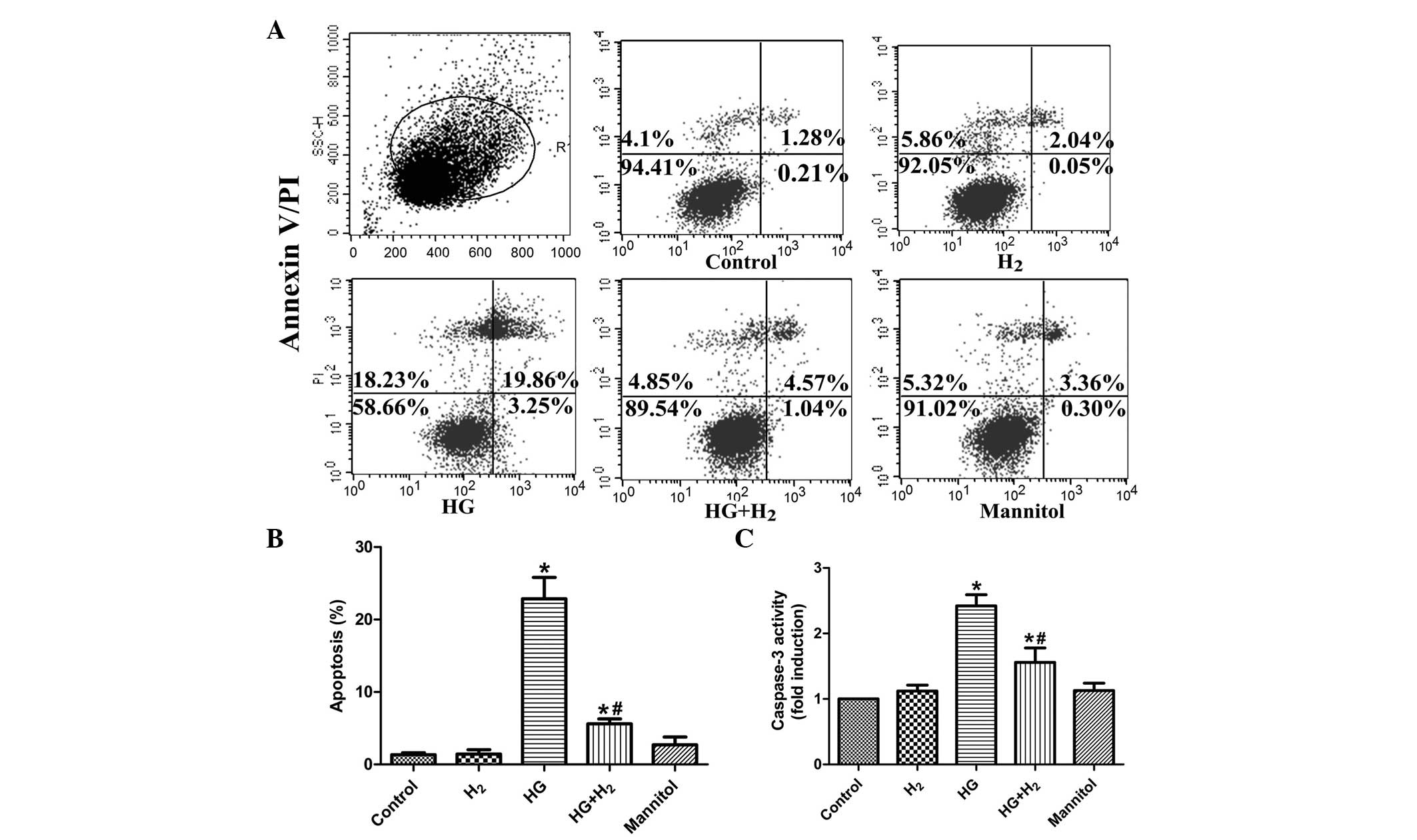
HM prevents apoptosis of SCs under HG
conditions
It is well known that HG-induced oxidative stress
promotes apoptosis (18,23). To further examine the curative
effects of HM on HG-induced apoptosis, the apoptotic rate and
caspase-3 activity of the SCs were determined using annexin
V-FITC/PI and caspase-3 activity assays, respectively. The
proportion of apoptotic cells in the HG group was markedly
increased, compared with the control cells cultured in primary DMEM
(P<0.05; Fig. 2A and B).
Treatment with HM significantly reduced the percentage of apoptotic
cells in the HG condition (P<0.05). Concordantly, caspase-3
activity was significantly increased in the HG-treated SCs
(P<0.05), which was alleviated by treatment with HM (P<0.05;
Fig. 2C), whereas treatment with
mannitol demonstrated no arresting effects (P>0.05) on either
the apoptotic rate or caspase-3 activity. These results indicated
that treatment with HM significantly suppressed HG-induced
apoptosis of SCs in vitro.
HM inhibits the production of
OH− and ONOO− under HG conditions
The levels of intracellular ROS, ONOO−
and OH− were detected in the treatment groups using
ELISA and DCFH-DA assays, respectively. The intracellular levels of
OH− (Fig. 3A and B) and
ONOO− (Fig. 3C) were
higher in the HG group, compared with the control group
(P<0.05). Treatment with HM suppressed the intracellular
concentrations of OH− and ONOO− under HG
conditions (P<0.05), whereas no significant effects of mannitol
on levels of ROS were observed (P>0.05).
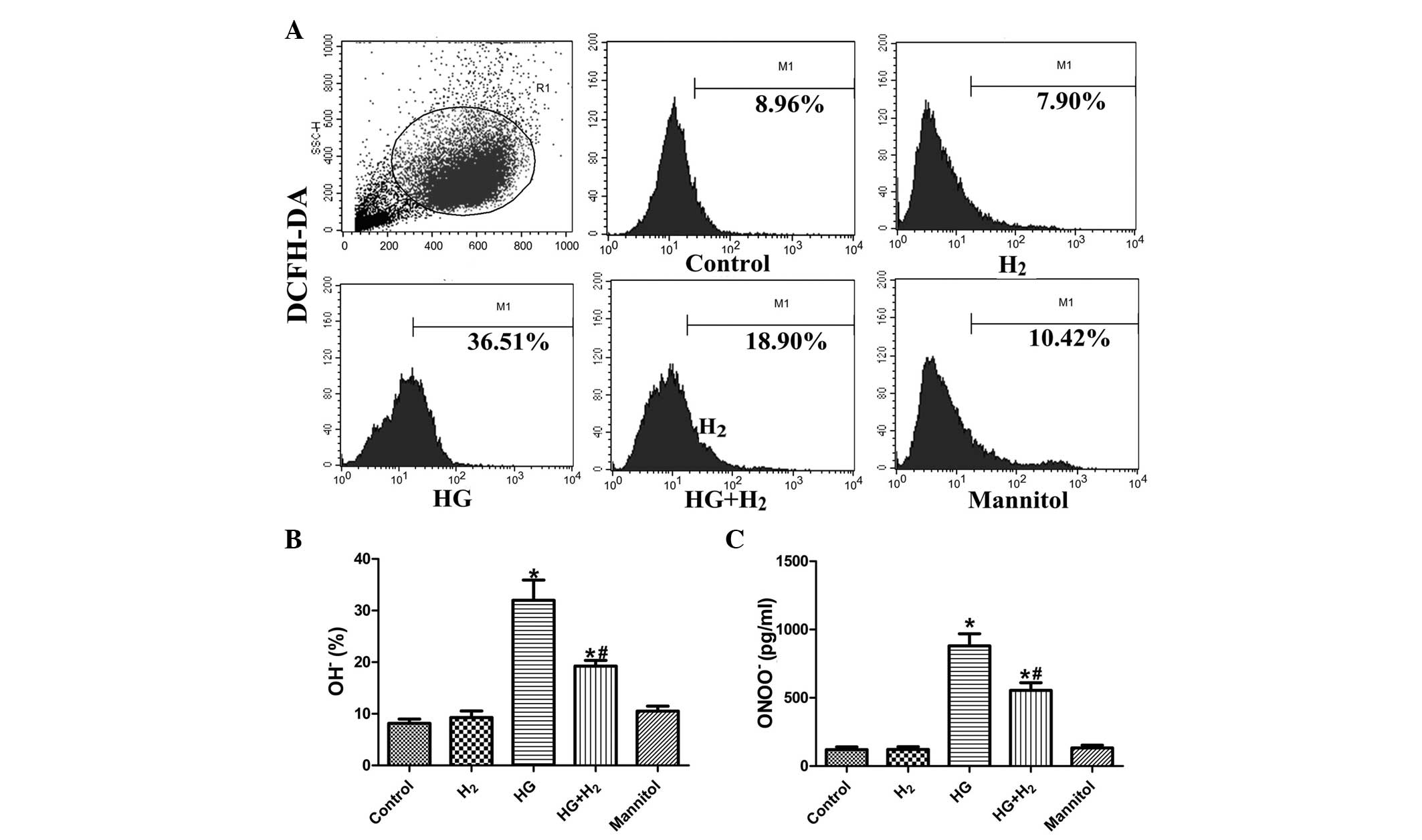 | Figure 3Determination of intracellular
OH− and ONOO− levels. Intracellular levels of
OH− and ONOO− were detected using flow
cytometry and enzyme-linked immunosorbent assays, respectively. (A)
Flow cytometric results of the DCFH-DA assay, 20,000 cells were
assessed from each group. Quantitative analysis of (B)
OH− and (C) ONOO− are expressed as the mean ±
standard deviation (n=3/group). *P<0.05, compared
with the control group; #P<0.05, compared with the HG
group. H2, hydrogen; HG, high glucose; OH−,
hydroxyl; ONOO−, peroxynitrite; M1, DCFH-DA labelled
cells. |
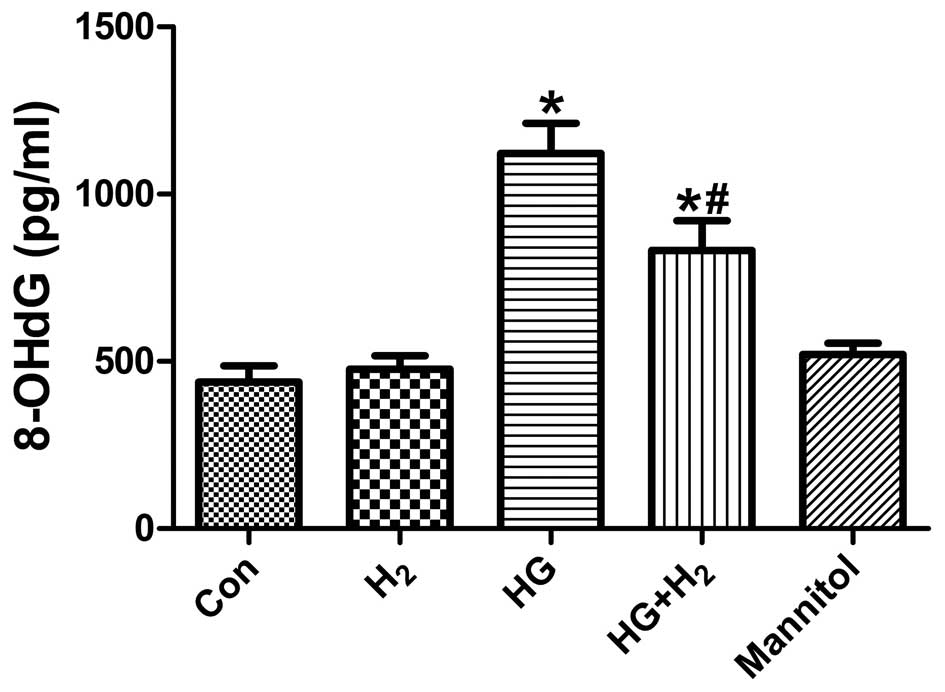
HM mitigates HG-induced 8-OHdG levels in
SCs
Treatment with HG significantly increased the levels
of 8-OHdG, a sensitive oxidative stress-induced DNA damage
biomarker, compared with the control group (Fig. 4; P<0.05). However, treatment
with HM markedly reduced the generation of 8-OHdG (P<0.05),
whereas treatment with mannitol had no effect (P>0.05). These
results suggested that HM inhibited oxidative stress-induced DNA
damage in the SCs under HG conditions.
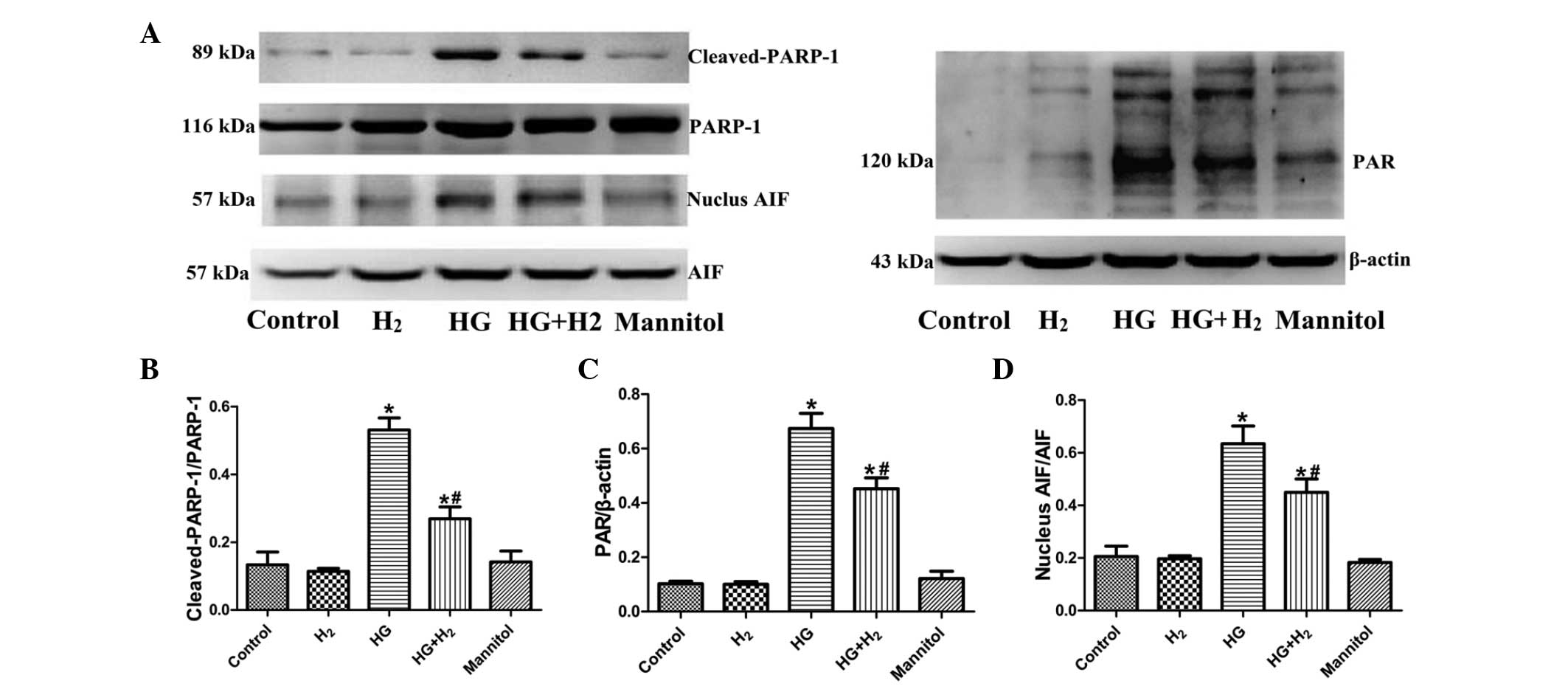
Effects of HM on the PARP-1/AIF
pathway
To clarify whether HM exhibits a protective effect
against HG-induced caspase-independent PARP-1/AIF apoptosis, the
activation of PARP-1 (ratio of cleaved-PARP-1/PARP-1), expression
of PAR and nuclear translocation of AIF (ratio nuclear AIF/AIF)
were detected in each treatment groups using western blot analysis
(Fig. 5A). The relative expression
levels of cleaved-PARP-1/PARP-1 (Fig.
5B), PAR (Fig. 5C) and nuclear
AIF/AIF (Fig. 5D) were markedly
increased in the HG group, compared with the control group
(P<0.05). Treatment with HM significantly reduced the activation
of PARP-1, expression of PAR and nuclear translocation of AIF
(P<0.05). However, no significant differences in the these
proteins were observed between the mannitol and control groups
(P>0.05).
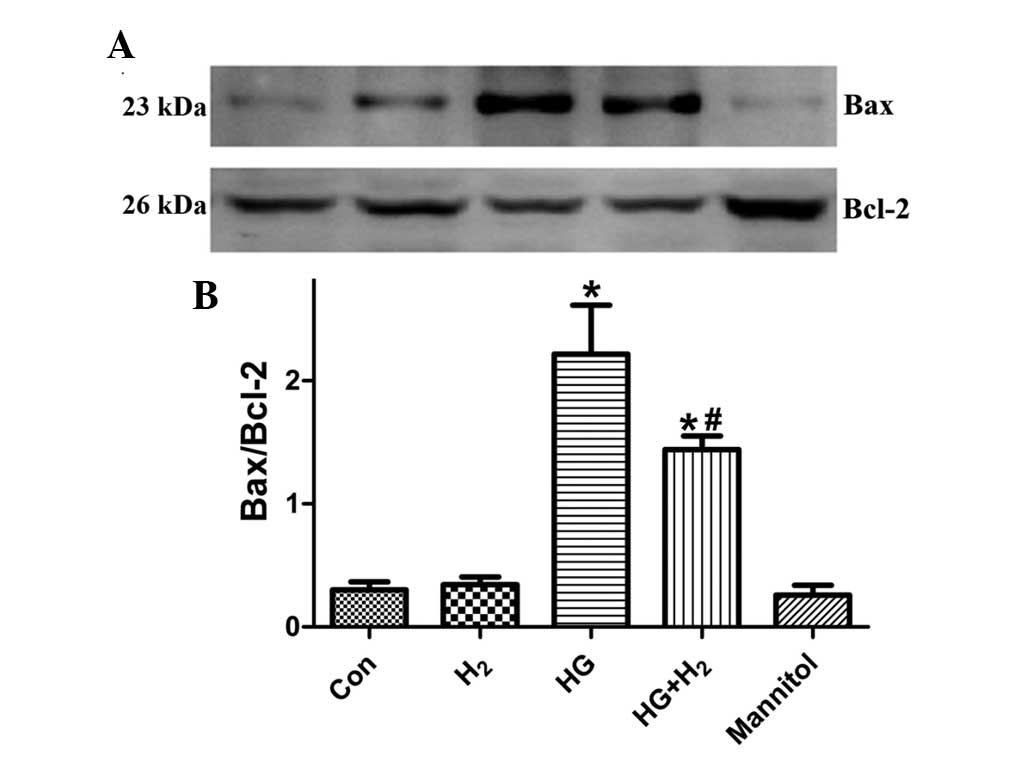
Effects of HM on the expression levels of
Bax and Bcl-2 in SCs
The expression levels of pro-apoptotic Bax and
anti-apoptotic Bcl-2 were detected using western blot analysis
(Fig. 6A) to examine the effects
of HM on HG-induced SC apoptosis. After 48 h treatment, the ratio
of Bax/Bcl-2 was significantly increased in the HG group, compared
with the control group (Fig. 6B;
P<0.05). However, treatment with HM significantly decreased the
HG-induced expression of Bax/Bcl-2 (P<0.05), and mannitol had no
effect (P>0.05).
Discussion
It is widely recognized that hyperglycemia, which
exists in all patients with diabetes mellitus, is the predominant
cause of diabetic complications (6). SCs are a unique type of glial and
myelin-forming cell in the peripheral nervous system, which have a
critical role in maintaining normal function and morphology and
also in the pathogenesis and development of peripheral nerves
(24). SCs can rapidly respond to
hyperglycemia, generating high levels of antioxidative enzymes, and
enhancing antioxidant defense mechanisms to relieve
hyperglycemia-induced oxidative stress (25). As injury to the DPN in SCs is
reversible (26), it is possible
that SCs is a promising target for the treatment of DPN. HG-induced
injury has also been widely investigated as a model of chronic
disease in vitro (27).
Therefore, the present study used SCs cultured under HG conditions
as an in vitro cellular model of DPN.
The present study aimed to investigate the
protective effects of H2-rich medium on oxidative
stress-mediated SC apoptosis under HG conditions in vitro.
The results of the present study indicated that (i) in normal SCs,
treatment with H2-rich medium exhibited little effect on
cell trauma and viability; (ii) treatment with HG induced severe
oxidative stress in SCs with significant injury to the peripheral
neural system, and this was significantly improved following
treatment with 0.6 mM HM for 48 h following HG administration;
(iii) HM inhibited oxidative stress-associated DNA damage under
hyperglycemic conditions in SCs; and (iv) treatment with HM
inhibited HG-induced caspase-dependent and caspase-independent
apoptosis in SCs.
Oxidative stress is considered to be an imbalance
between the production of ROS and activated anti-oxidative
mechanisms in cells (28).
Hyperglycemia-induced overproduction of free radicals is considered
to be the source of DPN complication through increased glycolysis
(29). The production of ROS,
including superoxide anions (O−) and OH−, is
unavoidable in all mammalian cells under normal circumstances. In
healthy cells, the generation of ROS is closely monitored, whereas
during metabolic disturbance, the overproduction of ROS may lead to
damage, dysfunction and deletion of nerve fibers in the PNS
(30). In DPN, the
hyperglycemia-induced overproduction of O− can result in
the combination of NO and O−, resulting in the formation
of the ONOO−, a potent antioxidant that can cause cell
death (31). In addition to the
formation of surplus ROS, hyperglycemia can also induce the
dysfunction of organelles and the nucleus, which may lead to the
activation of caspase-3, translocation of AIF and cytochrome
c, and mitochondrial biogenesis and fission (32). Accordingly, the four predominant
pathways associated with DPN include the AGE pathway, polyol
pathway, PKC pathway and hexosamine pathway (6), all of which lead to programmed cell
death. Our previous study demonstrated that, under hyperglycemic
conditions, SCs were a target of oxidative damage, and that
inhibition of ROS may inhibit the progression of neuropathy
(33).
PARP-1 is the most abundant protein in the PARP
family, and is a ubiquitous nuclear enzyme, the activation of which
in neurons and SCs of the PNS indicates the pathogenesis of
diabetic complications (34).
Hyperglycemia-associated oxidative stress can initiate excessive
DNA single or double strand breaks; however, PARP-1 can respond to
this damage by promoting DNA repair procedures via nicotinamide
adenine dinucleotide (NAD+)-dependent poly(ADP-ribos)
ylation on histones, or by itself, altering the mitochondrial
membrane potential and thus releasing apoptosis-inducing factor
from the mitochondria (35,36).
Furthermore, excessive PARP-1 activation can result in the
generation of increased PAR fragments in the nucleus, which are
released into the cytoplasm, promoting AIF nuclear translocation.
Therefore, it is possible to directly trigger apoptosis via a
caspase-independent pathway (19).
Superfluous activation of PARP-1 can markedly consume
NAD+ stores, deplete levels of adenosine triphosphate
and destroy the integrity of the oxidative respiratory chain, which
can lead to molecular death from necrosis or caspase-dependent
apoptosis (37). PARP-1 itself is
a substrate of caspase-3, can be decomposed from a 116 kDa protein
into two cleavage products (89 and 24 kDa), resulting in partial
loss of its activity (21). The
present study induced apoptosis of SCs using 50 mM glucose, and
demonstrated that HG significantly upregulated the levels of 8-OHdG
and increased the expression of cleaved PARP-1, release of PAR,
nuclear tanslocation of AIF and activation of caspase-3. These
results indicated that HG induced severe oxidative stress and
promoted caspase-independent and caspase-dependent apoptosis of the
SCs, both of which are associated with PARP-1.
Since oxidative stress is involved in all DPN
pathways, a logical therapeutic strategy may be to prevent
oxidative stress by increasing antioxidant defences. Numerous
clinical studies have demonstrated that H2 or HS exhibit
antioxidative (9,11,12,38),
anti-apoptotic (10) and
anti-inflammatory (14,15) effects in several disease models.
The protective effects of H2 or HS on zymosan-induced
organ impairment (14),
lipopolysaccharide (LPS)-associated lung injuries (39), LPS-associated sepsis (15,38,40)
and ouabain-induced auditory neuropathy (41) have also been observed in
vivo. The results of the present study indicated that HM
significantly inhibited oxidative damage in the SCs by selectively
suppressing the production of ONOO− and OH−,
formation of 8-OHdG and activation of PARP-1, leading to a
reduction in SC apoptosis via the caspase-independent and
caspase-dependent pathways. In addition, treatment with HM markedly
upregulated the expression of anti-apoptotic Bcl-2 and
downregulated the expression of pro-apoptotic Bax, indicating that
HM protected the SCs from HG-induced apoptosis.
The results of the present study indicated that HG
selectively induced the production of ROS and activation of PARP-1,
and promoted caspase-dependent and caspase-independent apoptosis of
SCs. Furthermore, the findings demonstrated that treatment with HM
inhibited HG-induced oxidative stress and the PARP-1
activation-associated apoptosis of SCs by down-regulating the
caspase-independent and caspase-dependent apoptotic pathways.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant nos. 81372033 to Dr Keliang Xie
and 81071533 to Professor Yonghao Yu).
References
|
1
|
Callaghan BC, Cheng HT, Stables CL, et al:
Diabetic neuropathy: Clinical manifestations and current
treatments. Lancet Neurol. 6:521–534. 2012. View Article : Google Scholar
|
|
2
|
Ajroud-Driss S, Christiansen M, Allen JA
and Kessler JA: Phase 1/2 open-label dose-escalation study of
plasmid DNA expressing two isoforms of hepatocyte growth factor in
patients with pain ful diabetic peripheral neuropathy. Mol Ther.
21:1279–1286. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zenker J, Ziegler D and Chrast R: Novel
pathogenic pathways in diabetic neuropathy. Trends Neurosci.
36:439–449. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cameron NA, Eaton SE, Cotter MA and
Tesfaye S: Vascular factors and metabolic interactions in the
pathogenesis of diabetic neuropathy. Diabetologia. 44:1973–1988.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Negi G, Kumar A, Joshi RP, et al:
Oxidative stress and diabetic neuropathy: current status of
antioxidants. IIOABJ. 2:71–78. 2011.
|
|
6
|
Brownlee M: Biochemistry and molecular
cell biology of diabetic complications. Nature. 414:813–820. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Obrosova IG: Diabetes and the peripheral
nerve. Biochim Biophys Acta. 1792:931–940. 2007. View Article : Google Scholar
|
|
8
|
Jiang H, Yu P, Qian DH, et al:
Hydrogen-rich medium suppresses the generation of reactive oxygen
species, elevates the Bcl-2/Bax ratio and inhibits advanced
glycation end product-induced apoptosis. Int J Mol Med.
6:1381–1387. 2013.
|
|
9
|
Terasaki Y, Ohsawa I, Terasaki M, et al:
Hydrogen therapy attenuates irradiation-induced lung damage by
reducing oxidative stress. Am J Physiol Lung Cell Mol Physiol.
301:L415–L426. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mather P, Salgado KF, Zivin JA and Lapchak
PA: A novel approach to screening for new neuroprotective compounds
for the treatment of stroke. Brain Res. 1173:117–125. 2007.
View Article : Google Scholar
|
|
11
|
Ohsawa I, Ishikawa M, Takahashi K, et al:
Hydrogen acts as a therapeutic antioxidant by selectively reducing
cytotoxic oxygen radicals. Nat Med. 13:688–694. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fan M, Xu X, Chen L, et al: Protective
effects of hydrogen-rich saline against erectile dysfunction in a
streptozotocin induced diabetic rat model. J Urol. 190:350–356.
2013. View Article : Google Scholar
|
|
13
|
Huang Y, Xie K, Li J, et al: Beneficial
effects of hydrogen gas against spinal cord ischemia-reperfusion
injury in rabbits. Brain Res. 1378:125–136. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xie K, Yu Y, Zhang Z, et al: Hydrogen gas
improves survival rate and organ damage in zymosan-induced
generalized inflammation model. Shock. 34:495–501. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen HG, Xie KL, Han HZ, et al: Heme
oxygenase-1 mediates the anti-inflammatory effect of molecular
hydrogen in LPS-stimulated RAW 264.7 macrophages. Int J Surg.
11:1060–1066. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ohsawa I, Nishimaki K, Yamagata K, et al:
Consumption of hydrogen water prevents atherosclerosis in
apolipoprotein E knockout mice. Biochem Biophys Res Commun.
377:1195–1198. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Viader A, Golden JP, Baloh RH, et al:
Schwann cell mitochondrial metabolism supports long-term axonal
survival and peripheral nerve function. J Neurosci. 31:10128–10140.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Russell JW, Sullivan KA, Windebank AJ, et
al: Neurons undergo apoptosis in animal and cell culture models of
diabetes. Neurobiol Dis. 6:347–363. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu SW, Wang H, Dawson TM and Dawson VL:
Poly(ADP-ribose) polymerase-1 and apoptosis inducing factor in
neurotoxicity. Neurobiol Dis. 14:303–317. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jergens A, Young J, Moore D, et al:
Bcl-2/caspase 3 mucosal imbalance favors T cell resistance to
apoptosis in dogs with inflammatory bowel disease. Vet Immunol
Immunopathol. 158:167–174. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lupachyk S, Shevalye H, Maksimchyk Y, et
al: PARP inhibition alleviates diabetes–induced systemic oxidative
stress and neural tissue 4-hydroxynonenal adduct accumulation:
Correlation with peripheral nerve function. Free Radic Biol Med.
50:1400–1409. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun LQ, Chen YY, Wang X, et al: The
protective effect of alpha lipoic acid on Schwann cells exposed to
constent or intermittent high glucose. Biochem Pharmacol.
84:961–973. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Russell JW, Golovoy D, Vincent AM,
Mahendru P, Olzmann JA, Mentzer A and Feldman EL: High
glucose-induced oxidative stress and mitochondrial dysfunction in
neurons. FASEB J. 16:1938–1748. 2002. View Article : Google Scholar
|
|
24
|
Véga C, Martiel JL, Drouhault D, et al:
Uptake of locally applied deoxyglucose, glucose and lactate by
axons and Schwann cells of rat vagus nerve. J Physiol. 546:551–564.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Askwith T, Zeng W, Eggo MC and Stevens MJ:
Oxidative stress and dysregulation of the taurine transporter in
high-glucose exposed human Schwann cells: Implications for
pathogenesis of diabetic neuropathy. Am J Physiol Endocrinol Metab.
297:E620–E628. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Eckeraley L: Role of the Schwann cell in
diabetic neuropathy. Int Rev Neurobiol. 50:293–321. 2002.
View Article : Google Scholar
|
|
27
|
Vincent AM, Russell JW, Low P and Feldman
EL: Oxidative stress in the pathogenesis of diabetic neuropathy.
Endocr Rev. 25:612–628. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Premkumar LS and Pabbidi RM: Diabetic
peripheral neuropathy: Role of reactive oxygen and nitrogen
species. Cell Biochem Biophys. 67:373–383. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun LQ, Xue B, Li XJ, et al: Inhibitory
effects of salvianolic acid B on apoptosis of Schwann cells and its
mechanism induced by intermittent high glucose. Life Sci.
90:99–108. 2012. View Article : Google Scholar
|
|
30
|
Head KA: Peripheral neuropathy: Pathogenic
mechanisms and alternative therapies. Altern Med Rev. 11:294–329.
2006.PubMed/NCBI
|
|
31
|
Ame JC, Spenlehauer C and de Murcia G: The
PARP super-family. Bioessays. 26:882–893. 2004. View Article : Google Scholar
|
|
32
|
Sun LQ, Zhao J, Zhang TT, et al:
Protective effects of Salvianolic acid B on Schwann cells apoptosis
induced by high glucose. Neurochem Res. 37:996–1010. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yu Y, Jiao Y, Li B, et al: Effects of
hydrogen on oxidative stress injury induced by high glucose in rat
Schwann cells: The relationship with parthanatos. Chin J
Anesthesiol. 35:36–39. 2015.
|
|
34
|
Zenker J, Ziegler D and Chrast R: Novel
pathogenic pathways in diabetic neuropathy. Trends Neurosci.
36:439–449. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schreiber V, Dantzer F, Ame JC and de
Murcia G: Poly(ADP-ribose): Novel functions for an old molecule.
Nat Rev Mol Cell Biol. 7:517–528. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Galluzzi L, Vitale I, Abrams JM, et al:
Molecular definitions of cell death subroutines: Recommendations of
the Nomenclature Committee on Cell Death 2012. Cell Death Differ.
19:107–120. 2012. View Article : Google Scholar :
|
|
37
|
Garcia Soriano F, Virág L, Jagtag P, et
al: Diabetic endothelial dysfunction: The role of poly(ADP-ribose)
polymerase activation. Nat Med. 7:108–113. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xie K, Yu Y, Pei Y, et al: Protective
effects of hydrogen gas on murine polymicrobial sepsis via reducing
oxidative stress and HMGB1 release. Shock. 34:90–97. 2010.
View Article : Google Scholar
|
|
39
|
Xie K, Yu Y, Huang Y, et al: Molecular
hydrogen ameliorates lipopolysaccharide-induced acute lung injury
in mice through reducing inflammation and apoptosis. Shock.
37:548–555. 2012.PubMed/NCBI
|
|
40
|
Wang X, Li J, Chen Y, et al: p28GANK
knockdown-derived reactive oxygen species induces apoptosis through
mitochondrial dysfunction mediated by p38 in HepG2 cells. Int J
Oncol. 33:743–750. 2008.PubMed/NCBI
|
|
41
|
Qu J, Gan YN, Xie KL, et al: Inhalation of
hydrogen gas attenuates ouabain-induced auditory neuropathy. Acta
Pharmacol Sin. 33:445–451. 2012. View Article : Google Scholar : PubMed/NCBI
|