Introduction
Natural menopause is a gradual process, which occurs
for the majority of women between the ages of 47 and 55 years
(1). During the menopause
transition, the majority of women report memory problems (2). Numerous studies have revealed that
females undergoing surgical or natural menopause may experience
memory impairment (3,4), which has been reproduced in adult
ovariectomized female rats and mice (5,6).
Previous studies have indicated that unilateral and bilateral
oophorectomy preceding the onset of the menopause is associated
with an increased risk of cognitive impairment or dementia
(3,4). This risk was observed to increase
with younger age at oophorectomy (7). However, the mechanisms underlying
memory impairment in the menopause remain to be elucidated. A
previous study demonstrated that a low level of endogenous estrogen
in the serum was associated with increased risk of poor verbal
memory (8), while other studies
have failed to confirm this (9,10).
These reports suggest that there are other mechanisms, which are
involved in cognitive impairments in menopause.
There is increasing evidence that oxidative damage
caused by excess reactive oxygen species (ROS) may correlate with
several neurodegenerative diseases, including Alzheimer's disease
and Parkinson's disease (11–13).
Excessive ROS can cause oxidative damage via the modification of
proteins, lipids and DNA, and the brain is particularly vulnerable
to the oxidative damage caused by ROS as it consumes large
quantities of oxygen and has an abundant lipid content, but a
relative paucity of antioxidant compounds, compared with other
organs (14,15).
There are multiple potential sources for ROS in
mammalian cells, including the mitochondrial electron transport
chain, xanthine oxidase, cyclooxygenases and monoamine oxidases
(16,17). However, the nicotinamide adenosine
dinucleotide phosphate oxidase enzyme (NADPH oxidase; NOX) is
important, as it is dedicated to the specific production of
superoxide. Superoxide production via NADPH oxidase has been
demonstrated to be important in a variety of neurological
disorders, including Alzheimer's disease. NOX is composed of
membrane-bound (gp91phox, also termed NOX2 and
p22phox) and cytoplasmic subunits [p40phox,
p47phox, p67phox and Ras-related C3 botulinum toxin
substrate 1 (RAC1)] (18). NOX2 is
expressed throughout the central nervous system and its levels are
particularly high in neurons, astrocytes and microglia (19). NOX2 has been implicated in nerve
growth factor signaling and may be important in the plasticity of
the nervous system, including learning and memory formation
(20). Previous studies have
demonstrated that the p67phox, p47phox and
p40phox NOX cytosolic subunit proteins are significantly
elevated with the progression of Alzheimer's disease (18,20).
In addition, a marked correlation has been observed between NOX
activity and cognitive status, in which as NOX activity increased,
cognitive performance decreased (18). The present study hypothesized that
oxidative damage caused by NADPH oxidase-derived ROS accumulation
may be involved in the learning and memory impairments in
menopause, and learning and memory abilities, the activity of
superoxide dismutase (SOD), the level of malondialdehyde (MDA) and
NADPH oxidase-derived ROS accumulation were investigated in 3-and
16-month-old female rats to confirm this hypothesis.
Materials and methods
Animals and treatment
The animal experiments were approved by the
Institutional Animal Care and Use Committee of Anhui Medical
University (Hefei, China). Sprague Dawley rats were obtained from
the Shanghai B&K Universal Group Limited (Shanghai, China).
Female adolescent rats (3 months; 210–260 g) and female aged rats
(16-months; 490–540 g) rats were used in the present study (17
animals in each group). All animals were housed in standard cages
with a 12 h light/dark cycle and had ad libitum access to
food and water throughout the investigation. The animals were
allowed to acclimate to their new surroundings for 1 week prior to
the commencement of the behavioral experiments.
Morris water maze
The water maze consisted of a plastic black circular
tank (160 cm in diameter; 60 cm high). An overhead video camera
connected to a computer and ANY-maze video tracking system software
(Stoelting Company, Wood Dale, IL, USA) was used to track the path
of movement of the rat. The water was maintained at a constant
temperature of 22–25°C. A circular escape platform (10 cm diameter)
was placed 2 cm below the surface of the water, in the middle of
one of the four quadrants. The general assessment process has been
described in detail previously (21,22).
In the training trials, the animals were placed into the tank from
the four quadrants each day, with a 5 min trial interval, for 4
days. Each trial lasted until the rats climbed onto the hidden
platform within 90 sec, the rats were guided to the platform if
they had not climbed onto the platform within 90 sec. The escape
latency onto the platform was recorded to indicate the learning
performance. Following the training trials, the platform was
removed from the pool and each rat received one swim probe trial of
60 sec. The swimming distance (m) and the mean speed (m/sec) were
recorded as an indication of motor behavior. The swimming duration
in the quadrant of the platform (sec), swimming distance in the
quadrant of the platform (m), number of crossings of the platform
and time of first entry onto the platform (sec) were recorded as an
indicator of memory aptitude.
Levels of serum estradiol and luteinizing
hormone (LH)
The levels of serum estradiol and LH were measured
using an automated microparticle enzyme immunoassay kit and
analysis instrument (AxSYM; Abbott Diagnostics, Inc., Abbott Park,
IL, USA). The AxSYM method is a heterogeneous immunoassay, in which
estradiol and LH from the specimens bind to rabbit polyclonal
anti-estradiol antibodies and anti-LH antibodies, which are linked
to microparticles. Following removal of the unbound materials,
estradiol and LH alkaline phosphatase conjugate were added and
bound to available sites. Following washing with PBS,
4-methylum-belliferyl phosphate was added and the fluorescent
product was measured. In this method, the intensity of the signal
is inversely proportional to the concentrations of estradiol and LH
in the specimens (23,24).
Activity of SOD and content of MDA in
brain tissue
The animals were sacrificed via cervical dislocation
(eight rats in each group). The brain was immediately dissected in
half along the coronal line, one half of which was frozen (−8°C)
for immunoblot analysis and the other was used to form a 10%
physiological saline homogenate. The homogenate was centrifuged at
4,000 x g for 10 min, and the supernatant was collected. The
activity of SOD and the level of MDA were detected using a
spectrophotometer (SpectraMax 190; Molecular Devices Corp.,
Sunnyvale, CA, USA), according to the SOD and MDA assay kits
(Niajing Jiancheng Bioengineering Institute, Nanjing, China).
Production of ROS in the frontal cortex
and hippocampus
Dihydroethidium (DHE) microfluorography (Beyotime
Institute of Biotechnology, Haimen, China) was used to determine
the production of ROS in the brain tissue (25). DHE is a cell permeable dye, which
is oxidized to ethidium bromide and associated products by
superoxide. Ethidium bromide is trapped intracellularly by
intercalation into DNA and can then be identified using red
fluorescence (26,27). In the present study, DHE was
injected via the vena caudalis (100 µM; 1 ml/100 g) for 30
min prior to sacrifice of the rat (n=8; four rats/group). The
brains were removed from the skull and transferred to a freezer at
−20°C. Subsequently, the brains were sectioned at a thickness of 20
µm using a cryostat section cutter (Leica, Wetzlar, Germany)
at −20°C and mounted onto glass slides. The sections were washed
with phosphate buffered saline three times and examined using a
fluorescence microscope (Olympus IX71; Olympus, Tokyo, Japan)
equipped with a custom filter set for detection of DHE oxidation
products (red). The production of ROS in the cortex and the
hippocampal CA1 and CA3 regions were assessed using previously
described methods (25). The
exposure time was 900 ms for all sections. A total of four sections
per group were assessed for ROS quantitative analysis. The mean
fluorescence intensities of three consecutive fields
(magnification, ×400) in the cortex, and hippocampal CA1 and CA3
regions, were performed in a blinded-manner using the Image-Pro
Plus 6.0 (Media Cybernetics, Inc., Silver Spring, MD, USA) analysis
system to indicate the production of ROS, with the data expressed
in relative fluorescence units.
Immunohistochemistry
Following the morris water maze assessment, the
animals (n=10; five rats/group) were sacrificed and the brains were
removed. The brains were fixed in 4% paraformaldehyde and embedded
in paraffin. The paraffin sections were cut at 5 µm and
mounted onto glass slides. Prior to immunostaining, the sections
were deparaffinized and rehydrated in water. The endogenous
peroxidase activity was inhibited by incubation with 0.3% hydrogen
peroxide for 30 min. Subsequently, the sections were incubated with
normal goat serum at room temperature for 30 min, following which
the sections were incubated with primary antibodies overnight at
4°C. The primary rabbit polyclonal antibodies were protein kinase C
α (PKCα; BS1577; 1:200; Bioworld Technology, St. Louis Park, MN,
USA), p47phox (BS4600; 1:200; Bioworld Technology), NOX2
(BS5674; 1:100; Bioworld Technology) and RAC1 (24072-1-AP; 1:100;
ProteinTech, Chicago, IL, USA). Immunostaining was visualized using
a peroxidase method with an ABC kit (Beijing Zhongshan Golden
Bridge Biotechnology Co., Ltd., Beijing, China). The sections were
resin-mounted and observed under a microscope (Olympus IX71;
Olympus). The positive cells were stained brown. A total of five
sections in each group were assessed for quantitative analysis and
a total of three consecutive fields (magnification, ×400) of the
cortex, and hippocampal CA1 and CA3 regions, in each section were
observed. The mean optical density of the positive neurons of the
hippocampal CA1 and CA3 regions and the cortex in each section were
measured using the Image-Pro Plus 6.0 analysis system to determine
the expression levels of PKCα, p47phox, NOX2 and RAC1
(22).
Immunoblot analysis
Tissue from the cortex and the hippocampus (100 mg)
were homogenized in 1 ml radioimmunoprecipitation assay lysis
buffer; Beyotime Institute of Biotechnology). Equal quantities of
protein (50 µg) were separated using SDS-PAGE and
transferred onto polyvinylidene difluoride membranes. The membranes
were washed in Tris-buffered saline with Tween 20 (TBS-T) and
further incubated with the antibodies against NOX2 (1:500),
p47phox (1:500), RAC1 (1:500) and β-actin (1:1,000)
overnight at 4°C. The membranes were thoroughly washed with TBS-T
and then incubated with anti-rabbit immunoglobulin G antibody
conjugated to horseradish peroxidase (1:10,000) for 1 h. Following
washing with TBS-T, the immunoreactive bands were visualized using
an enhanced chemiluminesense kit (Amersham Biosciences, Little
Chalfont, UK). The Tanon4500 imaging system (Shanghai Tanon Science
& Technology Co., Ltd., Shanghai, China) was used to visualize
protein bands, and densitometry was performed using ImageJ
software, version 1.43 (National Institutes of Health, Bethesda,
MD, USA). The ratio of the target band to that of β-actin was
determined to calculate the relative intensity of the expression of
p47phox, NOX2 and RAC1.
Statistical analysis
Data are expressed as the mean ± standard deviation.
The SPSS 17.0 (SPSS, Inc., Chicago, IL, USA) statistical software
package was used to analyze the results. Statistical comparisons
between groups were performed using Student's t-test with
Bonferroni's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
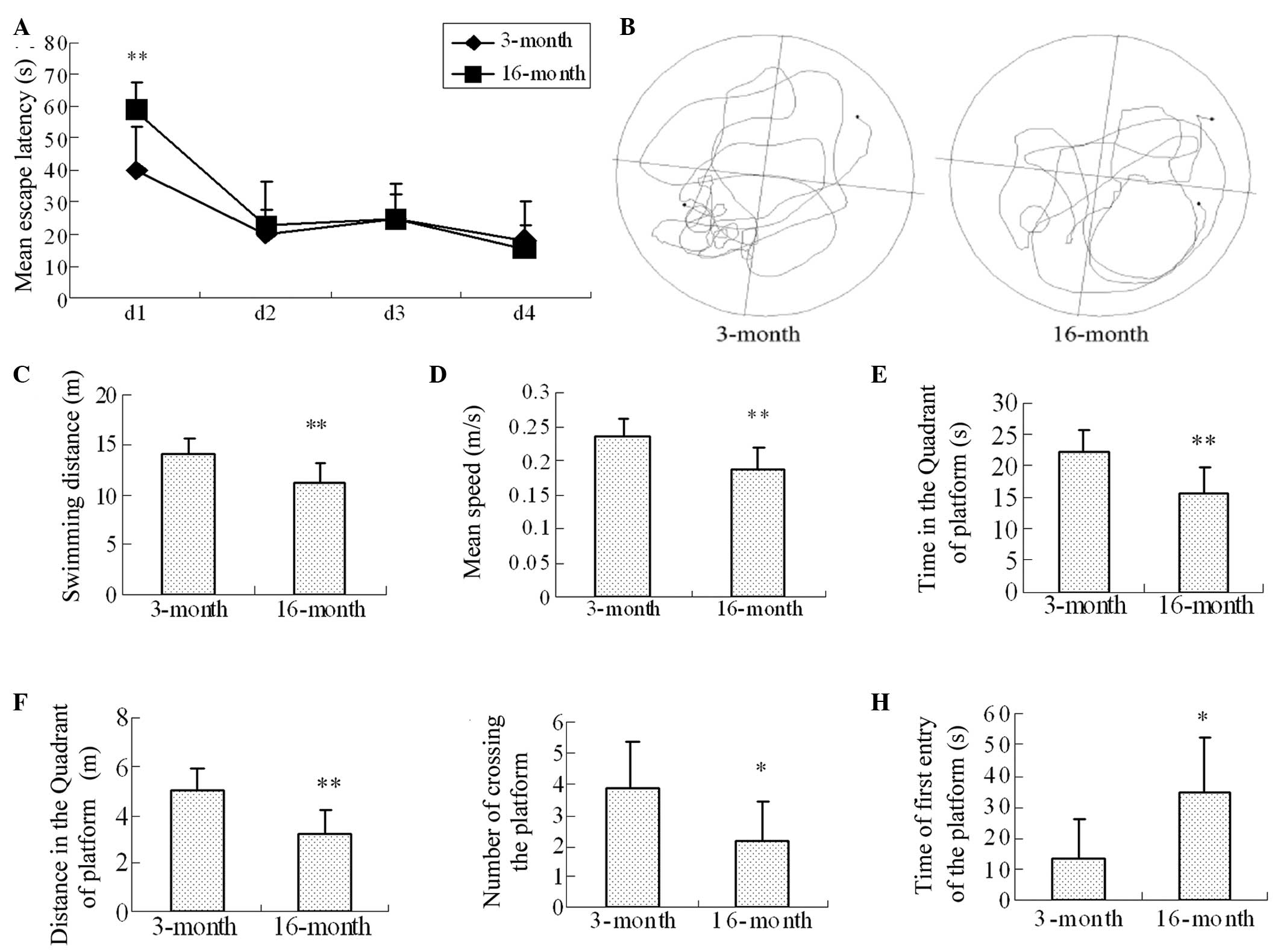
Learning and memory impairments in
16-month-old female rats, determined using a Morris water maze
A Morris water maze assessment was performed to
assess the learning and memory skills in the rats, as described
above. In the learning and memory training experiments, the mean
escape latencies in the 3-and 16-month-old groups on the first day
were significantly different (40.02±13.74, vs. 58.89±8.75,
respectively; P=0.005). No significant differences were observed in
the training experiments between day 2 and day 4 in the 3-month and
16-month-old groups (Fig. 1A). In
the probe trial experiments (Fig.
1B), the swimming distance (14.16±1.56, vs. 11.30±1.90;
P=0.007; Fig. 1C), mean swimming
speed (0.24±0.03, vs. 0.19±0.03; P=0.007; Fig. 1D), and the swimming duration and
distance to the platform (Fig. 1E
and 1F; P=0.006 and P=0.003) were
significantly different in the 3-month and 16-month-old groups,
respectively. In addition, the number of crossings (3.88±1.55, vs.
2.13±1.3; P=0.035; Fig. 1G) and
the time of first entry onto the platform (13.71±12.76, vs.
35.01±17.74; P=0.02; Fig. 1H) in
the 3-month and 16-month-old groups, respectively were
significantly different.
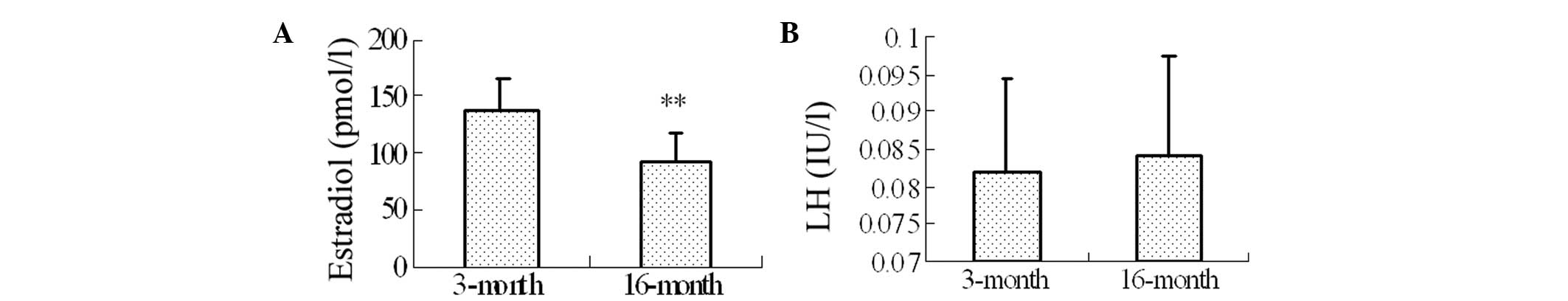
Levels of serum estradiol and LH in the
16-month-old rats
Levels of estradiol, which decline during the
menopause have been associated with learning and memory impairments
(28,29). To investigate the possible links
between memory impairments in the menopause and the levels of serum
estradiol and LH, the levels of serum estradiol and LH in the
3-month and 16-month-old rats were examined. The results revealed
that, compared with the 3-month-old rats, the level of serum
estradiol was significantly decreased in the 16-month-old rats
(137.86±26.10, vs. 91.15±25.99, respectively; P=0.0008; Fig. 2A). No significant differences were
identified in the level of serum LH between the 3-month and
16-month-old rats (Fig. 2B).
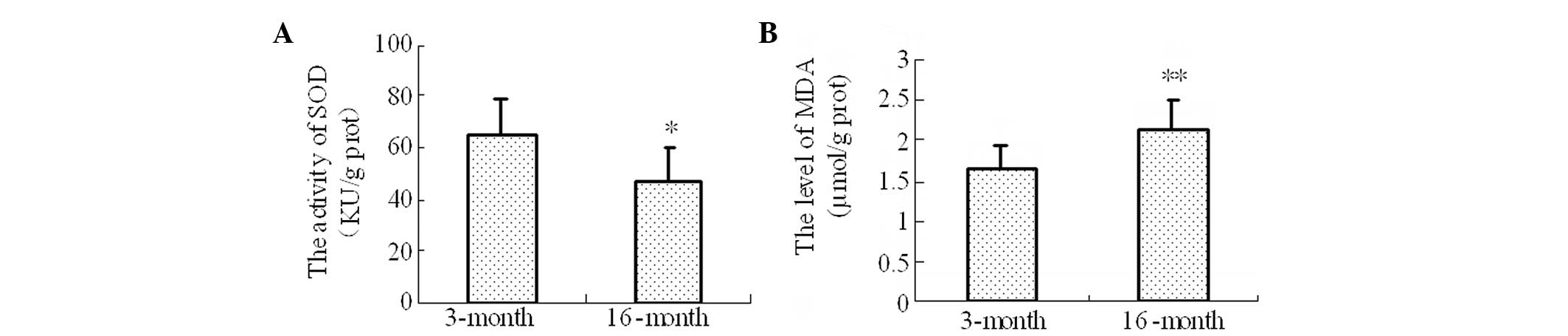
Activity of SOD and the level of MDA in
the brain tissues
Compared with the 3-month-old group, the SOD
activity was significantly decreased (P=0.03) and the MDA content
was significantly increased (P=0.009) in the 16-month-old rats
(Fig. 3).
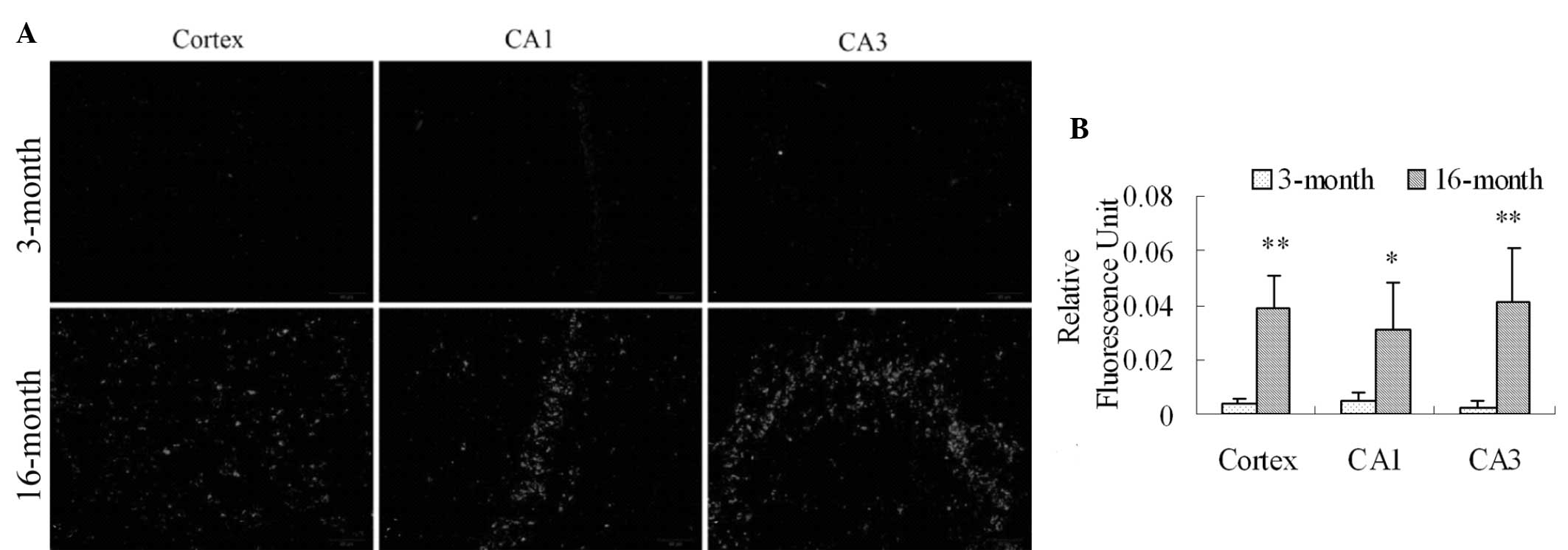
ROS production in the frontal cortex and
hippocampal CA1 and CA3 regions in 16-month old female rats
DHE was used to detect the production of ROS in the
cortex and hippocampal CA1 and CA3 regions in the 3-month and
16-month-old rats (27). A low
level of fluorescence was detected in the cortex and hippocampal
CA1 and CA3 regions in the 3-month rats (Fig. 4A), indicating mild ROS production.
However, in the 16-month-old group, the production of ROS was
significantly increased in the cortex and hippocampal CA1 and CA3
regions (Fig. 4A and 4B; P=0.001, P=0.024 and P=0.009,
respectively).
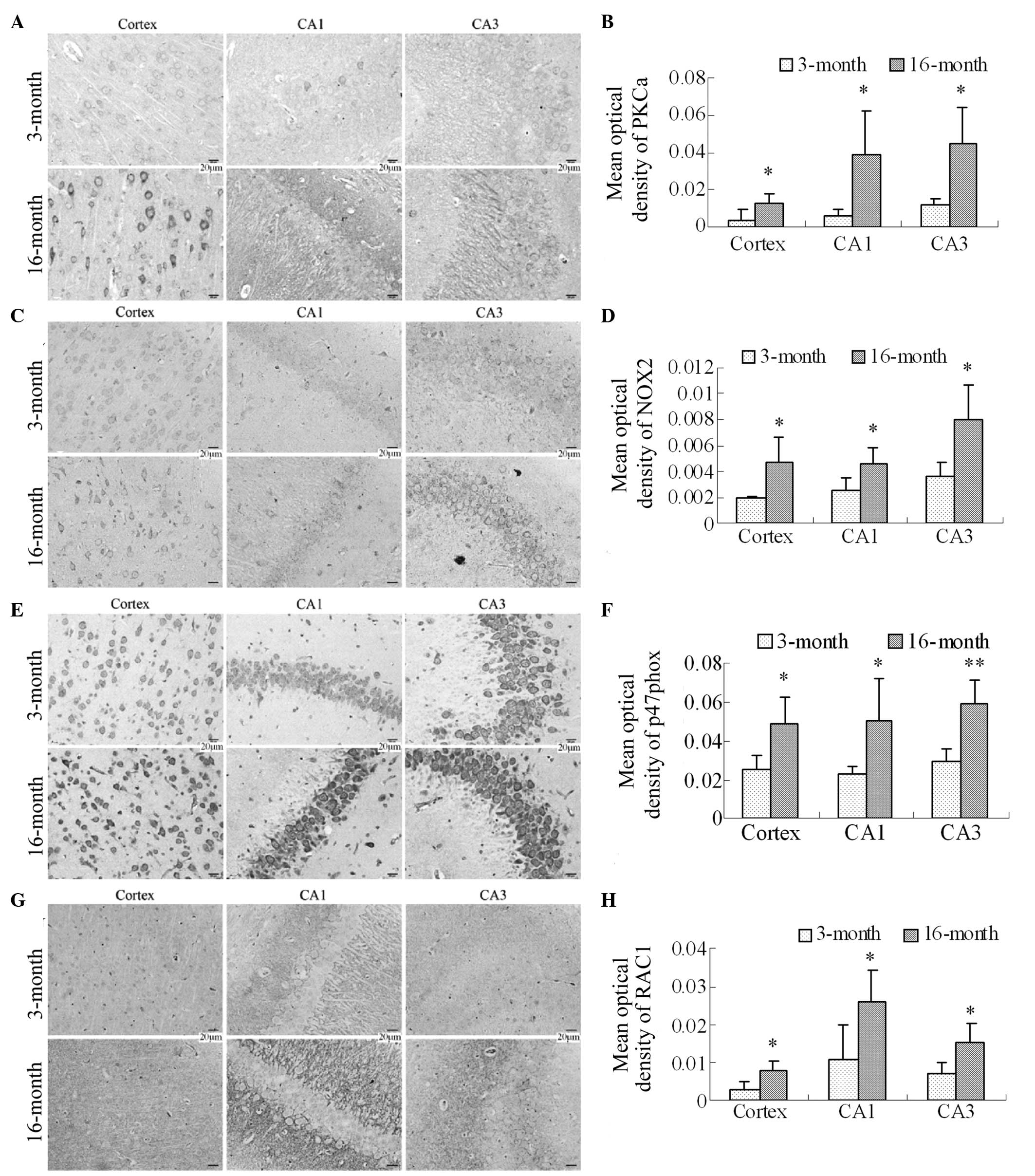
Expression levels of PKCα, NOX2, p47phox
and RAC1 in the frontal cortex and hippocampus in 16-month-old
rats
NOX family members are important sources for the
generation of ROS in neurodegenerative diseases. Therefore, the
expression levels of PKCα, which activates NOX2, the NOX2 membrane
subunit, the p47phox cytosolic subunit and RAC1 were
investigated in the frontal cortex and hippocampal CA1 and CA3
regions using immunohistochemistry. The PKCα immunostaining results
revealed that the expression of PKCα was significantly increased in
the cortex and the hippocampal CA1 and CA3 regions in the
16-month-old group (Fig. 5A and
5B; P<0.05). The NOX2
immunostaining results revealed lower expression levels of NOX2 in
the cortex and the hippocampal CA1 and CA3 regions in the
3-month-old rats, as the immunoreactive staining was light.
Compared with the 3-month old group, the numbers of NOX2
immunoreactive cells were significantly increased in the cortex and
the hippocampal CA1 and CA3 regions in the 16-month-old rats, in
which staining was marked (Fig. 5C
and 5D; P<0.05). The
p47phox and RAC1 immunostaining results also revealed that
the p47phox cytosolic subunit and RAC1, particularly
p47phox, were expressed in the cortex and hippocampus in the
two groups. However, compared with the 3-month group, the
expression levels of p47phox and RAC1, particularly
p47phox, were significantly increased in the cortex and the
hippocampal CA1 and CA3 regions in the 16-month old female rats
(Fig. 5E and 5F; P<0.05 and P<0.01 for
p47phox; Fig. 5G and
5H; P<0.05 for RAC1).
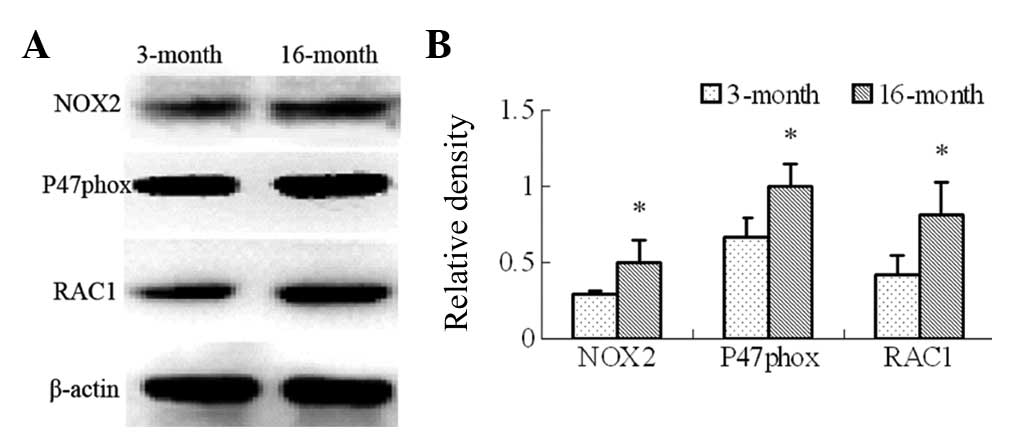
The expression levels of NOX2, p47phox and
RAC1 were also assessed in the frontal cortex and the hippocampus
using immunoblotting (Fig. 6A).
The results revealed that the expression levels of NOX2,
p47phox and RAC1 were also significantly increased in the
16-month-old rats (Fig. 6B;
P<0.05).
Discussion
In the present study, menopause-associated
differences in learning and memory ability, levels of serum
estradiol and LH, and NADPH oxidase-derived ROS production were
examined in the frontal cortex and hippocampal CA1 and CA3 regions
of 3-month and 16-month-old female rats. The natural menopause is
characterized by a progressive decline in the levels of estrogen
and is often accompanied by locomotor decline with age (8,28,30).
Numerous studies have demonstrated that there are significant
learning and memory impairments in females undergoing surgical or
natural menopause and in adult ovariectomized female rats and mice
(4,5,31,32).
In addition, estrogen levels can affect learning and memory,
inducing impairments in spatial reference memory, spatial working
memory and non-spatial memory, in aging and adult rodents (3,31–33).
The present study revealed that learning and memory abilities were
significantly decreased in the 16-month-old female rats. In
addition, the level of serum estradiol was found to be
significantly decreased in the 16-month-old rats. These results
were consistent with those of previous studies (3,31–33).
However, these changes are also associated with age and further
studies are required to elucidate the association between menopause
and age-associated learning and memory impairments.
In 1956, Harman (34) introduced the free radical theory of
aging, suggesting that constitutively produced ROS interact with
cellular components in a cumulatively deleterious manner.
Subsequent studies have demonstrated that ROS are central in the
pathogenesis of several diseases, including cardiovascular disease,
kidney damage and neurodegenerative diseases (35–37).
The cortex and the hippocampus are important brain areas, involved
in the process of learning and memory. Whether ROS levels in the
cortex and the hippocampus are involved in the modulation of
learning and spatial memory decline in the menopause remains to be
elucidated. In the present study, the activity of SOD, the level of
MDA and the production of ROS were examined in the cortex and
hippocampus of 3-month and 16-month-old rats. The results revealed
that the activity of SOD was decreased and the content of MDA was
increased in the brain tissues of the 16-month-old rats. ROS
production was also increased significantly in the cortex and the
hippocampal CA1 and CA3 regions in the 16-month-old female rats.
These data suggested that the accumulation of ROS in the cortex and
the hippocampus was important in the modulation of learning and
memory impairments in the menopause.
There is increasing evidence that NOX family members
are important sources of ROS in neurodegenerative diseases
(38,39). Previous studies have reported a
correlation between NOX activity and the progression of Alzheimer's
disease, suggesting that increased NADPH oxidase activity may be
one of the early events in the transition from normal cognition to
dementia (18,40,41).
Furthermore, the increased expression of NOX4 in human
renin/angiotensinogen chimeric transgenic mice is associated with
increased NOX activity and cognitive decline (42). In addition, NOX activity is also
significantly increased in aged amyloid precursor
protein/presinilin 1 mice, and shares a significant linear
correlation with deficits in cognitive function (17). However, whether NOX-mediated ROS
accumulation is involved in the cognitive impairments in the
menopause remains to be elucidated.
NOX, which is dedicated to the specific production
of ROS, consists of membrane (gp91phox, also termed NOX2 and
p22phox) and cytosolic (p47phox, p67phox,
p40phox and RAC1) components (43,44).
The NOX2 membrane-integrated protein is the catalytic core of the
enzyme responsible for electron transfer between NADPH and
molecular oxygen for superoxide production. In nonphagocytic cells,
NOX is constitutively activated, producing relatively low levels of
ROS under basal conditions and generating higher levels of ROS in
response to cytokines and growth factors. The increased ROS
accumulation can result in the stimulation of redox-sensitive
intracellular signaling pathways (45). Previous studies have revealed that
PKC can phosphorylate the gp91phox-cytosolic tail, and
phosphorylation of gp91phox enhances the catalytic activity
and assembly of the complex (46).
Upon activation, the cytosolic components translocate to the
membrane, as a result of interactions between the cytosolic
components and its phospholipid environment (47). A previous study demonstrated that
the elevated expression of p47phox was confirmed as NOX activation
(48). In the present study, the
immunohistochemistry results revealed that the expression levels of
PKCα, NOX2, p47phox and RAC1 were significantly increased in
the cortex and the hippocampus of 16-month-old female rats,
compared with 3-month-old rats. The immunoblotting results also
demonstrated that the expression levels of NOX2, p47phox and
RAC1 were significantly increased in the 16-month-old female rats.
These data suggested that NOX-derived ROS accumulation in the
frontal cortex and hippocampus may be involved in cognitive
impairments in 16-month-old female rats.
In conclusion, the present study demonstrated that
NOX-derived ROS accumulation may be involved in
menopause-associated learning and memory impairments. Although the
present study provided an experimental basis for the mechanism of
NOX-derived ROS accumulation and learning and memory impairments in
16 month old female rats, further investigations are required to
elucidate the mechanisms underlying menopause-associated learning
and memory impairments.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant no. 81371329), the Nature
Science Foundation of Anhui Province (grant. no. 1308085MH144) and
the Doctor Foundation of Anhui Medical University (grant no.
XJ201011). The authors would like to thank Mr. Shan Huang and Mrs.
Li Gui from the Synthetic Laboratory of Basic Medicine College,
Anhui Medical University for their technical assistance.
References
|
1
|
Greendale GA, Lee NP and Arriola ER: The
menopause. Lancet. 353:571–580. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sullivan Mitchell E and Fugate Woods N:
Midlife women's attributions about perceived memory changes:
observations from the Seattle Midlife Women's Health Study. J
Womens Health Gend Based Med. 10:351–362. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sherwin BB: Surgical menopause, estrogen
and cognitive function in women: what do the findings tell us? Ann
NY Acad Sci. 1052:3–10. 2005. View Article : Google Scholar
|
|
4
|
Henderson VW and Popat RA: Effects of
endogenous and exogenous estrogen exposures in midlife and
late-life women on episodic memory and executive functions.
Neuroscience. 191:129–138. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Luine VN, Richards ST, Wu VY and Beck KD:
Estradiol enhances learning and memory in a spatial memory task and
effects levels of monoaminergic neurotransmitters. Horm Behav.
34:149–162. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Frick KM, Fernandez SM and Bulinski SC:
Estrogen replacement improves spatial reference memory and
increases hippocampal synaptophysin in aged female mice.
Neuroscience. 115:547–558. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rocca WA, Bower JH, Maraganore DM, Ahlskog
JE, Grossardt BR, de Andrade M and Melton LJ: Increased risk of
cognitive impairment or dementia in women who underwent
oophorectomy before menopause. Neurology. 69:1074–1083. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Manly JJ, Merchant CA, Jacobs DM, Small
SA, Bell K, Ferin M and Mayeux R: Endogenous estrogen levels and
Alzheimer's disease among postmenopausal women. Neurology.
54:833–837. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Phillips SM and Sherwin BB: Effects of
estrogen on memory function in surgically menopausal women.
Psychoneuroendocrinology. 17:485–495. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Smith CC, Vedder LC, Nelson AR, Bredemann
TM and McMahon LL: Duration of estrogen deprivation, not
chronological age, prevents estrogen's ability to enhance
hippocampal synaptic physiology. Proc Natl Acad Sci USA.
107:19543–19548. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ansari MA and Scheff SW: Oxidative stress
in the progression of Alzheimer disease in the frontal cortex. J
Neuropathol Exp Neurol. 69:155–167. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Keller JN, Schmitt FA, Scheff SW, Ding Q,
Chen Q, Butterfield DA and Markesbery WR: Evidence of increased
oxidative damage in subjects with mild cognitive impairment.
Neurology. 64:1152–1156. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang W, Wang T, Pei Z, Miller DS, Wu X,
Block ML, Wilson B, Zhang W, Zhou Y, Hong JS and Zhang J:
Aggregated alpha-synuclein activates microglia: a process leading
to disease progression in Parkinson's disease. FASEB J. 19:533–542.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gutowicz M: The influence of reactive
oxygen species on the central nervous system. Postepy Hig Med Dosw.
65:104–113. 2011.In Polish. View Article : Google Scholar
|
|
15
|
Hu D, Serrano F, Oury TD and Klann E:
Aging-dependent alterations in synaptic plasticity and memory in
mice that overexpress extracellular superoxide dismutase. J
Neurosci. 26:3933–3941. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dröge W: Free radicals in the
physiological control of cell function. Physiol Rev. 82:47–95.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bruce-Keller AJ, Gupta S, Knight AG,
Beckett TL, McMullen JM, Davis PR, Murphy MP, Van Eldik LJ, St
Clair D and Keller JN: Cognitive impairment in humanized APPxPS1
mice is linked to Aβ(1–42) and NOX activation. Neurobiol Dis.
44:317–326. 2010. View Article : Google Scholar
|
|
18
|
Ansari MA and Scheff SW: NADPH-oxidase
activation and cognition in Alzheimer disease progression. Free
Radic Biol Med. 51:171–178. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sorce S and Krause KH: NOX enzymes in the
central nervous system: from signaling to disease. Antioxid Redox
Signal. 11:2481–2504. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tejada-Simon MV, Serrano F, Villasana LE,
Kanterewicz BI, Wu GY, Quinn MT and Klann E: Synaptic localization
of a functional NADPH oxidase in the mouse hippocampus. Mol Cell
Neurosci. 29:97–106. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fan G, Feng C, Li Y, Wang C, Yan J, Li W,
Feng J, Shi X and Bi Y: Selection of nutrients for prevention or
amelioration of lead-induced learning and memory impairment in
rats. Ann Occup Hyg. 53:341–351. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li WZ, Li WP, Huang DK, Kan HW, Wang X, Wu
WY, Yin YY and Yao YY: Dexamethasone and Aβ25–35
accelerate learning and memory impairments due to elevate amyloid
precursor protein expression and neuronal apoptosis in 12-month
male rats. Behav Brain Res. 227:142–149. 2012. View Article : Google Scholar
|
|
23
|
Cao Z, Swift TA, West CA, Rosano TG and
Rej R: Immunoassay of estradiol: unanticipated suppression by
unconjugated estriol. Clin Chem. 50:160–165. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Joffe H, Petrillo LF, Koukopoulos A,
Viguera AC, Hirschberg A, Nonacs R, Somley B, Pasciullo E, White
DP, Hall JE and Cohen LS: Increased estradiol and improved sleep,
but not hot flashes, predict enhanced mood during the menopausal
transition. J Clin Endocrinol Metab. 96:E1044–E1054. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Girouard H, Park L, Anrather J, Zhou P and
Iadecola C: Cerebrovascular nitrosative stress mediates
neurovascular and endothelial dysfunction induced by angiotensin
II. Arterioscler Thromb Vasc Biol. 27:303–309. 2007. View Article : Google Scholar
|
|
26
|
Dikalov S, Griendling KK and Harrison DG:
Measurement of reactive oxygen species in cardiovascular studies.
Hypertension. 49:717–727. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Girouard H, Wang G, Gallo EF, Anrather J,
Zhou P, Pickel VM and Iadecola C: NMDA receptor activation
increases free radical production through nitric oxide and NOX2. J
Neurosci. 29:2545–2552. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hedden T and Gabrieli JD: Insights into
the ageing mind: a view from cognitive neuroscience. Nat Rev
Neurosci. 5:87–96. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wilson IA, Gallagher M, Eichenbaum H and
Tanila H: Neurocognitive aging: prior memories hinder new
hippocampal encoding. Trends Neurosci. 29:662–670. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kondratova AA and Kondratov RV: The
circadian clock and pathology of the ageing brain. Nat Rev
Neurosci. 13:325–335. 2012.PubMed/NCBI
|
|
31
|
Daniel JM, Hulst JL and Berbling JL:
Estradiol replacement enhances working memory in middle-aged rats
when initiated immediately after ovariectomy but not after a
long-term period of ovarian hormone deprivation. Endocrinology.
147:607–614. 2006. View Article : Google Scholar
|
|
32
|
Duff SJ and Hampson E: A beneficial effect
of estrogen on working memory in postmenopausal women taking
hormone replacement therapy. Horm Behav. 38:262–276. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gibbs RB: Long-term treatment with
estrogen and progesterone enhances acquisition of a spatial memory
task by ovariectomized aged rats. Neurobiol Aging. 21:107–116.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Harman D: Aging: a theory based on free
radical and radiation chemistry. J Gerontol. 11:298–300. 1956.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dennis KE, Aschner JL, Milatovic D,
Schmidt JW, Aschner M, Kaplowitz MR, Zhang Y and Fike CD: NADPH
oxidases and reactive oxygen species at different stages of chronic
hypoxia-induced pulmonary hypertension in newborn piglets. Am J
Physiol Lung Cell Mol Physiol. 297:L596–L607. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Loukogeorgakis SP, van den Berg MJ, Sofat
R, Nitsch D, Charakida M, Haiyee B, de Groot E, MacAllister RJ,
Kuijpers TW and Deanfield JE: Role of NADPH oxidase in endothelial
ischemia/reperfusion injury in humans. Circulation. 121:2310–2316.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Perianayagam MC, Liangos O, Kolyada AY,
Wald R, MacKinnon RW, Li L, Rao M, Balakrishnan VS, Bonventre JV,
Pereira BJ and Jaber BL: NADPH oxidase p22phox and catalase gene
variants are associated with biomarkers of oxidative stress and
adverse outcomes in acute renal failure. J Am Soc Nephrol.
18:255–263. 2007. View Article : Google Scholar
|
|
38
|
Park KW, Baik HH and Jin BK: IL-13-induced
oxidative stress via microglial NADPH oxidase contributes to death
of hippocampal neurons in vivo. J Immunol. 183:4666–4674. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang QG, Raz L, Wang R, Han D, De Sevilla
L, Yang F, Vadlamudi RK and Brann DW: Estrogen attenuates ischemic
oxidative damage via an estrogen receptor alpha-mediated inhibition
of NADPH oxidase activation. J Neurosci. 29:13823–13836. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bruce-Keller AJ, Gupta S, Parrino TE,
Knight AG, Ebenezer PJ, Weidner AM, LeVine H, Keller JN and
Markesbery WR: NOX activity is increased in mild cognitive
impairment. Antioxid Redox Signal. 12:1371–1382. 2010. View Article : Google Scholar :
|
|
41
|
de la Monte SM and Wands JR: Molecular
indices of oxidative stress and mitochondrial dysfunction occur
early and often progress with severity of Alzheimer's disease. J
Alzheimers Dis. 9:167–181. 2006.PubMed/NCBI
|
|
42
|
Inaba S, Iwai M, Furuno M, Tomono Y, Kanno
H, Senba I, Okayama H, Mogi M, Higaki J and Horiuchi M: Continuous
activation of renin-angiotensin system impairs cognitive function
in renin/angiotensinogen transgenic mice. Hypertension. 53:356–362.
2009. View Article : Google Scholar
|
|
43
|
Bedard K and Krause KH: The NOX family of
ROS-generating NADPH oxidases: physiology and pathophysiology.
Physiol Rev. 87:245–313. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Masamune A, Watanabe T, Kikuta K, Satoh K
and Shimosegawa T: NADPH oxidase plays a crucial role in the
activation of pancreatic stellate cells. Am J Physiol Gastrointest
Liver Physiol. 294:G99–G108. 2008. View Article : Google Scholar
|
|
45
|
Weintraub NL: Nox response to injury.
Arterioscler Thromb Vasc Biol. 22:4–5. 2002.PubMed/NCBI
|
|
46
|
Raad H, Paclet MH, Boussetta T, Kroviarski
Y, Morel F, Quinn MT, Gougerot-Pocidalo MA, Dang PM and El-Benna J:
Regulation of the phagocyte NADPH oxidase activity: phosphorylation
of gp91phox/NOX2 by protein kinase C enhances its diaphorase
activity and binding to Rac2, p67phox and p47phox. FASEB J.
23:1011–1022. 2009. View Article : Google Scholar :
|
|
47
|
Mizrahi A, Berdichevsky Y, Casey PJ and
Pick E: A prenylated p47phox-p67phox-Rac1 chimera is a
Quintessential NADPH oxidase activator: membrane association and
functional capacity. J Biol Chem. 285:25485–25499. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Edlund J, Fasching A, Liss P, Hansell P
and Palm F: The roles of NADPH-oxidase and nNOS for the increased
oxidative stress and the oxygen consumption in the diabetic kidney.
Diabetes Metab Res Rev. 26:349–356. 2010. View Article : Google Scholar : PubMed/NCBI
|