Introduction
A previous study by our group demonstrated changes
in the glycosylation of serum proteins in tumor-bearing mice
(1). However, it was not possible
to establish a link between the various N-linked oligosaccharide
residues and the tumors of animals or cancer patients (1–3).
Another previous study by our group showed that the serpin
α-anti-trypsin from blood serum inhibits or blocks the growth of
tumors (4). The question that
arises from these studies is how proteins in the blood serum of
tumor-bearing mice as well as in the blood serum of tumor-free mice
acquire tumor-associated biological activity. It is known that the
blood serum of tumor-bearing animals contains a certain factor
which specifically accelerates tumor growth (2,5).
However, all attempts to identify this factor as a novel protein in
the blood serum of tumor-bearing animals were unsuccessful
(5,6). Significant progress has been made
toward comprehensive protein expression profiling, and numerous
biomarker candidates have been identified; however, none of the
reported biomarkers have been proven to be beneficial for patients
with cancer (7). It is therefore
necessary to identify these factors through their biological
activity in vivo. The above-mentioned N-linked
oligosaccharide residues are part of protein molecules. Changes in
these residues [in the previous study by our group they became
shorter (4)] are likely to lead to
conformational changes of the protein molecule. It is can therefore
be assumed that changes in the glycosylation of proteins occur in
order to modify the conformation of the protein molecule (8,9).
Conformational changes of a protein lead to changes in its
biological activity. In organisms displaying tumor growth, no novel
proteins were detected in the blood serum compared with those in
tumor-free organisms; this may be due to the existing proteins
undergoing conformational changes leading to the activation of
their tumor-associated activity (10).
The most informative method for studying proteins is
proteomic analysis. This analysis involves, in particular, the
proteolysis of proteins. It is known that certain proteolytic
enzymes have specific cleavage sites. These sites should fulfill
the following requirements: They are required to contain certain
amino acids, and furthermore, these sites should be available for
their specific cleavage enzymes. If the proteolysis of a protein
isolated from the serum of a tumor-bearing mouse and that of the
same protein isolated from the serum of a tumor-free mouse results
in products, it is indicated that this protein has a differential
conformation between the tumor-bearing and the tumor-free mouse.
However, until recently, a complete protein denaturation was
performed prior to proteomic analysis for better accessibility of
trypsin. After the complete denaturation of the proteins with
differential conformation, they are expected to have identical
availability for trypsin and the trypsinolysis products of these
denatured proteins would be identical, therefore not revealing any
information regarding differences in protein conformation. This
hypothesis explains why proteomic studies have never reported any
tumor-specific changes in blood serum proteins.
Tryptic cleavage under soft conditions is
challenging; therefore, to obtain a sufficient amount of peptide
ions, it is required to use 'semitrypsin' analysis (11,12).
This improves the quality and reliability of the identification of
proteins and allows for the identification of the protein
structure, including various post-translational modifications.
The aim of the present study was to examine the
hypothesis that 1) tumor-specific activity of proteins may be
associated with changes in their native conformation, e.g., due to
changes in their glycosylation and 2) it is possible to determine
changes in the native conformation via the proteolysis products of
the protein under soft conditions. Semi-tryptic peptide ions were
examined and the biological activity of albumin and
inter-alpha-trypsin inhibitor heavy chain 4 (ITIH4) in tumor-free
C57Bl/6 mice and those with B16 melanoma were assessed. The ITIH4
protein is a member of the serpin superfamily (13).
Materials and methods
Tumor and animal experimentation
B16 melanoma cells were obtained from the bank of
tumor strains of the N. N. Blokhin Russian Cancer Research Center
(Moscow, Russia). Melanoma cells were transplanted subcutaneously
to obtain solid tumors into the right hind limb of C57Bl/6 mice
(1×106 cells/mouse diluted in 200 µl RPMI-1640
medium; PanEcho, Moscow, Russia). The experiments were performed
using a total of 250 2- to 3-month-old male C57Bl/6 and F1
(C57Bl/6xCBA/Lac) mice (22–24 g) obtained from Stolbovaya Company
(Moscow, Russia). The mice received a standard laboratory diet and
tap water ad libitum and were kept under a natural
light/dark cycle. All experiments were performed in accordance with
the National Institutes of Health guidelines (14), the legal regulations for animal
experimentation in Russia, and the study was approved by the ethics
commiteeb of the Institute of Experimental Diagnosis and Therapy of
Tumors of the N. N. Blokhin Russian Cancer Research Center (Moscow,
Russia). Blood was extracted according to the protocol described by
Fisher et al (5).
Inductive effect of serum protein on
tumor growth
To determine the effect of blood serum proteins,
fractions of serum proteins were administered intraperitoneally to
healthy mice (100 µg/mouse in 400 µl 0.9% NaCl
solution; PanEcho) two weeks prior to tumor transplantation. Each
group included 10 animals.
Serum proteins
Blood serum proteins were collected from normal and
from tumor-bearing mice. Serum from B16 tumor-bearing mice was
collected 30 days after tumor cell injection. Serum proteins were
separated into fractions using ultrafiltration membranes
(Millipore, Billerica, MA, USA) under air pressure with a nominal
molecular weight limit of 300 kDa [PBMK04310; nominal molecular
weight limit (NMWL), 300,000] (fraction 1), 100 kDa (PBHK; NMWL,
100,000) (fraction 2) or 50 kDa (PBQK; NMWL, 50,000) (fraction 3).
Fraction-3 proteins were diluted in 0.01 M Tris buffer (pH 7.4,
containing 0.01% sodium azide) and transferred onto PD-10 columns
(Amersham Biosciences, GE Healthcare, Little Chalfont, UK)
(4). Proteins were applied to
Sepharose Q FF and Sepharose Blue FF columns (XK 16/20; GE
Healthcare). Non-bound serum proteins were eluted with 0.01 M Tris
buffer (pH 7.4, containing 0.01% sodium azide; Sigma-Aldrich, St
Louis, MO, USA), while the bound proteins were eluted using a
sodium chloride gradient (0.5 M, 0.01 M Tris buffer, pH 7.4, with
0.01% sodium azide; Sigma-Aldrich) (10) with the help of a GP-250 programmed
gradient pump (Pharmacia Biotech, GE Healthcare). Protein elution
was monitored in a flow cell (SN 20257; 2 mm; GE Healthcare) at
λ=280 nm. The samples containing albumin and serpins were collected
and analyzed using an HP 8452A diode array spectrophotometer
(Agilent Technologies, Santa Clara, CA, USA) measuring the
absorption value at λ=280 nm. Subsequently, proteins were
intraperitoneally injected at 100 µg/mouse in 400 µl
0.9% NaCl solution into healthy mice. After 14 days,
1×106 melanoma B16 tumor cells were transplanted into
these mice subcutaneously. Each group included 10 animals.
Protein gel electrophoresis
Serpin samples were separated by 12.5% SDS-PAGE and
stained with Coomassie blue (Sigma-Aldrich) according to Laemmli
(15). Samples subjected to
SDS-PAGE were solubilized in a sample buffer (Sigma-Aldrich)
containing 63 mM Tris/HCl, pH 6.8, 10% (v/v) glycerol, 2% (w/v) SDS
and 30 M bromophenol blue (Sigma-Aldrich). 2-Mercaptoethanol 5%
(v/v) (Sigma-Aldrich) was conditionally added or omitted in the
sample buffer. 12.5% acrylamide gels with a
bisacrylamide/acrylamide ratio of 0.8:30 were used. Samples were
applied in quantities of 10 and 50 µg protein/lane to
evaluate all components of the protein complexes. Protein gel
electrophoresis was used for determination of the mass of ITIH4
with tumor specific activity.
Assessment of albumin binding using
electron spin resonance (ESR)
ESR spectra were measured for each sample using a
commercially available ESR spectrometer (AXM-09; ESR-Analyzer/MMS;
MedInnovation GmbH, Berlin, Germany). The principle of this
technique is the measurement of albumin binding variables, achieved
by a fatty acid spin probe. The binding variables of the spin probe
were determined at different permutations of the ethanol
concentration as well as the ratio of spin probe and albumin
concentration. Variation of the ethanol concentration allowed for
the assessment of binding variables of the spin probe to albumin
under different hydrophobic conditions. Changes in the ratio of
spin probe to albumin enabled the measurement of the binding
affinity of albumin to the spin probe.
Commercial 16-doxyl stearic acid (Sigma-Aldrich) was
used as spin probe. This compound was selected due to the
exceptionally high binding constant of albumin to stearic acid
(6.9×107 l/mol), which produces >99.9% binding of the
spin probe to albumin. Ethanol, extra pure, (Merck Millipore) was
used for modifying the binding affinity of the fatty acid spin
probe to albumin. The final concentrations of ethanol (mol/l) and
spin probe (10−3 mol/l) were 2.9 and 0.83 in aliquot 1,
3.4 and 1.61 in aliquot 2, and 3.8 and 2.34 in aliquot 3,
respectively (16).
Mass spectrometry
For mass spectrometric analysis, samples of protein
were only used after purification using Sephadex Q FF columns.
Proteins were diluted in 50 mM ammonium bicarbonate solution and
transferred onto PD-10 columns (W359685; GE Healthcare). The final
concentration of the proteins in the samples was 1 mg/ml. Solely
following column chromatography without electrophoresis, the
samples were incubated at 37°C in 100 µl 20 mM ammonium
bicarbonate containing 5 ng/µl trypsin, chymotrypsin and 10%
acetonitrile overnight (all from Sigma-Aldrich). Peptides were
concentrated using SEP-PAK C18 cartridges (Millipore).
Mass spectra were recorded using an Ultraflex
Extreme MALDI-TOF/TOF mass spectrometer (Bruker Daltonics,
Billerica, MA, USA) equipped with a neodymium laser. To analyze
mass spectra, FlexAnalysis software, version 3.3 (Bruker Daltonics,
Bremen, Germany) was used. Aliquots of the samples were mixed on a
steel target with a solution of 2,5-dihydroxybenzoic acid (20
mg/ml) in 30% (v/v) acetonitrile (Sigma-Aldrich) with 0.5% (v/v)
trifluoroacetic acid. The [MH]+ molecular ions were
analyzed in linear (proteins) or reflector (peptides) mode, and the
m/z ratios were accurate to 30 ppm. Fragment ion spectra were
obtained in Lift mode with a mass accuracy of better than 1 Da.
Proteases cleaved peptides were used in the peptide mixtures. The
data were processed using Flex Analysis 2.2 software (Bruker
Daltonics), and peaks of trypsin fragments contained in samples
were used for calibration. The following search parameters were
used: Accuracy of mass determination = 100 ppm, NCBInr database,
Rodentia taxon (rodent), one missed cleavage and possible
methionine oxidation. The proteins were identified using the Mascot
version 2.2.07 search software (peptide fingerprint option;
http://www.matrixscience.com). The
search was conducted in the NCBI databases and/or EST vertebrates.
For the search of candidate proteins in combined ms + (ms-ms) data,
Biotools, version 3.0 (Bruker Daltonics) was used. If the value of
the Score parameter calculated for each protein exceeded 70,
identification was accepted as reliable.
Statistical analysis
Statistical evaluation was performed using Fisher's
exact test or Student's t-test. Data are presented as the mean ±
standard deviation and Microsoft Office Excel (14.0.6112.5000)
software was used for statistical analysis. P<0.05 was
considered to indicate a statistically significant difference
between values.
Results
Tumor-free mice and mice with B16
melanoma have differential proteolytic fragments of serum
albumin
By means of column chromatography, a fraction
corresponding to albumin was extracted from the blood serum of
tumor-free mice and the blood serum of mice with B16 melanoma.
Subsequent proteomic analysis identified the serum precursor of
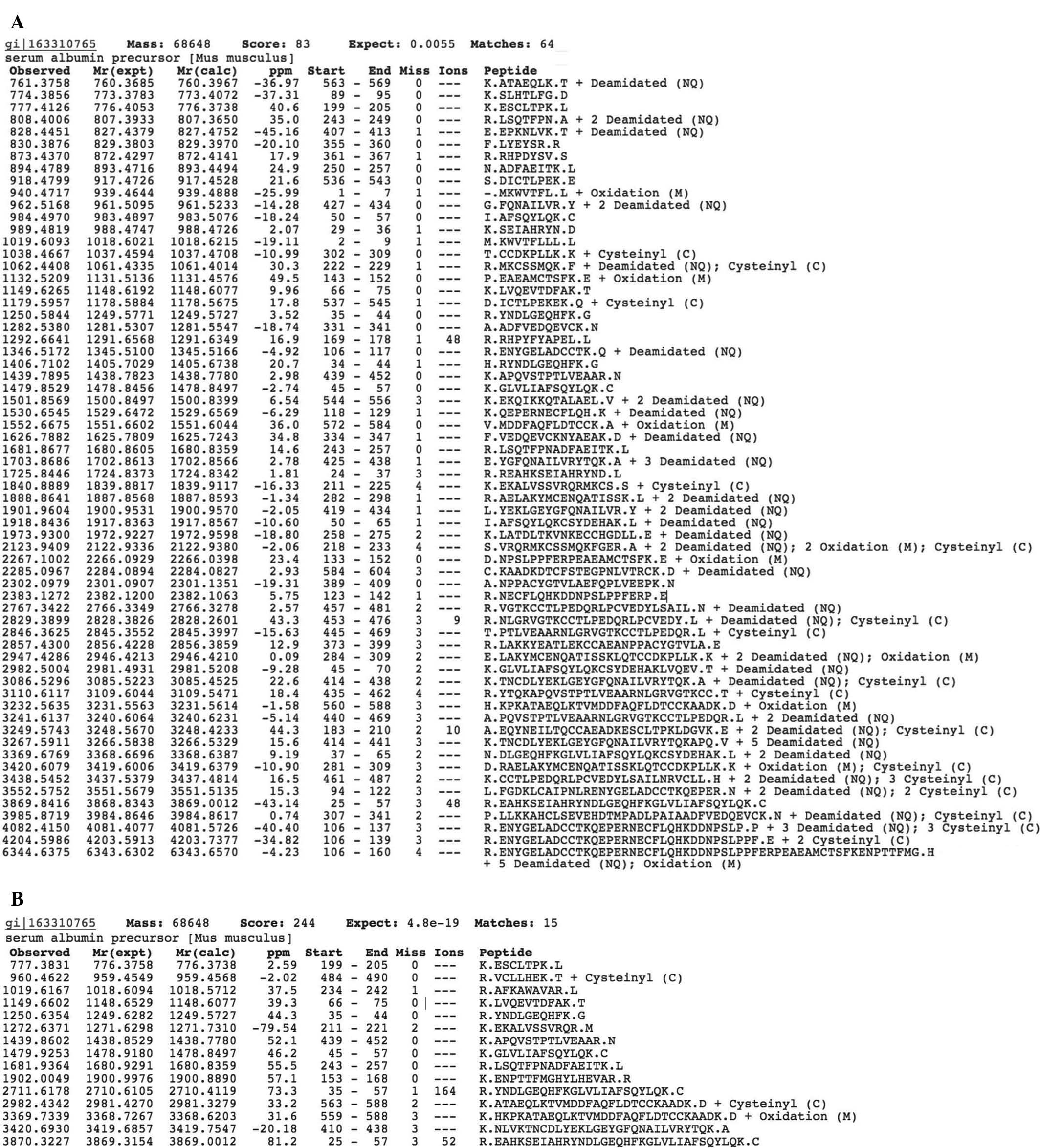
albumin in the two cases (Fig. 1A and
B). Thus, albumin extracted from mice with the tumor has the
same amino acid sequence as albumin extracted from the tumor-free
mice.
In addition, the data presented in Fig. 1A and B show that proteolytic
analysis of the albumin from tumor-free mice identified a greater
number of semi-tryptic peptide ions compared with that in mice with
B16 melanoma (64 vs 15 peptides, respectively). Normal mice and
those with B16 melanoma had seven peptides in common, and eight
peptides were found exclusively in mice with B16 melanoma (Tables I and II). However, a number of the peptides
which identified in mice with B16 melanoma partially or completely
overlapped with a significant number of the peptides identified in
tumor-free mice. This resulted in a larger number of identified
peptides in tumor-free mice compared with that in mice with B16
melanoma (Table III).
 | Table ISemi-tryptic peptides of albumin
identified in tumor-bearing mice and tumor-free mice. |
Table I
Semi-tryptic peptides of albumin
identified in tumor-bearing mice and tumor-free mice.
| B16 melanoma (m/z of
peptide ion) | Tumor-free mice (m/z
of peptide ion) | Amino acids
(start-end) |
|---|
| 777.3831 | 777.4126 | 199–205 |
| 1149.6602 | 1149.6265 | 66–75 |
| 1250.6354 | 1250.5844 | 35–44 |
| 1439.8602 | 1439.7895 | 439–452 |
| 1479.9253 | 1479.8529 | 45–57 |
| 1681.9364 | 1681.8677 | 243–257 |
| 3870.3227 | 3869.8416 | 25–57 |
 | Table IISemi-tryptic peptides of albumin
identified in tumor-bearing mice only. |
Table II
Semi-tryptic peptides of albumin
identified in tumor-bearing mice only.
| Peptide ion
(m/z) | Amino acids
(start-end) |
|---|
| 960.4622 | 484–490 |
| 1019.6167 | 234–242 |
| 1272.6371 | 211–221 |
| 1902.0049 | 153–168 |
| 2711.6178 | 35–57 |
| 2982.4342 | 563–588 |
| 3369.7339 | 559–588 |
| 342.693 | 410–438 |
 | Table IIIOverlapping peptides of albumin in
tumor-bearing mice and in tumor-free mice. |
Table III
Overlapping peptides of albumin in
tumor-bearing mice and in tumor-free mice.
| Animals | Peptide ion
(m/z) | Start-end | Amino acid
sequence |
|---|
| B16 melanoma | 2711.6178 | 35–57 |
R.YNDLGEQHFKGLVLIAFSQYLQK.C |
| Tumor-free mice | 1725.8446 | 24–37 |
R.REAHKSEIAHRYND.L |
| Tumor-free mice | 989.4819 | 29–36 | K.SEIAHRYN.D |
| Tumor-free mice | 1406.7102 | 34–44 | H.RYNDLGEQHFK.G |
| Tumor-free mice | 3369.6769 | 37–65 |
N.DLGEQHFKGLVLIAFSQYLQKCSYDEHAK.L +2
deamidated (NQ) |
| Tumor-free mice | 2982.5004 | 45–70 |
K.GLVLIAFSQYLQKCSYDEHAKLVQEV.T +
deamidated (NQ) |
| Tumor-free mice | 984.497 | 50–57 | I.AFSQYLQK.C |
| Tumor-free mice | 1918.8436 | 50–65 | I.AFSQYLQKCSYDEHAK.L
+ deamidated (NQ) |
| B16 melanoma | 1272.6371 | 211–221 | K.EKALVSSVRQR.M |
| Tumor-free mice | 1840.8889 | 211–225 | K.EKALVSSVRQRMKCS.S +
cysteinyl (C) |
| Tumor-free mice | 2123.9409 | 218–233 | S.VRQRMKCSSMQKFGER.A
+ 2 deamidated (NQ) |
| Tumor-free mice | 1681.8677 | 243–257 |
R.LSQTFPNADFAEITK.L |
| B16 melanoma | 1681.9364 | 243–257 |
R.LSQTFPNADFAEITK.L |
| Tumor-free mice | 808.4006 | 243–249 | R.LSQTFPN.A + 2
deamidated (NQ) |
| Tumor-free mice | 894.4789 | 250–257 | N.ADFAEITK.L |
| B16 melanoma | 3420.693 | 410–438 |
K.NLVKTNCDLYEKLGEYGFQNAILVRYTQK.A |
| Tumor-free
mice | 3086.5296 | 414–438 |
K.TNCDLYEKLGEYGFQNAILVRYTQK.A + Deamidated
(NQ) |
| Tumor-free
mice | 3267.5911 | 414–441 |
K.TNCDLYEKLGEYGFQNAILVRYTQKAPQ.V +5
deamidated (NQ) |
| Tumor-free
mice | 1901.9604 | 419–434 |
L.YEKLGEYGFQNAILVR.Y + 2 deamidated
(NQ) |
| Tumor-free
mice | 1703.8686 | 425–438 | E.YGFQNAILVRYTQK.A
+ 3 deamidated (NQ) |
| Tumor-free
mice | 962.5168 | 427–434 | G.FQNAILVR.Y + 2
deamidated (NQ) |
| Tumor-free
mice | 3110.6117 | 435–462 |
R.YTQKAPQVSTPTLVEAARNLGRVGTKCC.T +
cysteinyl (C) |
| Tumor-free
mice | 1439.7895 | 439–452 |
K.APQVSTPTLVEAAR.N |
| B16 melanoma | 1439.8602 | 439–452 |
K.APQVSTPTLVEAAR.N |
| Tumor-free
mice | 3241.6137 | 440–469 |
A.PQVSTPTLVEAARNLGRVGTKCCTLPEDQR.L + 2
deamidated (NQ) |
| Tumor-free
mice | 2846.3625 | 445–469 |
T.PTLVEAARNLGRVGTKCCTLPEDQR.L + cysteinyl
(C) |
| B16 melanoma | 3369.7339 | 559–588 |
K.HKPKATAEQLKTVMDDFAQFLDTCCKAADK. D +
oxidation (M) |
| B16 melanoma | 2982.4342 | 563–588 |
K.ATAEQLKTVMDDFAQFLDTCCKAADK. D +
cysteinyl (C) |
| Tumor-free
mice | 3232.5635 | 560–588 |
H.KPKATAEQLKTVMDDFAQFLDTCCKAADK. D +
oxidation (M) |
| Tumor-free
mice | 761.3758 | 563–569 | K.ATAEQLK.T +
deamidated (NQ) |
| Tumor-free
mice | 1552.6675 | 572–584 | V.MDDFAQFLDTCCK.A +
oxidation (M) |
| Tumor-free
mice | 2285.0967 | 584–604 |
C.KAADKDTCFSTEGPNLVTRCK.D + deamidated
(NQ) |
In tumor-free mice, seven peptides were identified
to partially overlap with those identified in mice with B16
melanoma with regard to their amino acid sequences at 35–57 and the
m/z ratio of 2,711.6178, and two peptides were identified to
partially overlap with regard to their amino acid sequences at
211–221 and the m/z ratio of 1,272.6371. Furthermore, in tumor-free
mice, two peptides were identified to partially overlap with those
found in mice with B16 melanoma with regard to their amino acid
sequences at 243–257 and the m/z ratio of 1,681.9364. In tumor-free
mice, six peptides were identified to partially or completely
overlap with those from mice with B16 melanoma in their amino acid
sequences at 410–438 and an m/z 3,420.693. In addition, in
tumor-free mice, two peptides were identified to partially overlap
with peptides from mice with B16 melanoma in their amino acid
sequences at 439–452 and the m/z ratios of 1,439.8602 and
1,439.7895. Finally, in tumor-free mice, four peptides were
identified to partially or fully overlap with two peptides in mice
with B16 melanoma regarding the amino acid sequences 559–588 with
the m/z ratio of 3,369.7339 and 563–588 with the m/z ratio of
2,982.4342. The large number and variation of proteolysis products
of the serum proteins from tumor-free mice compared to those from
mice with melanoma B16, indicates the mobility of these specific
regions in the former and the rigidity of the same parts of the
molecule in the latter. The increase in rigidity of the albumin
molecule during tumor growth may be due to the formation of
cross-links within the molecule. This process may be similar to the
transformation of fibrin to form internal cross-links (17). This process may make proteins in
animals with tumors more rigid compared to those of tumor-free
animals and leads to a decrease in the number of proteolysis
products.
Tumor-free mice and mice with B16
melanoma have differential proteolytic fragments of serum
ITIH4
Another protein isolated by column chromatography
was the protein fraction corresponding to ITIH4. Similarly to the
case of albumin, it was necessary to verify that proteins with the
same amino acid sequence were analyzed in the protein extracted
from the blood serum of the mice with B16 melanoma and that from
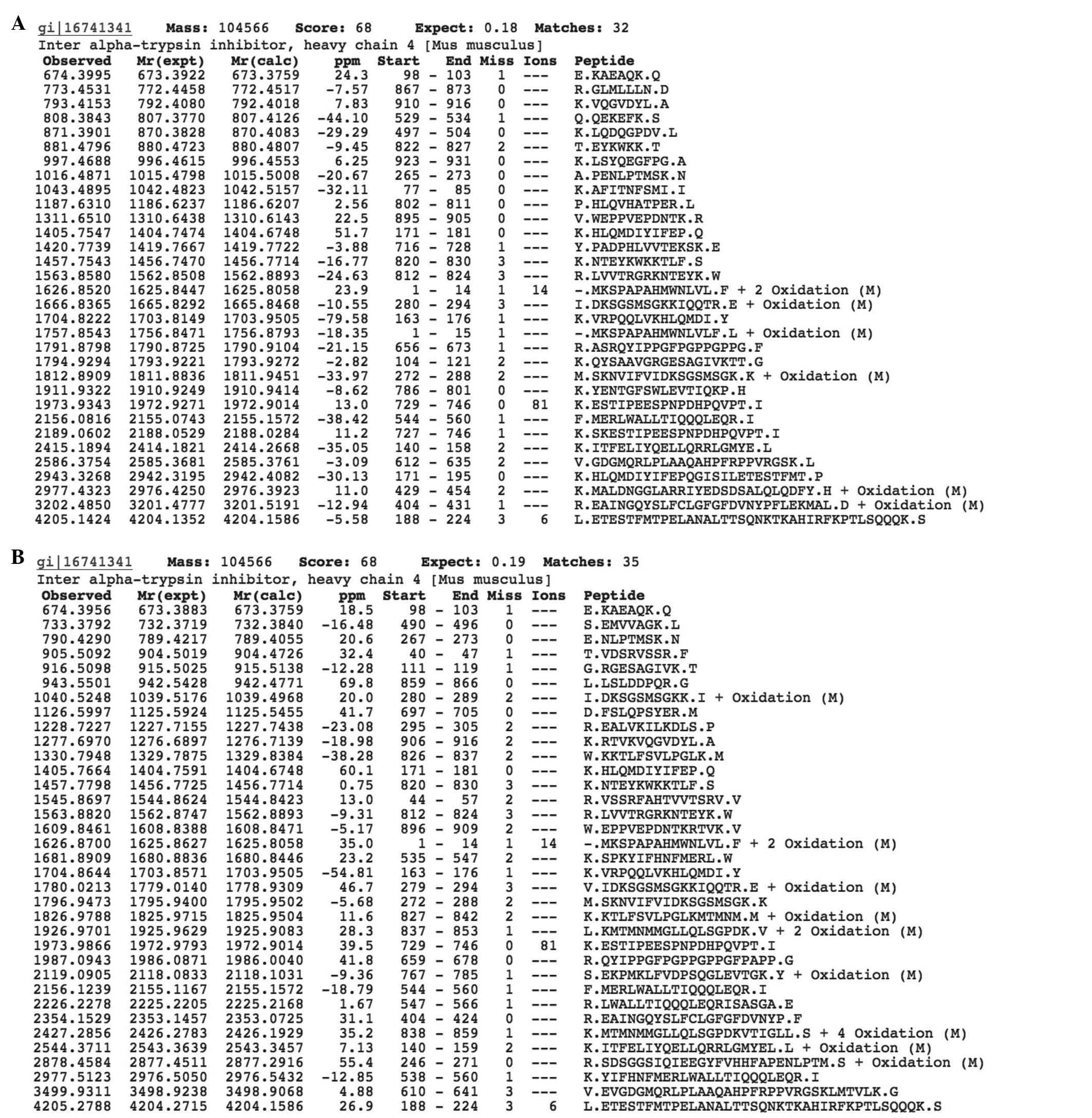
the blood serum of tumor-free mice. As shown in Fig. 2A and B, proteomic analysis
identified ITIH4 in the two groups.
The total quantity of identified peptides following
proteolysis of ITIH4 from the serum of tumor-free mice was 32, that
of mice with B16 melanoma was 35. Thus, by contrast with albumin,
in the case of serpin, no significant difference was observed in
the number of identified peptide fragments of ITIH4. As discussed
above, the lack of variations in the number of identified peptides
in plasma during tumor growth may be explained by various
mechanisms of modification of the proteins in the body. Serpin,
unlike albumin, is a glycosylated protein and its spatial structure
changes due to glycosylation, not due to internal cross-links.
Glycosylation-induced changes of blood serum proteins during tumor
growth are discussed above (2).
Among the identified peptide fragments of ITIH4
following proteolysis, 10 common peptides were identified among the
proteins derived from tumor-bearing mice and those obtained from
tumor-free mice (Table IV), while
the remaining peptide fragments were unique in the tumor-bearing
mice (Table V, n=22) and the
tumor-free mice (n=25).
 | Table IVSemi-tryptic peptides of the
inter-alpha-trypsin inhibitor heavy chain 4 identified in
tumor-bearing and tumor-free mice. |
Table IV
Semi-tryptic peptides of the
inter-alpha-trypsin inhibitor heavy chain 4 identified in
tumor-bearing and tumor-free mice.
| Peptide ion in B16
melanoma (m/z) | Peptide ion in
tumor-free mice (m/z) | Amino acids
(start-end) |
|---|
| 674.3956 | 674.3995 | 98–103 |
| 1405.7664 | 1405.7547 | 171–181 |
| 1457.7798 | 1457.7543 | 820–830 |
| 1563.882 | 1563.858 | 812–824 |
| 1626.8700 | 1626.852 | 1–14 |
| 1704.8644 | 1704.8222 | 163–176 |
| 1973.9866 | 1973.9343 | 729–746 |
| 2156.1239 | 2156.0816 | 544–560 |
| 1796.9473 | 1812.8909 | 272–288 |
| 4205.2788 | 4205.1424 | 188–224 |
 | Table VSemi-tryptic peptides of the
inter-alpha-trypsin inhibitor heavy chain 4 identified in
tumor-bearing mice only. |
Table V
Semi-tryptic peptides of the
inter-alpha-trypsin inhibitor heavy chain 4 identified in
tumor-bearing mice only.
| Peptide ion
(m/z) | Amino acids (start
to end) |
|---|
| 733.3792 | 490–496 |
| 790.4290 | 267–273 |
| 905.5092 | 40–47 |
| 916.5098 | 111–119 |
| 943.5501 | 859–866 |
| 1040.5248 | 280–289 |
| 1126.5997 | 697–705 |
| 1228.7227 | 295–305 |
| 1277.6970 | 906–916 |
| 1330.7948 | 826–837 |
| 1545.8697 | 44–57 |
| 1609.8461 | 896–909 |
| 1780.0213 | 279–294 |
| 1826.9788 | 827–842 |
| 1926.9701 | 837–853 |
| 1987.0943 | 659–678 |
| 2119.0905 | 767–785 |
| 2354.1529 | 404–424 |
| 2427.2856 | 838–859 |
| 2544.3711 | 140–159 |
| 2977.5123 | 538–560 |
| 3499.9311 | 610–641 |
Among the peptides identified in tumor-free mice,
twelve peptides were detected that partially or completely
overlapped with thirteen peptides detected in mice with B16
melanoma (Table VI). A peptide
fragment with an amino acid sequence of 104–121 and an m/z of
1,794.9294 was detected in tumor-free mice. This peptide completely
overlapped with the peptide fragment with the amino acid sequence
111–119 and an m/z of 916.5098 found in mice with B16 melanoma.
Furthermore, a peptide with the amino acid sequence of 140–158 and
an m/z ratio of 2,415.1894 detected in tumor-free mice partially
overlapped with a peptide with the amino acid sequence 140–159 and
the m/z of 2,544.3711 found in mice with B16 melanoma. The peptide
with the amino acid sequence of 171–195 and the m/z of 2,943.3268
detected in tumor-free mice completely overlapped with the peptide
with the amino acid sequence 171–181 found in mice with B16
melanoma (m/z, 1,405.7664) and in tumor-free mice (m/z 1,405.7547).
The peptide with the amino acid sequence of 265–273 and the m/z of
1,016.4871 detected in tumor-free mice partially overlapped with
the peptide with the amino acid sequence of 267–273 and the m/z of
790.429 found in mice with B16 melanoma. The peptide with the amino
acid sequence of 280–294 and the m/z of 1,666.8365 detected in
tumor-free mice partially overlapped with the peptide with the
amino acid sequence of 279–294 and the m/z of 1,780.0200 and
completely overlapped with the peptide with the amino acid sequence
of 280–289 and the m/z ratio of 1,040.5248 found in mice with B16
melanoma. The peptide with the amino acid sequence of 404–431 and
the m/z of 3,202.485 detected in tumor-free mice partially
overlapped with the peptide with the amino acid sequence of 404–424
and the m/z of 2,354.1529 from mice with B16 melanoma. The peptide
with the amino acid sequence of 544–560 which was detected in
tumor-free mice (m/z, 2,156.1239) and in mice with B16 melanoma
(m/z, 2,156.0816) partially overlapped with the peptide with the
amino acids sequence of 547–566 and the m/z of 2,226.2278 found in
mice with B16 melanoma. The peptide with the amino acid sequence of
612–635 and the m/z of 2,586.3754 detected in tumor-free mice
partially overlapped with the peptide with the amino acid sequence
of 610–641 and the m/z of 3,499.9311 found in mice with B16
melanoma. The peptide with the amino acid sequence 656–673 and the
m/z of 1,791.8798 detected in tumor-free mice partially overlapped
with the peptide with the amino acid sequence 659–678 and the m/z
1,987.0943 found in mice with B16 melanoma. The peptide with the
amino acid sequence of 822–827 and the m/z of 881.4796 detected in
tumor-free mice partially overlapped with the peptide with the
amino acid sequence of 826–837 and the m/z of 1,330.7948 found in
mice with B16 melanoma. The peptide with the amino acid sequence of
895–905 and the m/z of 1,311.651 detected in tumor-free mice
partially overlapped with the peptide with the amino acid sequence
of 896–909 and the m/z of 1,609.8461 found in mice with B16
melanoma. The peptide with the amino acid sequence of 910–916 and
the m/z of 793.4153 detected in tumor-free mice partially
overlapped with the peptide with the amino acid sequence of 906–916
and the m/z ratio of 1,277.697 found in mice with B16 melanoma.
Thus, the same pattern to that observed for albumin was confirmed
for ITIH4, i.e. ITIH4 obtained from mice with B16 melanoma provided
semi-tryptic peptide fragments that differed from the semi-tryptic
peptide fragments of ITIH4 from the serum of tumor-free mice. This
result indicated a difference in the availability of proteolysis
sites and therefore, a different molecular conformation of albumin
and ITIH4 in the serum of tumor-bearing and tumor-free mice.
 | Table VIOverlapping peptides of the
inter-alpha-trypsin inhibitor heavy chain 4 in tumor-bearing and in
tumor-free mice. |
Table VI
Overlapping peptides of the
inter-alpha-trypsin inhibitor heavy chain 4 in tumor-bearing and in
tumor-free mice.
| Animals | Peptide ion
(m/z) | Start-end | Amino acid
sequence |
|---|
| Tumor-free
mice | 1794.9294 | 104–121 |
K.QYSAAVGRGESAGIVKTT.G |
| B16 melanoma | 916.5098 | 111–119 | G.RGESAGIVK.T |
| Tumor-free
mice | 2415.1894 | 140–158 |
K.ITFELIYQELLQRRLGMYE.L |
| B16 melanoma | 2544.3711 | 140–159 |
K.ITFELIYQELLQRRLGMYEL.L + oxidation
(M) |
| Tumor-free
mice | 2943.3268 | 171–195 |
K.HLQMDIYIFEPQGISILETESTFMT.P |
| Tumor-free
mice | 1405.7547 | 171–181 |
K.HLQMDIYIFEP.Q |
| B16 melanoma | 1405.7664 | | |
| Tumor-free
mice | 1016.4871 | 265–273 | A.PENLPTMSK.N |
| B16 melanoma | 790.429 | 267–273 | E.NLPTMSK.N |
| Tumor-free
mice | 1666.8365 | 280–294 | I.DKSGSMSGKKIQQTR.E
+ oxidation (M) |
| B16 melanoma | 1780.0200 | 279–294 |
V.IDKSGSMSGKKIQQTR.E + oxidation (M) |
| B16 melanoma | 1040.5248 | 280–289 | I.DKSGSMSGKK.I +
oxidation (M) |
| Tumor-free
mice | 3202.485 | 404–431 |
R.EAINGQYSLFCLGFGFDVNYPFLEKMAL. +
oxidation (M) |
| B16 melanoma | 2354.1529 | 404–424 |
R.EAINGQYSLFCLGFGFDVNYP.F |
| Tumor-free
mice | 2156.0816 | 544–560 |
F.MERLWALLTIQQQLEQR.I |
| B16 melanoma | 2156.1239 | 544–560 |
F.MERLWALLTIQQQLEQR.I |
| B16 melanoma | 2226.2278 | 547–566 |
R.LWALLTIQQQLEQRISASGA.E |
| Tumor-free
mice | 2586.3754 | 612–635 |
V.GDGMQRLPLAAQAHPFRPPVRGSK.L |
| B16 melanoma | 3499.9311 | 610–641 |
V.EVGDGMQRLPLAAQAHPFRPPVRGSKLMTVLK.G |
| Tumor-free
mice | 1791.8798 | 656–673 |
R.ASRQYIPPGFPGPPGPPG.F |
| B16 melanoma | 1987.0943 | 659–678 |
R.QYIPPGFPGPPGPPGFPAPP.G |
| Tumor-free
mice | 881.4796 | 822–827 | T.EYKWKK.T |
| B16 melanoma | 1330.7948 | 826–837 |
W.KKTLFSVLPGLK.M |
| Tumor-free
mice | 1311.651 | 895–905 |
V.WEPPVEPDNTK.R |
| B16 melanoma | 1609.8461 | 896–909 |
W.EPPVEPDNTKRTVK.V |
| Tumor-free
mice | 793.4153 | 910–916 | K.VQGVDYL.A |
| B16 melanoma | 1277.697 | 906–916 |
K.RTVKVQGVDYL.A |
Serum albumin has differential
conformation-depending binding activity between tumor-bearing and
tumor-free mice
In the next stage of the present study, it was
required to test whether the biological activity of the assessed
proteins was altered upon their conformational changes. For the
assessment of the functional activity of albumin, ESR analysis was
used, allowing for the estimation of the conformational status and
transport parameters of albumin. For this purpose, the interaction
of albumin with the spin probe 16-doxyl stearic acid was examined.
As the results in Table VII
demonstrate, the spin-labeled acid interacted differently with
albumin depending on whether it was obtained from tumor-free or
tumor-bearing mice. The largest difference (almost 1.4 times) among
all values determined was observed in the discrimination parameter.
According to the developers of the ESR device, this the
discrimination parameter characterizes the conformation of the
molecule. Therefore, the results obtained supported the hypothesis
that albumin in tumor-bearing mice and tumor-free mice have
differential conformations, which may be involved in the regulation
of the tumor-associated activity of proteins.
 | Table VIIInteraction between albumin and the
fatty acid spin probe 16-doxyl stearate acid determined by electron
spin resonance analysis. |
Table VII
Interaction between albumin and the
fatty acid spin probe 16-doxyl stearate acid determined by electron
spin resonance analysis.
| Parameter | Tumor-free
mice | Tumor-bearing
mice | P-value |
|---|
| Discrimination
parameter | −3.08±0.06 | −2.2±0.09 | <0.05 |
| Binding efficiency
(%) | 28.4±5.1 | 22.0±5.0 | <0.05 |
| Real transport
quality (%) | 39.9±5.4 | 34.5±6.3 | >0.05 |
| Detoxification
efficiency (%) | 14.6±4.5 | 9.7±3.8 | >0.05 |
Prior injection of ITIH4 from mice with
B16 melanoma reduces melanoma growth in mice
Subsequently, a series of in vivo experiments
were performed to study the influence of inter ITIH4 (serpin) and
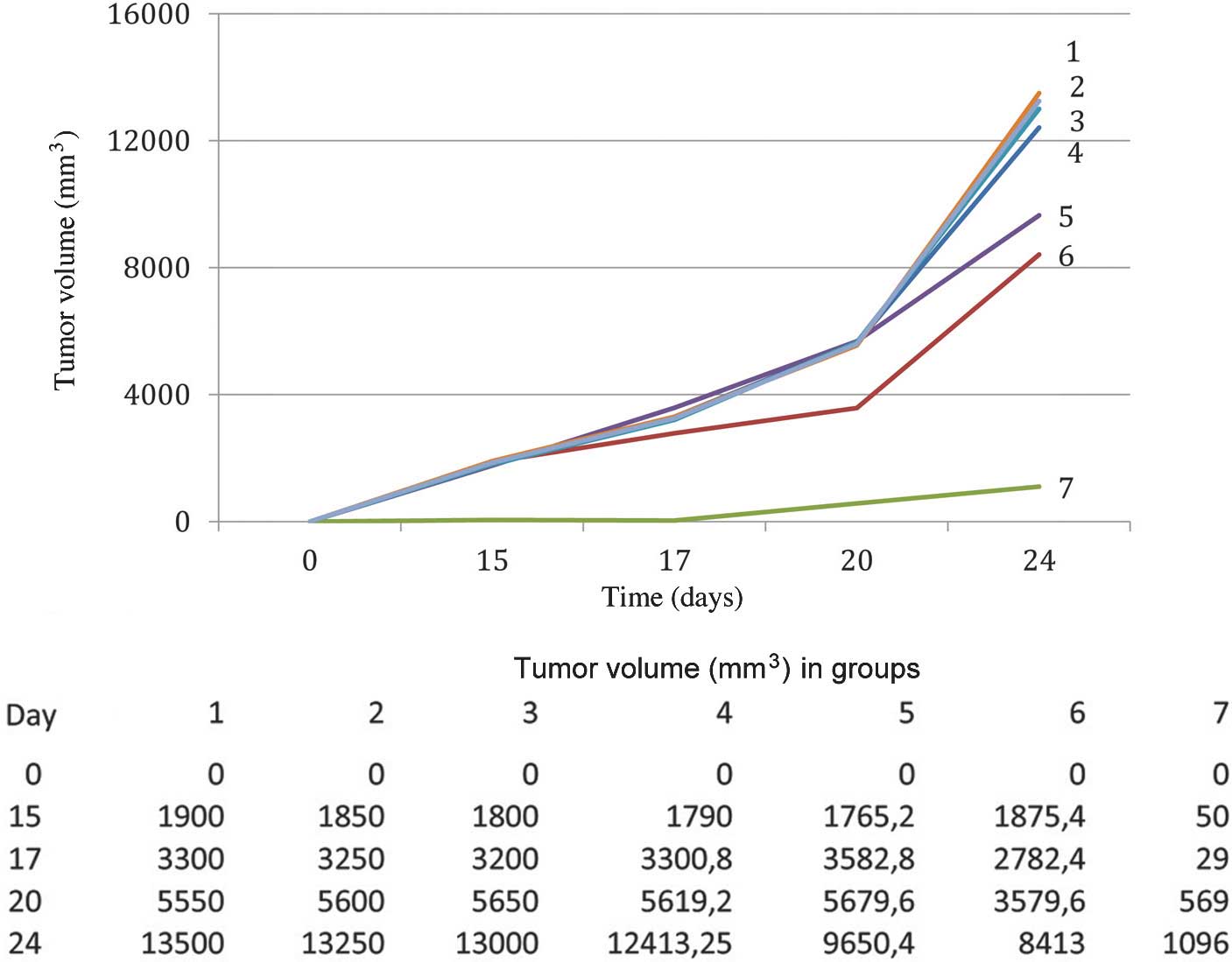
albumin on the growth of B16 melanoma (Fig. 3). For this purpose, mice were
injected with albumin or ITIH4 from plasma from mice with B16
melanoma or tumor-free mice two weeks prior to tumor cell
inoculation, and the effect on tumor growth was observed (4). It was discovered that albumin,
regardless of cancer status of the animal from which it was
obtained, had no effect on the growth of B16 melanoma in mice.
Serpin obtained from fresh blood serum of tumor-free animals or
from frozen blood serum of mice with B16 melanoma did not affect
the growth of B16 melanoma. As shown in Fig. 3, a significant inhibition of the
tumor growth was only achieved in group 7, in which animals had
been injected with ITIH4, which was obtained from fresh serum of
C57Bl/6 mice with B16 melanoma, prior to transplantation of the
tumor. Prior freezing of this serum or the use of allogeneic
protein led to the loss of the tumor-specific activity. Thus, only
the protein extracted from fresh blood serum of C57Bl/6 mice
significantly inhibited the growth of melanoma in C57Bl/6 mice.
The inhibition of the tumor growth by ITIH4 protein
was reflected by the lengthening of the period until the visual
appearance of the tumor: In the mice of the control group, the
appearance of the tumor was noted 10 days after tumor
transplantation, whereas in the experimental group, the tumors were
registered only at day 20 after tumor cell transplantation. This
may be interpreted as 100% tumor growth inhibition by ITIH4 over 10
days. The lifespan of animals with B16 melanoma in the control
group averaged 40.8±2.1 days, whereas in group 7, in which ITIH4
was injected, it was 65.3±3.4 days (P<0.05).
Discussion
To date, the biological effects of serum proteins
and their regulation have not been fully elucidated. In the present
study, the tumor-specific activity of serum proteins was assessed,
as well as their regulation by conformational changes. However, the
underlying mechanisms of this effect have yet to be elucidated. In
addition, it was not possible to reproduce the in vivo
results in an in vitro system; this may be due to the
metabolism and homeostasis in a living organism, which cannot be
reproduced by an in vitro cell model.
A previous study by our group demonstrated the
tumor-specific activity of the blood serum fractions of mice with
Ehrlich carcinoma, which contained proteins with a molecular weight
of 50–100 kDa (4). This fraction
contained 40 proteins and differences were most obvious by
electrophoretic mobility assay in the major band with a molecular
weight of ~65 kDa. The major changes in this band coincided with
the disappearance of alpha-1-anti-trypsin and the appearance in the
same sample of cathepsin L1. Inter-α-trypsin inhibitor was
additionally identificated in this fraction. This led to the
hypothesis that the tumor-specific activity of serpin is exerted by
α-1-anti-trypsin, even though the molecular weight of this protein
is significantly lower and was 45 kDa. In the present study, a
protein with a similar molecular weight of 65–70 kDa from the serum
of mice with B16 melanoma, identified as serpin ITIH4, also showed
a specific activity. The results of the previous studies by our
group as well as those obtained in the present study suggested that
in the two cases - Ehrlich carcinoma and B16 melanoma - the protein
with a tumor-specific activity was identical and that its
conformation determined its biological specificity.
In previous studies, our group as well as other
researchers, did not identify any significant differences in the
spectrum of proteins isolated from the serum of tumor-bearing and
tumor-free mice (3,4). In spite of this, these blood serum
proteins were observed to have a tumor-specific activity. This fact
can be explained by differences in sample preparation of these
proteins in the previous and present studies. In particular, in
prior studies, at least two methods were used which cause the
denaturation of proteins (prior freezing of samples or boiling in a
buffer for electrophoresis). The denaturation facilitates
proteolysis and the identification of proteins, but prevents the
evaluation of features of their conformation.
Therefore, in the present study, the protein
conformation was not investigated using these standard methods.
Only fresh serum samples were used, the separation of proteins was
performed by column chromatography and the changes in conformation
of the proteins were estimated via the soft proteolysis (with 10%
acetonitrile) product. It was discovered that identical sites of
the same protein yielded differential peptide fragment spectra
between tumor-free mice and those with melanoma. This result
demonstrated the difference in the availability of these sites for
the enzymes, and hence, the differential conformation of the
proteins between the two experimental groups. It is important to
note that the conformational changes of the proteins studied were
associated with changes in their biological activity. Thus, the
present study demonstrated for the first time, to the best of our
knowledge, that the tumor-specific activity of ITIH4 was based on
its differential conformation between tumor-free mice and those
with melanoma. This result confirmed the hypothesis that the
conformation of serpin determines its tumor-specific effect.
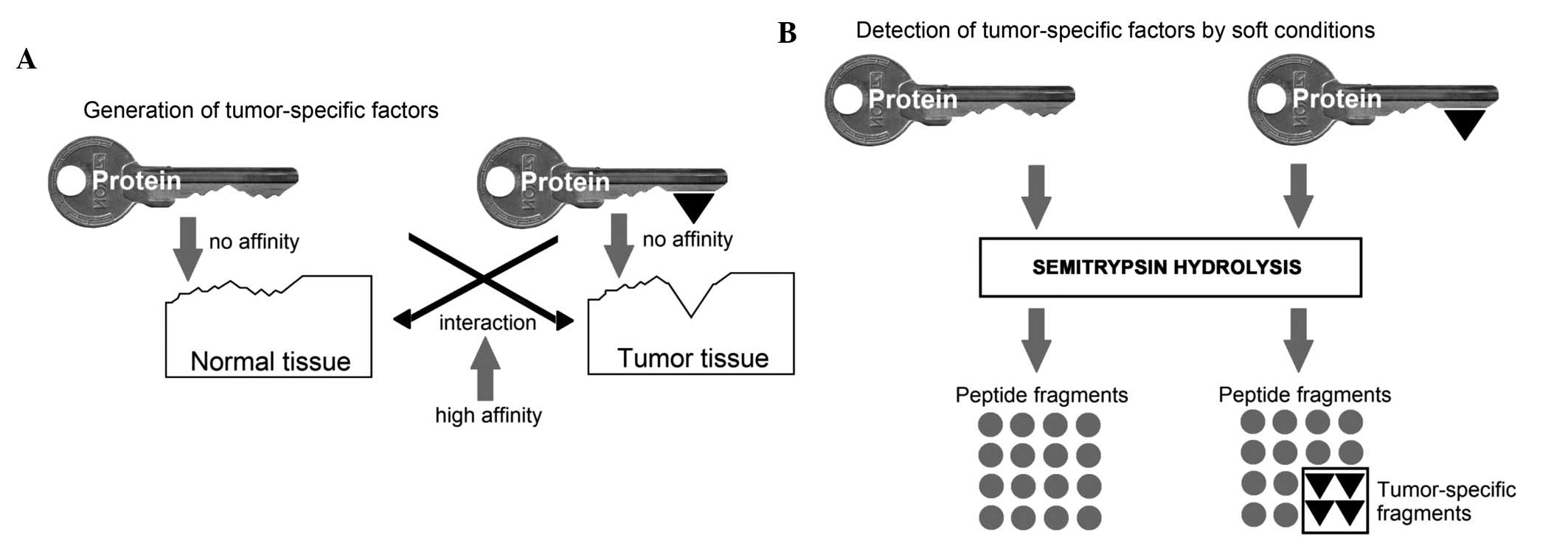
The results obtained can be figuratively explained
as illustrated in the scheme in Fig.
4A: Serum protein can be regarded as a key, which has a stable
part and a specific component. The stable part, represented in the
present study by common peptides, is present in all organisms of
the given species. In the present study, the specific component is
constituted by unique peptides in a particular individual and is
defined by its state, in particular, by the presence of a tumor.
The main condition for the functioning of this protein (key) is the
lack of high affinity to the tissues of the body and other serum
proteins. Tumor growth leads to an increased concentration of these
groups of antigenic determinants, which are located on the surface
of the tumor cells. In response to this increased concentration of
groups of antigenic determinants in cells, the availability of
antigenic determinants, which are capable of interacting with them,
are expected to decrease in the serum. This may be achieved in two
ways: The emergence of acute phase proteins among the blood serum
proteins (5), and conformational
changes in serum proteins, as shown in the present study. In order
to study this adaptation to tumor growth, the present study
performed a proteomics study, which required proteases to cleave
the native protein. Conventional methods of proteomic analyses of
proteins include the preliminary freezing of the samples and
boiling for electrophoresis. However, in the present study, this
denaturation resulted in the loss of the tumor-specific activity of
the samples. Therefore, the conditions of sample preparation were
modified by excluding any experimental conditions leading to
protein denaturation (Fig.
4B).
In conclusion, the results of the present study
confirmed the hypothesis that the conformation of serum proteins is
associated with their biological activity and with tumor growth in
the body. Injection of ITIH4 from the serum of mice with melanoma
was demonstrated to inhibit tumor growth in a mouse model of
melanoma under the condition that its conformation was
preserved.
References
|
1
|
Donenko FV, Ziganshin RK, Sitdikova SM,
Amandzholov BS, Kiselevskii MV and Efferth T: Induction of
resistance to murine tumor development is associated with
alterations in the glyco-sylation of blood serum proteins. Mol Med
Rep. 2:487–495. 2009.PubMed/NCBI
|
|
2
|
Donenko FV, Kabieva AO and Efferth T:
Tumor-specific blood serum factors as determinants of tumor growth.
Klin Lab Diagn. 50–52:13–15. 2013.In English, Russian.
|
|
3
|
Kormosh NG, Ziganshin RKh, Shender VO,
Voyushin KE and Donenko FV: Changes in the serum protein
composition in mice with transplanted Ehrlich's carcinoma. Bull Exp
Biol Med. 158:489–492. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Donenko FV, Ziganshin RH, Anisimova NY,
Voyushin KE, Sitdikova SM, Amandzholov BS, Kiselevskii MV and
Efferth T: Identification of serpin (alpha-1-antitrypsin) as serum
growth inhibitory factor in murine ehrlich carcinoma by proteomics.
Cancer Genomics Proteomics. 7:147–156. 2010.PubMed/NCBI
|
|
5
|
Fisher B, Gunduz N, Coyle J, Rudock C and
Saffer E: Presence of a growth-stimulating factor in serum
following primary tumor removal in mice. Cancer Res. 49:1996–2001.
1989.PubMed/NCBI
|
|
6
|
Sitdikova SM, Amandzholov BS, Kiselevskii
MV and Donenko FV: Specificity of relapses and metastases of
experimental transplanted Ehrlich carcinoma and B16 melanoma. Bull
Exp Biol Med. 143:80–82. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kondo T: Inconvenient truth: Cancer
biomarker development by using proteomics. Biochim Biophys Acta.
1844:861–865. 2014. View Article : Google Scholar
|
|
8
|
Sitdikova SM, Amandzholov BS, Kiselevskii
MV and Donenko FV: Lectin binding to mouse blood lymphocytes during
tumor growth. Bull Exp Biol Med. 140:445–448. 2005. View Article : Google Scholar
|
|
9
|
Donenko FV, Kabieva AO, Volkov IuT and
Moroz LV: Mouse serum inhibition of cytotoxicity of goat antibodies
against mouse thymocytes. Biull Eksp Biol Med. 113:642–644. 1992.In
Russian. PubMed/NCBI
|
|
10
|
Donenko FV, Sitdikova SM, Syrtsev AV,
Gradyushko AT, Kiselevsky MV, Serebryakova MV and Efferth T:
Hemoglobin-associated proteins isolated from blood serum of Ehrlich
carcinoma-bearing mice. Int J Oncol. 32:885–893. 2008.PubMed/NCBI
|
|
11
|
Sergeant K, Pinheiro C, Hausman JF,
Ricardo CP and Renaut J: Taking advantage of nonspecific trypsin
cleavages for the identification of seed storage proteins in
cereals. J Proteome Res. 8:3182–3190. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Alves G and Yu YK: Improving peptide
identification sensitivity in shotgun proteomics by stratification
of search space. J Proteome Res. 12:2571–2581. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Law RH, Zhang Q, McGowan S, Buckle AM,
Silverman GA, Wong W, Rosado CJ, Langendorf CG, Pike RN, Bird PI
and Whisstock JC: An overview of the serpin superfamily. Genome
Biol. 7:2162006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Goldin A, Kline I and Sofina ZP:
Experimental Evaluation of Antitumor Drugs in the USA and USSR and
Clinical Correlations. (National Cancer Institute Monograph 55. NIH
Publication no. 1933). U.S. Department of Health and Human
Services. National Institutes of Health, NCI; Bethesda, MD:
1980
|
|
15
|
Laemmli UK: Cleavage of structural
proteins during the assembly of head of bacteriophage T4. Nature.
227:680–685. 1970. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gurachevsky A, Shimanovitch E,
Gurachevskaya T and Muravsky V: Intra-albumin migration of bound
fatty acid probed by spin label ESR. Biochem Biophys Res Commun.
360:852–856. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dickneite G, Herwald H, Korte W, Allanore
Y, Denton CP and Matucci Cerinic M: Coagulation factor XIII: A
multifunctional transglutaminase with clinical potential in a range
of conditions. Thromb Haemost. 113:686–697. 2015. View Article : Google Scholar : PubMed/NCBI
|