Introduction
Aspirin is a well-known non-steroidal
anti-inflammatory drug (NSAID), which has been used worldwide for
hundreds of years. Studies have revealed that aspirin has numerous
pharmacological effects, including cardioprotective and anti-tumor
properties (1,2). In gastroenterology, numerous emerging
studies have suggested a potential application of aspirin in the
prevention of certain types of cancer (3). Results of recent meta-analyses have
estimated that long-term aspirin use may reduce the incidence of
esophageal and colorectal cancers by up to 30% (4). Furthermore, aspirin has demonstrated
growth-inhibiting and apoptosis-inducting abilities in numerous
colorectal cancer cell lines, including HCT116 and SW480 (5). However, aspirin has a major drawback
with regard to its routine administration, namely the risk of upper
gastrointestinal bleeding and peptic ulceration (6).
Isosorbide mononitrate (ISMN), an organic nitrate
compound, is a commonly used drug for the treatment of
cardiovascular diseases, including angina pectoris, acute
myocardial infarction and congestive heart failure. ISMN can be
metabolized in vivo to nitric oxide (NO), which is an
intercellular messenger with a variety of biological effects in the
cardiovascular, nervous, immune and other systems (7,8). NO
also has similar effects to those of prostaglandins on gastric
mucosa, which may reduce the gastric toxicity of aspirin (9). Furthermore, ISMN was shown to inhibit
angiogenesis, tumor growth and metastasis in a chick model of the
chorioallantoic membrane and a mouse model of Lewis Lung carcinoma
(10).
Although aspirin and ISMN have been frequently used
in the clinic, their effects on human colon cancer cells,
particularly their synergistic anti-tumor effects, have remained
elusive (11,12). In the present study, the growth
inhibitory effect of the combination of aspirin and ISMN was
assessed using an MTT assay in the colon cancer cell lines HCT116
and SW620 and the umbilical vein cell line EA.hy926.
The effects of the two drugs on cell apoptosis were
further demonstrated through nuclear morphology observation,
Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI)
double staining, caspase-3 activity assay and detection of
poly(adenosine triphosphate ribose) polymerase (PARP) cleavage.
Additional experiments for the elucidation of the underlying
mechanism of the apoptosis-inducing effects of the two drugs were
performed by NO activation assay, luciferase reporter assay and
analysis of Wnt pathway-associated signaling molecules. Finally,
the synergistic anti-tumor effects were validated in a nude mouse
HCT116 cell xenograft model in vivo.
Materials and methods
Compounds
Aspirin (no. A2093) and ISMN (no. I0775010) were
standard substances purchased from Sigma-Aldrich (St Louis, MO,
USA). They were pre-dissolved in dimethylsulfoxide (DMSO;
Sigma-Aldrich) at a concentration of 2 mol/l.
Cell culture and drug treatments
HCT116 (no. CCL-247) and SW620 (no. CCL-227) human
colon cancer cells and EA.hy926 (no. CRL-2922) human umbilical vein
cells (American Type Cell Collection, Manassas, VA, USA) were
cultured on Dulbecco's modified Eagle's medium (Gibco Life
Technologies, Carlsbad, CA, USA) supplemented with 10% fetal bovine
serum (Gibco Life Technologies), 100 U/ml penicillin and 100
µg/ml streptomycin (Gibco Life Technologies) in
25-cm2 culture flasks at 37°C in a humidified atmosphere
containing 5% CO2. All cells to be tested in assays had
a passage number of 3–6. For the drug treatment experiments, cells
were harvested from the culture during the exponential growth
phase, and then seeded into multi-well culture plates at
5×104−1×105 cells/ml in fresh medium. After
attachment of the cells overnight, they were treated with the
compounds at the indicated concentrations for 48 h.
MTT assay for cell viability
At the end of the drug treatment period (0.125,
0.25, 0.5 or 1 mM aspirin with 0, 2 or 4 mM ISMN) for 48 h, 10
µl 5 mg/ml MTT solution (Sigma-Aldrich) in
phosphate-buffered saline (PBS) (PBS without MTT as the blank) was
added to each well of the culture plate (containing 100 µl
medium). After 4 h of incubation, the formazan crystals that formed
in the wells were solubilized with 100 µl DMSO for optical
density reading at 570 nm with a spectrophotometer (Epoch; BioTek,
Winooski, VT, USA).
Detection of changes in nuclear
morphology
At the end of the drug treatment period (1 mM
aspirin with 4 mM ISMN for 48 h), the cells in each well were
washed once with PBS and fixed with 4% formaldehyde in PBS at 4°C
for 30 min. The cells were then washed with PBS and stained with 1
µg/ml Hoechst 33258 (no. C1018; Beyotime Institute of
Biotechnology, Shanghai, China) in PBS at 37°C for 15 min and then
viewed under a fluorescent microscope (DMI3000 B; Leica, Wetzlar,
Germany) to observe changes in nuclear morphology.
Caspase-3 activity assay
Caspase-3 activity of cells was determined using a
caspase-3 colorimetric assay kit (no. C1116; Beyotime Institute of
Biotechnology) according to the manufacturer' instructions.
Briefly, cells were re-suspended in the cell lysis buffer and
protein concentrations of the supernatants were measured. The
samples were then incubated at 37°C with reaction buffer and
substrate for 1–2 h. The optical density of the assay solutions was
measured at 405 nm with a spectrophotometer (Epoch; BioTek).
Annexin V-FITC/PI double staining
The apoptotic rate of the cells was assessed using
an Annexin V-FITC apoptosis detection kit (no. C1063; Beyotime
Institute of Biotechnology) according to the manufacturer's
instructions. Briefly, cells were collected, washed twice with PBS
and washed once with binding buffer. The cells were then stained
with Annexin V-FITC/PI at room temperature in the dark for 15 min,
and the fluorescence was quantified by flow cytometry (FACSCalibur;
BD Biosciences, Franklin Lakes, NJ, USA). Early apoptotic cells
were identified by Annexin V+/PI−
staining.
NO release assay
NO release of cells was determined in the culture
medium using a nitrate/nitrite colorimetric assay kit (no. S0024;
Beyotime Institute of Biotechnology) according to the
manufacturer's instructions. Briefly, the cell culture medium was
collected after drug treatment and incubated at 37°C with
nicotinamide adenine dinucleotide phosphate, flavin adenine
dinucleotide and nitrate reductase for 30 min, then at 37°C with
lactate dehydrogenase (LDH) buffer and LDH for 30 min, and then at
room temperature with Griess reagent I and II for 10 min. The
optical density of the assay solutions was measured at 540 nm with
a spectrophotometer (Epoch; BioTek).
Luciferase reporter assay for
transcription factor (TCF) binding
DNA transfections were performed on HCT116 cells in
the logarithmic growth phase. First, 0.2 µg TOPflash (no.
21-170) or FOPflash (no. 21-169) (Millipore, Billerica, MA, USA)
and 0.02 µg pRL-TK (no. E2241; Promega, Madison, WI, USA)
were co-transfected into 1×105 HCT116 cells in each well
of the culture plate using Lipofectamine 2000 reagent (no.
11668-019; Invitrogen Life Technologies, Carlsbad, CA, USA). After
4 h of transfection, aspirin and ISMN were added (1 mM aspirin with
4 mM ISMN) and incubated for 48 h. The luciferase activity was then
evaluated by the Dual-Luciferase Reporter Assay System (no. E1910;
Promega) using a microplate reader (TriStar2 LB942; Berthold, Bad
Wildbad, Germany).
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
At the end of the drug treatment period (1 mM
aspirin with 4 mM ISMN for 48 h), the cells in each well were lysed
in TRIzol solution (Invitrogen Life Technologies). RNA was
extracted with the RNAiso Plus kit (no. 9108; Takara, Otsu, Japan)
according to the manufacturer's instructions and quantitated
spectrophotometrically. Total RNA was used as a template for
reverse transcription using the following protocol: Each
20-µl reaction contained 1X Moloney's murine leukemia virus
(M-MLV) buffer, 125 µM deoxynucleotide triphosphate, 100
pmol oligo dT18 primer, 100 units M-MLV reverse transcriptase,
diethyl pyrocarbonate-treated water and 2 µg total RNA.
Briefly, RNA and oligo dT18 primer was incubated at 70°C for 10 min
and then immediately placed on ice, after which the other
components were added and incubated at 42°C for 1 h and then at
70°C for 15 min. qPCR was performed using the CFX96 Real-Time PCR
Detection System (Bio-Rad Laboratories, Inc., Hercules, CA, USA) by
using SYBR Premix Ex Taq (no. RR420A; Takara) according to the
manufacturer's instructions. Primer sequences for c-myc, cyclin D1
and GAPDH genes are shown in Table
I. The 20-µl qPCR reaction mixtures contained 10
µl SYBR Premix Ex Taq, 0.2 µM forward and reverse
primer each, 2 µl cDNA and nuclease-free water. The program
used for all genes consisted of a denaturing cycle of 30 sec at
95°C, 40 cycles of PCR (95°C for 5 sec and 60°C for 30 sec), and a
specific melting cycle (from 65°C to 95°C, 0.5°C increment for 5
sec) in order to confirm the specificity of the qPCR products. The
product sizes were confirmed by 3% agarose gel electrophoresis
using the DL500 DNA marker (Takara) and electrophoresis apparatus
(PowerPac Basic Power; Bio-Rad Laboratories, Inc.). and ethidium
bromide staining. Results were analyzed using the 2−ΔΔCT
method to compare the transcriptional levels of target genes
normalized to GAPDH in each sample relative to the non-treated
control.
 | Table ISequences of primers used for
polymerase chain reaction analysis. |
Table I
Sequences of primers used for
polymerase chain reaction analysis.
| Gene | Sequence |
|---|
| c-myc | Forward:
TGAACACAGCGAATGTTTCC |
| Reverse:
TTAGGAGCGCTCAGGTCTGT |
| Cyclin D1 | Forward:
CAGGTTGGACAGTTCACAGG |
| Reverse:
ACAGCTGGAGTTGGATGGAC |
| GAPDH | Forward:
GATGACATCAAGAAGGTGGTG |
| Reverse:
GCTGTAGCCAAATTCGTTGTC |
Western blot analysis
At the end of the drug treatment period (1 mM
aspirin with 4 mM ISMN for 48 h), the cells in each well were
disrupted using cell lysis buffer (no. P0013; Beyotime Institute of
Biotechnology). The suspension was centrifuged at 12,000 ×g and 4°C
for 5 min, and the protein content of the supernatant was
determined using a bicinchoninic acid assay (Beyotime Institute of
Biotechnology). Equal quantities of protein samples were loaded
onto 10% SDS-polyacrylamide gel (Bio-Rad Laboratories, Inc.) and
then transferred onto a microporous polyvinylidene difluoride
membrane (Bio-Rad Laboratories, Inc.). Western blotting was
performed using a mouse anti-human c-myc monoclonal antibody (no.
sc-40; 1:500 dilution), mouse anti-human cyclin D1 monoclonal
antibody (no. sc-450; 1:500 dilution) (both Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), rabbit anti-human caspase-3
polyclonal antibody (no. 9662; 1:1,000 dilution), rabbit anti-human
PARP polyclonal antibody (no. 9532; 1:1,000 dilution) or rabbit
anti-human β-actin monoclonal antibody (no. 8457; 1:1,000 dilution)
and horseradish peroxidase-conjugated anti-mouse or anti-rabbit
secondary antibodies (nos. 7076 and 7074; 1:1,500 dilution) (all
Cell Signaling Technology, Danvers, MA, USA). Protein bands were
visualized using an enhanced chemiluminescence substrate (no.
32109; Pierce, Thermo Fisher Scientific, Waltham, MA, USA) and a
FluorChem Q Western Blot Imaging System (ProteinSimple, Santa
Clara, CA, USA).
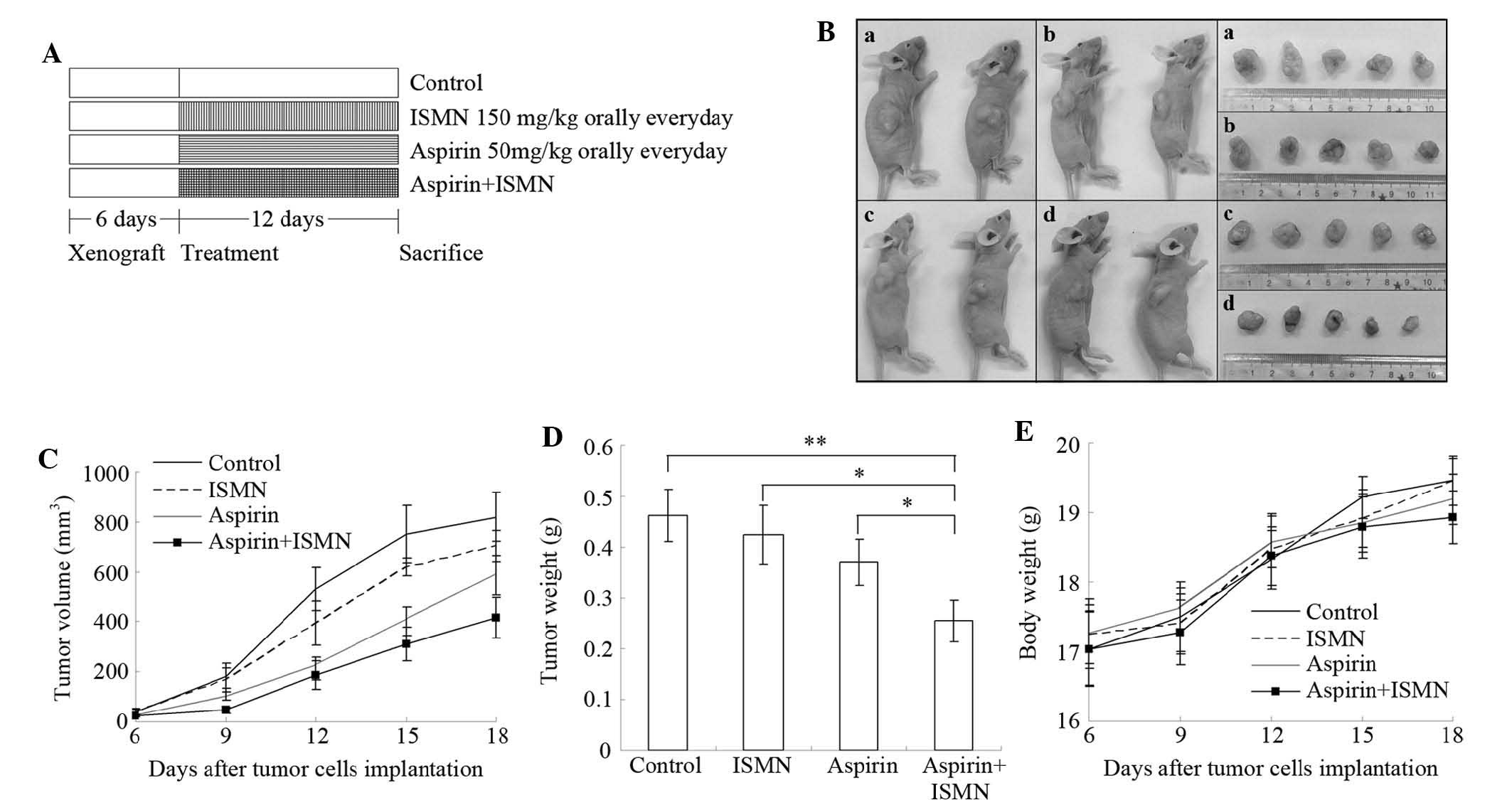
Tumor xenograft study
The protocol for the animal experiment performed in
the present study was approved by the Laboratory Animal Ethics
Committee of Shenzhen University (Shenzhen, China). A total of 20
female BALB/c nude mice (4-week-old; 18–20 g) were purchased from
the Medical Laboratory Animal Center (Guangzhou, China). All mice
were housed under constant laboratory conditions of a 12-h
light/dark cycle and specific pathogen-free conditions, and fed
with water and food ad libitum. After being acclimatized for
1 week, each nude mouse was inoculated subcutaneously into the
right-hand side of the back with 2×106 HCT116 cells in
order to establish xenograft tumors. After six days, all mice grew
visible tumors and were randomly assigned to four groups of five
mice each: Control (0.5% sodium carboxymethyl cellulose orally once
a day), ISMN (150 mg/kg orally once a day), aspirin (50 mg/kg
orally once a day), and aspirin + ISMN (two drugs used together
orally once a day). Aspirin and ISMN were ground into powder in a
mortar and pestle and suspended in 0.5% sodium carboxymethyl
cellulose (Sigma-Aldrich). The mice were treated for 12 days, and
body weights and tumor volumes were monitored every three days.
Tumor dimensions were measured with vernier calipers, and tumor
volumes were calculated using the following formula: (A ×
B2)/2, where A is the larger and B is the smaller
dimension of the tumor. Finally, all mice were anaesthetized using
pentobarbital (Sigma-Aldrich), and the tumors were removed. The
tumors were weighed and their images were captured.
Statistical analysis
Values are expressed as the mean ± standard
deviation of the indicated number of independently performed
experiments. Student's t-test was used for the determination of
statistical significance using SPSS 17.0 software (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference between values.
Results
Aspirin and ISMN synergically inhibit the
growth of colon cancer cells
After 48-h treatment of the HCT116 cells with
aspirin (0–1 mM), the cell growth was dose-dependently inhibited
relative to that of the untreated control. ISMN had a lower growth
inhibitory effect than aspirin, as no obvious effect on HCT116 cell
growth observed with 5 mM ISMN and a growth inhibition rate of
merely 10% was achieved with 10 mM ISMN (data not shown). In the
present study, a non-cytotoxic concentration of ISMN (2 or 4 mM)
was used to examine the synergic effect with aspirin on HCT116
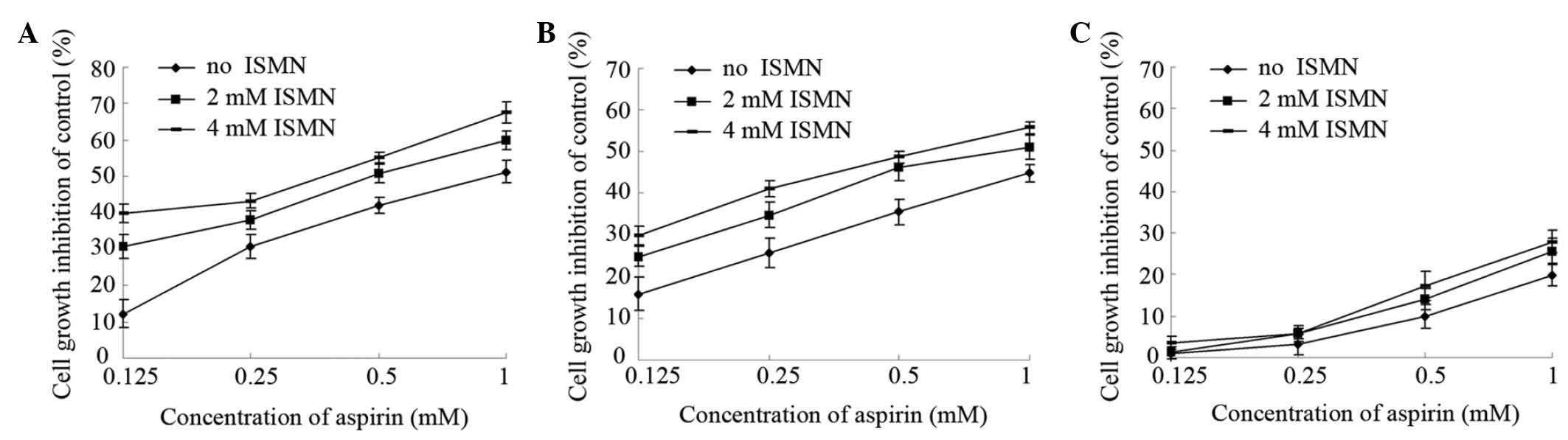
cells. As shown in Fig. 1A,
1 mM aspirin had an inhibition
rate of 51%, while simultaneous treatment with 2 and 4 mM ISMN
increased the inhibition rate to 60 and 68%, respectively. Similar
results were achieved with lower concentrations of aspirin (0.5,
0.25 and 0.125 mM). The IC50 (concentration causing 50%
growth inhibition of HCT116 cells) was 0.94±0.11 mM for aspirin
alone and 0.39±0.03 mM in the presence of 4 mM ISMN (Table II).
 | Table IIIC50 values (mM) of
aspirin in the absence or presence of ISMN on the human colon
cancer cell lines HCT116 and SW620 and the human umbilican vein
cell line EAhy.926. |
Table II
IC50 values (mM) of
aspirin in the absence or presence of ISMN on the human colon
cancer cell lines HCT116 and SW620 and the human umbilican vein
cell line EAhy.926.
| ISMN added
(mM) | HCT116 | SW620 | EAhy926 |
|---|
| 0 | 0.94±0.11 | 1.28±0.20 | >>1 |
| 2 | 0.50±0.08a | 0.73±0.17a | >>1 |
| 4 | 0.39±0.03b | 0.60±0.10b | >>1 |
Similar experiments were performed on SW620 cells,
another human colon cancer cell line, with similar results. Aspirin
had a slightly lower cytotoxity on SW620 than on HCT116 cells (1 mM
aspirin inhibited HCT116 growth by 51% and SW620 growth by 44%).
Furthermore, 4 mM ISMN had no obvious inhibitory effect on SW620
(data not shown). However, as shown in Fig. 1B, 2 and 4
mM ISMN enhanced the growth inhibition rate of 1 mM aspirin on
SW620 cells to 51 and 56%, respectively. The IC50 on
SW620 cells was 1.28±0.20 mM for aspirin alone and 0.60±0.10 mM in
the presence of 4 mM ISMN (Table
II).
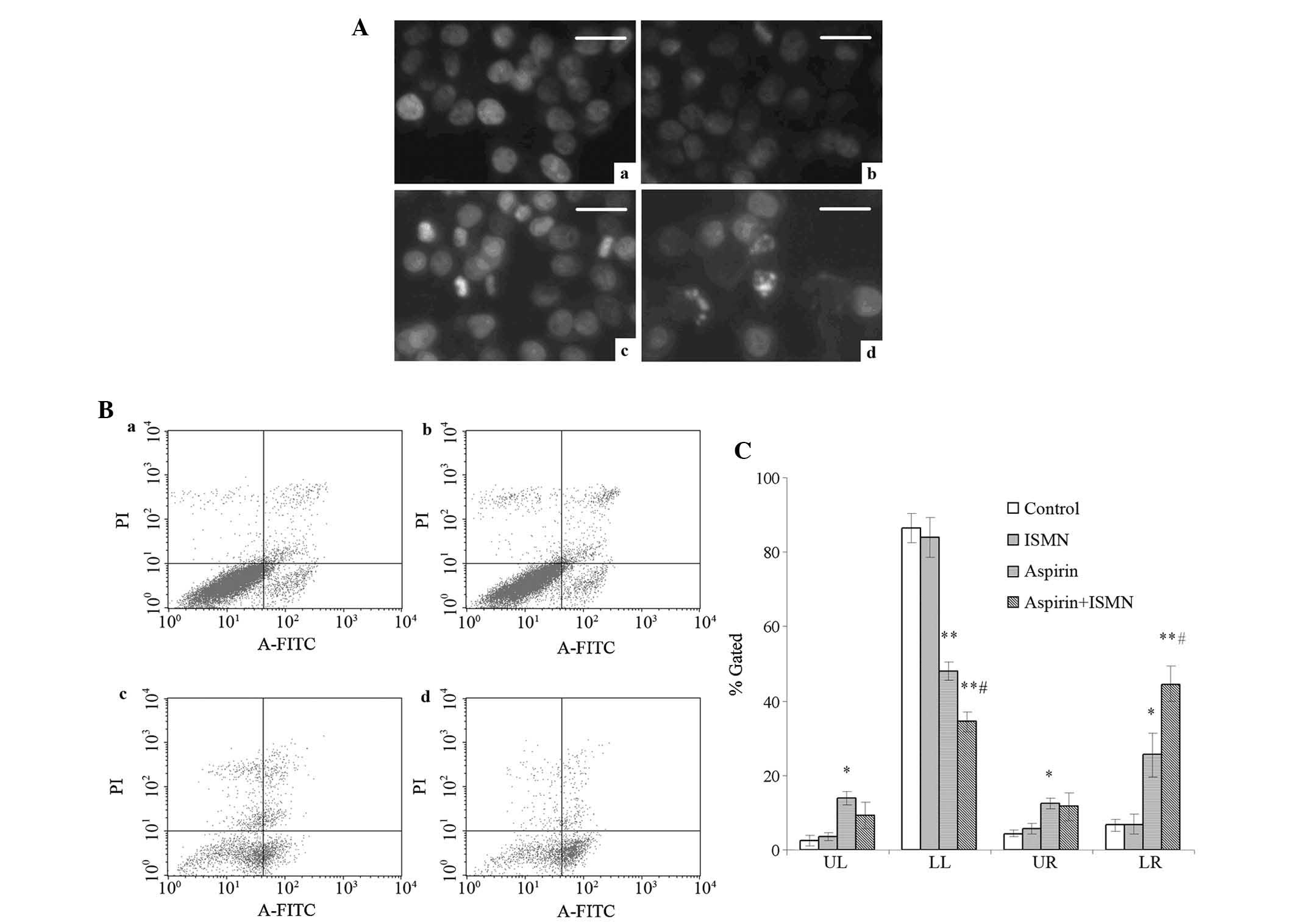 | Figure 2Apoptosis induction in HCT116 cells
treated with 1 mM aspirin and 4 mM ISMN for 48 h. (A) Nuclear
morphology of HCT116 cells in (a) the control group and the groups
treated with (b) ISMN, (c) aspirin and (d) aspirin + ISMN. Cells
were stained with Hoechst 33258 and observed by fluorescence
microscopy (scale bars, 20 µm). (B) Annexin V-FITC/PI
analysis of HCT116 cells in (a) the control group and the groups
treated with (b) ISMN, (c) aspirin and (d) aspirin + ISMN. (C)
Quantified results of Annexin V-FITC/PI staining, where LL, LR, UL
and UR quadrants represent normal, early apoptotic, necrotic and
late apoptotic populations, respectively. Values are expressed as
the mean ± standard deviation (n=3). *P<0.05;
**P<0.01 vs. control group; #P<0.05 vs.
aspirin group. FITC, fluorescein isothiocyanate; PI, propidium
iodide; UL, upper left; UR, upper right; LL, lower left; LR, lower
right; ISMN, isosorbide mononitrate. |
By contrast, markedly lower growth inhibitory
effects were observed on non-cancerous EA.hy926 human umbilical
vein cells treated with aspirin and/or ISMN. As shown in Fig. 1C, 1 mM aspirin inhibited the growth of
EA.hy926 cells by only 20%. Compared with aspirin treatment alone,
the inhibitory rate was not significantly altered upon co-treatment
with non-cytotoxic concentrations of ISMN (2 or 4 mM). In summary,
aspirin had a significantly greater growth inhibitory effect on
human colon cancer cells compared with that on human umbilical vein
cells. Furthermore, co-treatment with a non-cytotoxic concentration
of ISMN inhibited the growth and viability of colon cancer cells in
a synergistic manner with aspirin, while not affecting the growth
of umbilical vein cells.
Aspirin and ISMN induce apoptosis in
HCT116 cells
Changes of nuclear morphology, phosphatidylserine
(PS) translocation and capase-3 activity of colon cancer cells were
assessed to confirm cell apoptosis. After 48-h treatment, HCT116
cells were stained with Hoechst 33258 to observe chromatin
condensation, which is a hallmark of apoptosis. None of the control
cells showed specific chromatin condensation (Fig. 2A-a) and similarly, treatment with 4
mM ISMN had no visible effects (Fig.
2A-b). However, in the presence of 1 mM aspirin, hyperchromatic
nuclei were observed in a percentage of the cells (Fig. 2A-c). A large increase in the number
of cells with hyperchromatic nuclei cells was observed when 1 mM
aspirin and 4 mM ISMN were used in combination (Fig. 2A-d).
Phosphatidylserine (PS) translocation from the inner
to the outer side of the cell membrane is a characteristic event
occurring in the early stage of apoptosis. Annexin V is a
phospholipid-binding protein with high affinity to PS and is
frequently used to prove the exposure of PS on the plasma membrane.
To assess the apoptosis in the present study, flow cytometric
analysis of Annexin V/PI double stained cells was performed. In the
flow cytometry dot plots, Annexin V−/PI−
[lower left (LL)] cells were designated as normal cells, Annexin
V+/PI− [lower right (LR)] cells were
designated as early apoptotic cells, Annexin
V+/PI+ [upper right (UR)] cells were
designated as late apoptotic cells and Annexin
V−/PI+ (UL) cells were designated as necrotic
cells as shown in Fig. 2B and C.
After 48-h treatment, few apoptotic cells were present in the
control group and the 4 mM ISMN group (Fig. 2B-a and 2B–b). A significantly
greater number of apoptotic cells were observed after 1 mM aspirin
treatment (Fig. 2B-c).
Furthermore, when aspirin and ISMN were applied to HCT116 cells
simultaneously, the population of early apoptotic cells further
increased from 25.54±5.81 to 48.06±2.56%, compared to aspirin used
alone (Fig. 2B-d). The percentages
of late apoptotic cells (Annexin V+/PI+; UR)
and necrotic cells (Annexin V−/PI+; UL) were
not significantly changed by the drug treatments.
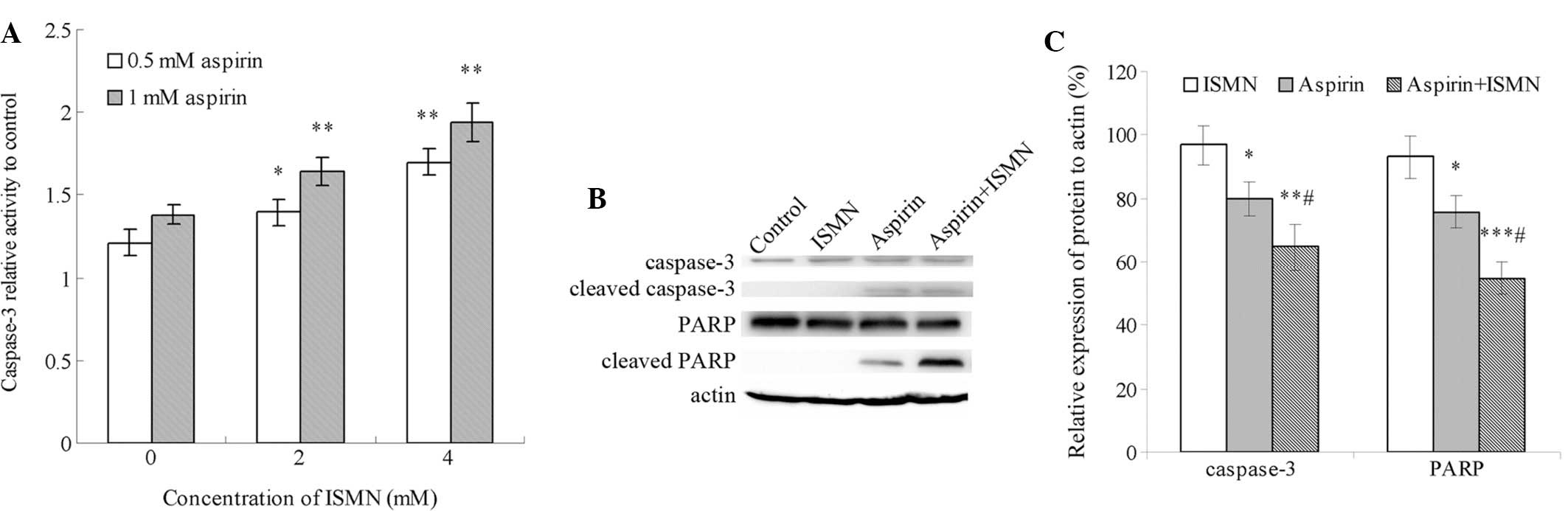
Caspase-3 is the major executor caspase at the
downstream of the apoptotic cascade, which is activated by other
initiators and upstream caspases. The caspase-3 activity of HCT116
cells treated with the drugs for 48 h relative to that of the
control group (no drug treatment) is shown in Fig. 3A. Treatment with 0.5 and 1 mM
aspirin caused a 1.2- and 1.4-fold increase in relative caspase-3
activity, respectively. By contrast, even 10 mM ISMN had no obvious
impact on caspase-3 activity (data not shown). Of note, caspase-3
was markedly activated when ISMN was used in combination with
aspirin. Co-treatment with 2 and 4 mM ISMN increased caspase-3
activity from 1.4-fold of the control group with 1 mM aspirin alone
to 1.65- and 1.9-fold, respectively. A similar synergistic effect
was achieved with lower concentrations of aspirin and ISMN.
Furthermore, caspase-3 activation and PARP cleavage were assessed
by western blot analysis. PARP is a nuclear enzyme involved in the
DNA repair process and is specifically cleaved by caspase-3 in the
process of apoptosis. As shown in Fig.
3B and C, the formation of cleaved caspase-3 and PARP was
induced by 1 mM aspirin alone, and was further increased by
co-treatment with 4 mM ISMN. In conclusion, co-treatment with a
non-cytotoxic concentration of ISMN synergistically enhanced HCT116
cell apoptosis induced by aspirin treatment through the caspase
pathway.
Aspirin enhances the NO release from ISMN
in the media of HCT116 cells
ISMN is a drug regularly used in the clinic for the
treatment of cardiovascular diseases, and which is able to release
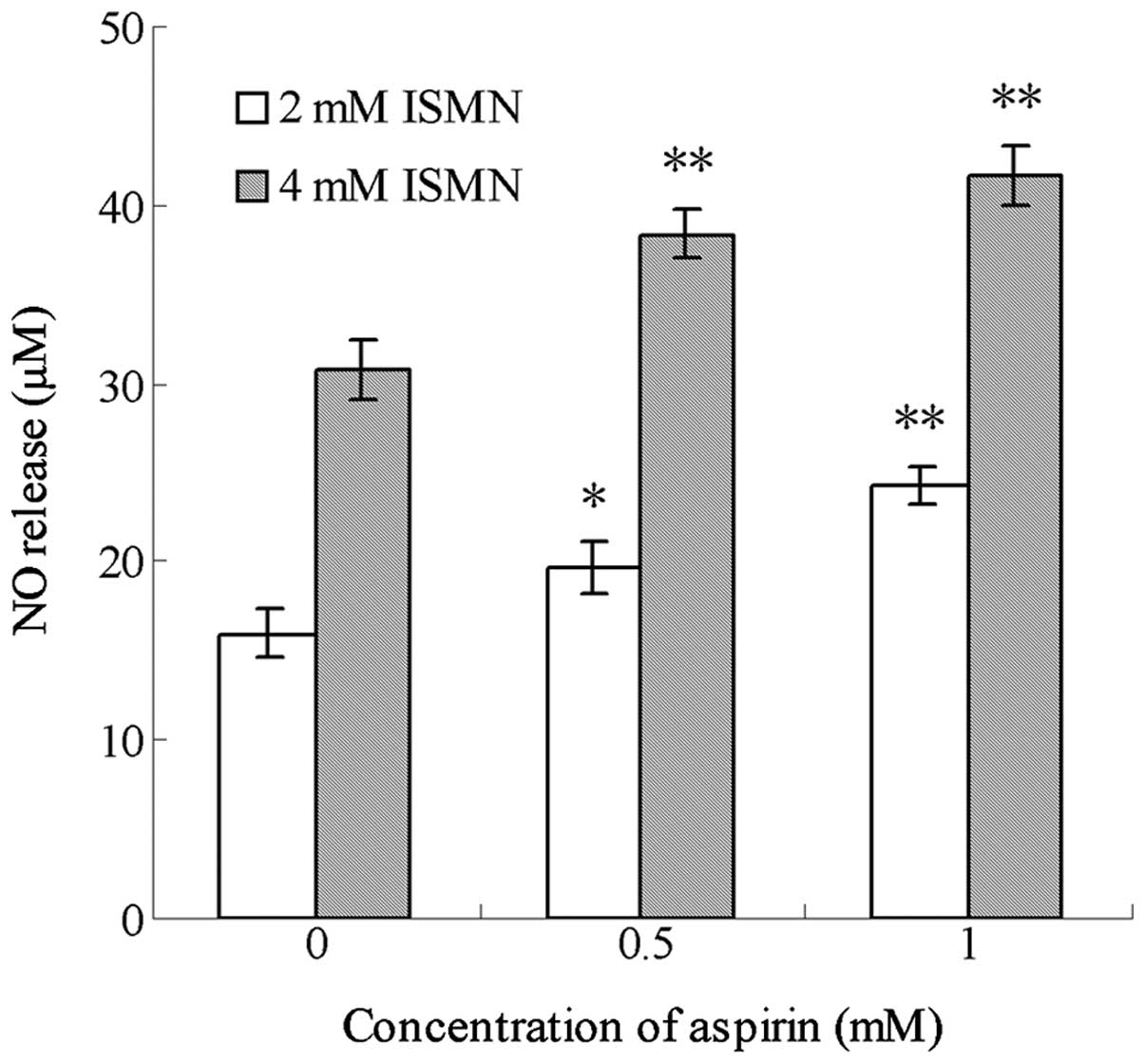
a certain amount of NO in vivo. In the present study,
culture medium containing 0.5 and 1 mM ISMN was shown to contain 15
and 31 µM NO, while media treated with 1 mM aspirin alone
contained an insignificant amount of NO (data not shown). As shown
in Fig. 4, after 48-h treatment,
aspirin significantly enhanced the NO release from ISMN when the
two drugs were used in combination. For example, 0.5 mM aspirin
increased the amount of NO released from 4 mM ISMN from 30.72±1.74
to 38.36±1.31 µM, while 1 mM aspirin increased NO levels
from 30.72±1.74 to 41.64±1.60 µM. Similar trends were
observed with 2 mM ISMN.
ISMN enhances the inhibitory effects of
aspirin on the β-catenin/TCF signaling pathway in HCT116 cells
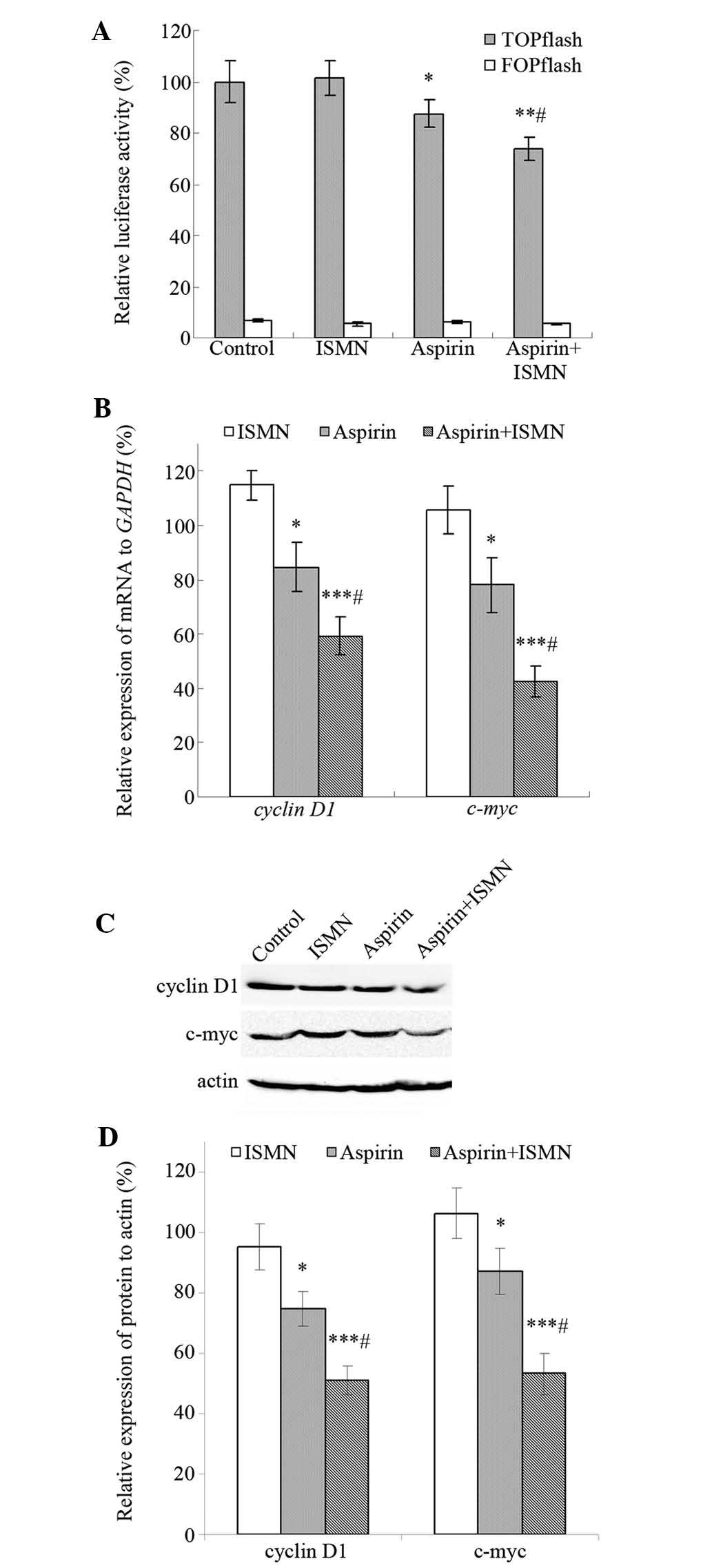
In colon cancer cells, such as HCT116, Wnt signaling
is frequently activated as a result of the cellular accumulation of
β-catenin. The TOPflash/FOPflash reporter gene assay was performed
to investigate the effect of aspirin and ISMN on the
transcriptional activity of β-catenin/TCF in HCT116 cells. TOPflash
(TCF reporter plasmid) contains two sets of three copies of the TCF
binding site (wild-type) upstream of the thymidine kinase minimal
promoter, and FOPflash contains mutated TCF binding sites as a
negative control. As shown in Fig.
5A, cells transfected with TOPflash showed significantly higher
luciferase activity than those transfected with FOPflash, and
TOPflash was markedly downregulated by the drugs rather than
FOPflash. The luciferase activity of TOPflash decreased to 88%
after treatment with 1 mM aspirin, while 4 mM ISMN had no impact on
TOPflash. In addition, luciferase activity was further reduced to
73% when aspirin and ISMN were used in combination.
Cyclin D1 and c-myc are well-established target
genes of the β-catenin-dependent pathway and have an important role
in tumor cell growth and proliferation. RT-qPCR and western blot
analyses were used to assess the transcription and translation of
these β-catenin/TCF target genes. After 48-h treatment, 4 mM ISMN
did not alter the expression of the respective genes, while 1 mM
aspirin suppressed the mRNA and protein expression of cyclin D1 and
c-myc. Furthermore, co-treatment of ISMN in addition to aspirin
further reduced the transcription and translation of cyclin D1 and
c-myc (Fig. 5B–D).
Tumor growth inhibition of aspirin and
ISMN in xenograft mice
The synergistic inhibitory effect of aspirin and
ISMN on colon cancer growth was further examined in vivo in
a nude mouse xenograft model inoculated with HCT116 cells (Fig. 6). 50 mg/kg aspirin and/or 150 mg/kg
ISMN were administered orally to the mice once a day starting from
days 6–18 following tumor cell inoculation, as illustrated in
Fig. 6A. Combined treatment of
aspirin and ISMN resulted in smaller tumors compared with those in
the single treatment or control groups (Fig. 6B–D). Even though aspirin or ISMN
alone inhibited tumor volumes in the xenograft mice, combined
treatment had a significantly more potent inhibitory effect
(Fig. 6B–D). As shown in Fig. 6D, although treatment with ISMN or
aspirin alone decreased the tumor weights, the differences compared
with the control group were not significant. However, the average
tumor weight in the combination treatment group (255 mg) was
significantly lower than that in the control group (463 mg;
P<0.01), ISMN group (424 mg; P<0.05) or aspirin group (370
mg; P<0.05). Throughout the experiment, no significant
difference of body weight was observed between the control and any
treated group, indicating that no observable side effects emerged
from the drug treatment (Fig.
6E).
Discussion
NO, first described as an endothelium-derived
relaxation factor, is a free radical acting as a gaseous messenger
that affects various biological functions (13). At low concentrations, NO functions
as a signal transducer in numerous physiological processes,
including as blood flow regulation, smooth muscle relaxation and
iron homeostasis. At high concentrations, it also has a cytotoxic
defensive role against pathogens and tumors (14). On the other hand, numerous studies
indicated that chronic NO-level elevation may lead to certain
pathological conditions in humans, including inflammatory bowel
disease and cancer (15,16). Although the association between NO
and the biology of cancer has remained to be fully elucidated,
NO-based anti-cancer drugs are being studied in vitro and
in vivo to develop novel therapies (17). One of the successful examples are
NO-NSAIDs, which consists of traditional NSAIDs covalently bound to
an NO-donating moiety. It has been demonstrated that NO-NSAIDs not
only possess a lower gastric toxicity, but are also more potent
against colon carcinoma and other tumor types than parent NSAIDs
(18). Among them, NO-aspirin is
the most effective known candidate as indicated by studies which
reported its efficacy in an animal model of cancer prevention
(19) and its apoptosis-inducing
ability in leukemia cells (20).
In the present study, ISMN was used as an NO donor and its ability
to promote the cytotoxicity of aspirin on tumor cells was
examined.
The results of the present study showed that aspirin
and ISMN had a synergistic inhibitory effect on HCT116 and SW620
cells, but not on EA.hy926 cells, indicating the specificity of the
two drugs for human colon cancer cells. Aspirin alone exerted a
certain cytotoxicity to HCT116 and SW620 cells, and was able to
induce apoptosis in HCT116 cells after 48-h treatment, which is
consistent with the findings of previous studies (5). ISMN alone only had a minor effect on
cell growth and proliferation. However, when 4 mM ISMN was used in
combination with aspirin the IC50 value of aspirin on
colon cancer cell lines dropped by 50% (from 0.94 to 0.39 mM for
HCT116, from 1.28 to 0.60 mM for SW620). In addition, ISMN enhanced
the apoptosis-inducing effect of aspirin on HCT116 cells, as
evidenced by changes in nuclear morphology, PS translocation,
caspase-3 activation and PARP cleavage. In the presence of 4 mM
ISMN, aspirin treatment resulted in a significantly larger amount
of chromatin condensation, an elevated apoptotic rate and increased
caspase-3 activity. These results indicated that the induction of
cell apoptosis, through a caspase-dependent pathway, is an
underlying mechanism of the cytotoxicity of ISMN and aspirin to
colon cancer cells.
Next, the present study performed an NO release
assay in an attempt to provide an explanation for the molecular
mechanism of the anti-tumor effects of ISMN. In the cell culture
media, treatment with ISMN alone caused a release of NO in a
concentration-dependent manner, while aspirin alone had no impact
on NO levels in the media. However, in the presence of ISMN, a
correlation was found between the concentration of aspirin and the
amount of NO in the culture medium. Although ISMN is an NO donor,
the NO increase from two drugs compared with ISMN alone was likely
to originate from the cells through signaling pathway activation.
When the two drugs were used at the same time, the amount of NO
release was not completely in parallel with the cytotoxicity and
apoptosis-inducing ability, indicating that other mechanisms may
exist besides the activation of the NO pathway.
Furthermore, the effect of the drugs on the Wnt
signaling pathway was thoroughly examined using a luciferase
reporter assay, RT-qPCR and western blot analysis. In recent years,
aberrant regulation of the Wnt pathway has been a prevalent theme
in cancer biology. The Wnt/β-catenin pathway, also called the
canonical pathway, is well understood and controls cellular
processes, including the cell cycle, apoptosis and differentiation
(21). When Wnt signaling is
activated, which leads to inactivation of the β-catenin destruction
complex, β-catenin phosphorylation is reduced and stabilized. The
stabilized β-catenin then translocates to the nucleus where it
regulates downstream gene expression, cyclin D1 and c-myc as
typical examples, by binding to lymphoid enhancer-binding
factor/TCF factors (22). NSAIDs,
including aspirin, have been shown to repress the function of
β-catenin as a Wnt inhibitor to overcome malignant cells (23). NO-NSAIDs have also been reported to
have marked growth-inhibitory and apoptotic effects on human
epidermoid carcinoma cells by targeting β-catenin (24). In a reporter assay performed in the
present study, HCT116 cells were transfected with TOPflash (TCF
reporter plasmid) containing two sets of three copies of the TCF
binding site (wild-type) upstream of the thymidine kinase minimal
promoter, and FOPflash, containing mutated TCF binding sites as a
negative control (25). It was
demonstrated that aspirin inhibited the promoter activity of
TOPflash, but not that of FOPflash, as well as the transcription
and translation of cyclin D1 and c-myc. Despite the fact that ISMN
alone did not influence Wnt signaling, TOPflash activity and cyclin
D1 or c-myc expression in HCT116 cells were further reduced in a
synergistic manner when aspirin and ISMN were used in combination.
These results implied that inhibition of the Wnt/β-catenin pathway
is an underlying mechanism of the synergistic apoptosis-inducing
effect of the two drugs.
Finally, a nude mouse xenograft model inoculated
with HCT116 cells was applied to examine the anti-tumor efficacy of
aspirin and ISMN in vivo. Combined treatment with the two
drugs led to a considerable reduction in the volumes and weights of
xenografted colon tumors without any obvious side effects, compared
to the effects of vehicle control or single drug treatment.
In conclusion, the present study demonstrated for
the first time, to the best of our knowledge, that two commonly
used drugs, aspirin and ISMN, synergistically exerted anti-tumor
effects on human colon cancer cells, while being less cytotoxic to
normal human umbilical vein cells. The underlying mechanisms of
action included the induction of cell apoptosis, the activation of
the NO pathway and the inhibition of the Wnt pathway. The potent
anti-tumor effect of the drug combination was also validated in
nude mouse xenograft model in vivo. Therefore, the combined
treatment with aspirin and ISMN is potentially and potent method
for treating human colon cancer. Compared with novel drugs,
well-known approved drugs, such as aspirin and ISMN, have more
clinical applications and levels of acceptance, due to their
well-defined pharmacokinetic/dynamic properties and side effects.
Continued efforts should be made to elucidate the signal
transduction pathways in more detail. Further clinical
investigations are required to validate the efficacy of the drug
combination in humans.
Acknowledgments
The authors would like to thank the Natural Science
Foundation of China (grant no. 81202396), the Science Foundation of
Shenzhen (grant no. JCYJ20130326112757843) and the Science
Foundation of Shenzhen University (grant no. 201146) for financial
support of this study.
References
|
1
|
Hossain MA, Kim DH, Jang JY, Kang YJ, Yoon
JH, Moon JO, Chung HY, Kim GY, Choi YH, Copple BL, et al: Aspirin
induces apoptosis in vitro and inhibits tumor growth of human
hepatocellular carcinoma cells in a nude mouse xenograft model. Int
J Oncol. 40:1298–1304. 2012.
|
|
2
|
Fuster V and Sweeny JM: Aspirin: A
historical and contemporary therapeutic overview. Circulation.
123:768–778. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dovizio M, Bruno A, Tacconelli S and
Patrignani P: Mode of action of aspirin as a chemopreventive agent.
Recent Results Cancer Res. 191:39–65. 2013. View Article : Google Scholar
|
|
4
|
Jankowska H, Hooper P and Jankowski JA:
Aspirin chemoprevention of gastrointestinal cancer in the next
decade. A review of the evidence. Pol Arch Med Wewn. 120:407–412.
2010.PubMed/NCBI
|
|
5
|
Goel A, Chang DK, Ricciardiello L, Gasche
C and Boland CR: A novel mechanism for aspirin-mediated growth
inhibition of human colon cancer cells. Clin Cancer Res. 9:383–390.
2003.PubMed/NCBI
|
|
6
|
Cooper K, Squires H, Carroll C,
Papaioannou D, Booth A, Logan RF, Maguire C, Hind D and Tappenden
P: Chemoprevention of colorectal cancer: Systematic review and
economic evaluation. Health Technol Assess. 14:1–206. 2010.
View Article : Google Scholar
|
|
7
|
Chen C, Hu JT, Tu YJ, Wu JC, Liang J, Gao
LL, Wang ZG, Yang BF and Dong DL: Effects of isosorbide mononitrate
on the restoration of injured artery in mice in vivo. Eur J
Pharmacol. 640:150–156. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kaufman R, Nunes I, Bolognese JA, Miller
DL, Salotti D, McCarthy JM, Smith WB, Herman GA and Feig PU:
Single-dose effects of isosorbide mononitrate alone or in
combination with losartan on central blood pressure. J Am Soc
Hypertens. 4:311–318. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wallace JL and Del Soldato P: The
therapeutic potential of NO-NSAIDs. Fundam Clin Pharmacol.
17:11–20. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pipili-Synetos E, Papageorgiou A, Sakkoula
E, Sotiropoulou G, Fotsis T, Karakiulakis G and Maragoudakis ME:
Inhibition of angiogenesis, tumour growth and metastasis by the
NO-releasing vasodilators, isosorbide mononitrate and dinitrate. Br
J Pharmacol. 116:1829–1834. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Usman MW, Luo F, Cheng H, Zhao JJ and Liu
P: Chemopreventive effects of aspirin at a glance. Biochim Biophys
Acta. 1855:254–263. 2015.PubMed/NCBI
|
|
12
|
Tan Z, Shang X, Li L, Tian L, Ma Y, Peng Y
and Gao L: Clinical study of isosorbide mononitrate treatment for
angina pectoris in coronary heart disease. Exp Ther Med.
5:1133–1136. 2013.PubMed/NCBI
|
|
13
|
Palmer RM, Ferrige AG and Moncada S:
Nitric oxide release accounts for the biological activity of
endothelium-derived relaxing factor. Nature. 327:524–526. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ignarro L: Nitric oxide biology and
pathobiology. 2nd edition. Academic Press; New York: 2009
|
|
15
|
Shah V, Lyford G, Gores G and Farrugia G:
Nitric oxide in gastrointestinal health and disease.
Gastroenterology. 126:903–913. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu W, Liu LZ, Loizidou M, Ahmed M and
Charles IG: The role of nitric oxide in cancer. Cell Res.
12:311–320. 2002. View Article : Google Scholar
|
|
17
|
Mocellin S, Bronte V and Nitti D: Nitric
oxide, a double edged sword in cancer biology: Searching for
therapeutic opportunities. Med Res Rev. 27:317–352. 2007.
View Article : Google Scholar
|
|
18
|
Rigas B and Kashfi K:
Nitric-oxide-donating NSAIDs as agents for cancer prevention.
Trends Mol Med. 10:324–330. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Williams JL, Nath N, Chen J, Hundley TR,
Gao J, Kopelovich L, Kashfi K and Rigas B: Growth inhibition of
human colon cancer cells by nitric oxide (NO)-donating aspirin is
associated with cyclooxygenase-2 induction and beta-catenin/T-cell
factor signaling, nuclear factor-kappaB, and NO synthase 2
inhibition: Implications for chemoprevention. Cancer Res.
63:7613–7618. 2003.PubMed/NCBI
|
|
20
|
Khan NI, Cisterne A, Baraz R, Bradstock KF
and Bendall LJ: Para-NO-aspirin inhibits NF-kappaB and induces
apoptosis in B-cell progenitor acute lymphoblastic leukemia. Exp
Hematol. 40:207–215. e12012. View Article : Google Scholar
|
|
21
|
Yang Y, Yang JJ, Tao H and Jin WS: New
perspectives on beta-catenin control of cell fate and proliferation
in colon cancer. Food Chem Toxicol. 74:14–19. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang Y: Wnt signaling in development and
disease. Cell Biosci. 2:142012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lu D, Cottam HB, Corr M and Carson DA:
Repression of beta-catenin function in malignant cells by
nonsteroidal antiin-flammatory drugs. Proc Natl Acad Sci USA.
102:18567–18571. 2005. View Article : Google Scholar
|
|
24
|
Nath N, Liu X, Jacobs L and Kashfi K:
Flurbiprofen benzyl nitrate (NBS-242) inhibits the growth of A-431
human epidermoid carcinoma cells and targets β-catenin. Drug Des
Devel Ther. 7:389–396. 2013.
|
|
25
|
Kang YJ, Park HJ, Chung HJ, Min HY, Park
EJ, Lee MA, Shin Y and Lee SK: Wnt/β-catenin signaling mediates the
antitumor activity of magnolol in colorectal cancer cells. Mol
Pharmacol. 82:168–177. 2012. View Article : Google Scholar : PubMed/NCBI
|