Introduction
Lipoteichoic acids (LTA) are polymers composed of
alternating units of polyhydroxy alkanes, including glycerol and
ribitol, and phosphoric acids, which together form phosphodiester
units in the envelop of gram-positive bacteria (1). LTA is released from bacterial cells
following bacteriolysis induced by leukocyte lysozymes or β-lactam
antibiotics, and binds to target cells either non-specifically via
membrane phospholipids, or specifically via CD14 and Toll-like
receptor 2, resulting in the release of reactive oxygen and
nitrogen species, acid hydrolase, proteinase, bactericidal
peptides, and proinflammatory cytokines (2). Through these mechanisms LTA is
involved in the pathophysiology of inflammation and post-infectious
sequelae such as septic shock, adult respiratory distress syndrome,
and toxic shock syndrome, thus suggesting that LTA is one of the
major virulence factors of gram-positive bacteria (2–4).
The bronchial epithelium is continuously exposed to
gram-positive bacteria that induce inflammation, such as
Streptococcus pneumoniae, which initiate the release of
interleukin (IL)-8 in human lung epithelial cells via nuclear
factor (NF)-κB recruitment to the IL-8 promoter (5). LTA is a major virulence factor of
Streptococcus pneumoniae, and activates NF-κB in order to
induce inflammatory responses in the respiratory system, via a
complex signaling pathway involving Toll-like receptors (6,7). In
numerous respiratory diseases, the bronchial epithelium induces an
inflammatory response through the production of proinflammatory
cytokines (8). Therefore,
ascertaining which genes and proteins are involved in the
regulation of the inflammatory response of bronchial epithelial
cells is crucial for the prevention and treatment of respiratory
diseases (8).
β-catenin is a member of the WNT/β-catenin signaling
pathway, which regulates various biological processes including
cellular proliferation, differentiation, and development (9). A previous study showed that β-catenin
modulated the inflammatory response of bronchial epithelial cells
treated with lipopolysaccharide (LPS), which is a virulence factor
of gram-negative bacteria (10).
The present study was conducted to investigate if β-catenin also
has a significant role in the LTA-induced inflammatory response in
BEAS-2B human bronchial epithelial cells.
Materials and methods
Cell culture
The BEAS-2B human bronchial epithelial cell line was
purchased from the American Type Culture Collection (Manassas, VA,
USA), and cultured in Dulbecco's modified Eagle's medium
(Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% fetal
bovine serum (Gibco Life Technologies, Grand Island, NY, USA), 100
µg/ml streptomycin, and 100 U/ml penicillin (Lonza,
Walkersville, MD, USA) at 37°C in an atmosphere containing 5%
CO2. The BEAS-2B human bronchial epithelial cells were
treated with LTA from Staphylococcus aureus (Sigma-Aldrich),
in order to induce an inflammatory response. LTA was treated at a
concentration of 100 µg/ml for up to 3 h after suitable
treatment conditions were experimentally determined.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The mRNA expression levels of the proinflam-matory
cytokines were measured by RT-qPCR, as previously described
(10). Briefly, the BEAS-2B human
bronchial epithelial cells were cultured in 12-well plates, and
total RNA was extracted using an RNeasy kit (Qiagen GmbH, Hilden,
Germany). Total RNA (1 µg) was then reverse-transcribed at
37°C using a cDNA Reverse Transcription kit (Applied Biosystems
Life Technologies, Foster City, CA, USA). Briefly, the reaction was
performed in a final volume of 20 µl, which included reverse
transcriptase reaction buffer, 100 mM dNTP mix, random primers,
MultiScribe reverse transcriptase, RNase inhibitor and total RNA.
The reaction mixtures were heated at 25°C for 10 min, 37°C for 120
min and 85°C for 5 min. RT-qPCR was performed in triplicate using a
StepOne PCR system (Applied Biosystems Life Technologies), in a
final volume of 20 µl containing TaqMan® Gene
Expression Master mix (Applied Biosystems Life Technologies), an
optimized concentration of each primer, 250 nM TaqMan®
probe, and 2.0 µl cDNA reaction mixture. The reaction
mixtures were preheated at 95°C for 10 min in order to activate the
enzyme, and then subjected to 40 cycles of melting at 95°C for 15
sec, prior to annealing/extension at 60°C for 1 min. RT-qPCR
efficiencies were ~100%. Assay-on-demand gene expression products
(Applied Biosystems Life Technologies) were used to evaluate the
mRNA expression levels of IL-6 (Hs00174131/m1), IL-8
(Hs99999034/m1), IL-1β (Hs01555410/m1), tumor necrosis factor
(TNF)-α (Hs01113624/m1), monocyte chemoat-tractant protein (MCP)-1
(Hs00234140/m1), and 18S rRNA (Hs99999901/m1). For each sample, the
mRNA expression levels were normalized to 18S rRNA expression
levels, and the ratios of normalized mRNA to untreated control
sample mRNA were determined using the comparative cycle threshold
(Ct) method (11).
Promoter reporter assay
The transcriptional activity levels induced by NF-κB
or β-catenin were determined using a promoter reporter assay as
previously described (10). In
order to measure NF-κB-dependent transcriptional activity,
2×105 BEAS-2B human bronchial epithelial cells were
co-transfected at a 1:50 ratio with a pGL 4.32 vector (Promega
Corporation, Madison, WI, USA) containing a firefly luciferase
reporter gene linked to a promoter containing the NF-κB response
element, and a pGL 4.17 vector (Promega Corporation) containing a
Renilla luciferase reporter gene using Lipofectamine 2000 reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA). The cells were
harvested 24 h post-transfection, and luciferase activity was
measured using a Dual-Luciferase Reporter Assay system (Promega
Corporation). In order to measure β-catenin-dependent
transcriptional activity, the BEAS-2B human bronchial epithelial
cells were co-transfected at a 1:50 ratio with a TOP flash vector
(EMD Millipore, Billerica, MA, USA) containing a firefly luciferase
reporter gene linked to a promoter containing the β-catenin
response element, and a pGL 4.17 vector containing a Renilla
luciferase reporter gene. For each assay, the firefly luciferase
activity was normalized to the Renilla luciferase activity in order
to identify variations in transfection efficiency.
Western blotting
The BEAS-2B human bronchial epithelial cells were
lysed with ice-cold radioimmunoprecipitation assay buffer
containing 25 mM Tris-HCl pH 7.6, 150 mM NaCl, 1% Nonidet P-40, 1%
sodium deoxycholate, 0.1% SDS, and a protease inhibitor cocktail
(Sigma-Aldrich), and the insoluble materials were subsequently
removed by centrifugation at 20,000 × g for 20 min at 4°C. The
protein concentrations were determined using a bicinchoninic acid
protein assay kit (Pierce, Rockford, IL, USA). A total of 50
µg lysate proteins were electrophoresed in 12% SDS-PAGE and
transferred onto nitrocellulose membranes (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). Protein expression was detected using
1:1,000 dilutions of primary antibodies, including anti NF-κB (cat.
no. 13681, Cell Signaling Technology, Inc., Danvers, MA, USA), anti
I-κB (cat. no. 9242, Cell Signaling Technology, Inc.), anti phospho
I-κB (cat. no. 9246, Cell Signaling Technology, Inc.) and anti
β-catenin (cat. no. 610153, BD Biosciences, San Jose, CA, USA)
overnight at 4°C, followed by 1:1,000 dilutions of horseradish
peroxidase-conjugated anti-mouse (cat. no. 7076S, Cell Signaling
Technology, Inc.) or anti-rabbit (cat. no. 7074S, Cell Signaling
Technology, Inc.) secondary antibody for 2 h at room temperature.
Peroxidase activity was visualized using an ECL kit (Bio-Rad
Laboratories Inc.). Anti-β-actin antibody (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) was used as the loading
control for total cell lysates.
Small interfering (si)RNA
transfection
The BEAS-2B human bronchial epithelial cells were
grown to 70% confluence and transfected with 50 nM control siRNA or
β-catenin siRNA (Santa Cruz Biotechnology, Inc.) mixed with
Lipofectamine® RNAiMAX Transfection reagent (Invitrogen
Life Technologies), all of which were suspended in
Opti-MEM® medium (Invitrogen Life Technologies).
Following an incubation period of 18 h, the transfected cells
(2×105 cells) were treated with LTA in order to induce
an inflammatory response.
Statistical analysis
All data are expressed as the mean ± standard
deviation, and all experiments were performed in triplicate.
Statistically significant differences between treated and untreated
samples were detected using unpaired t-tests. P<0.05 was
considered to indicate a statistically significant difference. All
statistical analyses were performed using SPSS 18.0 (SPSS, Chicago,
IL, USA).
Results
LTA-induces mRNA expression levels of
proinflammatory cytokines in bronchial epithelial cells
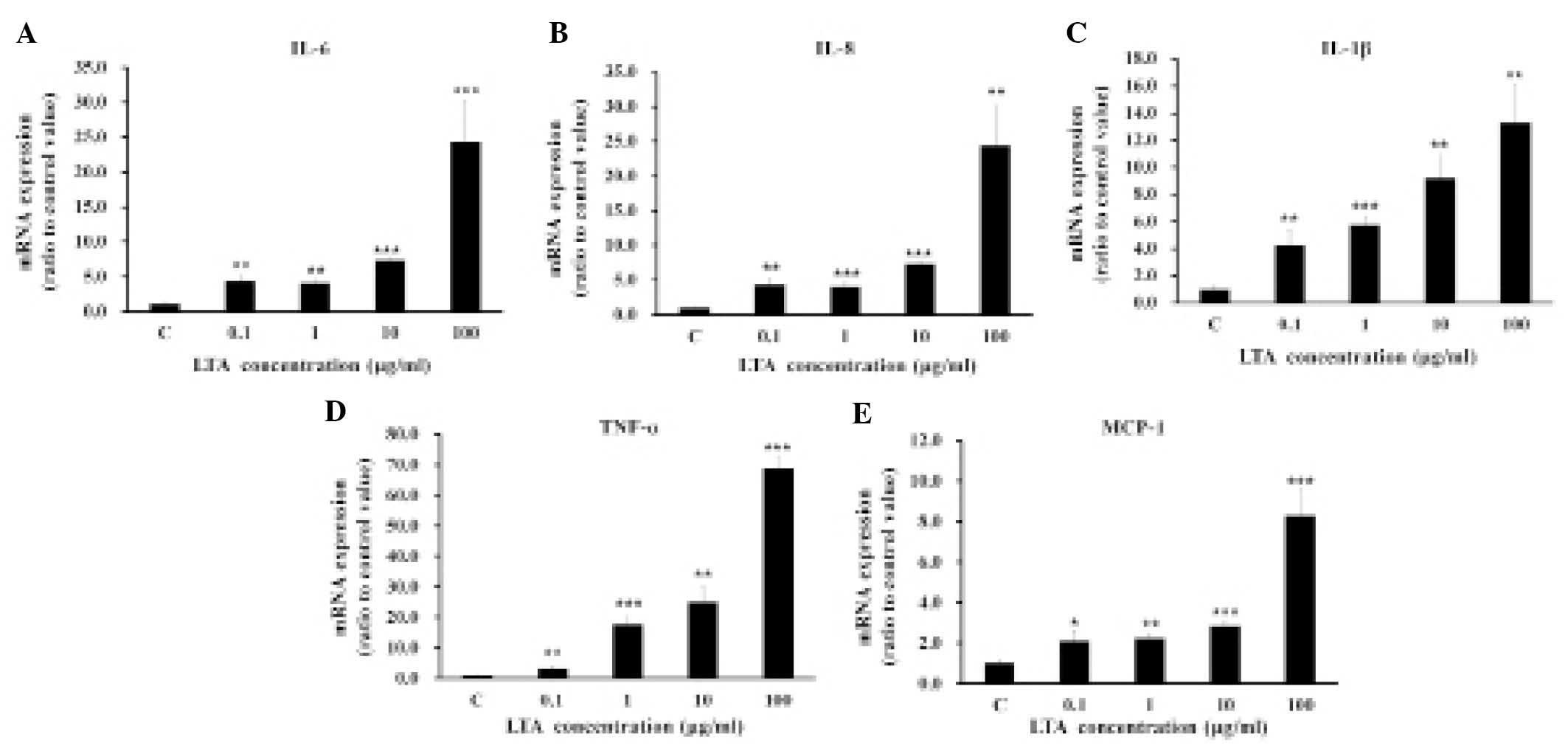
The mRNA expression of proinflammatory cytokines
IL-6, IL-8, IL-1β, TNF-α, and MCP-1 was induced in a dose-dependent
manner by treating the BEAS-2B human bronchial epithelial cells
with various concentrations of LTA, ranging from 0.1–100
µg/ml (Fig. 1). All
subsequent experiments were conducted using LTA at a concentration
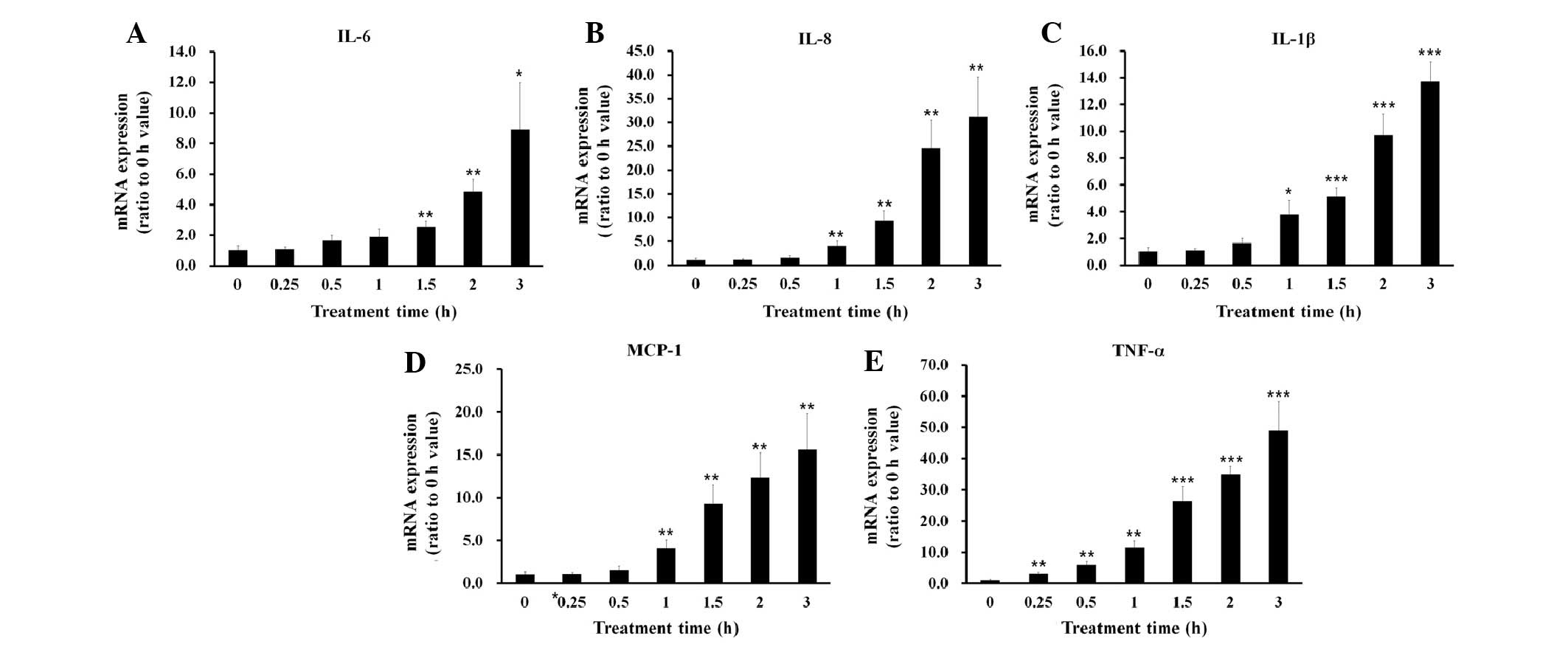
of 100 µg/ml. Treatment with 100 µg/ml LTA increased
the mRNA expression levels of the proinflammatory cytokines
following ~1 h incubation (Fig.
2).
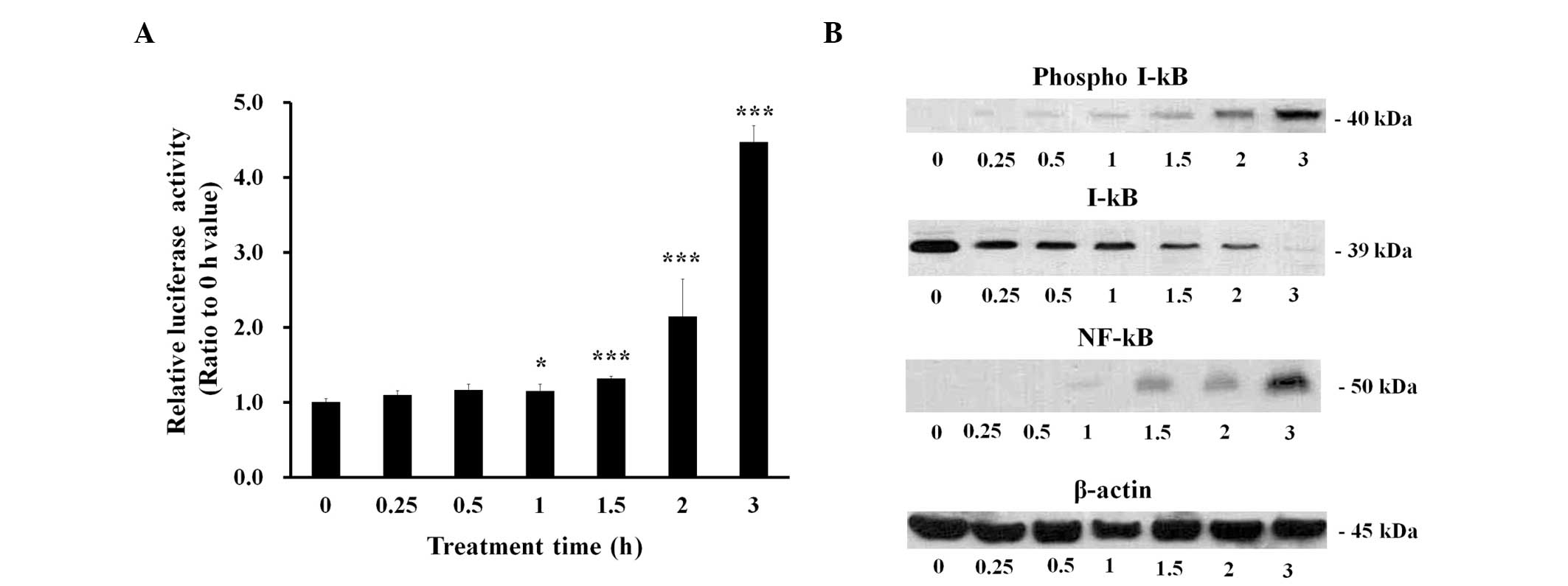
LTA induces NF-κB-driven transcriptional
activity in BEAS-2B human bronchial epithelial cells
The role of NF-κB, which is the principal
transcription factor responsible for proinflamma-tory cytokine
expression, was investigated in the BEAS-2B human bronchial
epithelial cells treated with 100 µg/ml LTA. NF-κB-driven
transcriptional activity was measured at various time points in the
LTA-treated cells following transfection with a pGL 4.32 vector
containing a luciferase gene linked to an NF-κB-dependent promoter.
The results demonstrated that NF-κB-driven transcription increased
~1 h following LTA treatment (Fig.
3A). Under the same experimental conditions, phosphorylation of
I-κB increased 0.25–0.5 h following LTA treatment, whereas the
protein expression levels of I-κB were decreased (Fig. 3B). In addition, the protein
expression levels of NF-κB increased 1 h following LTA treatment
(Fig. 3B).
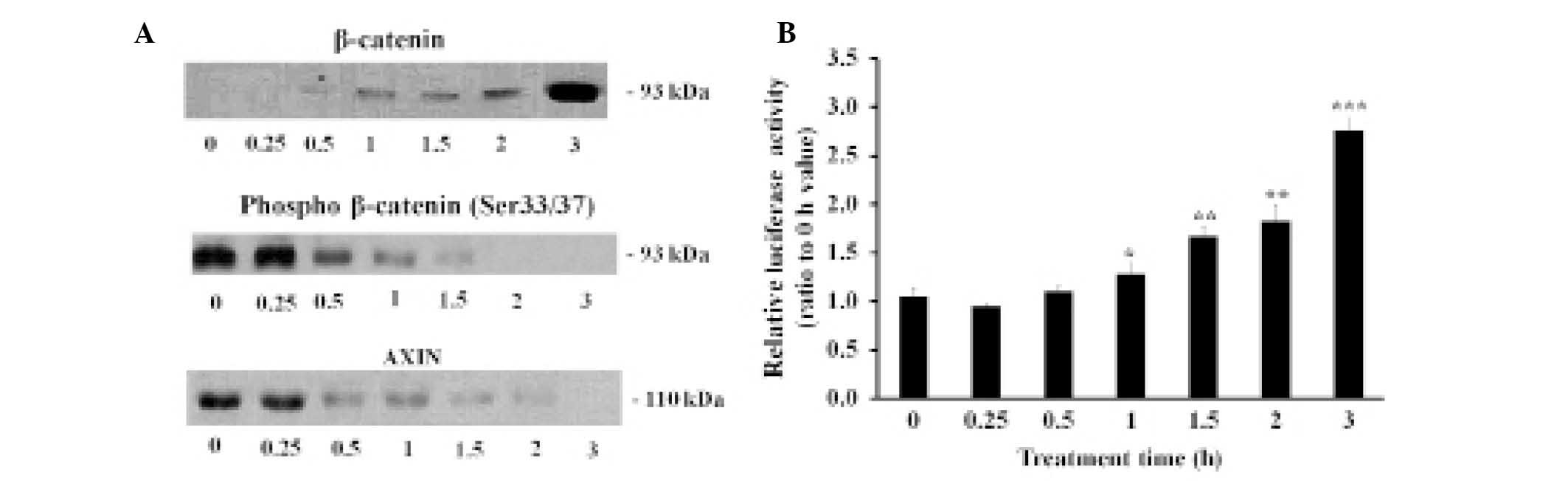
LTA upregulates β-catenin in BEAS-2B
human bronchial epithelial cells
The BEAS-2B human bronchial epithelial cells were
treated with 100 µg/ml LTA for various time periods. The
results showed that the protein expression levels of β-catenin
increased 0.5 h following treatment with LTA (Fig. 4A). Conversely, the phosphorylation
levels of β-catenin at Ser 33/37 residues, as well as the
expression levels of AXIN, a major component of the β-catenin
degradation complex, were downregulated following treatment with
LTA (12,13). The transcriptional coactivator
activity levels of β-catenin were measured using a TOP flash vector
containing a luciferase gene linked to a β-catenin-dependent
promoter (Fig. 4B). LTA
upregulated both the activity and protein expression levels of
β-catenin.
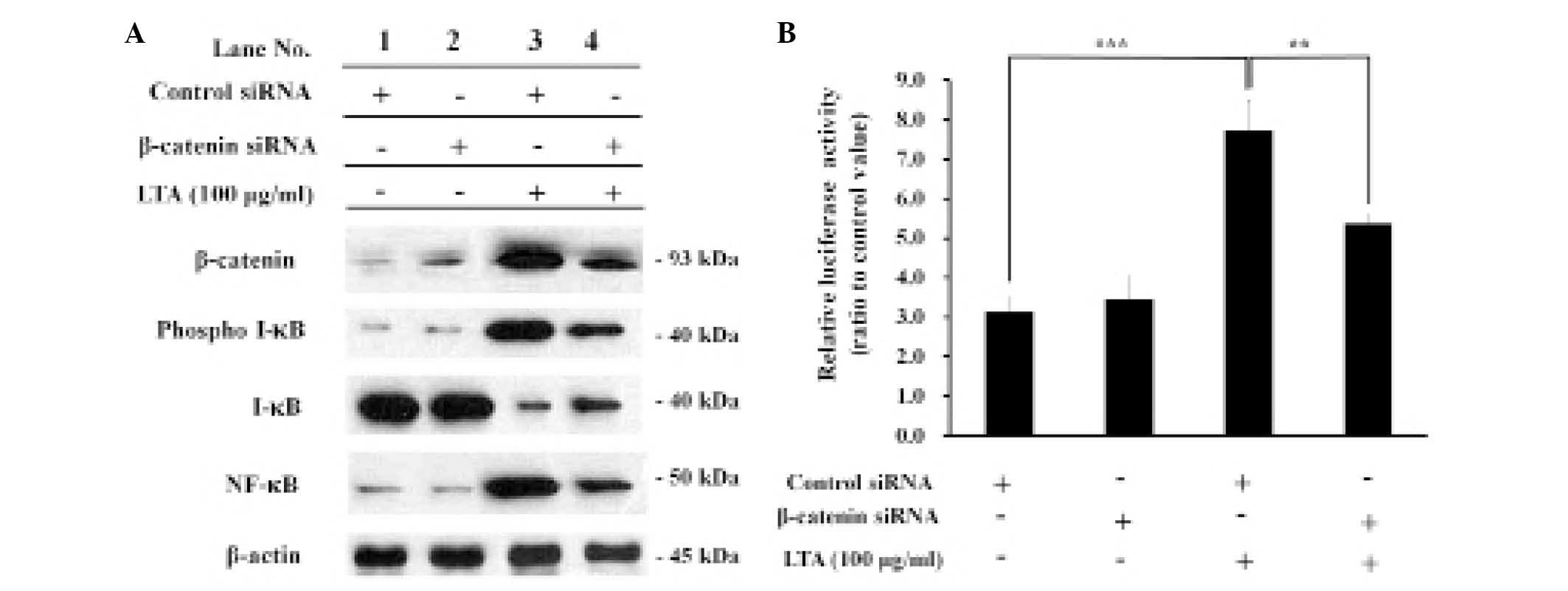
β-catenin knockdown decreases the
activity and expression levels of NF-κB in LTA-treated BEAS-2B
human bronchial epithelial cells
In order to investigate the effects of β-catenin on
LTA-induced NF-κB activity and expression, the BEAS-2B human
bronchial epithelial cells were transfected with either control or
β-catenin siRNA, and treated with LTA in order to induce NF-κB
activity. The expression levels of β-catenin upregulated by LTA
treatment were reduced following β-catenin siRNA transfection, as
compared with control siRNA transfection, demonstrating that
β-catenin is knocked down by β-catenin siRNA. Under the same
experimental conditions, the expression levels of phospho I-κB were
significantly reduced following β-catenin knockdown, whereas the
expression levels of I-κB were increased, demonstrating an inverse
correlation between the expression levels of I-κB and phospho-I-κB
(Fig. 5A, lanes 3 and 4).
Similarly, the expression levels of NF-κB upregulated by LTA
treatment were significantly reduced following β-catenin knockdown,
demonstrating an inverse correlation between the expression levels
of NF-κB and I-κB. The transcriptional activity of the
NF-κB-dependent promoter, which was upregulated by LTA treatment,
was also significantly reduced following β-catenin knockdown,
demonstrating a correlation between the expression levels of the
NF-κB-dependent promoter and NF-κB (Fig. 5B).
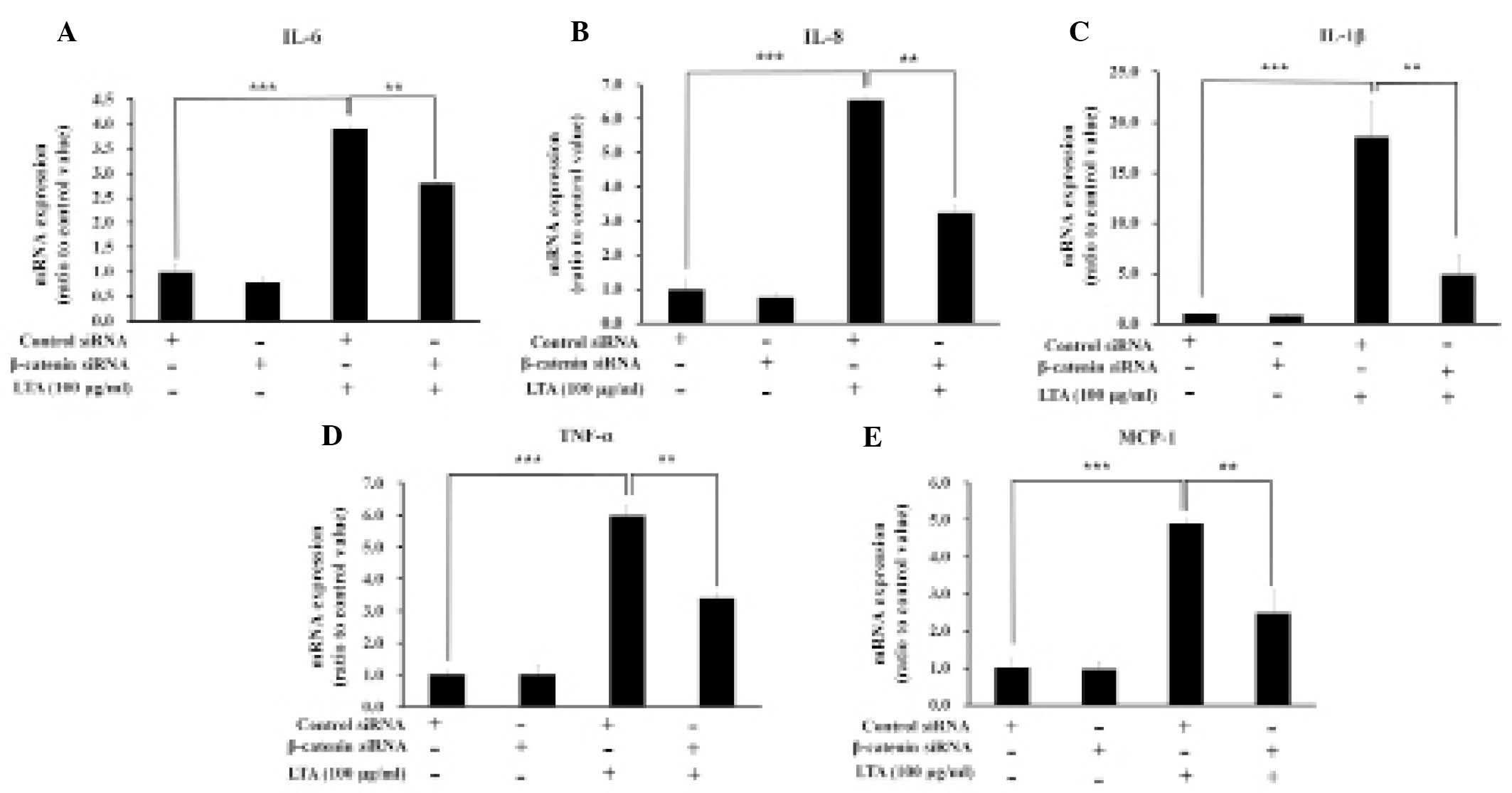
β-catenin knockdown decreases the
expression levels of proinflammatory cytokines in LTA-treated
cells
In order to investigate the effects of β-catenin on
the expression levels of LTA-induced proinflammatory cytokines, the
expression levels of IL-6, IL-8, IL-1β, TNF-α and MCP-1 were
measured in the BEAS-2B human bronchial epithelial cells
transfected with either control or β-catenin siRNA, both with and
without LTA treatment (Fig. 6).
The expression levels of the proinflammatory cytokines upregulated
by LTA treatment were significantly reduced following β-catenin
knockdown. These results indicate a correlation between the
expression levels of NF-κB, the NF-κB-dependent promoter, and
proinflammatory cytokines (Figs. 5
and 6).
Discussion
LTA is a major virulence factor that has an
important role in infection and post-infection pathological events
caused by gram-positive bacteria (2). The results of the present study
confirmed that LTA induces the expression of proinflammatory
cytokines in BEAS-2B human bronchial epithelial cells. In addition,
LTA was able to increase the transcriptional activity of NF-κB, as
determined by a reporter assay of the NF-κB-dependent promoter, and
increase the phosphorylation/degradation of I-κB. These results
demonstrated that LTA may modulate the expression levels of I-κB
and NF-κB, thereby upregulating proinflammatory cytokine expression
in BEAS-2B human bronchial epithelial cells. NF-κB is an important
factor in the inflammation of bronchial epithelial cells exposed to
various toxic compounds and pathogenic microorganisms (14–19).
Therefore, ascertaining which genes or proteins modulate NF-κB may
help identify therapeutic targets for the prevention and treatment
of respiratory diseases mediated by bronchial inflammation.
In the present study, both the activity and protein
expression levels of β-catenin were significantly increased in
LTA-treated BEAS-2B human bronchial epithelial cells. Conversely,
the phosphorylation levels of β-catenin at Ser 33/37 residues as
well as the protein expression levels of AXIN, were decreased
following treatment with LTA. Previous studies have reported that
the activity and protein expression levels of β-catenin are
post-translationally regulated via the WNT/β-catenin signaling
pathway (12,13). When the WNT/β-catenin signaling
pathway is in the resting state, β-catenin is degraded by a
degradation complex composed of AXIN, adenomatous polyposis coli,
and glycogen synthase kinase 3β, which phosphorylates β-catenin at
Ser 33/37 residues creating a binding site for E3 ubiquitin, which
leads to the ubiquitination and proteolytic degradation of
β-catenin (12,13). Conversely, activation of the
WNT/β-catenin signaling pathway results in the upregulation of
β-catenin via its dephosphorylation at Ser 33/37 residues,
preventing ubiquitination and proteolytic degradation (12,13).
The results of the present study demonstrated that β-catenin was
upregulated by LTA treatment via the downregulation of AXIN, a
component of the β-catenin degradation complex.
In order to confirm the role of β-catenin in the
LTA-induced inflammatory response of bronchial epithelial cells,
β-catenin was knocked down by β-catenin siRNA transfection. When
β-catenin was knocked down, the protein expression levels of NF-κB
were significantly reduced, suggesting the important role of
β-catenin in the modulation of NF-κB expression. NF-κB
downregulation following β-catenin knockdown in LTA-treated cells
was accompanied by reduced phosphorylation/degradation of I-κB. The
transcriptional activity of the NF-κB-dependent promoter, which was
upregulated by LTA, was also significantly reduced following
β-catenin knockdown. In addition, the expression levels of
LTA-induced proinflamma-tory cytokines were significantly reduced
following β-catenin siRNA transfection, demonstrating a correlation
between the expression levels of NF-κB, the NF-κB-dependent
promoter, and proinflammatory cytokines. These results confirm that
β-catenin has a significant role in the upregulation of NF-κB and
proinflammatory cytokine expression during the inflammatory
response to LTA.
Previous studies have suggested the importance of
β-catenin in the inflammatory response induced by various
pathogenic agents. Kim et al (20) reported that LPS caused β-catenin
accumulation and nuclear translocation, followed by NADPH oxidation
in RAW 264.7 macrophage cells, and murine bone marrow-derived
macrophages. Furthermore, a previous study demonstrated that
LPS-induced proinflammatory cytokine expression in bronchial
epithelial cells was suppressed by β-catenin knockdown, suggesting
an important role for β-catenin in the LPS-induced inflammatory
response (10). In addition,
β-catenin upregulated the expression levels of inflammatory
cytokines in THP-1 human monocytic cells stimulated by Der p 1, a
major house dust mite allergen (21). TNF-α-induced proinflammatory
cytokine expression in bronchial epithelial cells has also been
shown to be suppressed by β-catenin siRNA transfection (unpublished
data). The present study provides evidence that β-catenin modulates
the expression levels of NF-κB and inflammatory cytokines in
BEAS-2B human bronchial epithelial cells stimulated by LTA, a major
virulence factor of gram-positive bacteria. The results of the
present study are concordant with those of previous studies,
suggesting a major role for β-catenin in the regulation of the
inflammatory response.
The precise mechanism underlying β-catenin
modulation of inflammatory signaling remains unclear. However, the
similarities in the effects of β-catenin, and the variety in
upstream signaling molecules between diverse inflammatory inducers
such as LTA, LPS, and TNF-α, suggest that the target of β-catenin
may be located downstream of the inflammatory signaling pathway.
LPS (10), LTA (present study),
and TNF-α (unpublished data) induce the phosphorylation and
degradation of I-κB, followed by the upregulation of NF-κB
expression, suggesting that the target of β-catenin may be
associated, directly or indirectly, to the
phosphorylation/degradation of I-κB. Further studies are required
in order to elucidate the precise molecular mechanism underlying
β-catenin activity, but the results of the present study suggest
that β-catenin may be a regulator of bronchial inflammation. These
results may prove useful in the prevention and treatment of
respiratory diseases.
Acknowledgments
The present study was supported by a grant from the
Basic Science Research Program of the National Research Foundation
of Korea (grant no. NRF-2013R1A1A2A10006146).
References
|
1
|
Schneewind O and Missiakas D: Lipoteichoic
acids, phosphate-containing polymers in the envelope of
gram-positive bacteria. J Bacteriol. 196:1133–1142. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ginsburg I: Role of lipoteichoic acid in
infection and inflammation. Lancet Infect Dis. 2:171–179. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Opal SM and Cohen J: Clinical
gram-positive sepsis: Does it fundamentally differ from
gram-negative bacterial sepsis? Crit Care Med. 27:1608–1616. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sriskandan S and Cohen J: Gram-positive
sepsis. Mechanisms and differences from gram-negative sepsis.
Infect Dis Clin North Am. 13:397–412. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schmeck B, Huber S, Moog K, Zahlten J,
Hocke AC, Opitz B, Hammerschmidt S, Mitchell TJ, Kracht M, Rosseau
S, et al: Pneumococci induced TLR- and Rac1-dependent
NF-kappaB-recruitment to the IL-8 promoter in lung epithelial
cells. Am J Physiol Lung Cell Mol Physiol. 290:L730–L737. 2006.
View Article : Google Scholar
|
|
6
|
Kawai T and Akira S: Toll-like receptors
and their crosstalk with other innate receptors in infection and
immunity. Immunity. 34:637–650. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee IT, Lee CW, Tung WH, Wang SW, Lin CC,
Shu JC and Yang CM: Cooperation of TLR2 with MyD88, PI3K, and Rac1
in lipoteichoic acid-induced cPLA2/COX-2-dependent airway
inflammatory responses. Am J Pathol. 176:1671–1684. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bradding P, Roberts JA, Britten KM,
Montefort S, Djukanovic R, Mueller R, Heusser CH, Howarth PH and
Holgate ST: Interleukin-4, -5, and -6 and tumor necrosis
factor-alpha in normal and asthmatic airways: Evidence for the
human mast cell as a source of these cytokines. Am J Respir Cell
Mol Biol. 10:471–480. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Moon RT, Bowerman B, Boutros M and
Perrimon N: The promise and perils of Wnt signaling through
beta-catenin. Science. 296:1644–1646. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jang J, Ha JH, Chung SI and Yoon Y:
B-catenin regulates NF-κB activity and inflammatory cytokine
expression in bronchial epithelial cells treated with
lipopolysaccharide. Int J Mol Med. 34:632–638. 2014.PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
12
|
Cadigan KM and Liu YI: Wnt signaling:
Complexity at the surface. J Cell Sci. 119:395–402. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Willert K and Nusse R: Beta-catenin: A key
mediator of Wnt signaling. Curr Opin Genet Dev. 8:95–102. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Profita M, Bonanno A, Montalbano AM,
Ferraro M, Siena L, Bruno A, Girbino S, Albano GD, Casarosa P,
Pieper MP and Gjamarkaj M: Cigarette smoke extract activates human
bronchial epithelial cells affecting non-neuronal cholinergic
system signalling in vitro. Life Sci. 89:36–43. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tal TL, Simmons SO, Silbajoris R, Dailey
L, Cho SH, Ramabhadran R, Linak W, Reed W, Bromberg PA and Samet
JM: Differential transcriptional regulation of IL-8 expression by
human airway epithelial cells exposed to diesel exhaust particles.
Toxicol Appl Pharmacol. 243:46–54. 2010. View Article : Google Scholar
|
|
16
|
Pylkkänen L, Stockmann-Juvala H, Alenius
H, Husgafvel-Pursiainen K and Savolainen K: Wood dusts induce the
production of reactive oxygen species and caspase-3 activity in
human bronchial epithelial cells. Toxicology. 262:265–270. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bossios A, Gourgiotis D, Skevaki CL,
Saxoni-Papageorgiou P, Lötvall J, Psarras S, Karpathios T,
Constandopoulos AG, Johnston SL and Papadopoulos NG: Rhinovirus
infection and house dust mite exposure synergize in inducing
bronchial epithelial cell interleukin-8 release. Clin Exp Allergy.
38:1615–1626. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ishibashi Y and Nishikawa A: Role of
nuclear factor-kappa B in the regulation of intercellular adhesion
molecule 1 after infection of human bronchial epithelial cells by
Bordetella pertussis. Microb Pathog. 35:169–177. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thomas LH, Friedland JS, Sharland M and
Becker S: Respiratory syncytial virus-induced RANTES production
from human bronchial epithelial cells is dependent on nuclear
factor-kappa B nuclear binding and is inhibited by
adenovirus-mediated expression of inhibitor of kappa B alpha. J
Immunol. 161:1007–1016. 1998.PubMed/NCBI
|
|
20
|
Kim JS, Yeo S, Shin DG, Bae YS, Lee JJ,
Chin BR, Lee CH and Baek SH: Glycogen synthase kinase 3beta and
beta-catenin pathway is involved in toll-like receptor 4-mediated
NADPH oxidase 1 expression in macrophages. FEBS J. 277:2830–2837.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jang J, Ha JH, Kim SM, Kim W, Kim K, Chung
SI and Yoon Y: β-catenin mediates the inflammatory cytokine
expression induced by the Der p 1 house dust mite allergen. Mol Med
Rep. 9:633–638. 2014.
|