Introduction
Neuropsychological stimulation has been shown to
affect tumor progression and therapeutic response. Neural
stimulation is associated with increased tumor incidence and
metastasis (1). β-adrenergic
activation of the cyclic adenosine monophosphate-protein kinase A
signaling pathway has been reported to enhance tumor angiogenesis
in vivo and promote malignant cell growth (2). β-blockers have been shown to reduce
recurrence rates and mortality in patients with breast, melanoma
and prostate cancer (3–5). A recent report found that cholinergic
parasympathetic signaling regulates prostate cancer invasion
(6). Furthermore, transplantation
of beta-endorphin neurons into the hypothalamus has been reported
to increase the activity of natural killer (NK) cells and
macrophages, reduce inflammation and epithelial to mesenchymal
transition (EMT) in tumor tissues and suppress mammary tumor growth
and progression (7).
Neuropsychological factors also influence the effect of anticancer
therapies. Nonmyelinating Schwann cells are components of a
hematopoietic niche and maintain hematopoietic stem cell (HSC)
hibernation (8). Sympathetic
nerves in the marrow of mice promote the survival of constituents
of the stem cell niche which initiate recovery, thus
chemotherapy-induced nerve injury in the bone marrow has been found
to impair hematopoietic regeneration (9). Adrenergic nerve protection
strategies, for example the administration of 4-methylcatechol or
glial-derived neurotrophic factor, have been reported to promote
hematopoietic recovery (9).
Dopamine receptors (DRs) are involved in several
physiological and pathological processes, including age, emotion,
opiate addiction and vascular activity. Five DRs have been
identified and divided into two families: The D1 (including D1R and
D5R) and D2 (including D2R, D3R and D4R) receptor families
(10). DRs expressed on
lymphocytes are involved in pathogenesis. D3R expressed on
CD4+ T cells has also been reported to have an important
role in the pathogenesis of
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson's
disease (11). D3R-deficient mice
were observed to be protected against the loss of dopaminergic
neurons and microglial activation during MPTP-induced Parkinson's
disease; however, upon transfer of wild-type CD4+ T
cells, mice became susceptible to MPTP-induced neuronal
degeneration and activation of microglia (11). The role of dopamine, not only in
mediating interactions in the nervous system, but as an
immunomodulator in the pathogenesis of disease, has been the focus
of much research (12).
Circulating dopamine levels have been reported to be higher in
patients with lung cancer compared with healthy donors and dopamine
has been found to effectively inhibit proliferation and
cytotoxicity in T lymphocytes through D1 DRs (13).
Sachlos et al (14) identified that DRs are expressed in
lymphoid stem cells and CD44+ CD24−/low
breast cancer stem cells (CSCs). However, DRs have not been
observed in primitive HSCs or progenitor populations. Differential
DR expression enables potential DR drug targeting for cancer
(14). Thioridazine is a well
established anti-psychotic and -anxiety agent which acts through
DR1-5 (15). The present study
aimed to investigate the anti-tumor effect of thioridazine in a
murine breast cancer model.
Materials and methods
Cell culture
The 4T1 cancer cell line (American Type Culture
Collection, Manassas, VA, USA) was maintained in RPMI-1640
(Gibco-BRL, Carlsbad, CA, USA) supplemented with 10% fetal bovine
serum at 37°C in a humidified atmosphere containing 5%
CO2.
Cell cycle and apoptosis analysis
The 4T1 cell line was treated with 5, 10 and 20
µM thioridazine. To assess apoptosis, cells were collected
and stained with fluorescein isothiocyanate (FITC)-labeled Annexin
V and propidium iodide, according to the manufacturer's
instructions (Roche, Mannheim, Germany). Apoptotic cells were
analyzed using flow cytometry (FCM) using CellQuest™ software (BD
Biosciences, San Jose, CA, USA). Thioridazine was purchased from
Sigma-Aldrich (St. Louis, MO, USA).
MTT assay
MTT assay was performed in 96-well plates to
determine cell growth inhibition. A total of 3,000 cells/well were
seeded in 96-well plates and treated with 20 µM thioridazine
the following day. After 24–72 h drug incubation, MTT was added to
each well and incubated for 4 h at 37°C. The supernatant was then
removed and formazan precipitates in the cells were dissolved using
150 µl dimethyl sulfoxide. Absorbance was read at 570 nm and
a growth curve was plotted.
Western blot analysis
Subsequent to treatment with 20 µM
thioridazine for 24 h, 4T1 tumor cells were harvested and lysed
using radioimmunoprecipitation assay buffer containing protease
inhibitors. Equal quantities of protein were loaded and resolved
using SDS-PAGE and transferred onto polyvinylidene difluoride
membranes. Membranes were then incubated with primary antibodies
against STAT3, phosphorylated (p)-STAT3, Akt, p-Akt and p-p65 (all
rabbit mAb; cat. nos. 4904, 9145, 4685, 4051 and 3033 respectively;
Cell Signaling Technology Inc., Danvers, MA USA), followed by
incubation with horseradish peroxidase-conjugated secondary
antibodies (Goat pAb; cat. no. SP-9001; ZSGB-BIO ORIGENE, Beijing,
China). Immunoreactive bands were visualized using an enhanced
chemiluminescence detection system.
Tumor challenge and treatment
In order to determine the anti-tumor activity of
thioridazine, flank syngeneic breast tumors were established in
six- to eight-week-old female BALB/c mice through subcutaneous
injection with 1×106 4T1 cells. When the tumors reached
~100 mm3 on day six following inoculation, the mice with
established tumors were stratified by tumor volume and randomly
divided into two groups, which were treated with vehicle and
thioridazine (32 mg/kg body weight), respectively. Thioridazine was
intraperitoneally administered daily. Tumor diameters were measured
using a caliper and tumor volumes were calculated by the formula
0.52 × a ×b2, where a is the larger diameter and b is
the smaller diameter.
Immunohistochemistry
Tumors were harvested and paraffin-embedded, then
tumor sections were stained with hematoxylin and eosin. For the
analysis of cell proliferation, tumor sections were incubated with
anti-Ki67 antibodies (cat. no. AB9260; Millipore Corporation,
Billerica, MA, USA) at 4°C overnight, followed by incubation with
biotin-conjugated secondary antibodies and streptavidin-horseradish
peroxidase complexes. For the analysis of apoptosis, the TUNEL
assay (Promega Corporation, Madison, WI, USA) was performed on the
tumor sections, according to the manufacturer's instructions.
Frozen tumor sections were incubated with CD31 antibodies (cat. no.
ab7388; Abcam PLC, Cambridge, UK) and phycoerythrin-conjugated
secondary antibodies (cat. no. ab7010; Abcam PLC) for microvessel
analysis.
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical comparisons were performed using analysis of variance
or the student's t-test. SPSS V13.0 software (SPSS Inc., Chicago,
IL, USA) was used for statistical analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
Dopamine receptor antagonist thioridazine
reduces breast tumor growth
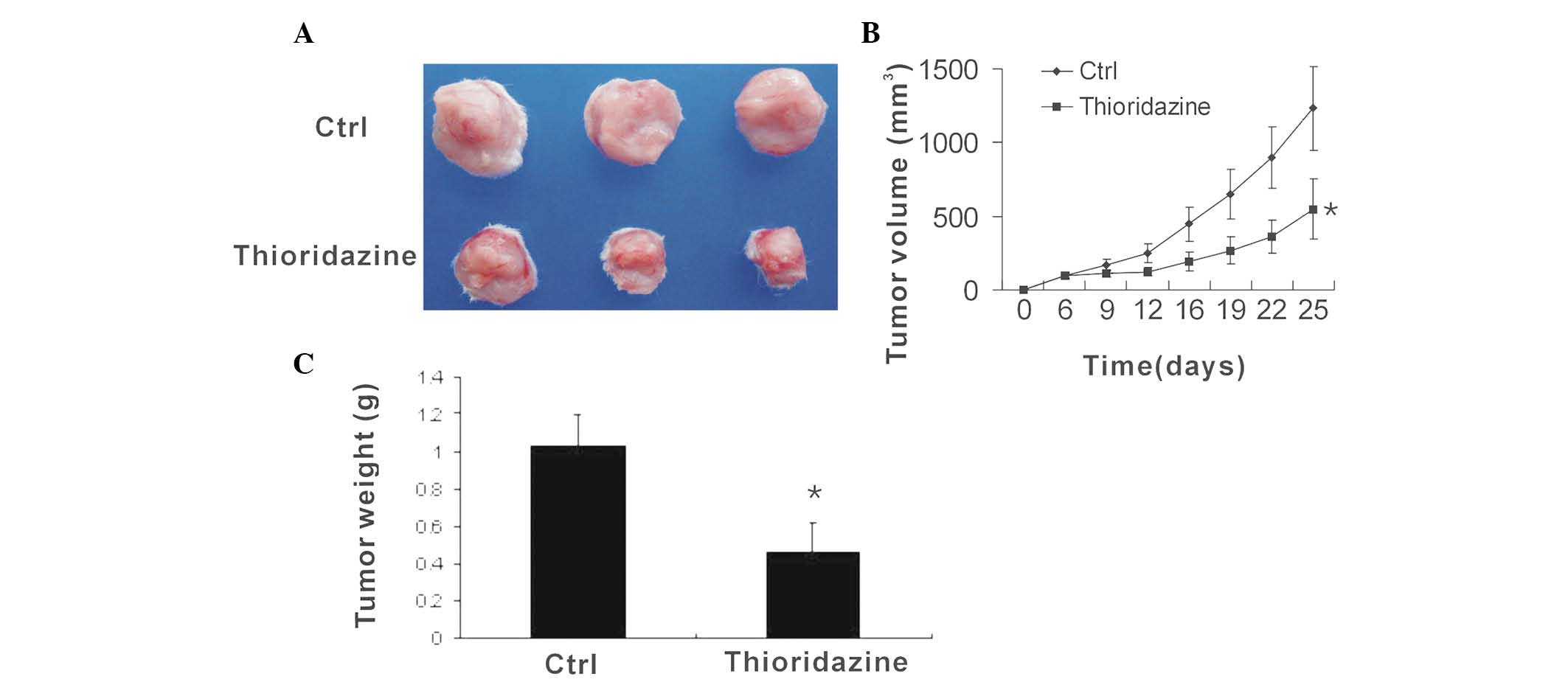
To determine the anti-tumor efficacy of thioridazine
in vivo, flank syngeneic 4T1 breast tumors were established
in BALB/c mice. The mice with then treated with vehicle or
thioridazine. The control tumors were observed to grow rapidly,
with a volume of 1232.8±282.8 mm3 at day 25
post-inoculation. However, following treatment with thioridazine,
the tumor volume was found to be 549.3±205.2 mm3
(Fig. 1A and B). Thus,
thioridazine reduced tumor volume by 55% (Fig. 1C), suggesting that the dopamine
receptor antagonist thioridazine effectively reduces breast tumor
growth.
Thioridazine induces apoptosis and
inhibits tumor cell growth in vitro
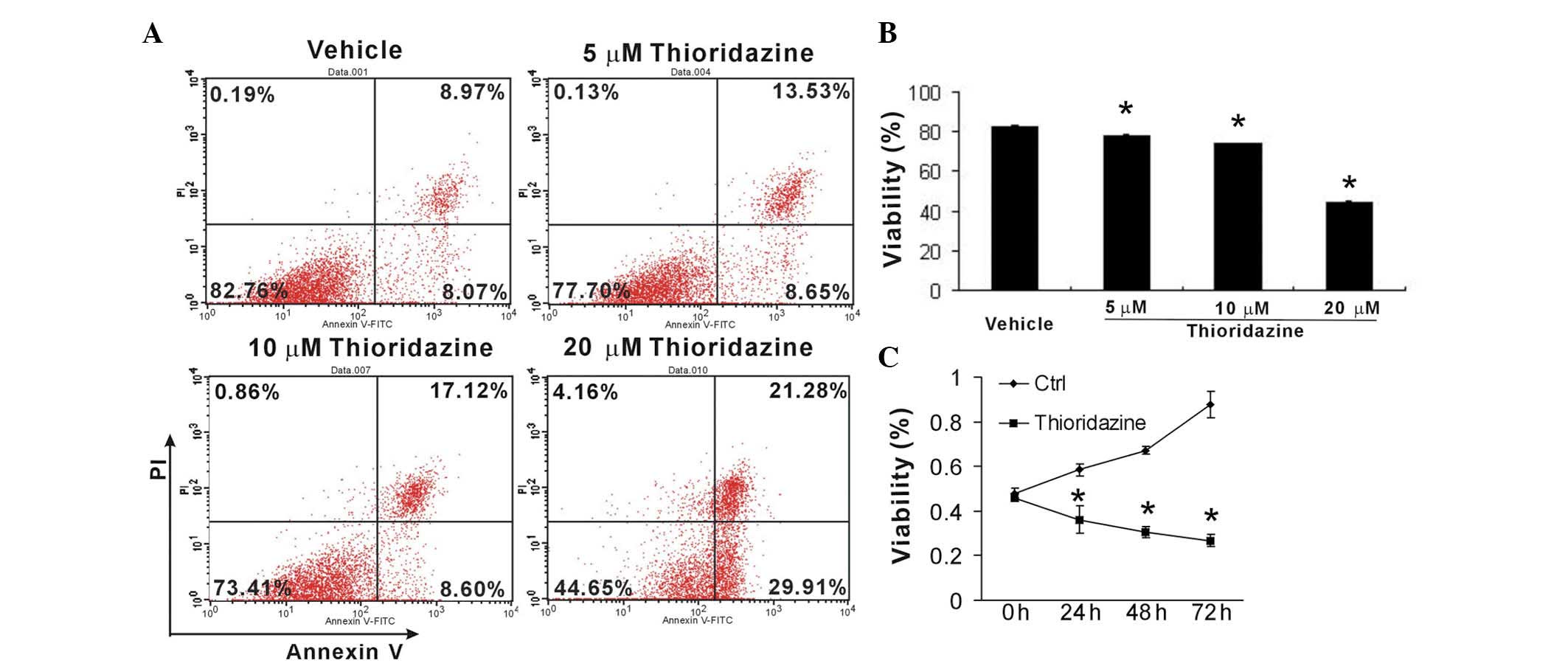
To investigate the cytotoxic and cytostatic effects
of thioridazine on breast cancer cells, murine 4T1 cells were
treated with various concentrations of thioridazine for 24 h. Cell
apoptosis was then analyzed using FCM. Thioridazine was observed to
markedly induce 4T1 cell apoptosis in a dose-dependent manner
(Fig. 2A and B). Furthermore, an
MTT assay revealed that thioridazine significantly inhibited tumor
growth (Fig. 2C). These findings
suggested that thioridazine effectively reduced tumor cell growth
and induced tumor apoptosis.
Thioridazine induces apoptosis and
inhibits tumor cell growth in vivo
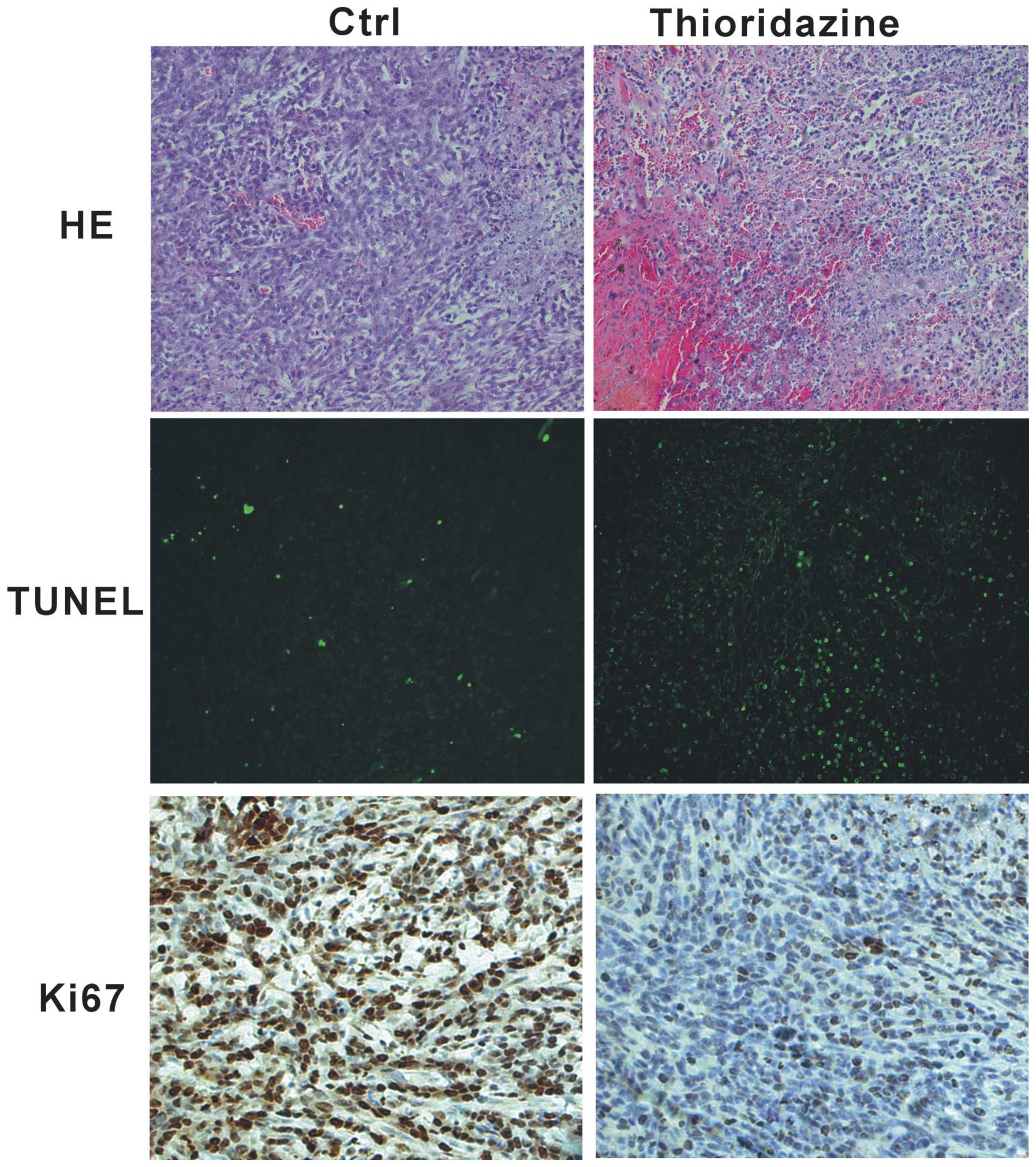
In order to investigate whether thioridazine
inhibits tumor cell proliferation in vivo, histological
assessment was performed using tumor samples from 4T1 tumors. The
4T1 tumors from the mice in the control group showed poor
differentiation and limited necrosis (Fig. 3, upper panel). By contrast,
treatment with thioridazine was found to induce marked hemorrhagic
necrosis (Fig. 3, upper panel).
The TUNEL assay revealed that apoptosis was higher in the
thioridazine treatment group compared with that in the control
group (Fig. 3, middle panel).
Furthermore, immunohistochemistry showed a decrease in cell
proliferation in the thioridazine therapy group, as indicated by a
decrease in the cell cycle marker Ki67, with the tumors from the
control mice exhibiting higher proliferation (Ki-67+)
indexes (Fig. 3, lower panel).
Thioridazine-induced suppression of the
nuclear factor κ-light-chain-enhancer of activated B cells (NFκB)
pathway in breast cancer cells
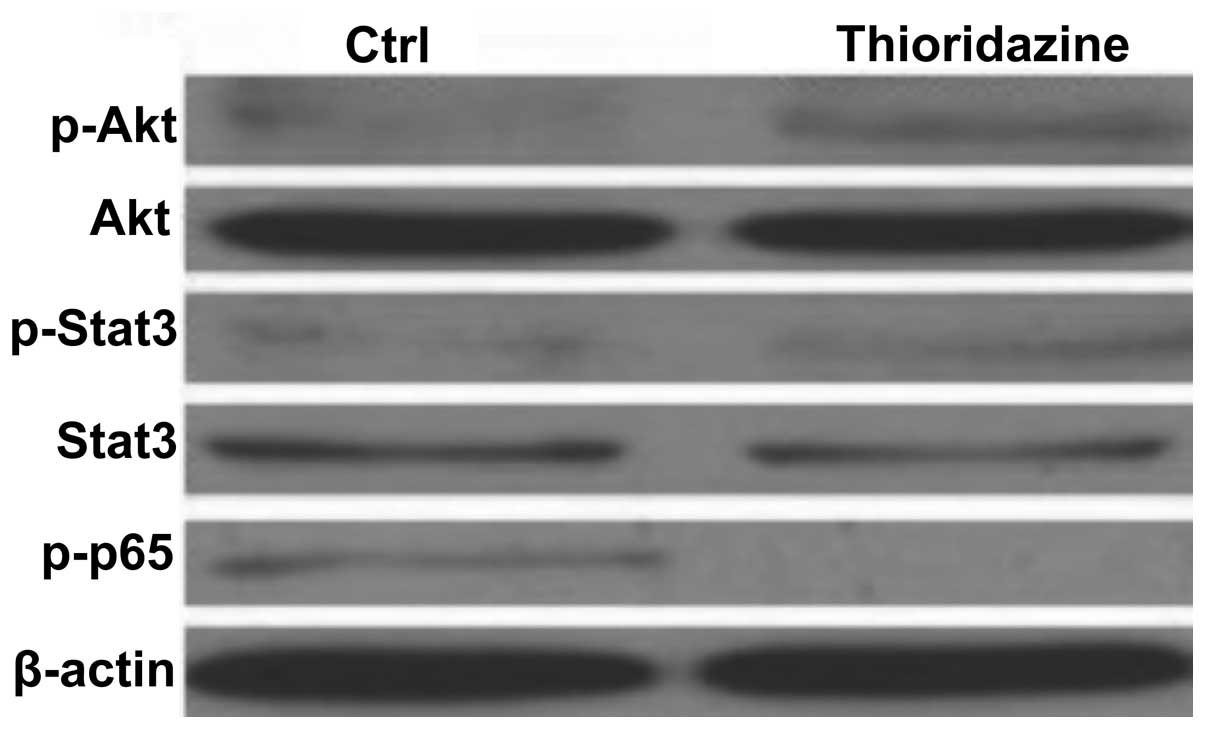
In order to determine the cell signaling pathways
mediating the thioridazine-induced tumor inhibition in the 4T1
breast cancer cells, the levels of Akt, STAT3 and NFκB p65 were
analyzed. As shown in Fig. 4,
thioridazine was not observed to reduce the activity of Akt or
STAT3 in 4T1 cells. However, thioridazine was found to
significantly inhibit the phosphorylation of NFκB p65 (Fig. 4). These findings suggested that
thioridazine inhibited cell proliferation and induced cell
apoptosis through inhibiting the NFκB pathway.
Thioridazine inhibits tumor
angiogenesis
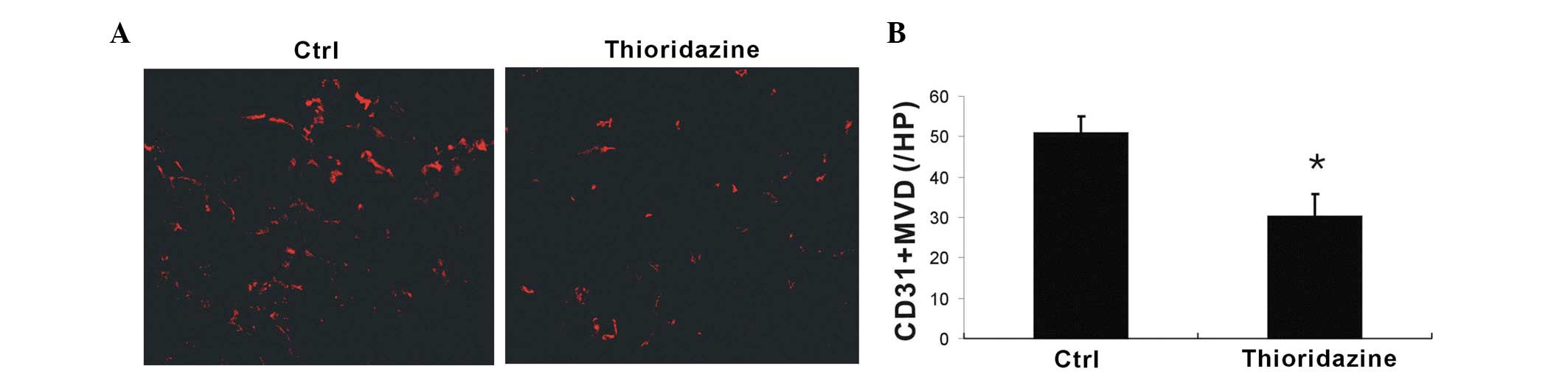
In order to assess whether tumor blood vessel
formation was inhibited by thioridazine treatment, CD31+
microvessels were analyzed using immunohistochemistry. Thioridazine
was observed to markedly reduce vascularization compared with the
control (Fig. 5A). Further more,
the vessels in the thioridazine-treated tumors were found to be
smaller in diameter than those in the control. Quantitative
analysis of microvessel density revealed that thioridazine
treatment had a significant inhibitory effect on tumor
neovascularization (Fig. 5B).
These findings suggested that thioridazine treatment inhibited
tumor angiogenesis.
Discussion
The present study investigated the anti-tumor
efficacy of the DR inhibitor thioridazine in a mouse breast cancer
model. Thioridazine was observed to significantly reduce tumor
growth, inhibit proliferation and angiogenesis, as well as induce
apoptosis. Furthermore, these thioridazine-induced effects were
associated with inhibition of the canonical NFκB pathway.
Thioridazine has been extensively used to treat
mental disorders. In the case of cancer, thioridazine has been used
to treat tumor-associated depression (16) and sweating (17). In the present study, thioridazine
was found to effectively suppress breast tumor growth in a
preclinical model. Thioridazine has previously been identified to
be an inhibitor of the phosphatidylinositol-3-kinase (PI3K)/Akt
pathway in ovarian cancer cells (18). The PI3K pathway has an important
role in tumorigenesis and therapeutic response (19). Almost every aspect of cell
regulation, including apoptosis, proliferation, differentiation,
oncogenic transformation, tumorigenesis and angiogenesis have been
associated with PI3K activity (20). Thus, the present study investigated
the effect of thioridazine on the inhibition of the PI3K/Akt
pathway in breast cancer cells; however, thioridazine was not
observed to affect Akt phosphorylation. Furthermore, thioridazine
was not found to activate STAT3, another transcription factor
associated with tumor proliferation and angiogenesis (21,22).
The NFκB pathway has been identified to have a central role in
apoptosis, proliferation and differentiation (23). The present study assessed the
phosphorylation of the NFκB p65 subunit following treatment with
thioridazine and found that thioridazine markedly reduced p65
phosphorylation. There are numerous NF-κB-dependent targets which
are involved in different aspects of tumorigenesis, including
c-Myc, cyclinD1, cyclinE and cyclin-dependent kinase 2 which are
involved in tumor cell proliferation, X-linked inhibitor of
apoptosis protein and survivin, which are involved in cell
survival, matrix metalloproteinase 2/9 and intercellular adhesion
molecule 1, which are involved in metastasis, interleukin (IL)-1,
inducible nitric oxide synthase and cyclooxygenase 2, which are
involved in inflammation, vimentin and twist, which are involved in
EMT and vascular endothelial growth factor and IL-8, which are
involved in angiogenesis (23).
Several NFκB pathway inhibitors have been shown to be effective for
cancer therapy (24).
Angiogenesis is essential for tumor growth and
metastasis and targeting angiogenesis is a promising strategy for
cancer therapy (25). The present
study showed that thioridazine is an angiostatic agent which was
found to effectively inhibit angiogenesis in a murine tumor model.
There have been several studies on the anti-angiogenic effect of
thioridazine, which have reported findings consistent with the
results of the present study. In vitro, thioridazine has
been observed to inhibit vascular endothelial growth factor
(VEGF)-induced proliferation, invasion and endothelial cell tube
formation of (26). In the present
study, thioridazine was found to reduce microvessel density in
vivo. Focal adhesion kinase (FAK) is upregulated in various
types of cancer and has been reported to regulate tumor
angiogenesis, which has been associated with endothelial cell
survival, proliferation and migration (27). Thioridazine has been found to exert
its anti-angiogenic effects through the inhibition of integrin
αV-mediated VEGF expression in tumor cells and the inhibition of
endothelial cell migration by suppressing the phosphorylation of
Src/FAK (26). Furthermore, the
angiostatic properties of thioridazine may be partially mediated
through the suppression of CSCs. DRs are expressed on the CSCs
(14), which secrete VEGF and have
high angiogenic activity (28).
In conclusion, the present study provided evidence
that the DR antagonist thioridazine is an effective drug against
breast cancer. The potential for this drug to be used in cancer
therapy requires further investigation in clinical settings.
Acknowledgments
This study was supported by a grant from the
National Natural Science Foundation of China (no. 81272523) and the
National Major Project (no. 2011ZX09302-001-01).
References
|
1
|
Schuller HM, Al-Wadei H, Ullah MF and
Plummer HK III: Regulation of pancreatic cancer by
neuropsychological stress responses: a novel target for
intervention. Carcinogenesis. 33:191–196. 2012. View Article : Google Scholar :
|
|
2
|
Thaker PH, Han LY, Kamat AA, et al:
Chronic stress promotes tumor growth and angiogenesis in a mouse
model of ovarian carcinoma. Nat Med. 12:939–944. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Melhem-Bertrandt A, Chavez-MacGregor M,
Lei X, et al: Beta-blocker use is associated with improved
relapse-free survival in patients with triple-negative breast
cancer. J Clin Oncol. 29:2645–2652. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lemeshow S, Sørensen HT, Phillips G, et
al: β-blockers and survival among Danish patients with malignant
melanoma: a population-based cohort study. Cancer Epidemiol
Biomarkers Prev. 20:2273–2279. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Grytli HH, Fagerland MW, Fosså SD, et al:
Use of β-blockers is associated with prostate cancer-specific
survival in prostate cancer patients on androgen deprivation
therapy. Prostate. 73:250–260. 2013. View Article : Google Scholar
|
|
6
|
Magnon C, Hall SJ, Lin J, et al: Autonomic
nerve development contributes to prostate cancer progression.
Science. 341:12363612013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sarkar DK, Zhang C, Murugan S, et al:
Transplantation of β-endorphin neurons into the hypothalamus
promotes immune function and restricts the growth and metastasis of
mammary carcinoma. Cancer Res. 71:6282–6291. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamazaki S, Ema H, Karlsson G, et al:
Nonmyelinating Schwann cells maintain hematopoietic stem cell
hibernation in the bone marrow niche. Cell. 147:1146–1158. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lucas D, Scheiermann C, Chow A, et al:
Chemotherapy-induced bone marrow nerve injury impairs hematopoietic
regeneration. Nat Med. 19:695–703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sibley DR and Monsma FJ Jr: Molecular
biology of dopamine receptors. Trends Pharmacol Sci. 13:61–69.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
González H, Contreras F, Prado C, et al:
Dopamine receptor D3 expressed on CD4+ T cells favors
neurodegeneration of dopaminergic neurons during Parkinson's
Disease. J Immunol. 190:5048–5056. 2013. View Article : Google Scholar
|
|
12
|
Pacheco R, Prado CE, Barrientos MJ and
Bernales S: Role of dopamine in the physiology of T-cells and
dendritic cells. J Neuroimmunol. 216:8–19. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saha B, Mondal AC, Basu S and Dasgupta PS:
Circulating dopamine level, in lung carcinoma patients, inhibits
proliferation and cytotoxicity of CD4+ and CD8+ T cells by D1
dopamine receptors: an in vitro analysis. Int Immunopharmacol.
1:1363–1374. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sachlos E, Risueño RM, Laronde S, et al:
Identification of drugs including a dopamine receptor antagonist
that selectively target cancer stem cells. Cell. 149:1284–1297.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Seeman P and Lee T: Antipsychotic drugs:
direct correlation between clinical potency and presynaptic action
on dopamine neurons. Science. 188:1217–1219. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ly KL, Chidgey J, Addington-Hall J and
Hotopf M: Depression in palliative care: a systematic review. Part
2. Treatment. Palliat Med. 16:279–284. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhukovsky DS: Fever and sweats in the
patient with advanced cancer. Hematol Oncol Clin North Am.
16:579–588. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rho SB, Kim BR and Kang S: A gene
signature-based approach identifies thioridazine as an inhibitor of
phosphatidylinositol-3′-kinase (PI3K)/AKT pathway in ovarian cancer
cells. Gynecol Oncol. 120:121–127. 2011. View Article : Google Scholar
|
|
19
|
Engelman JA: Targeting PI3K signalling in
cancer: opportunities, challenges and limitations. Nat Rev Cancer.
9:550–562. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Osaki M, Oshimura M and Ito H: PI3K-Akt
pathway: its functions and alterations in human cancer. Apoptosis.
9:667–676. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Corvinus FM, Orth C, Moriggl R, et al:
Persistent STAT3 activation in colon growth. Neoplasia. 7:545–555.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sherry MM, Reeves A, Wu JK and Cochran BH:
STAT3 is required for proliferation and maintenance of multipotency
in glioblastoma stem cells. Stem Cells. 27:2383–2392. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bassères DS and Baldwin AS: Nuclear
factor-kappaB and inhibitor of kappaB kinase pathways in oncogenic
initiation and progression. Oncogene. 25:6817–6830. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lam LT, Davis RE, Pierce J, et al: Small
molecule inhibitors of IkappaB kinase are selectively toxic for
subgroups of diffuse large B-cell lymphoma defined by gene
expression profiling. Clin Cancer Res. 11:28–40. 2005.PubMed/NCBI
|
|
25
|
Weis SM and Cheresh DA: Tumor
angiogenesis: molecular pathways and therapeutic targets. Nat Med.
17:1359–1370. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Byun HJ, Lee JH, Kim BR, et al:
Anti-angiogenic effects of thioridazine involving the FAK-mTOR
pathway. Microvasc Res. 84:227–234. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lechertier T and Hodivala-Dilke K: Focal
adhesion kinase and tumour angiogenesis. J Pathol. 226:404–412.
2012. View Article : Google Scholar
|
|
28
|
Ponti D, Costa A, Zaffaroni N, et al:
Isolation and in vitro propagation of tumorigenic breast cancer
cells with stem/progenitor cell properties. Cancer Res.
65:5506–5511. 2005. View Article : Google Scholar : PubMed/NCBI
|