Introduction
Liver fibrosis results from chronic damage to the
liver in conjunction with the accumulation of extracellular matrix
(ECM) proteins, including collagen, which also occurs in the
majority of types of chronic liver disease (1). Advanced liver fibrosis results in
cirrhosis, liver failure and portal hypertension, and often
requires liver replacement. The build upof ECM proteins distorts
hepatic morphology by developing a fibrous scar, and the consequent
development of nodules of regenerating hepatocytes describes
cirrhosis (2). Cirrhosis produces
hepatocellular dysfunction and amplified intrahepatic opposition to
blood flow, which results in hepatic inadequacy and portal
hypertension, respectively (3).
Hepatic fibrosis occurs as the result of the continued wound
healing response of the liver to toxic, infectious or metabolic
agents. Chronic hepatitis C infection, alcohol abuse and
non-alcoholic steatohepatitis are also major causes of hepatic
fibrosis (4–5). Early clinical reports in the 1970s
suggested that advanced liver fibrosis is reversible (6), however, in the 1980s, hepatic
stellate cells (HSCs), formerly known as lipocytes or peri
sinusoidal cells, were recognized as the predominant
collagen-producing cells in the liver (7). This cell type undergoes marked
phenotypic activation in chronic liver disease, with the
acquisition of fibrogenic properties (8). Activation of HSCs leads to retinoid
storage, remodeling of the ECM and the production of growth factors
and cytokines (9,10). Suppression of HSC activation has
been suggested as a therapeutic target against hepatic fibrosis
(11–13).
Oxidative stress is a key pathogenic factor in
several types of liver disease, which can cause hepatocyte
destruction through lipid peroxidation and protein alkylation.
Superoxide dismutase (SOD) and catalase are central antioxidant
enzymes, which function as endogenous free radical scavengers
(14). However, previous studies
have identified metallothionein(MT) as a more competent scavenger
for reactive oxygen species (ROS) (15,16).
Quinones in the plant kingdom represent a broad
category of widely distributed quinoid compounds in nature. Several
quinones have been associated with a wide range of bioactivities.
At present, a number of clinically important pharmaceutical agents
containing a quinone nucleus with significant anticancer activity
have been confirmed, including anthracycline, mitoxantrones and
saintopin. These quinoid compounds predominantly target DNA,
however, the exact contribution of quinone moiety to the anticancer
effect remains to be fully elucidated. In general, quinone toxicity
is attributed to the ability to undergo reversible
oxidation-reduction reactions and to its electrophilic nature,
leading to the formation of free radicals (17). Juglone, also termed
5-hydroxy-l,4-naphthalenedione, belongs to the quinoid family of
compounds and occurs naturally in the leaves, roots, husks, fruit
and bark of plants in the Juglandaceae family, in particular, the
black walnut (Juglansnigra) (18). Juglone is an example of an
allelopathic compound, which is a substance that is synthesized by
one type of plant and affects the growth of another (19–22).
At present, there are no effective drugs to avert or
treat liver fibrosis, and certain natural products with antioxidant
potential are being investigated for developing novel therapeutic
reagents. In view of the lack of effective remedial measures for
hepatic fibrosis, the present study aimed to investigate the
antifibrotic effects of jugalone, and established that treatment of
rats with juglone reduced DMN-induced hepatic fibrosis. The present
study also demonstrated that jugalone increased the activity of SOD
and decreased oxidative stress in the liver, suggesting that liver
fibrosis protection by juglone may occur by increasing the
antioxidative capability of the liver. It was also observed that
serum levels of alanine aminotransferase (ALT), aspartate
amino-transferase (AST), hyaluronic acid (HA), laminin (LN), type
III procollagen (PCIII) and type IV collagen (CIV) were
significantly reduced by treatment with juglone. The effect of
juglone on the expression levels of α-smooth muscle actin (α-SMA)
and collagen (Col) III in the liver were also examined, which
revealed that juglone significantly reduced the expression levels
of α-SMA and Col III in the liver.
Materials and methods
Chemicals and animal treatments
Juglone was purchased from the Shandong Engineering
Research Center for Natural Drugs (Yantai, China), the purity of
which was 99.5%, according to high-performance liquid
chromatography (acetonitrile: water, 90:10; C18 column).
Analytical grade dimethylnitrosamine (DMN) was purchased from
Chengdu Kelong Chemical Reagent Factory (Chengdu, China) and
silymarin was purchased from Tianshili Pharmaceutical Company
(Tianjing, China). A total of 30 male Sprague-Dawley rats, weighing
150±25 g, were obtained from the Experimental Animal Center of
Guiyang Medical College (Guiyang, China; Approval no. SCXK
2001–0022). The present study was performed according to
instructions approved by the Institutional Ethical Committee of the
General Hospital of Chengdu Military Region (Chengdu, China). The
rats were randomly divided into the following five groups, each
containing six rats: Controlgroup, DMN-induced hepatic fibrosis
(model) group, juglone prevention (JP) group, silymarin prevention
(SP) group and juglone + silymarin prevention (JP+SP) group. The
rats in the model, JP, SP and JP+SP groups received subcutaneous
injections of DMN solution at a dose of 0.5 ml/kg twice weekly for
4 weeks, with an initial dose of 0.2 ml/kg. These rats were fed a
high-lipid/low-protein diet. The rats in the control group were fed
a normal diet. Juglone (200 mg/kg), silymarin (1.0 g/kg) and
juglone (200 mg/kg) + silymarin (1.0 g/kg), were administered once
a day by gastric gavage to the rats in the In the JP, SP and JP+SP
groups, respectively. After 4 weeks, the rats were sacrificed using
anesthesia (anaestheticpropofol, 0.1 ml/100 mg) and a midline
incision was made to remove the liver; ~2 ml blood was collected
from each rat, as well as 0.5–1 mm liver lobe sections. The same
section of each liver was removed and fixed in 10% neutral formalin
(Sigma-Aldrich, St. Louis, MA, USA). The residual portion of liver
was stored at −50°C. Rat serum was prepared by centrifugation at
224 x g for 15 min at room temperature and stored at −50°C.
Biochemical parameters for the assessment
of liver function
The serum levels of ALT, AST, HA, LN, PCIII and CIV
were measured using commercially available kits as follows: Alanine
Aminotransferase BioAssay™ ELISA kit, Aspartate Aminotransferase
BioAssay™ ELISA kit, Hyaluronic Acid BioAssay™ ELISA kit,
LamininBioAssay™ ELISA kit, Procollagen III BioAssay™ ELISA kit and
Collagen Type IV (CIV) BioAssay™ ELISA Kit (Wuhan Boster Biological
Engineering Co., Ltd., Wuhan, China) and were performed, according
to the manufacturer's instructions.
SOD and malondialdehyde (MDA) in liver
tissue
The tissue samples were homogenized in cold 20 mM
HEPES buffer, containing 1 mM EGTA, 150 mMmannitol and 30 mM
sucrose (pH 7.1; all Sigma-Aldrich), using a tephlon homogenizer
(Polytron RZR 1; Heidolph, Schwabach, Germany). The cell debris was
detached by centrifugation at 2,000 × g for 5 min at 4°C. The
supernatants were used for the assessment of SOD activity, with
assays conducted according to the manufacturer's instructions
(Cayman Chemical Company, Ann Arbor, MI, USA). The levels of MDA
were also estimated using a thiobarbituric acid method, according
to the manufacturer's instructions (Cayman Chemical Company).
Histopathology of liver tissues
Following fixation in 10% formalin for 24 h, the
liver samples were embedded in paraffin (Sigma-Aldrich). The
samples were then cut into 5-mm tissue sections and mounted onto
slides (Sigma-Aldrich). The sections were processed for hematoxylin
and eosin (H&E) and Masson's trichrome (both Sigma-Aldrich)
staining for assessment of the degree of liver fibrosis. A second
set of tissue sections (size, 0.5×0.5 cm) were prepared for
immunohistochemical assessment of the tissues and mitotic indexing
(MI). The liver tissues were further assessed for histopathological
examination by a single observer in a blinded-manner.
Immunohistochemical analysis and MI
The liver sections were incubated in 2%
H2O2 (Qingdao HiseaChem Co., Ltd., Qingdao,
China) for 15 min following deparaffinization in xylene,
rehydration in graded ethanol and antigenunmasking by heat
treatment using citrate buffer. The sections were subsequently
incubated with mouse anti-α-SMA (cat. no. SC-32251; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), mouse anti-Col III (cat.
no.SC-80564; Santa Cruz Biotechnology) and rabbit anti-MT (cat.
no.SC-11377; Santa Cruz Biotechnology, Inc.)antibodies (all at
1:500 dilution) overnight at 4°C Anti-mouse IgG in rabbit (cat no.
M7023; 1:500; Sigma-Aldrich) was used as the secondary antibody.
The samples were subsequently processed using an EnVision kit (Dako
Denmark A/S, Glostrup, Denmark), according to the manufacturer's
instructions. Phosphate-buffered saline (Sigma-Aldrich) served as a
negative control. The cells with brown staining in the
cytoplasm/nucleus were considered to be positive. A total of five
high power microscopic fields (magnification, x500) were randomly
selected in each slide and the numbers of positive cells in each
field were counted under a microscope (Eclipse E600; Nikon
Corporation, Tokyo, Japan (α-SMA and Masson's
trichromeimmunopositivity). For the Col III staining, five high
power fields were randomly selected in each tissue section and
images were captured using a BioMias 2000 image analysis instrument
(Image and Figure Research Institute, Sichuan University, China),
to quantify the transparency of the immune-positive areas in the
tissue section.
Cell proliferation was assessed by counting the
number of mitotic cells per high-power field at a magnification of
x200. For each slide, 10 randomly selected fields were counted. The
MI was defined as the number of mitotic cells per 1,000 hepatocytes
in paraffin-embedded liver samples stained with H&E.
Western blot analysis
The liver tissues were treated with TRIzol protein
extraction reagent (Invitrogen Life Technologies, Carlsbad, CA,
USA), according to the manufacturer's instructions, and the protein
concentrations were determined using Lowry's method (23). The protein samples were separated
using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(Sigma-Aldrich) and electroblotted onto polyvinylidenedifluoride
membranes (Millipore Corporation, Billerica, MA, USA). Following
blocking with 1.5% bovine serum albumin (Sigma-Aldrich), the
membranes were incubated overnight at 4°C with primary antibodies
against peroxisome proliferator-activated receptor (PPAR)-γ (mouse
anti-PPAR-γ; cat. no. SC-7273), α-SMA (mouse anti-α-SMA; cat.
no.SC-32251), TGF-β1 (rabbit anti-TGF-β1; cat. no.SC-146), TIMP-1
(rabbit anti-TIMP-1; cat. no.SC-5538) and MMP-2 (rabbit anti-MMP-2;
cat. no. SC-10736) obtained from Santa Cruz Biotechnology, Inc. The
membranes were further incubated with secondary antibody for 1 h at
room temperature. The protein bands were detected using an enhanced
chemiluminescence detection system (Pierce Biotechnology, Inc.,
Rockford, IL, USA) and the protein expression levels were
determined using Quantity One software version 4.5 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Statistical analyses were performed using one-way
analysis of variance followed by Bonferroni's post-hoc test, using
SPSS 20.0 software (SPSS, Inc., Chicago, IL USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Juglone treatment decreases the activity
of functional liver enzymes
The effect of juglone treatment on functional liver
enzymes is shown in Fig. 1.
Biochemical analysis revealed that the DMN-treated rats exhibited a
marked increase in the serum levels of ALT and AST, which were
significantly higher, compared with those in the normal rats
(P<0.01; n=10). However, the increased levels of these liver
enzymes were efficiently reduced by treatments with silymarin and
juglone (P<0.01; n=10). Juglone significantly restored the
biochemical parameters to levels comparable with those following
treatment with the silymarin, used as a standard drug in the
present study.
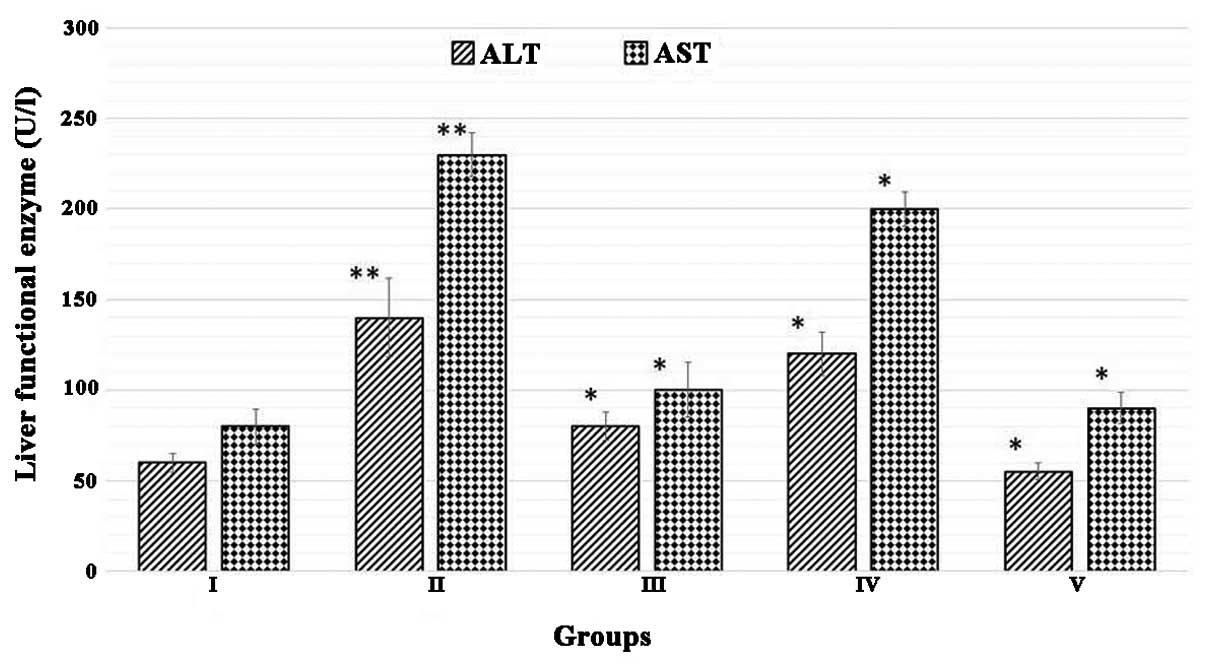 | Figure 1Juglone treatment decreases the
activity of functional liver enzymes. Group I, normal control;
Group II, model control + dimethylnitrosamine; Group III, 200 mg/kg
silymarin treatment; Group IV, 200 mg/kg juglone treatment; Group
V, 400 mg/kg juglone treatment. The results were analyzed using
one-way analysis of variance, followed by Tukey's post-hoc test.
The data are expressed as the mean ± standard deviation (n=10;
**P<0.01, vs. normal control group; *P<0.01, vs. model
control). ALT, alanine amino-transferase; AST, aspartate
amino-transferase. |
Juglone treatment inhibits DMN-induced
collagen accumulation
The results of the effect of juglone on collagenic
accumulation in the livers of rats are shown in Fig. 2. The results demonstrated that, in
rats treated with DMN (the liver fibrosis model) significantly
increased serum levels of HA, LN, PCIII and CIV were observed,
compared with the control (P<0.01; n=10). However, following
treatment with silymarin and juglone, a gradual decrease in the
activities of HA, LN, PCIII and CIV were observed in the fibrotic
tissues (P<0.01, vs. model group; n=10). These results suggested
that treatment with juglone inhibited DMN-induced collagenic
changes in these animals. The effect of juglone effect was more
marked, compared with that of silymarin, which was used as the
standard.
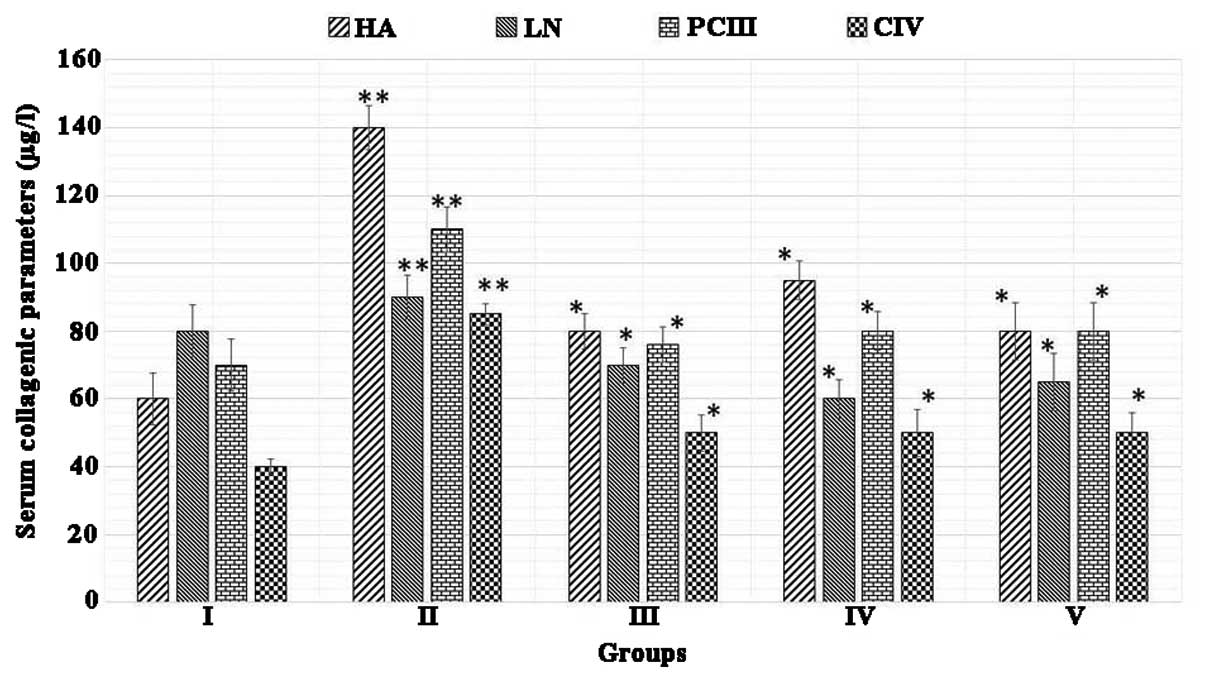 | Figure 2Juglone treatment reduces the
accumulation of collagen. Group I, normal control; Group II, model
control + dimethylnitrosamine; Group III. 200 mg/kg silymarin
treatment; Group IV, 200 mg/kg juglone treatment; Group V, 400
mg/kg juglone treatment. The results were analyzed using one-way
analysis of variance, followed by Tukey's post-hoc test. The data
are expressed as the mean ± standard deviation (n=10; **P<0.01,
vs. normal control, *P<0.01, vs. model control). HA, hyaluronic
acid; LN, laminin; PCIII, type III procollagen; CIV, type IV
collagen. |
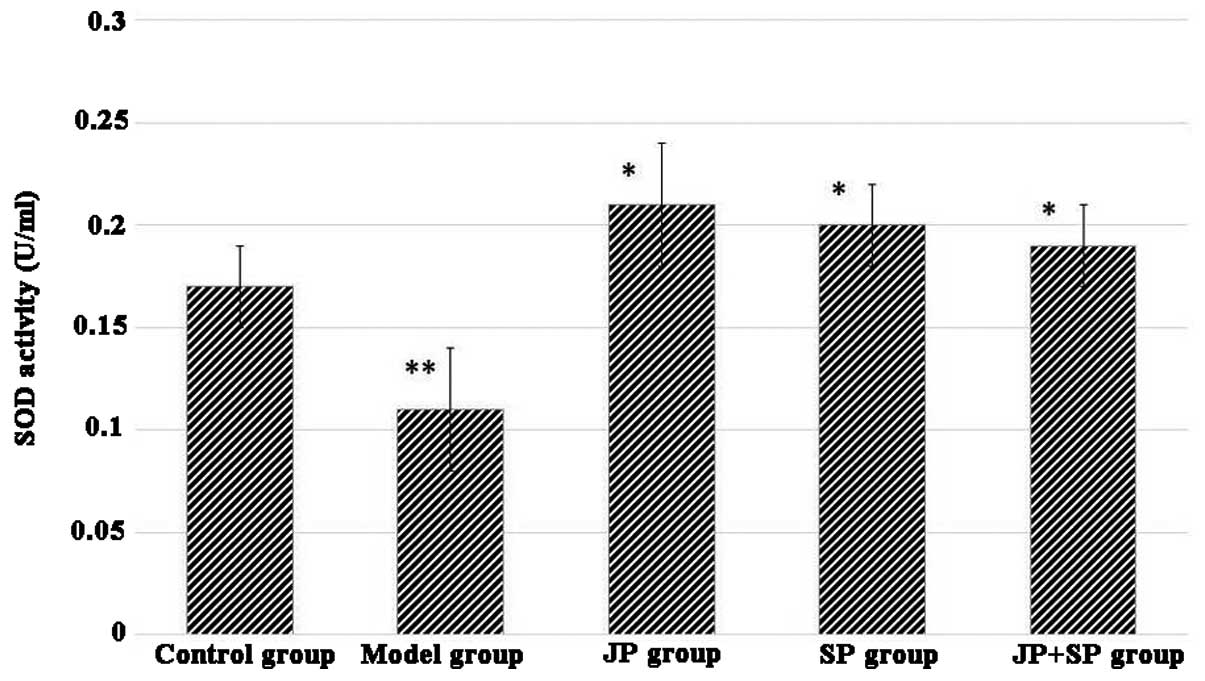
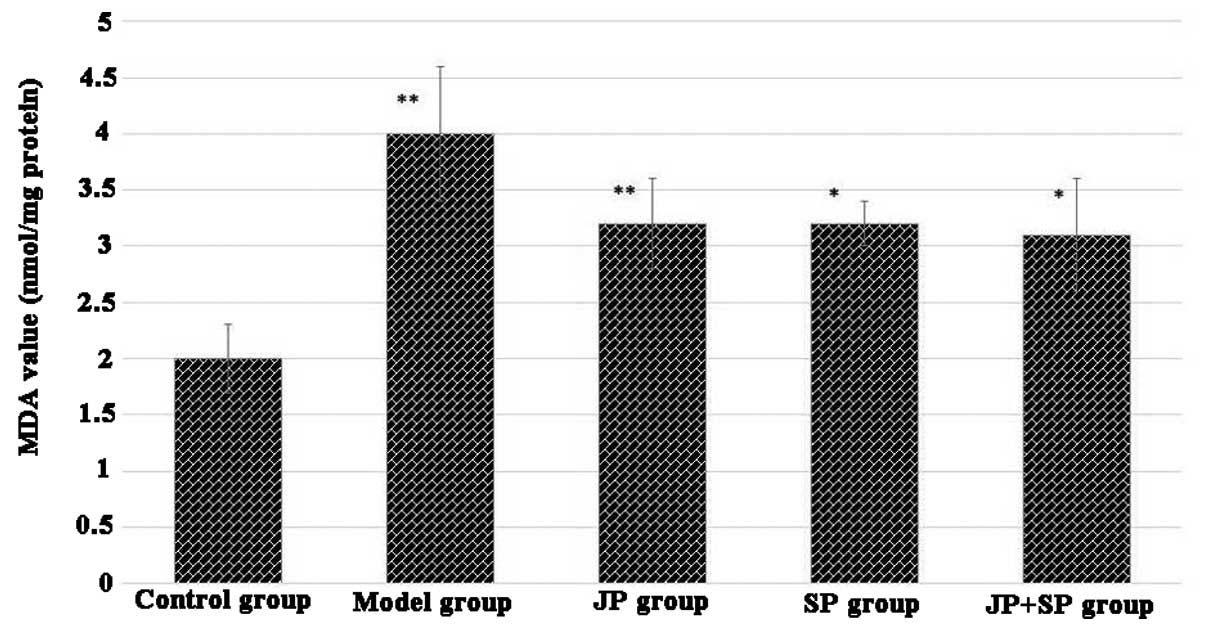
Effect of juglone on SOD and MDA contents
of liver
The antioxidant activity of the DMN group indicated
that SOD was significantly reduced compared with the normal group.
The JP group or SP group significantly restored the SOD level
(Fig. 3). MDA level of liver
homogenate was significantly high in the model group (DMN-treated
group), however, administration of juglone or silymarin
significantly reduced the level of MDA. The results of JP group
were comparable with the SP group (Fig. 4). The combination of juglone and
silymarin demonstrated a significant effect on the decrease of MDA
in rat liver homogenate.
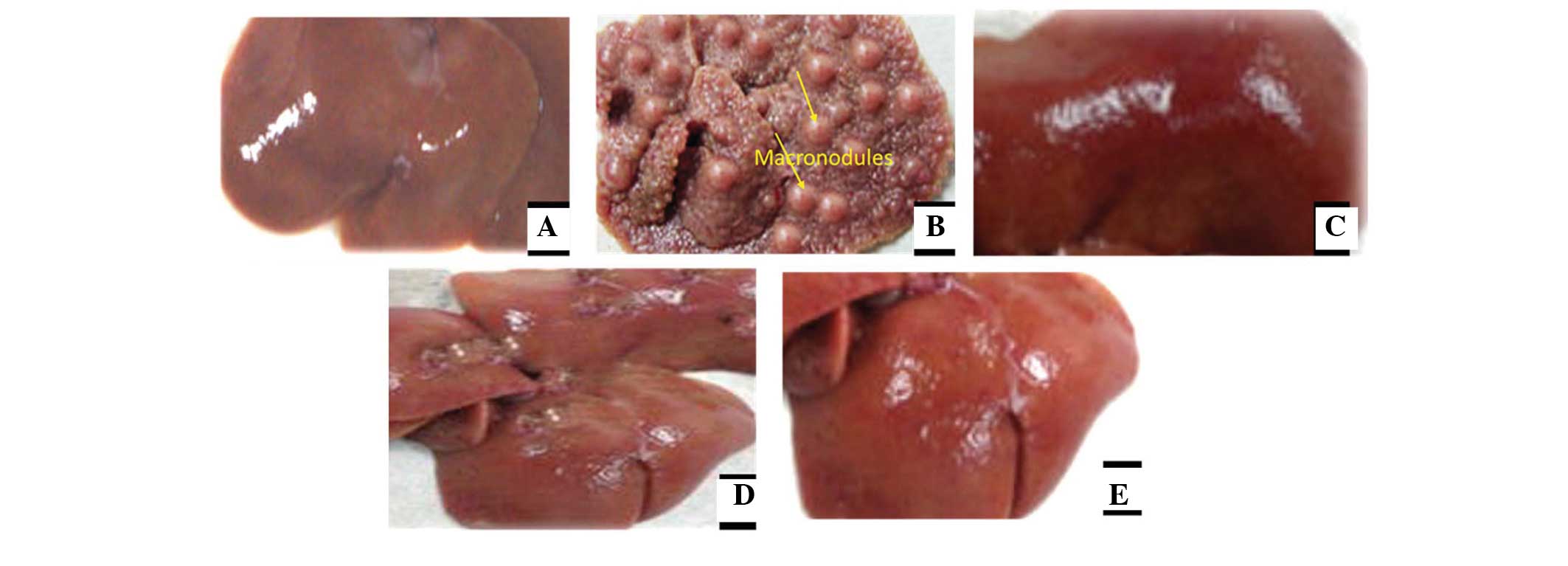
Effects of juglone treatment on liver
injury and morphology
The appearance of the liver tissues in each group
are shown in Fig. 5. It was
evident that, with the exception of the DMN-treated group, which
exhibited considerable nodules and irregularities, the liver
tissues in all the groups generally exhibited smooth and even
surfaces with no signs of nodules. Furthermore, the normal group
exhibited normal growth, whereas the rats in the DMN-treated model
group exhibited lower body weights. The protective effects of
juglone on liver injury were comparable to those of silymarin.
These two groups exhibited similar smoothness of the surface of the
liver.
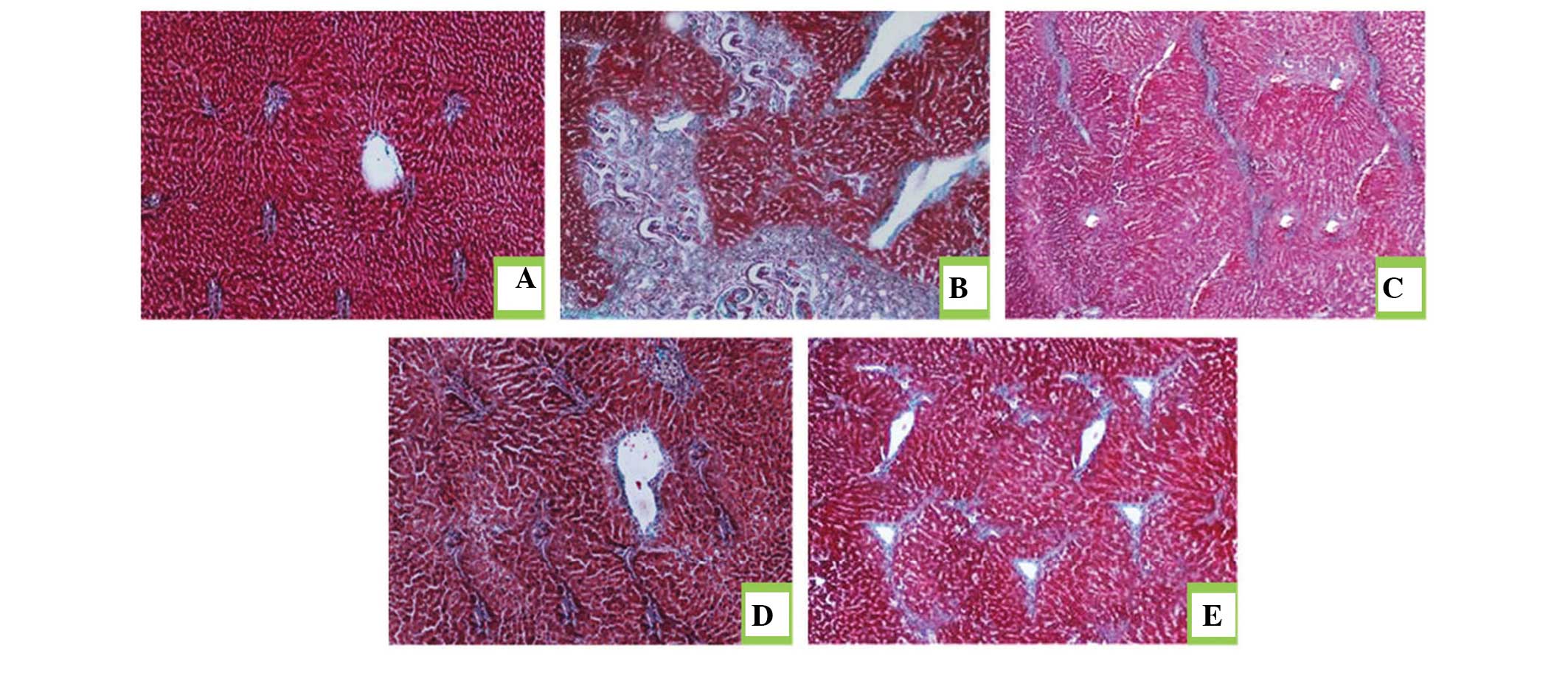
Histopathological evaluation of the rat
livers following drug treatment
Masson's trichrome staining was performed to
estimate the extent of fibrosis following treatment with DMN. As
shown in Fig. 6A, histological
analysis of the rat liver sections revealed that those from the
normal group exhibited no collagen deposition. The DMN-treated
model groupexhibited proliferation of the bile duct with thick
fibrous septa and increased deposition of collagen fibers around
the clogged central vein, indicative of significant fibrosis
(Fig. 6B). Theliver sections from
the rats in the JP group exhibited fewer fibrous septa and uneven
regenerating nodules (Fig. 6C).
The protective effect of silymarin on liver fibrosis was observedin
the SP group (Fig. 6D), treated
with silymarin as a standard drug for liver fibrosis. The collagen
deposition patterns appeared comparable between the JP group, SP
group and JP+SP (Fig. 6E) group.
These observations demonstrated the hepatoprotective effect of
juglone on hepatic fibrosis in rats.
Immunohistochemical analysis of juglone
treatment revealing decreased expression levels of α-SMA and Col
III in the liver
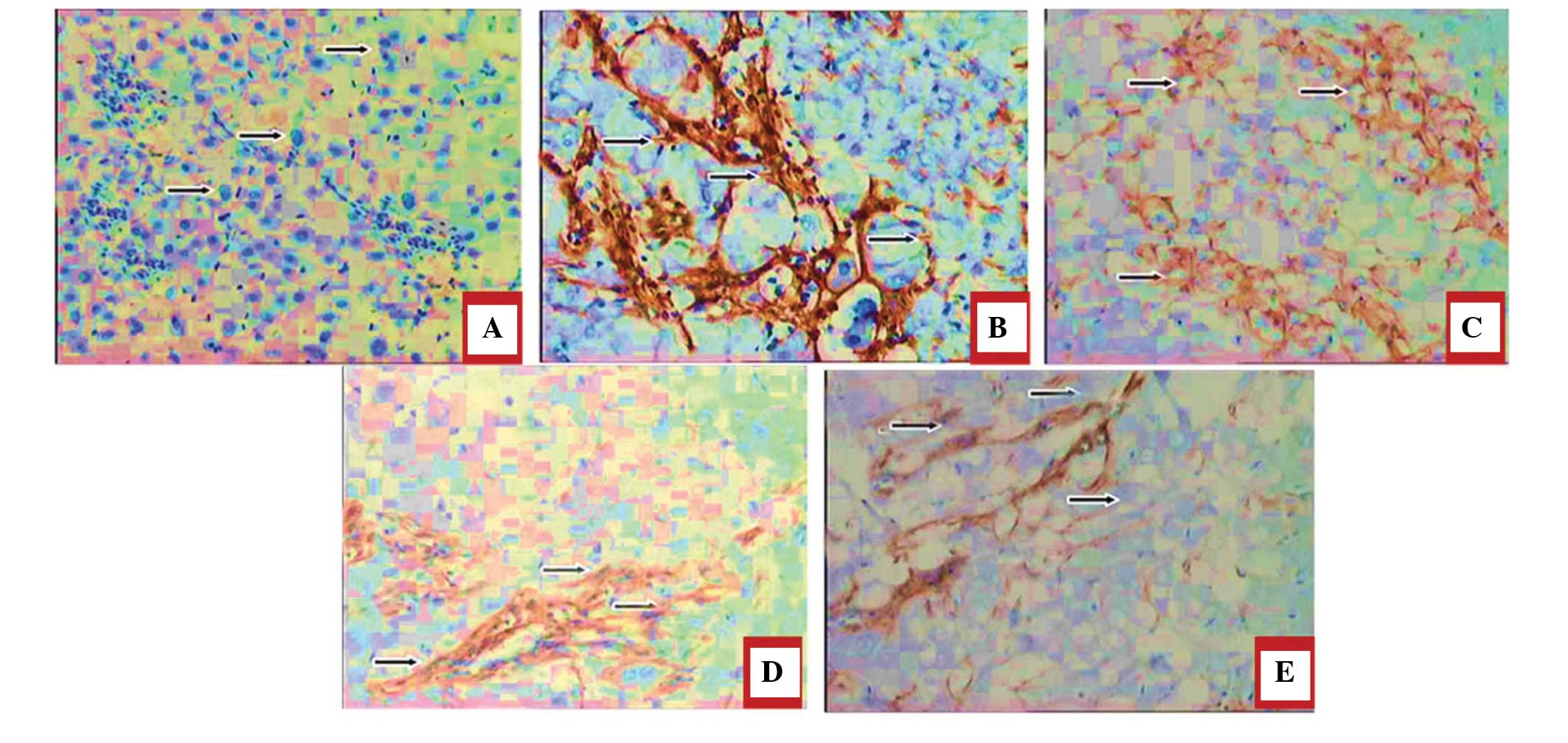
The effect of treatment with juglone on the
expression ofα-SMA is shown in Fig.
7A–E. Since the expression levels of α-SMA and CoI III are
markers for liver fibrosis, the present study investigated the
effect of juglone on the expression levels of α-SMA and CoI III.
The results of the immunohistochemical staining demonstrated that,
in the normal group, α-SMA-positive staining was limited to the
vascular walls in the central veins and portal areas, whereas
significantly marked immune staining was observed in the central
veins, portal areas and surrounding the bile ductules in the
DMN-treated model group. In the JP, SP and JP+SP groups, the
expression levels of α-SMA were considerably decreased, compared
with those in the model group (P<0.05; Fig. 7).
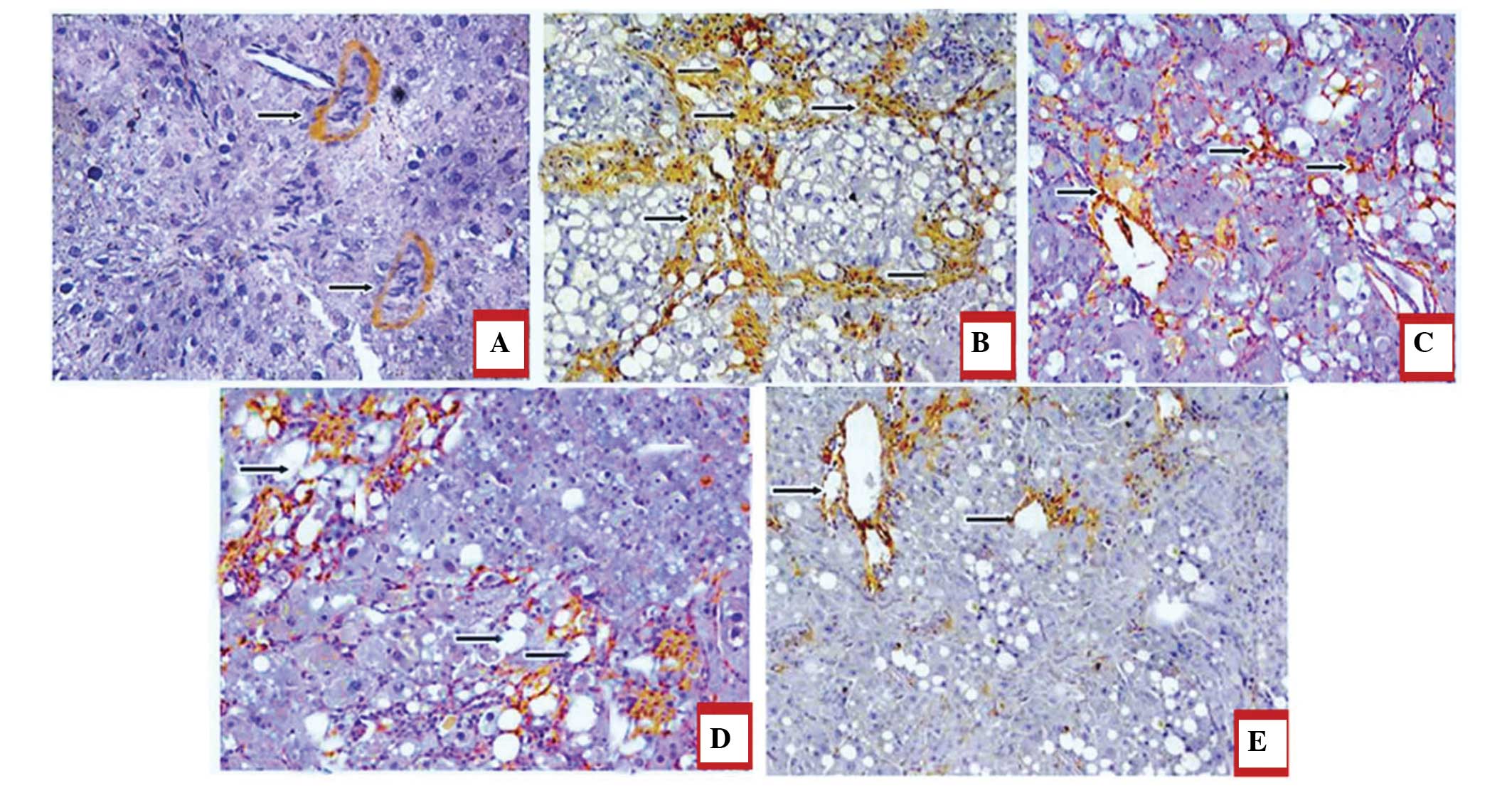
In terms of the expression levels of Col III, an
increase in the numbers of Col III positive fibers were observed in
the hepatic sinusoids and periportal areas in the model group,
whereas a lower expression level of Col III was identified in the
normal control group. In the JP, SP and JP+SP treatment groups, the
expression levels of Col III were also significantly decreased,
compared with the levels in the model group (P<0.05; Fig. 8A–E).
Effect of juglone on the mRNA expression
levels of PPAR-γ and α-SMA
As shown in Fig. 9,
treatment with DMNsignificantly suppressed the mRNA expression of
PPAR-γ and increased the mRNA expression of α-SMA in the liver
tissues (P<0.01). Compared with the model group, silymarin
promoted the effect of DMN on the mRNA expression levels of PPAR-γ
and α-SMA. By contrast, juglone increased the mRNA expression of
PPAR-γ and reduced the mRNA expression of α-SMA, compared with the
model and silymarin groups (P<0.01; Fig. 9).
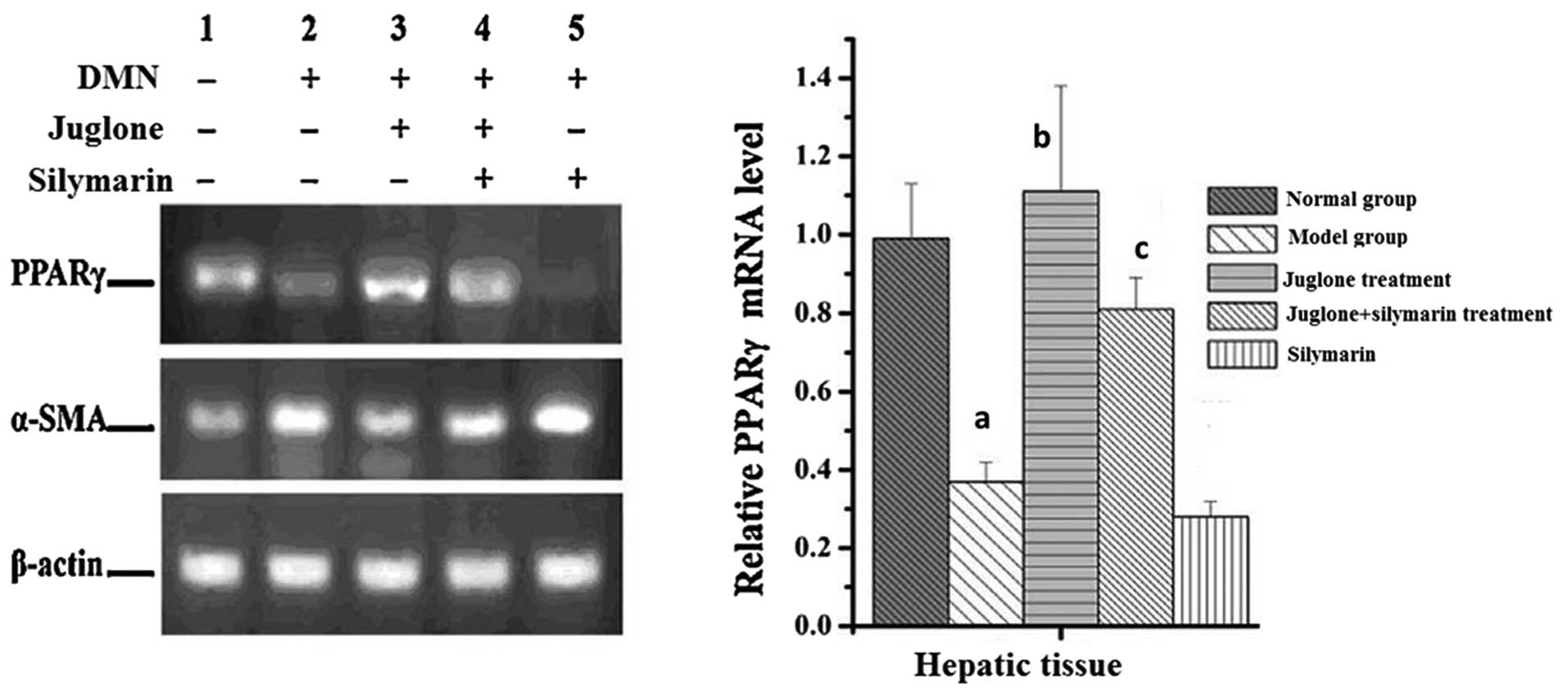 | Figure 9Effect of juglone on the mRNA
expression levels of PPAR-γ and α-SMA. The rats were treated with
DMN, juglone, silymarin or juglone+silymarin and, after 8 weeks,
liver samples were collected. The mRNA expression levels of PPAR-γ
and α-SMA in the livers were analyzed and assessed using reverse
transcription-quantitative polymerase chain reaction and agarose
gel electrophoresis with ethidium bromide. The data are presented
as the mean ± standard deviation (n=6). aP<0.01, vs.
normal group; bP<0.01, vs. model group;
cP<0.05. SMA, smooth muscle actin; PPAR, peroxisome
proliferator-activated receptor, DMN, dimethylnitrosamine. |
Discussion
Hepatic fibrosis is a common pathological finding in
liver cirrhosis and liver cancer, and is characterized by liver
function failure (24). The ECM is
largely comprised of collagen proteins, and ECM components,
including HA, LN, PCIII and CIV, may be altered by metabolic
collagen, which parallels with the degree of hepatic fibrosis
(25). Raised serum levels of ALT
and AST, triggered by chemical hepatotoxicity, are accompanied by
hepatic structural lesions, resulting in functional liver enzymes,
which are abnormally present in the cytoplasm and are released into
the circulation (26,27). Therefore, elevated levels of liver
enzymes are a mark of liver damage, are important for diagnosis by
clinicians and are a reflection of liver function (28).
Oxidative stress, as an important primary factor,
has been extensively investigated in an increasing number of liver
diseases (29). Previous studies
have demonstrated that hepatic failure is associated with the
production of ROS, collagen synthesis and cellular proliferation,
due to oxidative stress, which aggravates inflammation and induces
the pathogenesis of hepatic fibrosis (30–32).
Oxidative stress augments liver fibrosis through the activation of
HSCs, and lipid peroxidationtriggers transcription of the collagen
gene. The expression of α-SMA is a representative feature of
activated HSCs and this is considered a hallmark for liver fibrosis
(33). HSCs are activated
following liver damage and segregate into myofibroblast-like cells,
which proliferate and contribute to collagen accumulation in the
ECM (34). Collagen is responsible
for ~55% of the total protein in fibrous liver tissues (35).
The present study demonstrated that juglone markedly
decreased the Col III content and decreased the expression levels
of α-SMA and CoI III in the liver, indicating an inhibitory effect
on the activation of HSCs. It was also demonstrated that juglone
increased the activity of SOD and decreased oxidative stress in the
liver. These data suggested that the protective effect of juglone
in fibrosis may be due to its antioxidative effect in theliver. The
results also indicated that juglone markedly increased the speed of
recovery of the liver damage. Liver fibrosis caused hepatomegaly
and treatment with juglone significantly prevented the effect of
DMN on the liver.
DMN increases the levels of serum biochemical
parameters, including ALT and AST, which is in accordance with the
extent of liver damage (36), and
the increase in AST and ALT reflects hepatocellular injury.
Treatment with juglone markedly reduced the increased levels of AST
and ALT. Following treatment with juglone, the serum enzymatic
levels and visceral indices wereconsiderably decreased, signifying
that juglone improved liver function and immunocompetence in the
DMN-injured rats. The use of Masson's trichrome staining enabled
observation and analysis of the developmentof liver fibrosis. The
results revealed that treatment with juglone efficiently reversed
the liver fibrosis processes in the presence of DMN.
Histological assessment indicated a protective
effect of juglone against DMN-induced liver fibrosis. A normal
liver has a regular and even surface, but in liver fibrosis it
appeared rough and nodular with micronodule and macronodule
formation. In the histopathological evaluation, severe structural
damage, formation of dense fibrotic septa and proliferation of bile
ducts in the presence of inflammatory cells were observed.
Treatment with silymarin and juglone recovered the liver structure
from fibrosis.
In conclusion, the present study established that
the protective effect of juglone in liver fibrosis was associated
with increased activity of SOD, reduced oxidative stress and
decreased levels of α-SMA and Col III in the liver. Histological
data demonstrated that juglone slowed the progression liver
fibrosis. The results also demonstrated that the serum levels of
ALT, AST, HA, LN, PCIII and CIV were significantly reduced
following treatment with juglone. These findings suggested that
juglone increased the antioxidative ability of the liver by
increasing the activity of SOD, which decreased the ECM collagen
accumulation in the liver.
References
|
1
|
Friedman SL: Liver fibrosis-from bench to
bedside. J Hepatol. 38(Suppl 1): 38–53. 2003. View Article : Google Scholar
|
|
2
|
Bataller R and Brenner DA: Liver fibrosis.
J Clin Invest. 115:209–218. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gines P, Cardenas A, Arroyo V and Rodes J:
Management of cirrhosis and ascites. N Engl J Med. 350:1646–1654.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Friedman SL: Liver firosis - from bench to
bedside. J Hepatol. 38(Suppl 1): S38–S53. 2003. View Article : Google Scholar
|
|
5
|
Chen SL and Morgan TR: The natural history
of hepatitis C virus (HCV) infection. Int J Med Sci. 3:47–52. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Soyer MT, Ceballos R and Aldrete JS:
Reversibility of severe hepatic damage caused by jejunoileal bypass
after re-establishment of normal intestinal continuity. Surgery.
79:601–604. 1976.PubMed/NCBI
|
|
7
|
Friedman SL, Roll FJ, Boyles J and Bissell
DM: Hepatic lipocytes: the principal collagen-producing cells of
normal rat liver. ProcNatlAcadSci USA. 82:8681–8685. 1985.
View Article : Google Scholar
|
|
8
|
Geerts A: History, heterogeneity,
developmental biology and functions of quiescent hepatic stellate
cells. Semin Liver Dis. 21:311–335. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li D and Friedman SL: Liver fibrogenesis
and the role of hepatic stellate cells: new insights and prospects
for therapy. J GastroenterolHepatol. 14:618–633. 1999.
|
|
10
|
Tsukada S, Parsons CJ and Rippe RA:
Mechanisms of liver fibrosis. ClinChimActa. 364:33–60. 2006.
|
|
11
|
Wu J and Zern MA: Hepatic stellate cells:
a target for the treatment of liver fibrosis. J Gastroenterol.
35:665–672. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bataller R and Brenner DA: Liver fibrosis.
J Clin Invest. 115:209–218. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee MK, Ha NR, Yang H, Sung SH, Kim GH and
Kim YC: Antiproliferative activity of triterpenoids from
Ecliptaprostrata on hepatic stellate cells. Phytomedicine.
15:775–780. 2008. View Article : Google Scholar
|
|
14
|
Cichoz-Lach H and Michalak A: Oxidative
stress as a crucial factor in liver diseases. World J
Gastroenterol. 20:8082–8091. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tahan V, Ozaras R, Canbakan B, et al:
Melatonin reduces dimethylnitrosamine-induced liver fibrosis in
rats. J Pineal Res. 37:78–84. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Poli G: Pathogenesis of liver fibrosis:
role of oxidative stress. Mol Aspects Med. 21:49–98. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li Q, Zhao XL, Sun J, Jiang SG and Gong
XF: Anti-proliferative and apoptosis-inducing activities of juglone
in LS-174T cells. Bangladesh J Pharmacol. 8:65–72. 2013. View Article : Google Scholar
|
|
18
|
Aithal BK, Kumar MR, Rao BN, Udupa N and
Rao BS: Juglone, a naphthoquinone from walnut, exerts cytotoxic and
genotoxic effects against cultured melanoma tumor cells. Cell
BiolInt. 33:1039–1049. 2009.
|
|
19
|
Thomson RH: Naturally Occurring Quinones.
Academic Press; New York, NY: pp. 257–276. 1971
|
|
20
|
Seshadri P, Rajaram A and Rajaram R:
Plumbagin and juglone induce caspase-3-dependent apoptosis
involving the mitochondria through ROS generation in human
peripheral blood lymphocytes. Free RadicBiol Med. 51:2090–2107.
2011. View Article : Google Scholar
|
|
21
|
Funt RC and Martin J: Black walnut
toxicity to plants, humans and horses. Ohio State University
Extension Fact sheet HYG-1148-93; 1993
|
|
22
|
Ji YB, Qu ZY and Zou X: Juglone-induced
apoptosis in human gastric cancer SGC-7901 cells via the
mitochondrial pathway. ExpToxicolPathol. 63:69–78. 2011.
|
|
23
|
Lowry OH, Rosebrough NJ, Farr AL and
Randall RJ: Protein measurement with the Folin phenol reagent. J
BiolChem. 193:265–275. 1951.
|
|
24
|
Jiao J, Friedman SL and Aloman C: Hepatic
fibrosis. CurrOpinGastroenterol. 25:223–229. 2009.
|
|
25
|
Ruehl M, Muche M, Freise C, et al:
Hydroxyproline-containing collagen analogs trigger the release and
activation of collagen-sequestered proMMP-2 by competition with
prodomain-derived peptide P33-42. Fibrogenesis Tissue Repair.
4:12011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen JC, Tsai CC, Chen LD, Chen HH and
Wang WC: Therapeutic effect of gypenoside on chronic liver injury
and fibrosis induced by CC14 in rats. Am J Chin Med. 28:175–185.
2000. View Article : Google Scholar
|
|
27
|
Meng Z, Wang Y, Wang L, et al: FXR
regulates liver repair after CC14-induced toxic injury.
MolEndocrinol. 24:886–897. 2010.
|
|
28
|
Giannini EG, Testa R and Savarino V: Liver
enzyme alteration: A guide for clinicians. CMAJ. 172:367–379. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Radosavljevic T, Mladenovic D and Vucevic
D: The role of oxidative stress in alcoholic liver injury. Med
Pregl. 62:547–553. 2009.In Serbian. View Article : Google Scholar
|
|
30
|
Casini A, Ceni E, Salzano R, et al:
Neutrophil derived superoxide anion induceslipid peroxidation and
stimulates collagen synthesis in human hepatic stellate cells: role
of nitric oxide. Hepatology. 25:361–367. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nieto N, Friedman SL and Cederbaum AI:
Cytochrome P450 2El-derived reactive oxygen species mediate
paracrine stimulation of collagen I protein synthesis byhepatic
stellate cells. J BiolChem. 277:9853–9864. 2002.
|
|
32
|
SvegliatiBaroni G, D'Ambrosio L, Ferretti
G, et al: Fibrogenic effect of oxidative stress on rat hepatic
stellate cells. Hepatology. 27:720–726. 1998. View Article : Google Scholar
|
|
33
|
Campbell JS, Hughes SD, Gilbertson DG, et
al: Platelet derived growth factor C induces liver fibrosis,
steatosis and Hepatocellular carcinoma. ProcNatlAcadSci USA.
102:3389–3394. 2005. View Article : Google Scholar
|
|
34
|
Friedman SL: Molecular regulation of
hepatic fibrosis, an integrated cellular response to tissue injury.
J BiolChem. 275:2247–2250. 2000.
|
|
35
|
Wang YD, Jia LW and Li CM: Hepatic content
of collagens and laminin in rat model of experimental liver
fibrosis. World J Gastroenterol. 6:732000.
|
|
36
|
Islam S, Antonsson L, Westin J and Lagging
M: Cirrhosis in hepatitis C virus-infected patients can be excluded
using an index of standard biochemical serum markers. Scand J
Gastroenterol. 40:867–872. 2005. View Article : Google Scholar : PubMed/NCBI
|