Introduction
Hepatocellular carcinoma (HCC) is the most common
type of liver cancer, which accounts for ~85–90% of all types of
primary liver cancer, and is one of the most life-threatening forms
of cancer (1). Despite marked
progress in the treatment of HCC, the survival rate remains poor
and the molecular pathogenesis of HCC remains to be fully
elucidated. The development and progression of HCC is a complex
process, which involves the dysregulation of oncogenes and tumor
suppressor genes. It has previously been reported that microRNAs
(miRNAs) are essential in oncogenesis by the regulation of
oncogenes and tumor suppressor genes (2–4).
Previous studies have reported a link between the aberrant
expression of miRNAs and malignant tumor development, including HCC
(5–8), increasing understanding of the
underlying molecular mechanisms and novel therapeutic targets of
HCC.
miRNAs are widely distributed, non-coding, small
RNAs, which regulate target gene expression through binding to the
3′-untranslated regions (3′UTRs) of target mRNAs at the
translational level (9). It has
previously been reported that ≤30% of human genes are regulated by
miRNAs (10). miRNAs have been
suggested to be biomarkers for HCC diagnosis (11). Among those miRNAs, miR-21 was one
of the first to be discovered, and is considered one of the most
important miRNAs, which is overexpressed in various types of
carcinomas, including prostate, gastric, colon, breast and lung
cancer (12–17). As a biomarker, miR-21 has a higher
sensitivity, compared with carcinoembryonic antigen and cancer
antigen 15-3 in the diagnosis of breast cancer (18,19).
In colorectal cancer, miR-21 has been suggested as a potential
biomarker for diagnosis and as a target for therapy (20) and radiotherapy (21). In addition, miR-21 has been
observed to stimulate gastric cancer growth and invasion through
the inhibition of phosphatase and tensin homolog (PTEN) and
programmed cell death protein 4 (PDCD4) (22). The inhibition of miR-21 decreases
the proliferation and invasion of lung cancer cells by increasing
the expression of PTEN (17),
indicating that PTEN is a direct target gene of miR-21.
Previous studies have demonstrated that B-cell
translocation gene 2 (BTG2) is regulated by miR-21 in laryngeal
cancer and prostate cancer cells (13,23).
BTG2 was the first gene to be identified in the BTG/Tob
anti-proliferation gene family (24). BTG2 is expressed in the majority of
normal tissues, with high levels being detected in the lungs,
kidneys, intestines, pancreas, and prostate (25). BTG2 is an instantaneous early
response protein, which is involved in cell differentiation,
anti-proliferation, DNA damage repair and cell apoptosis (26–29).
In addition, our previous study demonstrated that p53 positively
regulates BTG2 and negatively regulates cyclin D1 and cyclin E,
indicating a critical role in hepatocarcinogenesis (30,31).
The expression of BTG2 is reduced or absent in various types of
cancer, including melanoma, breast, gastric and lung cancers
(26,32–34).
It has also been suggested that BTG2 is regulated by miR-21 in
laryngeal and prostate cancer cells (13,23,34)
However, whether low expression levels of BTG2 are associated with
high expression levels of miRNA-21 in liver cancer cells remains to
be elucidated. The aim of the present study was to investigate the
mechanism by which BTG2 is regulated by miR-21, and determine the
role of miR-21 in the growth and progression of HCC.
Materials and methods
Cell lines
The Hep3B, Huh-7, QGY7701 and HepG2 human HCC cell
lines, and the L02 normal human liver cell line, were obtained from
the Liver Cancer Institute of Zhongshan Hospital, Fudan University
(Shanghai, China). The cells were cultured in Dulbecco's modified
Eagle's medium (DMEM) supplemented with 10% fetal bovine serum
(FBS; Invitrogen Life Technologies, Carlsbad, CA, USA) at 37°C in a
humidified incubator containing 5% CO2. The media were
replaced every other day and cells were passaged every 2 or 3 days
once they had reached 70–80% confluence. The cells in the
exponential growth phase were harvested and used for the subsequent
experiments.
Cell transfection
An miR-21 inhibitor, anti-miR-21 (sequence,
5′-UCAACAUCAGUCUGAUAAGCUA-3′); which is a chemically modified
single strand RNA that is a competitive inhibitor of miR-21, and a
mismatched negative control (miR-21 inhibitor NC) were obtained
from Shanghai GenePharma Co., Ltd. (Shanghai, China).
Lipofectamine® 2000 was purchased from Invitrogen Life
Technologies. Transfection of the cells with anti-miR-21 or NC was
performed using Lipofectamine® 2000 in Opti-MEM,
according to the manufacturer's instructions. Briefly, diluted
Lipofectamine® 2000 (3.75 µl) was mixed with
diluted miR-21 inhibitor or NC (150 nmol/l), and incubated at room
temperature for 20 min with gentle agitation. The mixture was then
added to the HepG2 cell culture plates (60% confluent), and
incubated at 37°C in an atmosphere containing 5% CO2.
Following a 6 h incubation, the medium was replaced with fresh
medium, supplemented with 10% FBS, and the cells were cultured for
a further 48 h prior to performing the subsequent experiments.
Luciferase-reporterassay
Target genes of miR-21 were predicted by PicTar
(http://pictar.mdc-berlin.de/cgi-bin/PicTar_vertebrate.cgi)
and Targetscan (http://www.targetscan.org/). The HepG2 cells
(Changsha-run Win Biotechnology, Inc., Hunan, China) were
co-transfected with a pYr-MirTarget-BTG2-3U plasmid (0.8 µg;
Changsha-run Win Biotechnology, Inc.) and either the miRNA NC (75
nmol/l), miR-21 mimics (75 nmol/l; sequence,
5′-UAGCUUAUCAGACUGAUGUUGAAAC AUCAGUCUGAUAAGCUAUU-3′; Shanghai
GenePharma Co., Ltd.) or miR-21 inhibitor (150 nmol/l; Shanghai
GenePharma Co., Ltd.). In addition, HepG2 cells were transfected
with miR-21 mimiwcs and pYr-Mir Target plasmid, pYr-Mir
Target-BTG2-3U plasmid, or pYr-Mir Target-BTG2-3U-Delete site
plasmid (Changsha-run Win Biotechnology, Inc.). Transfection was
performed using Lipofectamine® 2000, on 60% confluent
cells seeded in 24-well plates, at 37°C for 48 h. At 24 h
post-transfection, the activities of firefly and Renilla luciferase
were measured using a Dual-Luciferase Reporter assay (Promega
Corporation, Madison, WI, USA). The activity of Renilla luciferase
for each sample was normalized to that of firefly luciferase. Each
experiment was repeated twice and assessed in triplicate.
Western blotting
The cells were lysed and the total protein was
extracted using radioimmunoprecipitation (RIPA) buffer, containing
150 mM NaC1, 25 mM Tris-HCl (pH 7.6), 1% sodium deoxycholate, 0.1%
SDS and 1% NP-40 (Thermo Fisher Scientific, Inc., Rockford, IL,
USA). Protease inhibitor (cat. no. 78410; Thermo Fisher Scientific,
Inc.) was added to the RIPA buffer prior to use. Total protein
concentrations were quantified using the Bicinchoninic Acid Protein
Assay kit (Beyotime Institute of Biotechnology, Shanghai. China).
Samples with equal quantities of total protein (100 µg) were
fractionated by 12% SDS-PAGE and transferred onto nitrocellulose
membranes (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The
expression levels of BTG2 were probed using primary monoclonal
mouse anti-human BTG2 (1:500; cat. no. ab58219; Abcam, Cambridge,
MA, USA), and the internal control β-actin was detected using
primary monoclonal mouse anti-human β-actin (1:1,000; cat. no.
sc-47778; Santa Cruz Biotechnology, Inc., Dallas, TX, USA). The
secondary antibody used was goat anti-mouse IgG (H+L), HRP
conjugate (1:5,000; cat. no. 31430; Thermofisher Technology Co.,
Ltd, Shanghai, China). The signals of the bands were visualized
using an enhanced chemiluminescence method (Thermo Fisher
Scientific, Inc.). Protein expression levels were quantified using
a Gel Documentation system (Gel Doc™ EQ; cat. no. 170-8060; Bio-Rad
Laboratories, Inc.).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the cells using
TRIzol® reagent (Invitrogen Life Technologies),
according to the manufacturer's instructions. Reverse transcription
was conducted using RevertAid Reverse Transcriptase (Shanghai
GenePharma Co., Ltd). Quantification of mature miRNAs was performed
using a Quantitect SYBR Green PCR kit (Changsha-run Win
Biotechnology, Inc.) and a 7500 Multiplex Quantitative PCR system
(Applied Biosystems Life Technologies, Foster City, CA, USA),
according to the manufacturer's instructions. The PCR reaction mix
consisted of: 5 µl 2X PCR Master mix, 0.3 µl forward
primer (10 µM), 0.3 µl reverse primer (10 µM),
2 µl cDNA, and 2.4 µl H2O. The PCR cycling
conditions were as follows: 95°C for 5 min, followed by 42 cycles
at 95°C for 30 sec, 59°C for 30 sec and 72°C for 20 sec, and 95°C
for 10 sec. The U6 gene was used as an internal control and each
reaction was performed in triplicate. The primer (Shanghai
GenePharma Co., Ltd) sequences were as follows: miR-21, forward
5′-GCGGCGTAGCTTATCAGACTGA-3′, reverse 5′-GTGCAGGGTCCGAGGT-3′; and
U6, forward 5′-CTC GCTTCGGCAGCACA-3′, and reverse 5′-AACGCTTC
ACGAATTTGCGT-3′. The fold-change of miR-21 expression in HepG2
cells was compared with that in the L02 normal liver cells using
the 2−ΔΔCT method (35).
Measurement of cell proliferation
An MTT assay was used to detect cell proliferation.
Briefly, the cells were made into a single cell suspension using
DMEM, supplemented with 10% FBS, and seeded into 96-well plates at
a density of 1×104 cells in 200 µl/well. The
cells were then cultured for 24 h at 37°C, to allow them to adhere
to the plate. Subsequently, 20 µl MTT solution (5 mg/ml;
Beyotime Institute of Biotechnology) was added to each well and
incubated for 4 h at 37°C. The culture medium was then discarded,
and 150 µl DMSO (Sigma-Aldrich, St. Louis, MO, USA) was
added to each well, and the plates were agitated for 15 min in
order to let the crystalized precipitates fully dissolved. The
optical density values were measured at 490 nm using an ELISA
monitor (Multiskan MK3; Thermo Fisher Scientific). Each
experimental group contained nine replicate wells, and the
experiment was performed in triplicate.
Detection of cell migration using a
scratch wound assay
Once the cells had reached 70~80% confluence, a
wound (1–1.5 mm wide) was made using a sterile tip. The wells were
gently washed three times with PBS. The cells were subsequently
cultured in serum-free DMEM at 37°C. At 0, 24, 30 and 48 h
post-wounding, the distance of the scratch between the cells was
evaluated under an Olympus inverted microscope (CKX41; Olympus
Corporation, Tokyo, Japan).
Measurement of cell invasion using a
Transwell invasion assay
A Transwell invasion assay was used to detect the
invasive ability of the treated HepG2 cells and control cells. The
cells (1×105 cells suspended in 300 µl serum-free
DMEM) were seeded onto the upper chamber of a Transwell invasion
system (Changsha-run Win Biotechnology, Inc.), and 500 µl
DMEM supplemented with 10% FBS was added to the lower chamber. The
chamber was then incubated at 37°C in a humidified incubator
containing 5% CO2 for 24 h. The cells on the upper
surface of the base membrane were removed using a sterile cotton
ball. The cells, which had invaded to the lower surface of the base
membrane were stained with 1% crystal violet (Changsha-run Win
Biotechnology, Inc.). The cells that had successfully crossed the
Transwell polycarbonate membrane were counted under an Olympus
inverted microscope (Olympus Corporation). A total of of five
fields were randomly selected for the calculation of each sample,
and the experiment was repeated at least three times. The invasive
ability of the cells was determined by the number of cells that
crossed the Transwell polycarbonate membrane.
Analysis of cell cycle distribution
Once the cells had reached 70~80% confluence, the
medium was replaced with serum-free medium. The cells were cultured
for 48 h, in order to reach synchronization. The treated cells were
then digested, fixed with anhydrous alcohol overnight and stained
with propidium iodide (100 µg/ml; Changsha-run Win
Biotechnology, Inc.) for 30 min in the dark. The cell cycle
distribution was detected by flow cytometry (FACSCalibur; BD
Biosciences, Franklin Lakes, NJ, USA). Each experiment was repeated
in triplicate and the average values of each cell cycle phase were
calculated.
Analysis of apoptosis
The HepG2 cells (1–5×105) were harvested
and gently re-suspended in 500 µl binding buffer (Nanjing
KeyGEN Biotech. Co., Ltd., Nanjing, China), to which 5 µl
annexin V-allophycocyaninand 5 µl 7-aminoacti-nomycin D
(Nanjing KeyGEN Biotech. Co., Ltd.) were added. The cells were
maintained at room temperature for 15 min in the dark and then
analyzed using flow cytometry (BD Biosciences). Each experiment was
performed in triplicate.
Statistical analysis
All data were obtained from at least three
independent experiments and are presented as the mean ± standard
deviation. SPSS 20.0 statistical analysis software (IBM SPSS,
Armonk, NY, USA) was used for data analysis. Student's t test was
used to compare data between two groups. P<0.05 was considered
to indicate a statistically significant difference.
Results
Expression levels of BTG2 and miR-21 in
HCC cells
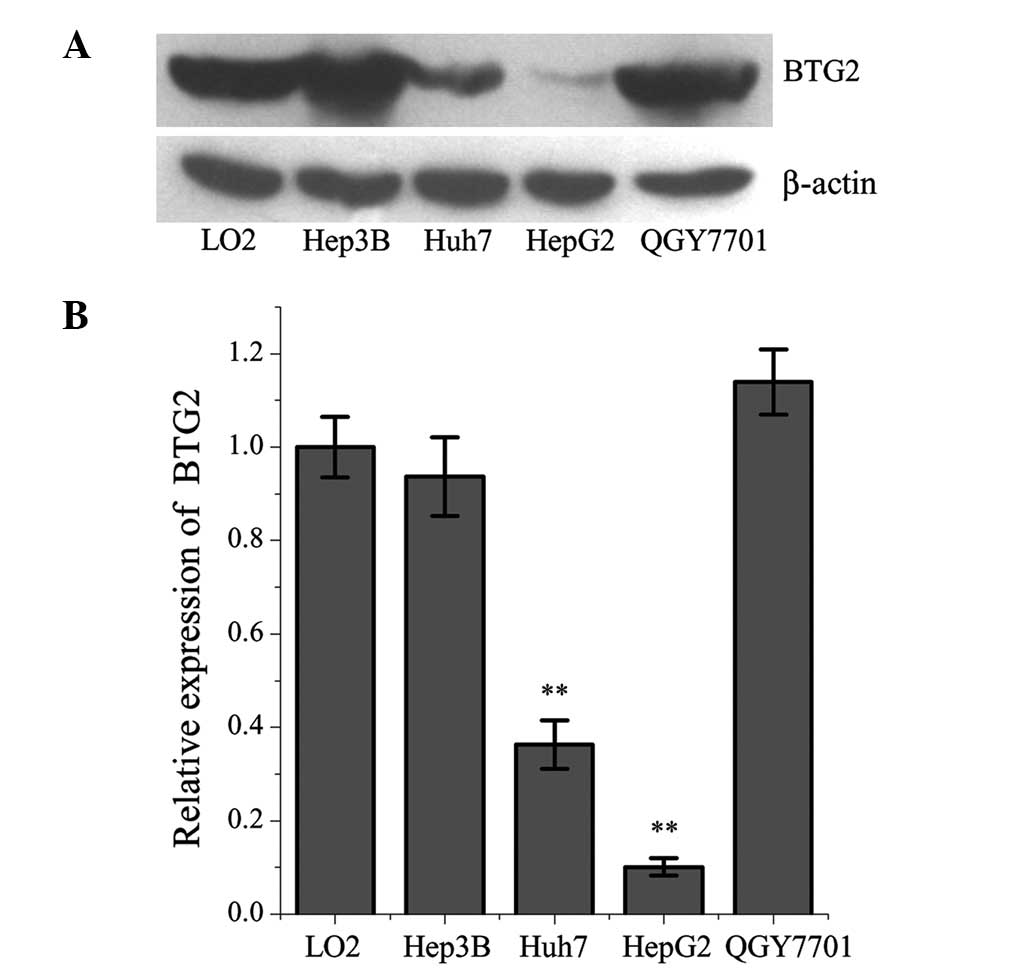
The expression levels of BTG2 were significantly
lower in the Huh-7 and HepG2 HCC cells, compared with in the L02
normal human liver cell line (Fig.
1). Among the cancer cell lines, the expression levels of BTG2
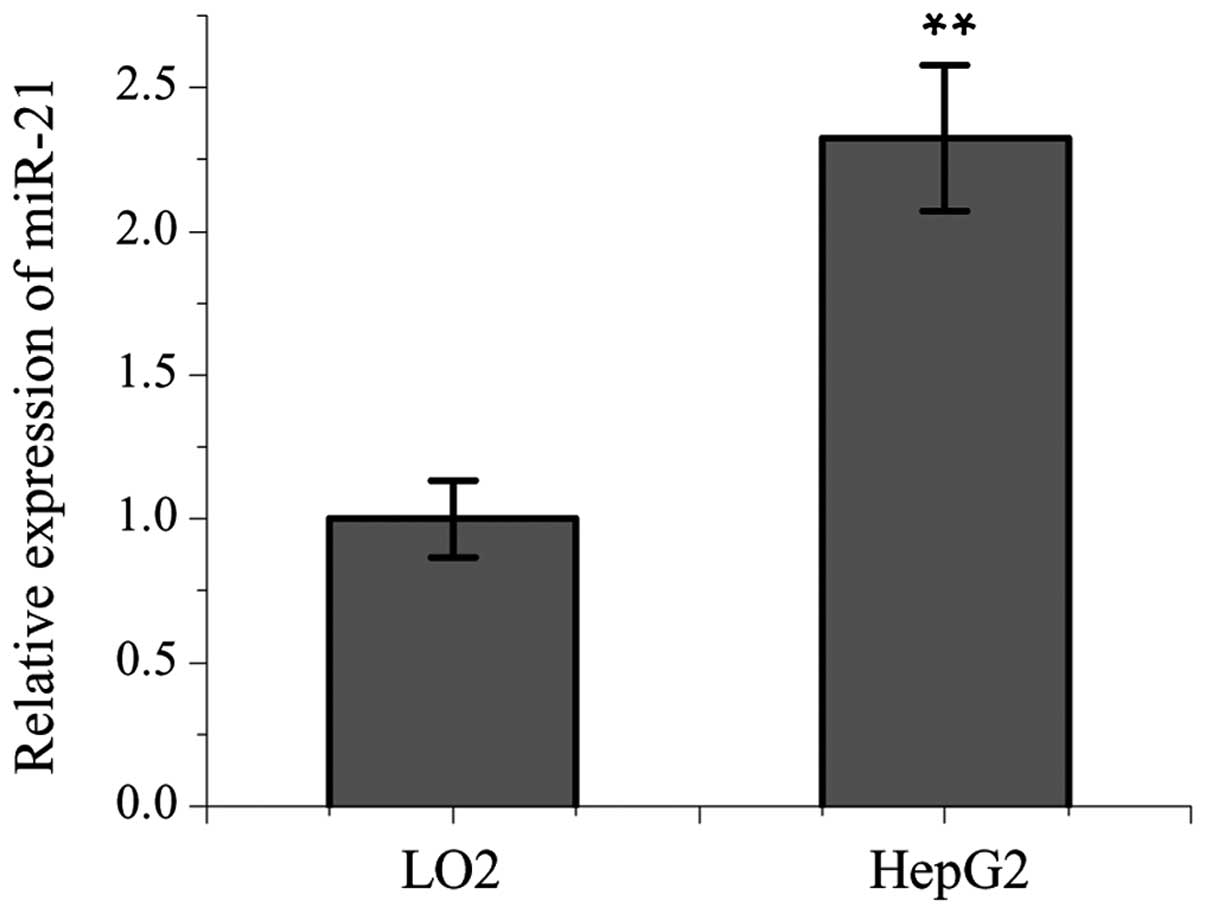
were lowest in the HepG2 cells. The expression levels of miR-21
were significantly higher in the HepG2 cells, compared with in the
L02 cells (Fig. 2).
Effects of miR-21 on the growth of HepG2
cells
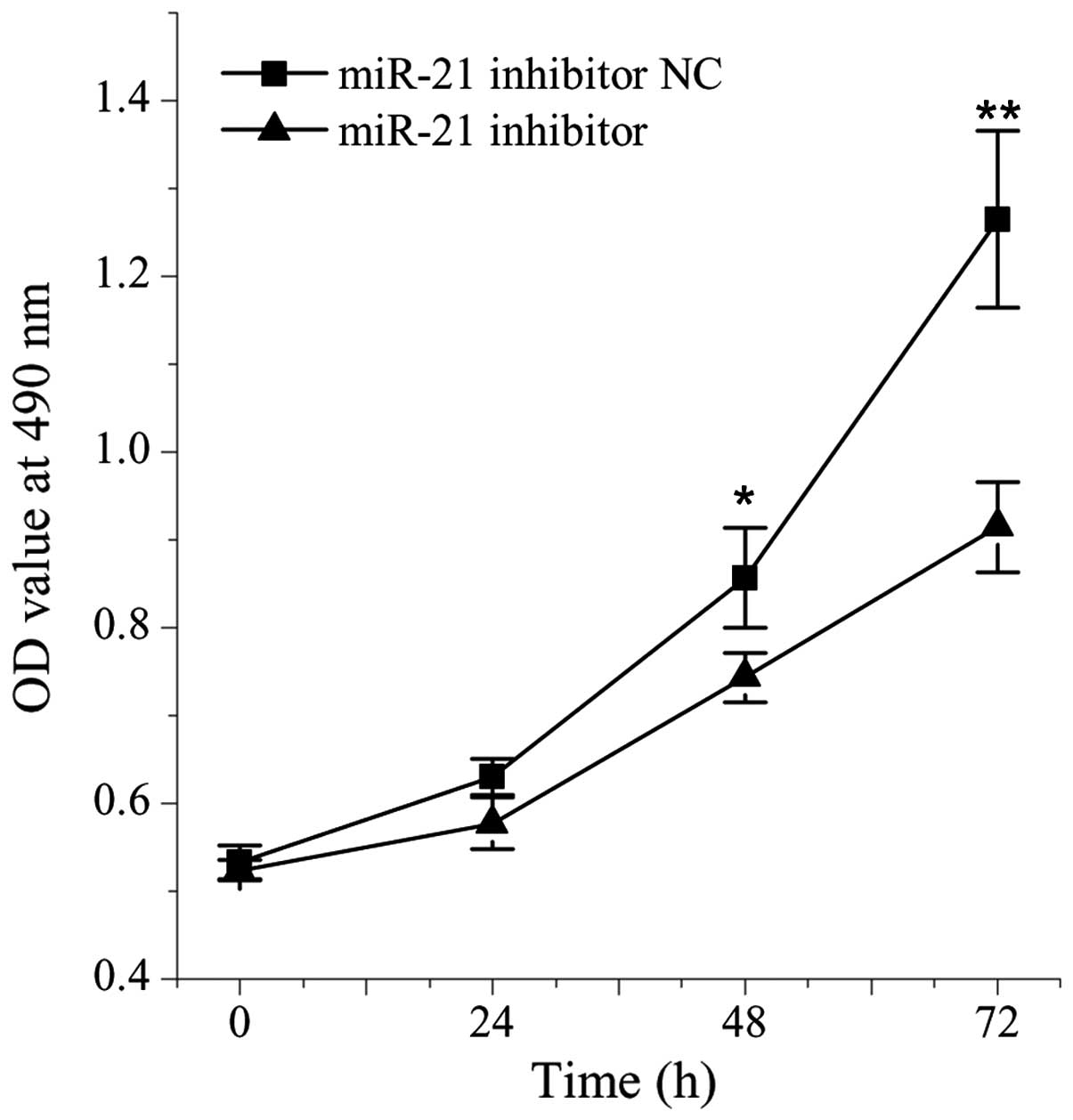
The present study aimed to further analyze the
association between miR-21 and cell growth by comparing the cell
viabilities between cells treated with miR-21 inhibitor and NC. The
viability of the HepG2 cells transfected with the miR-21 inhibitor
was significantly lower, compared with the cells transfected with
the NC (P<0.05; Fig. 3).
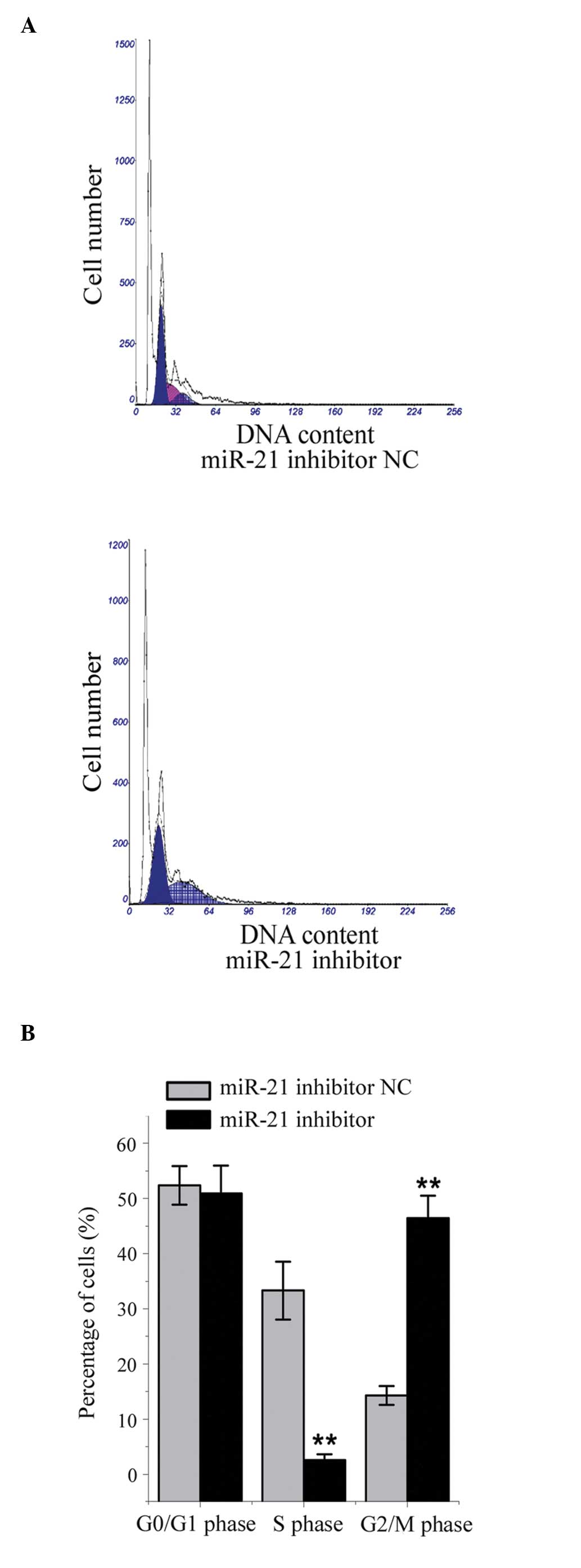
Effect of miR-21 inhibition on cell cycle
distribution in HepG2 cells
Compared with the NC-transfected cells, the mean
number of cells in the G2 phase was significantly
higher, and the mean number of cells in the S phase was
significantly lower, in the HepG2 cells transfected with the miR-21
inhibitor (all P<0.05; Fig. 4).
These results indicated that miR-21 facilitated cell cycle
progression through the G2 phase.
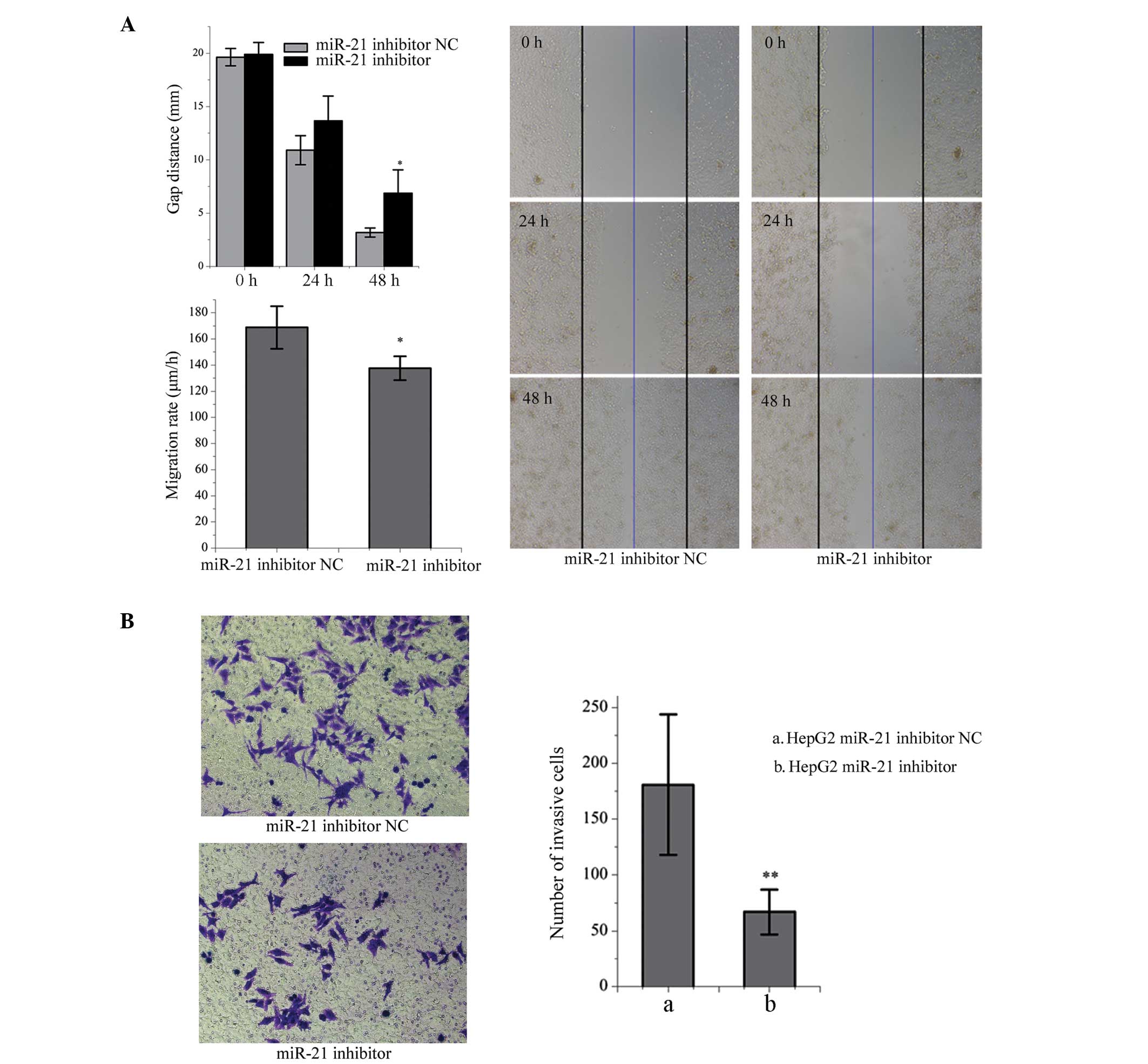
Effects of miR-21 inhibition on the
migration and invasion of HepG2 cells
Cell migration was significantly inhibited following
transfection of the HepG2 cells with the miR-21 inhibitor, measured
by the distance of the scratch between the cells and the rate of
migration, compared with the NC-transfected cells (P<0.05;
Fig. 5A). In addition, the number
of HepG2 cells transfected with the miR-21 inhibitor that crossed
the polycarbonate membrane of the Transwell invasion chamber was
significantly lower, compared with the NC-transfected cells
(P<0.01; Fig. 5B).
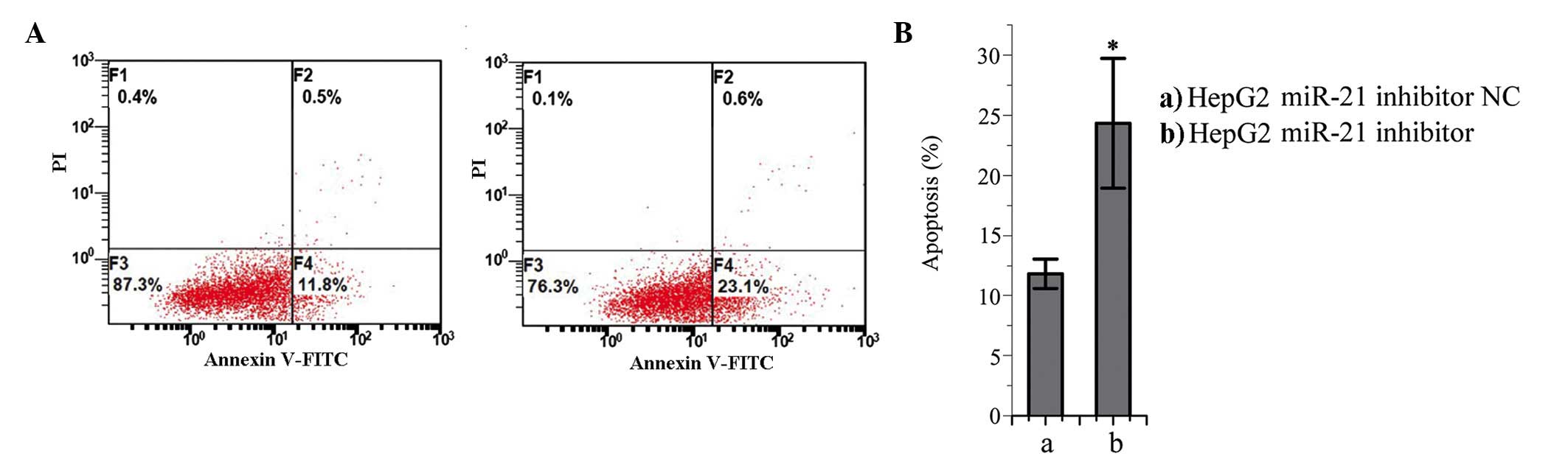
Effects of miR-21 inhibition on the
apoptosis of HepG2 cells
The number of apoptotic cells in the miR-21
inhibitor-trans-fected group was significantly higher, compared
with number in the NC-transfected group (P<0.05; Fig. 6). These results suggested that
miR-21 may prevent apoptosis in HepG2 cells.
BTG2 as a direct target gene of miR-21 in
HepG2 cells
According to publicly available databases, including
TargetScan and PicTar (36,37),
the BTG2 gene is a predicted target gene of miR-21. As shown in
Fig. 7A, inhibition of the
expression of miR-21 promoted the protein expression of BTG2 in
HepG2 cells, compared with the NC-transfected cells. In the
luciferase reporter assay, the signal in the HepG2 cells, which
were co-transfected with the BTG2-3′-UTR plasmid and miR-21 mimic
was significantly decreased, whereas the luciferase signal was
increased in the cells co-transfected with the BTG2-3′-UTR plasmid
and miR-21 inhibitor (Fig. 7B and
C). Following deletion of the predicted binding site of the
BTG2 3′UTR, theHepG2 cells were co-transfected with miR-21 mimics
and pYr-MirTarget plasmid, pYr-MirTarget-BTG2-3U plasmid, or
pYr-MirTarget-BTG2-3U-Delete site plasmid. As shown in Fig. 7D, the luciferase activities were
significantly enhanced following deletion of the predicted binding
site, compared with the wild-type group, suggesting that BTG2 is a
direct target gene of miR-21.
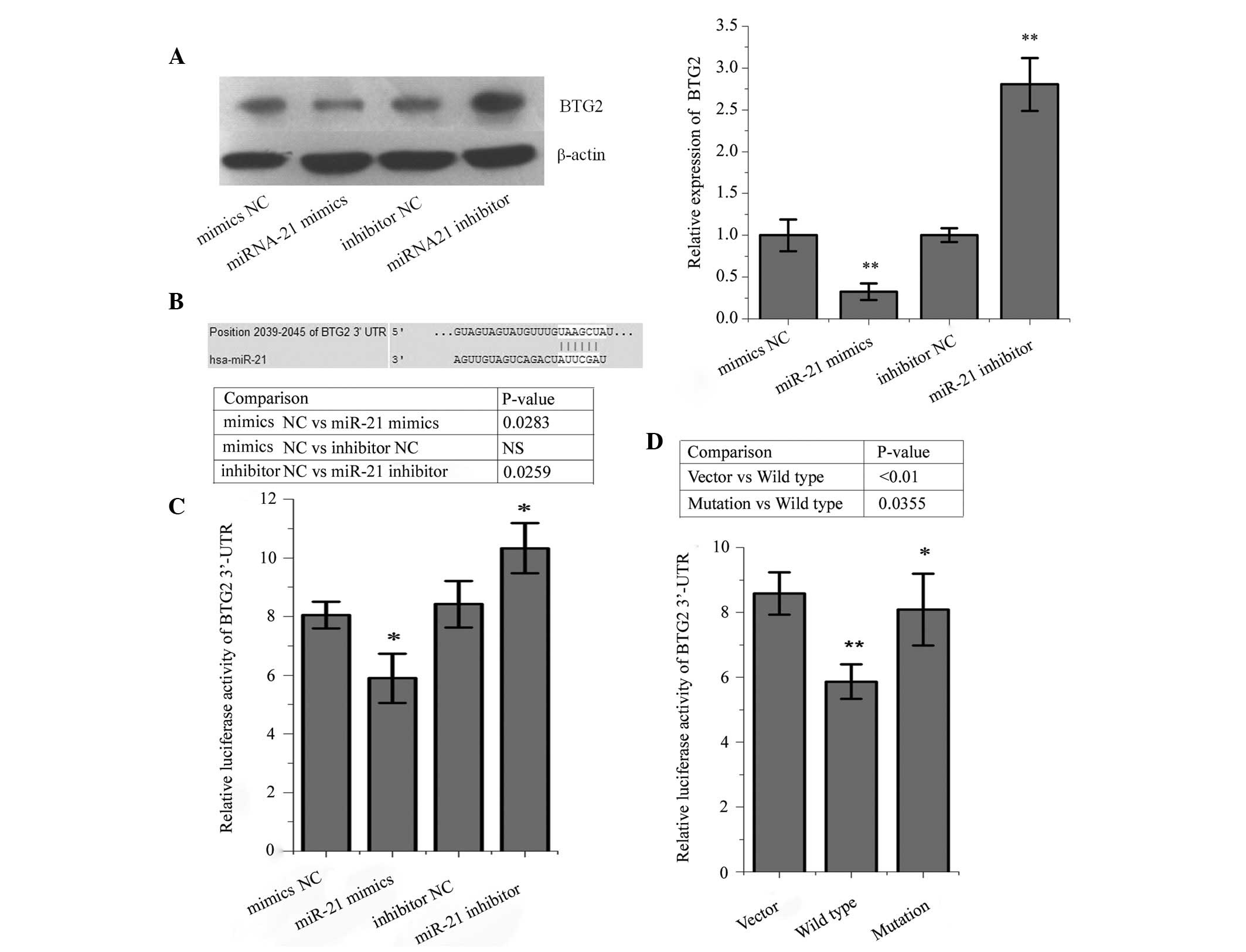 | Figure 7BTG2 is a direct target gene of
miR-21. (A) Protein expression levels of BTG2 were increased in
HepG2 liver cancer cells transfected with the miR-21 inhibitor,
compared with those transfected with the miR-21 inhibitor NC. The
protein expression levels of BTG2 decreased in the HepG2 cells
transfected with miR-21 mimics, compared with those transfected
with the NC. (B) Position of the 3′UTR of BTG2 binding with miR-21.
(C) Luciferase activity was significantly decreased in the HepG2
cells following co-ntransfection with the pYr-MirTarget-BTG2-3′U
plasmid and miR-21 mimics, but was increased following
co-transfection with the pYr-MirTarget-BTG2-3′U plasmid and miR-21
inhibitor, compared with the NC group. (D) Following deletion of
the predicted binding site of BTG2 3′UTR, the HepG2 cells were
co-transfected with miR-21 mimics and a pYr-MirTarget plasmid
(vector), pYr-MirTarget-BTG2-3U plasmid (wild type) or
pYr-MirTarget-BTG2-3U-Delete site plasmid (mutation). The results
revealed that the luciferase activities were significantly enhanced
following deletion of the predicted binding site, compared with the
wild-type (mimic) group. *P<0.05,
**P<0.01, the mimics NC group compared with miR-21
mimics, and the inhibitor group compared with miR-21 inhibitor
group. BTG2, B-cell translocation gene 2; miR-21, microRNA-21; NC,
negative control; UTR, untranslated region. |
Discussion
As a novel class of regulatory molecules, miRNAs are
important in cancer development and progression. It has been
reported that almost half of miRNAs are located in
cancer-associated gene regions and vulnerable regions (38,39).
The present study aimed to determine the roles of miRNAs in liver
carcinogenesis. Our previous studies demonstrated that miR-224 is
significantly upregulated in HepG2 cells, and is able to regulate
cell migration and invasion via the homeobox
D10/phosphorylated-PAK4/matrix metalloproteinase (MMP)-9 signaling
pathway (40,41). In the present study, inhibition of
miR-21 suppressed cell proliferation, induced G2/M phase
arrest, inhibited cell migration and promoted apoptosis in the
HepG2 cells.
miRNAs regulate cellular biological functions by
affecting the expression of several target genes. It has previously
been reported that miR-21 has various target genes, including PTEN,
PDCD4 and tropomyosin 1 (22,42).
BTG2 has also been suggested as a target gene of miR-21 in
laryngeal and prostate cancer cells (13,23).
In the present study, it was demonstrated that BTG2 was a target
gene of miR-21. Firstly, the BTG2 gene was predicted to be a target
gene of miR-21 following database searches, including TargetScan
and PicTar, which indicated that the 3′UTR of BTG2 included a
sequence matching the miR-21 seed region. Secondly, the protein
expression levels of BTG2 were negatively correlated with the
expression levels of miR-21 in the HepG2 cells. Thirdly, the
overexpression of miR-21 decreased the luciferase activity in the
HepG2 cells, whereas inhibition of miR-21 enhanced the luciferase
signal. Finally, a deletion mutation in the BTG2 3′UTR affected the
regulatory effects of miR-21.
It has been reported that overexpression of BTG2
inhibits the expression of several genes in lung cancer cells,
including cyclin D1, MMP-1 and MMP-2 (26). In addition, BTG2 suppresses the
growth and proliferation of gastric cancer cells (34). BTG2 promotes cell apoptosis and
inhibits cancer cell invasion (29), however, Wagener et al
(27) reported that endogenous
expression of BTG2 contributes to the migration of bladder cancer
cells. The results of the present study demonstrated that the
expression of BTG2 was downregulated in hepatocarcinoma cells,
indicating that the loss of BTG2 may promote carcinogenesis. The
overexpression of miR-21 may contribute to the downregulated
expression of BTG2 in hepatocarcinoma, however, alternative
molecular mechanisms cannot be ruled out.
BTG2 acts as a cell cycle regulatory molecule and is
important in inducing G1/S arrest through downregulation
of cyclin D1. The activation of BTG2 depends on p53 (24). However, in the present study,
suppression of the expression of miR-21 prevented cell cycle
progression via inducing G2/M phase arrest, and no
significant effect on G1 phase was observed. It has
previously been reported that BTG2 induces G2/M arrest
and cell death by inhibiting cyclin B1-Cdc2 binding (24,43).
Therefore, it was hypothesized that inhibition of cell
proliferation and growth partly depend on BTG2-induced
G2/M phase arrest, through inhibition of cyclin B1-Cdc2
binding. Further investigations are required to understand the
underlying molecular mechanisms.
In conclusion, the results of the present study
suggested that miR-21 may be important in the development and
progression of hepatocarcinoma. In the present study, miR-21 was
found to be overexpressed and BTG2 was found to to be downregulated
in HepG2 liver cancer cells. Inhibition of miR-21 suppressed cell
proliferation, migration and invasion, and promoted cell apoptosis.
In addition, the inhibition of cell growth partly depended on
BTG2-induced G2/M phase arrest through inhibition of
cyclinB1-Cdc2 binding. BTG2 was a direct target gene of miR-21 in
the liver cancer cells. These results suggested that understanding
the miR-21/BTG2 pathway may elucidate a novel regulatory mechanism
for liver tumorigenesis, and provide a novel diagnostic and
therapeutic target for HCC in the future.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81272498, 81301631,
and 30973457) and the Chongqing National Natural Science Foundation
of China (grant no. cstc 2013jjB9901).
References
|
1
|
Saito Y, Hibino S and Saito H: Alterations
of epigenetics and microRNA in hepatocellular carcinoma. Hepatol
Res. 44:31–42. 2014. View Article : Google Scholar
|
|
2
|
Liu J, Liu X, Cui F, Chen G, Guan Y and He
J: The efficacy of the inhalation of an aerosolized Group A
streptococcal preparation in the treatment of lung cancer. Chin J
Cancer Res. 24:346–352. 2012. View Article : Google Scholar
|
|
3
|
Skinner HD and Komaki R: Proton
radiotherapy in the treatment of lung cancer. Transl Cancer Res.
1:264–270. 2012.
|
|
4
|
Quintavalle C and Condorelli G: Dulanermin
in cancer therapy: Still much to do. Transl Lung Cancer Res.
1:158–159. 2012.PubMed/NCBI
|
|
5
|
Yamashita S, Yamamoto H, Mimori K, Nishida
N, Takahashi H, Haraguchi N, Tanaka F, Shibata K, Sekimoto M, Ishii
H, et al: MicroRNA-372 is associated with poor prognosis in
colorectal cancer. Oncology. 82:205–212. 2012.PubMed/NCBI
|
|
6
|
Weiland M, Gao XH, Zhou L and Mi QS: Small
RNAs have a large impact: Circulating microRNAs as biomarkers for
human diseases. RNA Biol. 9:850–859. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tjensvoll K, Svendsen KN, Reuben JM,
Oltedal S, Gilje B, Smaaland R and Nordgård O: miRNA expression
profiling for identification of potential breast cancer biomarkers.
Biomarkers. 17:463–470. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Papaconstantinou I, Karakatsanis A,
Gazouli M, Polymeneas G and Voros D: The role of microRNAs in liver
cancer. Eur J Gastroenterol Hepatol. 24:223–228. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang B, Pan X, Cobb GP and Anderson TA:
microRNAs as oncogenes and tumor suppressors. Dev Biol. 302:1–12.
2007. View Article : Google Scholar
|
|
10
|
Iorio MV and Croce CM: MicroRNAs in
cancer: Small molecules with a huge impact. J Clin Oncol.
27:5848–5856. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu QY, Jiang H, Su J and Jia YQ: MicroRNAs
as biomarkers for hepatocellular carcinoma: A diagnostic
meta-analysis. Clin Lab. 59:1113–1120. 2013.PubMed/NCBI
|
|
12
|
Pass HI: Biomarkers and prognostic factors
for mesothelioma. Ann Cardiothorac Surg. 1:449–456. 2012.
|
|
13
|
Coppola V, Musumeci M, Patrizii M,
Cannistraci A, Addario A, Maugeri-Saccà M, Biffoni M,
Francescangeli F, Cordenonsi M, Piccolo S, et al: BTG2 loss and
miR-21 upregulation contribute to prostate cell transformation by
inducing luminal markers expression and epithelial-mesenchymal
transition. Oncogene. 32:1843–1853. 2013. View Article : Google Scholar
|
|
14
|
Niu J, Shi Y, Tan G, Yang CH, Fan M,
Pfeffer LM and Wu ZH: DNA damage induces NF-κB-dependent
microRNA-21 up-regulation and promotes breast cancer cell invasion.
J Biol Chem. 287:21783–21795. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang BG, Li JF, Yu BQ, Zhu ZG, Liu BY and
Yan M: microRNA-21 promotes tumor proliferation and invasion in
gastric cancer by targeting PTEN. Oncol Rep. 27:1019–1026.
2012.PubMed/NCBI
|
|
16
|
Shibuya H, Iinuma H, Shimada R, Horiuchi A
and Watanabe T: Clinicopathological and prognostic value of
microRNA-21 and microRNA-155 in colorectal cancer. Oncology.
79:313–320. 2010. View Article : Google Scholar
|
|
17
|
Zhang JG, Wang JJ, Zhao F, Liu Q, Jiang K
and Yang GH: MicroRNA-21 (miR-21) represses tumor suppressor PTEN
and promotes growth and invasion in non-small cell lung cancer
(NSCLC). Clin Chim Acta. 411:846–852. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gao J, Zhang Q, Xu J, Guo L and Li X:
Clinical significance of serum miR-21 in breast cancer compared
with CA153 and CEA. Chin J Cancer Res. 25:743–748. 2013.
|
|
19
|
Petrović N, Mandušić V, Stanojević B,
Lukić S, Todorović L, Roganović J and Dimitrijević B: The
difference in miR-21 expression levels between invasive and
non-invasive breast cancers emphasizes its role in breast cancer
invasion. Med Oncol. 31:8672014. View Article : Google Scholar
|
|
20
|
Li T, Leong MH, Harms B, Kennedy G and
Chen L: MicroRNA-21 as a potential colon and rectal cancer
biomarker. World J Gastroenterol. 19:5615–5621. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu J, Zhu H, Yang X, Ge Y, Zhang C, Qin
Q, Lu J, Zhan L, Cheng H and Sun X: MicroRNA-21 is a novel
promising target in cancer radiation therapy. Tumour Biol.
35:3975–3979. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li L, Zhou L, Li Y, Lin S and Tomuleasa C:
MicroRNA-21 stimulates gastric cancer growth and invasion by
inhibiting the tumor suppressor effects of programmed cell death
protein 4 and phosphatase and tensin homolog. J BUON. 19:228–236.
2014.PubMed/NCBI
|
|
23
|
Liu M, Wu H, Liu T, Li Y, Wang F, Wan H,
Li X and Tang H: Regulation of the cell cycle gene, BTG2, by miR-21
in human laryngeal carcinoma. Cell Res. 19:828–837. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lim IK: TIS21 (/BTG2/PC3) as a link
between ageing and cancer: Cell cycle regulator and endogenous cell
death molecule. J Cancer Res Clin Oncol. 132:417–426. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Melamed J, Kernizan S and Walden PD:
Expression of B-cell translocation gene 2 protein in normal human
tissues. Tissue Cell. 34:28–32. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wei S, Hao C, Li X, Zhao H, Chen J and
Zhou Q: Effects of BTG2 on proliferation inhibition and
anti-invasion in human lung cancer cells. Tumour Biol.
33:1223–1230. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wagener N, Bulkescher J, Macher-Goeppinger
S, Karapanagiotou-Schenkel I, Hatiboglu G, Abdel-Rahim M,
Abol-Enein H, Ghoneim MA, Bastian PJ, Müller SC, et al: Endogenous
BTG2 expression stimulates migration of bladder cancer cells and
correlates with poor clinical prognosis for bladder cancer
patients. Br J Cancer. 108:973–982. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Choi KS, Kim JY, Lim SK, Choi YW, Kim YH,
Kang SY, Park TJ and Lim IK: TIS21(/BTG2/PC3) accelerates the
repair of DNA double strand breaks by enhancing Mre11 methylation
and blocking damage signal transfer to the Chk2(T68)-p53(S20)
pathway. DNA Repair (Amst). 11:965–975. 2012. View Article : Google Scholar
|
|
29
|
Zhang YJ, Wei L, Liu M, Li J, Zheng YQ,
Gao Y and Li XR: BTG2 inhibits the proliferation, invasion, and
apoptosis of MDA-MB-231 triple-negative breast cancer cells. Tumour
Biol. 34:1605–1613. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang Z, Chen C, Wang G, Yang Z, San J,
Zheng J, Li Q, Luo X, Hu Q, Li Z and Wang D: Aberrant expression of
the p53-inducible antiproliferative gene BTG2 in hepatocellular
carcinoma is associated with overexpression of the cell
cycle-related proteins. Cell Biochem Biophys. 61:83–91. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang ZM, Wang G, Yang ZX, Shan JL, Chen
C, Jin F, Xu W, Li Q, Luo XZ, Wang D and Li ZP: The expression of
B-cell translocation gene 2 in diethylnitrosamine-induced primary
hepatocellular carcinoma rat model. Zhonghua Gan Zang Bing Za Zhi.
17:107–111. 2009.In Chinese. PubMed/NCBI
|
|
32
|
Yang CH, Yue J, Pfeffer SR, Handorf CR and
Pfeffer LM: MicroRNA miR-21 regulates the metastatic behavior of
B16 melanoma cells. J Biol Chem. 286:39172–39178. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Takahashi F, Chiba N, Tajima K, Hayashida
T, Shimada T, Takahashi M, Moriyama H, Brachtel E, Edelman EJ,
Ramaswamy S and Maheswaran S: Breast tumor progression induced by
loss of BTG2 expression is inhibited by targeted therapy with the
ErbB/HER inhibitor lapatinib. Oncogene. 30:3084–3095. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang L, Huang H, Wu K, Wang M and Wu B:
Impact of BTG2 expression on proliferation and invasion of gastric
cancer cells in vitro. Mol Biol Rep. 37:2579–2586. 2010. View Article : Google Scholar
|
|
35
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
36
|
Krek A, Grün D, Poy MN, Wolf R, Rosenberg
L, Epstein EJ, MacMenamin P, da Piedade I, Gunsalus KC, Stoffel M
and Rajewsky N: Combinatorial microRNA target predictions. Nat
Genet. 37:495–500. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lewis BP, Shih IH, Jones-Rhoades MW,
Bartel DP and Burge CB: Prediction of mammalian microRNA targets.
Cell. 115:787–798. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nair VS, Maeda LS and Ioannidis JP:
Clinical outcome prediction by microRNAs in human cancer: A
systematic review. J Natl Cancer Inst. 104:528–540. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ross SA and Davis CD: MicroRNA, nutrition,
and cancer prevention. Adv Nutr. 2:472–485. 2011. View Article : Google Scholar :
|
|
40
|
Li Q, Wang G, Shan JL, Yang ZX, Wang HZ,
Feng J, Zhen JJ, Chen C, Zhang ZM, Xu W, et al: MicroRNA-224 is
upregulated in HepG2 cells and involved in cellular migration and
invasion. J Gastroenterol Hepatol. 25:164–171. 2010. View Article : Google Scholar
|
|
41
|
Li Q, Ding C, Chen C, Zhang Z, Xiao H, Xie
F, Lei L, Chen Y, Mao B, Jiang M, et al: miR-224 promotion of cell
migration and invasion by targeting Homeobox D 10 gene in human
hepatocellular carcinoma. J Gastroenterol Hepatol. 29:835–842.
2014. View Article : Google Scholar
|
|
42
|
Zhu S, Si ML, Wu H and Mo YY: MicroRNA-21
targets the tumor suppressor gene tropomyosin 1 (TPM1). J Biol
Chem. 282:14328–14336. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ryu MS, Lee MS, Hong JW, Hahn TR, Moon E
and Lim IK: TIS21/BTG2/PC3 is expressed through PKC-delta pathway
and inhibits binding of cyclin B1-Cdc2 and its activity,
independent of p53 expression. Exp Cell Res. 299:159–170. 2004.
View Article : Google Scholar : PubMed/NCBI
|