Introduction
Although the incidence of gastric cancer (GC) has
decreased substantially in most parts of the world due to advances
in early diagnosis, eradication of Helicobacter pylori and
changes in lifestyle, GC is one of the most frequently diagnosed
malignant tumor types (1,2). In Europe, GC was the fourth most
common cause of cancer-associated mortality in 2012 (3). However, almost two-thirds of these
cancers occur in developing countries, the incidence in China
accounts for ~42% of all cases and GC was the second most common
cancer in 2009 (4,5). The prognosis for gastric cancer
patients with advanced cancer is generally poor and hard to
predict. To date, surgery has remained the primary treatment,
following which the average five-year survival rate is only 20–25%
(6). Although diagnostic
techniques have improved, most patients with GC already have
locally advanced disease, and early recurrence and metastasis after
surgery still remain a major challenge (7,8).
Recently, epithelial-mesenchymal transition (EMT) has been noted as
a critical event in the early step of cancer metastasis (9). EMT is considered to be correlated
with the malignancy of cancer cells and responsible for cancer
invasion and metastasis, as it promotes the detachment of cancer
cells from the primary tumor areas, leading to invasion of the
vasculature and colonization of distant organs with secondary
tumors (10,11).
Nuclear transcription factor CDX2 is a significant
regulator of in the proliferation and differentiation of intestinal
epithelial cells in fetal and adult tissues. CDX2 has specificity
for enterocytes and has been employed for the diagnosis of primary
and metastatic colorectal cancers (12). Moskaluk et al (13) assessed the expression of CDX2 in
745 cancer specimens from various anatomic sites by microarray
analysis, which showed that 20–30% of carcinomas of the stomach
were positive for CDX2, particularly intestinal-type GC (14). Several studies have reported that
CDX2-positive GC patients had a higher survival rate than those who
were CDX2-negative (15). In
addition, a negative correlation between CDX2 expression and the
depth of tumor invasion and lymph node metastasis was noted
(16–18). A recent meta-analysis showed that
CDX2 is a prognostic factor, which may be used as a marker of good
outcome in patients with GC (19).
However, the molecular mechanisms leading to the improved
biological behavior and outcome due to CDX2 over-expression in GC
patients have remained elusive. To address this issue, the present
study assessed whether CDX2 was expressed on GC cells and whether
overexpression of CDX2 was able to inhibit GC-cell growth and
reverse the EMT in vitro and in vivo.
Materials and methods
Cell culture and transfection
The human GC cell lines AGS and MKN-45 (Cell Bank of
the Chinese Academy of Sciences, Shanghai, China) was maintained in
Dulbecco's modified Eagle's medium (DMEM; HyClone, UT, USA)
supplemented with 10% fetal bovine serum (FBS; Tianhang Biological
Technology Co., Ltd, Zhejiang, China), penicillin (100 U/ml) and
streptomycin (100 µg/ml) (Gibco-BRL, Invitrogen Life
Technologies, Inc., Carlsbad, CA, USA) at 37°C in a humidified 5%
CO2 incubator. The pEGFP-N1 plasmid (Clontech
Laboratories, Inc., Mountainview, CA, USA) was used to construct
the CDX2 expression vector. Total RNA was extracted from human GC
AGS cells using TRIzol® (Invitrogen Life Technologies,
Inc.). The resulting RNAs were treated with RNase-free DNase
(Promega Corporation, Madison, WI, USA) and 800 ng RNA was reverse
transcribed into cDNA using the PrimeScript RT Reagent kit (Takara
Biotechnology Co., Ltd., Dalian, China), according to the
manufacturer's instruc tions. CDX2 cDNA was amplified using a
reverse transcription-polymerase chain reaction (RT-PCR) kit
(Invitrogen Life Technologies, Inc.), according to the
manufacturer's instructions. The complete cDNA sequence of CDX2 was
amplified by RT-PCR using primers, which were synthesized by Sangon
Biotech Co., Ltd. (Shanghai, China). The sequences of the primers
were as follows: Forward 5′-TTTTCTCGAG ATGTACGTGAGCTACCTCCTG-3′,
which introduced an XhoI cleavage site sequence (CTGCTC),
and reverse 5′-TTTT AAGCTTCTGGGTGACGGTGGGGTTTAG-3′ which introduced
a HindIII cleavage site sequence (CTTAAG). The CDX2 cDNA PCR
product and pEGFP-N1 were digested using XhoI/HindIII
(Fermentas, Thermo Fisher Scientific, Inc., Vilnius, Lithuania).
The PCR amplification (Bio-Rad T100, Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) conditions were as follows: Initial denaturation
at 94°C for 2 min, followed by 25 cycles of 94°C for 60 sec, 55°C
for 60 sec and 72°C for 2 min, followed by a final extension step
at 72°C for 5 min. Subsequently, 5 µl PCR products were
electrophoresed on 1% agarose gel. The PCR products were purified
using the DNA Purification kit (Beyotime Institute of
Biotechnology, Nantong, China), according to the manufacturer's
instructions, and ligated into the cut vector to form
pEGFP-N1-CDX2. Following ligation, the plasmid was transformed into
E. coli DH5α cells (Takara Biotechnology Co., Ltd.), and
plated on solid lysogeny broth medium (containing kanamycin;
Yaopharma Co., Ltd., Chongqing, China). Kanamycin-resistant
colonies were cultured at 37°C overnight with agitation. The
recombinant plasmid was prepared, and the sequences were verified
by electrophoresis of the digested product. pEGFP-N1-CDX2 was
transfected into GC MKN-45 cells using Lipofectamine™ 2000 reagent
(Invitrogen Life Technologies, Inc.) according to the
manufacturer's instructions. Briefly, 24 h prior to transfection,
cells were seeded into six-well plates (5×105
cells/well) and inoculated in complete medium without antibiotics
(2 ml/well). At 70–80% confluence, the plasmid DNA (4.0
µg/well) and transfection reagent (10 µl/well) were
diluted with RPMI-1640 (250 µl) and incubated at room
temperature for 5 min. The transfection reagent and dilution of the
plasmid DNA were then mixed and incubated at room temperature for
20–30 min. Next, the transfection complexes were filled into
six-well plates, supplementary medium was added at 2 ml/well and
plates were cultured at 37°C in a humidified 5% CO2
incubator. Four to six hours after transfection, the supernatant
was replaced with DMEM containing 10% FBS followed by further
cultivation.
RT-quantitative (q)PCR
The total RNA from cultured cells was extracted
using the TRIzol® reagent extraction kit (Invitrogen
Life Technologies, Inc.) according to the manufacturer's
instructions. Amplification was performed in a 7500 Real-Time PCR
System (Applied Biosystems, Thermo Fisher Scientific, Waltham, MA,
USA) using the QuantiTect SYBR Green PCR kit (Qiagen, Hilden,
Germany). PCR was performed as previously described using primers
specific for β-actin and CDX2 (20). The β-actin gene was adopted as an
internal control for all RT-qPCR reactions.
Western blot analysis
The cells were lysed using radioimmunoprecipitation
buffer and phenylmethylsulfonyl fluoride (all Beyotime Institute of
Biotechnology) for 30 min on ice. The protein extracts were
subsequently centrifuged at 12,800 x g for 20 min at 4°C, and the
protein concentration was determined using a bicinchoninic acid
protein assay kit (Beyotime Institute of Biotechnology). Equal
amounts (40 µg) of protein from various samples were
separated by 12% SDS-PAGE (Beyotime Institute of Biotechnology) and
electro-transferred onto polyvinylidene fluoride (PVDF) membranes
(EMD Millipore, Billerica, MA, USA) using the Mini Protean 3 system
(Bio-Rad Laboratories, Inc.). PVDF membranes were blocked with
phosphate-buffered saline (PBS) containing 5% skimmed milk powder
for 2 h and incubated with the following primary antibodies: Mouse
anti-CDX2 monoclonal antibody (cat. no. 60243-1-Ig), rabbit
anti-E-cadherin polyclonal antibody (cat. no. 20874-1-AP) and
rabbit anti-vimentin polyclonal antibody (cat. no. 10366-1-AP)
(1:2,000; all Proteintech Group, Inc., Chicago, IL, USA), as well
as the internal control mouse anti-β-actin monoclonal antibody
(1:2,000; cat. no. M20010; Abmart, Inc., Shanghai, China) at 4°C
overnight. The membranes were then incubated for 2 h at room
temperature with horseradish peroxidase-conjugated anti-mouse (cat.
no. 115-032-003) or anti-rabbit (cat. no. 111-035-003)
immunoglobulin G secondary antibodies (Jackson ImmunoResearch
Europe Ltd., Newmarket, UK), diluted at 1:5,000. The protein was
visualized using an enhanced chemiluminescent detection system,
according to the manufacturer's instructions (GE Healthcare Life
Sciences, Little Chalfont, UK). Following chemiluminescence, the
membrane was exposed to X-ray film (Eastman Kodak, Rochester, NY,
USA), and ImageJ 1.37 software (National Institutes of Health,
Bethesda, MD, USA) was used for grey value analysis. Relative
amounts of proteins were quantified by absorbance analysis, and the
level was normalized to β-actin.
Cell counting kit (CCK)-8 assay
Cell proliferation was determined using a CCK-8
(Beyotime Institute of Biotechnology) according to the
manufacturer's instructions. Briefly, cells (5×107
cells/l) suspended in DMEM (100 µl) containing 10% FBS were
seeded in 96-well plates and incubated for 24, 48 or 72 h. CCK-8
solution (10 µl) was added to each well and the cultures
were incubated at 37°C and 5% CO2 for 2.5 h. The
absorbance was determined at 450 nm using a multi-mode microplate
reader (Varioskan Flash; Thermo Fisher Scientific).
Colony formation assay
The colony formation assay was performed as
described previously (21).
Briefly, 100 cells were seeded onto each of the 35-mm dishes and
allowed to grow for two weeks to assess colony formation on the
culture plates. Cell colonies were stained with 0.5% crystal violet
(Amresco LLC, Solon, OH, USA) for 5 min at room temperature.
Colonies of >50 cells were counted under an upright light
microscope (BX51; Olympus Corporation, Tokyo, Japan; magnification,
×100) and the mean value was calculated.
Migration and invasion assays
For the cell migration ability assays,
5×105 cells transiently transfected for 24 h in the
logarithmic growth phase were seeded onto six-well plates. The
cells were cultured to form confluent cell monolayers and then
wounded by performing a horizontal scratch with a pipette tip.
Non-adherent cells were gently removed by rinsing with PBS. Wound
closure was monitored using an inverted fluorescence microscope
(IX71; Olympus Corporation; magnification, ×100) at various
time-points. Migration activity was calculated as the mean distance
between the edges of three points. Healing rate = (mean original
distance - mean distance at a time-point)/mean original distance
×100%.
For the invasion assays, 1×105 cells
transiently transfected for 48 h were seeded in the top chamber of
a Transwell plate containing a Matrigel-coated membrane (24-well
insert; 8 mm pore size; BD Biosciences, Franklin Lakes, NJ, USA).
Serum-free medium was used for the cells in the upper chamber
containing the cells, and medium supplemented with 20% (v/v) FBS
was used as a chemoattractant in the lower chamber. The cells were
incubated for 24 h at 37°C and in a 5% (v/v) CO2
incubator. Following incubation, the non-invading cells were
removed from the upper side of the Transwell membrane filter
inserts using cotton-tipped swabs. The invaded cells on the lower
side of the inserts were stained with Giemsa, according to the
manufacturer's instructions (Wright-Giemsa Stain kit; Nanjing
Jiancheng Bioengineering Institute, Nanjing, China). Visual fields
(n=15) of each insert were randomly counted under an upright light
microscope (BX51; Olympus Corporation) and the mean value was
calculated.
Tumorigenic assay in nude mice
The present study was approved by the ethics
committee of the Affiliated Hospital of Nantong University
(Nantong, China). The experiments were performed according to the
Nantong University Institutional Animal Care and Use Committee. To
analyze the tumorigenicity of GC cells in vivo, MKN45 cells
at twenty-four hours after transfection were diluted to 1:4 for
passage and MKN45/CDX2 stable cell lines were screened by
administration of G418 (Invitrogen Life Technologies, Inc.).
Four-week-old male or female BALB/c nu/nu nude mice were purchased
from the Center of Laboratory Animal Science, Chinese Academy of
Medical Science (Shanghai, China). According to the experimental
animal guidelines, the mice were maintained in 24°C
temperature-controlled and specific pathogen-free conditions; they
were housed separately, and were fed sterilized food and autoclaved
water. The mice were subcutaneously injected in the dorsal flanks
with 2×107 MKN45/CDX2, empty-vector (EV) transfected
MKN45/EV or native MKN45 cells, respectively, suspended in 200
µl PBS. Mice were weighed, and tumor width and length were
measured with calipers three times a week. Mice were sacrificed at
seven weeks after cell inoculation. Tumor volume was calculated
using the formula V (mm3) = (width2 ×
length)/2. The mice were sacrificed by cervical dislocation 7 weeks
after cell inoculation, and the tumor tissue samples were taken to
assess the protein expression levels of vimentin and E-cadherin.
The tumor tissues were homogenized using an ultrasonic processor
(S-450D; Branson Ultrasonics Corporation, Danbury, CT, USA) for
tissue lysate extraction, the tissue lysates were centrifuged at
12,800 × g for 20 min at 4°C and the supernatants were collected.
Equal quantities (150 µg) of protein were loaded per lane
for western blot analysis.
Statistical analysis
All experiments were repeated at least three times.
Statistical analysis was performed using SPSS 19.0 (SPSS, Inc.,
International Business Machines, Armonk, NY, USA). Values are
expressed as the mean ± standard deviation and groups were compared
using one-way analysis of variance. P<0.05 was considered to
indicate a statistically significant difference.
Results
Construction of the pEGFP-N1-CDX2
recombinant vector and transient transfection
A previous study showed that the expression of CDX2
on MKN-45 cells was low (19). To
examine the effects of CDX2 on gastric cancer cells in
vitro, the plasmid pEGFP-N1 was used to construct the CDX2
expression vector, pEGFP-N1-CDX2, to be transfected into the MKN45
cells to create the engineered cell line MKN45/CDX2 with forced
overexpression of CDX2 protein. A control cell line was transfected
with the empty vector and named as MKN45/EV. The transfection
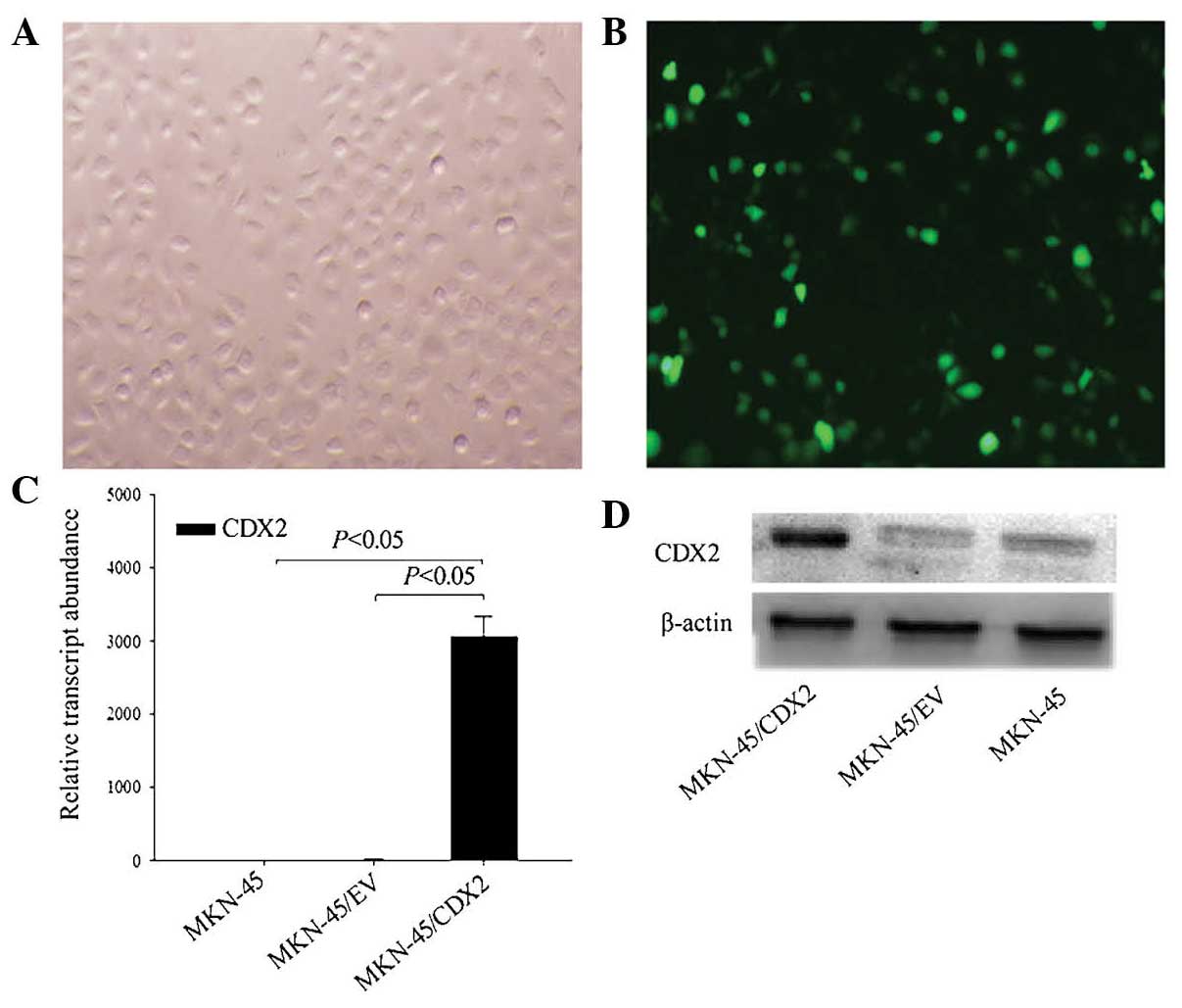
efficiency was confirmed by fluorescence microscopy observation.
The results showed that >80% cells were labeled with EGFP,
indicating that MKN45 cells had been successfully transfected with
pEGFP-N1-CDX2 (Fig. 1A and B).
mRNA and protein levels of CDX2 in MKN45 cells were validated by
RT-qPCR and western blot analysis, respectively. Compared with the
control groups (MKN45/EV and MKN45), CDX2 mRNA and protein
expression were significantly enhanced in MKN45/CDX2 cells
(P<0.05; Fig. 1C and D).
CDX2 suppresses gastric cancer cell
proliferation
To investigate the effects of CDX2 overexpression on
the growth of MKN45 cells, cell viability was assessed using the
CCK-8 assay. The results showed that high expression of CDX2 in the
MKN45/CDX2 group significantly inhibited the cell proliferation
compared with that in the MKN45/EV group and the untransfected
MKN45 group (P<0.05; Table I).
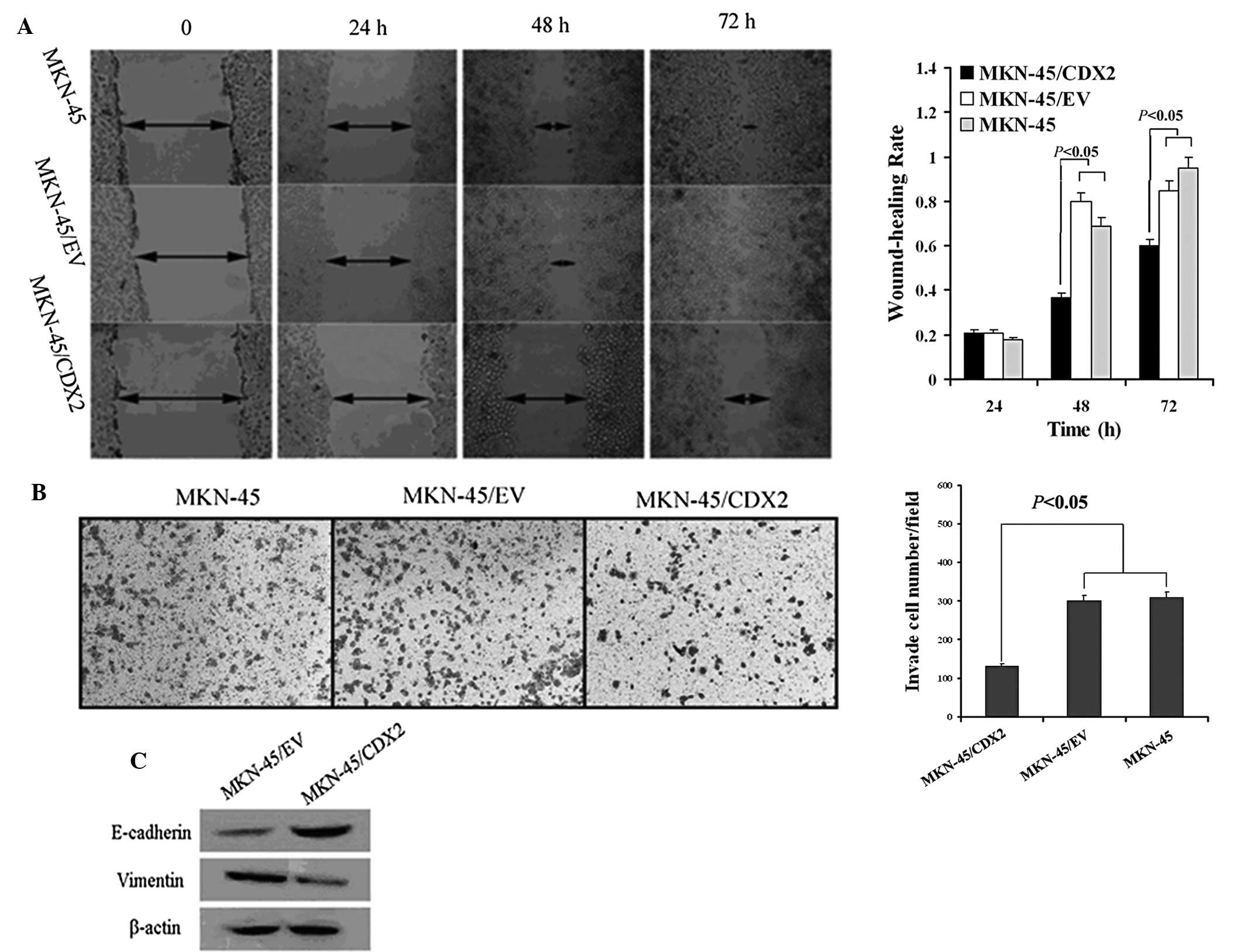
These results were further supported by the colony formation assay.
The three cell lines were all able to form colonies in soft
agarose; however, the number of colonies formed by MKN45/CDX2 cells
after two weeks of culture was significantly decreased compared
with that in the MKN45/EV and MKN45 cells (P<0.05; Fig. 2A and B).
 | Table ICell viability measured by cell
counting kit 8 assay at various times. |
Table I
Cell viability measured by cell
counting kit 8 assay at various times.
| Group | 0 h
| 24 h
| 48 h
| 72 h
|
|---|
| A450 value | A450 value | IR (%) | A450 value | IR (%) | A450 value | IR (%) |
|---|
| MKN-45 | 0.714±0.040 | 0.968±0.046 | 0 | 1.247±0.030 | 0 | 1.376±0.040 | 0 |
| MKN-45/EV | 0.693±0.023 | 0.961±0.048 | 1.166±2.292 | 1.209±0.046 | 3.206±2.487 | 1.324±0.026 | 3.590±1.150 |
| MKN-45/CDX2 | 0.687±0.054 | 0.840±0.044 |
13.430±1.097a | 0.922±0.050 |
25.749±3.224a | 1.023±0.065 |
25.834±3.139a |
CDX2 inhibits migration and invasion and
reverses the EMT phenotype of gastric cancer cells in vitro
The scratch-wound assay was used to compare the
scratch width at 0, 24, 48 and 72 h. The results showed that
MKN45/CDX2 cells had a decreased migratory ability at the 48 and 72
h time-points compared to that of the control groups MKN45/EV and
MKN45 (P<0.05; Fig. 3A).
Furthermore, the invasion ability of three groups was assessed
using a Transwell® assay. The number of cells that had
transgressed through the membrane was counted following
transfection for 48 h. The results showed that the numbers of
MKN45/EV and MKN45 cells that had invaded through the membrane of
the Matrigel chamber were ~2.3-fold higher than that in the
MKN45/CDX2 group (P<0.05; Fig.
3B).
To study the effects of CDX2 on the EMT of MKN45
cells, the expression of E-cadherin and vimentin was determined
using western blot analysis. MKN45/CDX2 cells overexpressing CDX2
exhibited a significant upregulation of E-cadherin protein
expression and a significant downregulation of vimentin protein
expression (P<0.05; Fig.
3C).
CDX2 regresses gastric tumor xenograft
growth and inhibits EMT in nude mice
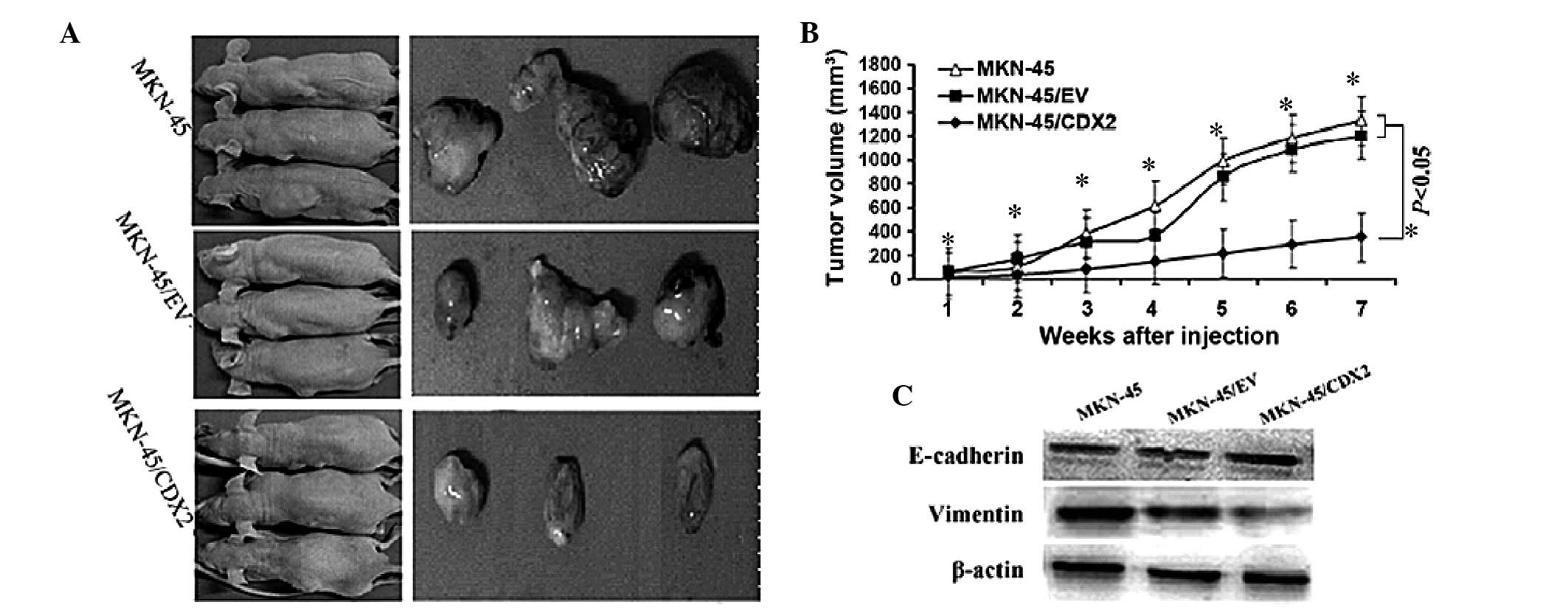
To assess the pathophysiological role of CDX2 in
gastric tumor xenograft growth, in vivo tumorigenicity
assays were performed, which showed that mice injected with
MKN45/CDX2 cells developed smaller and slower-growing tumors when
compared to those in animals injected with the control MKN45/EV and
MKN45 cells (P<0.05; Fig. 4A and
B; Table II). Furthermore,
western blot analysis of the tumor tissue showed that MKN45/CDX2
cells exhibited a significant upregulation of E-cadherin protein
and a significant downregulation of vimentin protein in nude mice
(P<0.05; Fig. 4C).
 | Table IITumor volume. |
Table II
Tumor volume.
| Weeks after
injection | MKN-45/CDX2
(mm3) | MKN-45/EV
(mm3) | MKN-45
(mm3) |
|---|
| 1 | 28.02±3.52a | 62.09±1.89 | 59.40±5.88 |
| 2 | 38.44±3.13a | 170.60±4.98 | 103.96±8.10 |
| 3 | 83.69±4.36a | 313.10±13.09 | 379.4±18.30 |
| 4 | 151.15±4.66a | 361.27±32.12 | 620.08±16.65 |
| 5 | 218.58±8.68a | 857.15±18.20 | 989.09±24.20 |
| 6 | 291.62±7.97a | 1092.84±19.53 | 1181.01±21.97 |
| 7 |
352.88±26.11a | 1205.06±34.08 | 1329.99±24.16 |
Discussion
The present study used the plasmid pEGFP-N1 to
construct a CDX2 expression vector. MKN45 cells were successfully
transfected with pEGFP-N1-CDX2, and CDX2 was overexpressed in
MKN45/CDX2 cells in vitro. Compared with the MKN45/EV group
and MKN45 group, the CCK-8 assay revealed that high expression of
CDX2 in the MKN45/CDX2 group significantly inhibited cell
proliferation, and a colony formation assay also showed that the
number of colonies formed by MKN45/CDX2 cells after two weeks of
culture was significantly decreased. Furthermore, migration and
invasion assays demonstrated that MKN45/CDX2 cells showed a lower
migration and invasion ability compared to those in the MKN45/EV
and MKN45 control groups. All of these results suggested that in
vitro, forced expression of CDX2 in MKN45/CDX2 cells inhibited
GC-cell growth and proliferation, as well as reduced migration and
invasion. These results are consistent with those of a previous
study (22), while the
pathophysiological role of CDX2 in gastric tumors had not been
reported previously. In the present study, in vivo
tumorigenicity assays showed that mice injected with MKN45/CDX2
cells developed smaller and slower-growing tumors compared to those
in animals injected with MKN45/EV and MKN45 cells.
Abnormal proliferation of epithelial cells and
angiogenesis are hallmarks of the initiation and early growth of
primary epithelial cancer (23).
With further onset of the disease, cancer cells acquire invasive
properties at the last stages of the multi-step process eventually
leading to metastatic dissemination, which has life-threatening
consequences. Several studies have proposed the activation of an
EMT program as the crucial mechanism for the acquisition of
malignant phenotypes by epithelial cancer cells (24,25).
During the EMT, a polarized epithelial cell, which normally
interacts with the basement membrane via its basal surface,
undergoes multiple biochemical changes in order to transform into a
mesenchymal cell phenotype, which is characterized by an enhanced
migratory capacity, invasiveness, elevated resistance to apoptosis
and markedly increased production of ECM components (9). As cancer cells lose their epithelial
phenotype and acquire a mesenchymal phenotype, intercellular
adhesions are decreased, leading to their detachment from the tumor
mass and invasion of neigh-boring tissue, blood or lymph vessels,
which is a basis for the development of secondary tumors (25,26).
Cells undergoing EMT lose their structural adhesion
components, including E-cadherin, and gain mesenchymal cell markers
such as vimentin, which are hallmarks of the EMT (9,27).
It has been reported that aberrant activation of the EMT
contributes to tumour progression and metastasis, and the EMT has
been associated with poor prognosis in several types of cancer
(28–32).
CDX2 is a nuclear homeobox transcription factor that
belongs to the caudal-related family of CDX homeobox genes
(11). Several studies have
reported that loss of the expression of epithelial marker CDX2 in
colorectal cancer correlated with high tumor grade, microsatellite
instability, positive lymph node metastasis or advanced tumor stage
(33–35). Furthermore, loss of CDX2 and
E-cadherin as well as vimentin overexpression were observed in
rhabdoid colorectal tumors but not in normal mucosa or adenomas
(36). These results indicated
that loss of CDX2 contributed to aggressive tumor behavior and
increase the likelihood of metastatic disease. A recent study
suggested that there was a significant link between sialyl Lewis x
and sialyl Lewis a (sLex/a) expression and the EMT in
colon cancer cells; furthermore c-Myc and CDX2 were shown to have a
pivotal role in regulating sLex/a expression during the
EMT (37).
Although certain evidence has indicated that CDX2 is
a tumor suppressor in GC (38),
the association between CDX2 and metastasis has remained to be
fully elucidated. A clinical study by Okayama et al
(17) reported that the loss of
CDX2 expression in GC tissues was significantly correlated with
lymph node metastasis. Forced expression of CDX2 in GC cells has
been reported to inhibit proliferation and induce apoptosis in
vitro (22), while the
association between CDX2 expression and EMT has remained
elusive.
The present study demonstrated in vitro and
in vivo that MKN45/CDX2 cells overexpressing CDX2 exhibited
a significant upregulation of E-cadherin protein expression and a
significant downregulation of vimentin protein expression. Numerous
animal studies and cell experiments have demonstrated that cancer
cells can acquire a mesenchymal phenotype and express mesenchymal
markers, including α-smooth muscle actin, fibroblast-specific
protein 1, vimentin and desmin (39). These cells are typically found at
the invasive front of primary tumors and are considered to be the
cells that eventually enter into subsequent steps of the
invasion-metastasis cascade, including intravasation, transport
through the circulation, extravasation, formation of
micrometastases, and ultimately the growth of small colonies into
macroscopic metastases (24,40,41).
In conclusion, the results of the present study
demonstrated that forced expression of CDX2 in human GC cells is
capable to inhibit growth and invasion, and reverse the EMT in
vitro and in vivo. The reduced capacities of migration
and invasion may be correlated with the reversal of the EMT.
However, the precise underlying molecular mechanisms remain elusive
and require further study.
Acknowledgments
This study was supported by the Technologies
Research Program of the Affiliated Hospital of Nantong University
(no. TDFY0343).
References
|
1
|
Bertuccio P, Chatenoud L, Levi F, Praud D,
Ferlay J, Negri E, Malvezzi M and La Vecchia C: Recent patterns in
gastric cancer: A global overview. Int J Cancer. 125:666–673. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferlay J, Steliarova-Foucher E,
Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D and
Bray F: Cancer incidence and mortality patterns in Europe:
Estimates for 40 countries in 2012. Eur J Cancer. 49:1374–1403.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang L: Incidence and mortality of gastric
cancer in China. World J Gastroenterol. 12:17–20. 2006.PubMed/NCBI
|
|
5
|
Chen W, Zheng R, Zhang S, Zhao P, Li G, Wu
L and He J: Report of incidence and mortality in China cancer
registries, 2009. Chin J Cancer Res. 25:10–21. 2013.PubMed/NCBI
|
|
6
|
Zeng H, Zheng R, Guo Y, Zhang S, Zou X,
Wang N, Zhang L, Tang J, Chen J, Wei K, et al: Cancer survival in
China, 2003–2005: A population-based study. Int J Cancer.
136:1921–1930. 2015. View Article : Google Scholar
|
|
7
|
Shi Y and Zhou Y: The role of surgery in
the treatment of gastric cancer. J Surg Oncol. 101:687–692. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Meyer HJ and Wilke H: Treatment strategies
in gastric cancer. Dtsch Arztebl Int. 108:698–705. 2011.PubMed/NCBI
|
|
9
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Celià-Terrassa T, Meca-Cortés O, Mateo F,
de Paz AM, Rubio N, Arnal-Estapé A, Ell BJ, Bermudo R, Díaz A,
Guerra-Rebollo M, et al: Epithelial-mesenchymal transition can
suppress major attributes of human epithelial tumor-initiating
cells. J Clin Invest. 122:1849–1868. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chung JY, Davis JA, Price BD, Staley DM,
Wagner MV, Warner SL, Bearss DJ and Hansen MD: Competitive
enhancement of HGF-induced epithelial scattering by accessory
growth factors. Exp Cell Res. 317:307–318. 2011. View Article : Google Scholar
|
|
12
|
Saad RS, Ghorab Z, Khalifa MA and Xu M:
CDX2 as a marker for intestinal differentiation: Its utility and
limitations. World J Gastrointest Surg. 3:159–166. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moskaluk CA, Zhang H, Powell SM, Cerilli
LA, Hampton GM and Frierson HF Jr: Cdx2 protein expression in
normal and malignant human tissues: An immunohistochemical survey
using tissue microarrays. Mod Pathol. 16:913–919. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Park do Y, Srivastava A, Kim GH,
Mino-Kenudson M, Deshpande V, Zukerberg LR, Song GA and Lauwers GY:
CDX2 expression in the intestinal-type gastric epithelial
neoplasia: Frequency and significance. Mod Pathol. 23:54–61. 2010.
View Article : Google Scholar
|
|
15
|
Song JH, Kim CJ, Cho YG, Chae JS, Cao Z,
Nam SW, Lee JY and Park WS: Genetic alterations of the Cdx2 gene in
gastric cancer. APMIS. 116:74–80. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mizoshita T, Tsukamoto T, Nakanishi H,
Inada K, Ogasawara N, Joh T, Itoh M, Yamamura Y and Tatematsu M:
Expression of Cdx2 and the phenotype of advanced gastric cancers:
Relationship with prognosis. J Cancer Res Clin Oncol. 129:727–734.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Okayama H, Kumamoto K, Saitou K, Hayase S,
Kofunato Y, Sato Y, Miyamoto K, Nakamura I, Ohki S, Sekikawa K, et
al: CD44v6, MMP-7 and nuclear Cdx2 are significant biomarkers for
prediction of lymph node metastasis in primary gastric cancer.
Oncol Rep. 22:745–755. 2009.PubMed/NCBI
|
|
18
|
Qin R, Wang NN, Chu J and Wang X:
Expression and significance of homeodomain protein Cdx2 in gastric
carcinoma and precancerous lesions. World J Gastroenterol.
18:3296–3302. 2012.PubMed/NCBI
|
|
19
|
Wang XT, Wei WY, Kong FB, Lian C, Luo W,
Xiao Q and Xie YB: Prognostic significance of Cdx2
immunohistochemical expression in gastric cancer: A meta-analysis
of published literatures. J Exp Clin Cancer Res. 31:982012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang JF, Zhang JG, Kuai XL, Zhang H,
Jiang W, Ding WF, Li ZL, Zhu HJ and Mao ZB: Reactivation of the
homeotic tumor suppressor gene CDX2 by
5-aza-2′-deoxycytidine-induced demethylation inhibits cell
proliferation and induces caspase-independent apoptosis in gastric
cancer cells. Exp Ther Med. 5:735–741. 2013.PubMed/NCBI
|
|
21
|
Franken NA, Rodermond HM, Stap J, Haveman
J and van Bree C: Clonogenic assay of cells in vitro. Nat Protoc.
1:2315–2319. 2006. View Article : Google Scholar
|
|
22
|
Xie Y, Li L, Wang X, Qin Y, Qian Q, Yuan X
and Xiao Q: Overexpression of Cdx2 inhibits progression of gastric
cancer in vitro. Int J Oncol. 36:509–516. 2010.PubMed/NCBI
|
|
23
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Książkiewicz M, Markiewicz A and Zaczek
AJ: Epithelial-mesenchymal transition: A hallmark in metastasis
formation linking circulating tumor cells and cancer stem cells.
Pathobiology. 79:195–208. 2012. View Article : Google Scholar
|
|
26
|
Matsuoka J, Yashiro M, Doi Y, Fuyuhiro Y,
Kato Y, Shinto O, Noda S, Kashiwagi S, Aomatsu N, Hirakawa T, et
al: Hypoxia stimulates the EMT of gastric cancer cells through
autocrine TGFβ signaling. PLoS One. 8:e623102013. View Article : Google Scholar
|
|
27
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Elloul S, Vaksman O, Stavnes HT, Trope CG,
Davidson B and Reich R: Mesenchymal-to-epithelial transition
determinants as characteristics of ovarian carcinoma effusions.
Clin Exp Metastasis. 27:161–172. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kumarswamy R, Mudduluru G, Ceppi P,
Muppala S, Kozlowski M, Niklinski J, Papotti M and Allgayer H:
MicroRNA-30a inhibits epithelial-tomesenchymal transition by
targeting Snai1 and is downregulated in non-small cell lung cancer.
Int J Cancer. 130:2044–2053. 2012. View Article : Google Scholar
|
|
30
|
Chen ZL, Zhao XH, Wang JW, Li BZ, Wang Z,
Sun J, Tan FW, Ding DP, Xu XH, Zhou F, et al: microRNA-92a promotes
lymph node metastasis of human esophageal squamous cell carcinoma
via E-cadherin. J Biol Chem. 286:10725–10734. 2011. View Article : Google Scholar :
|
|
31
|
Sarrio D, Rodriguez-Pinilla SM, Hardisson
D, Cano A, Moreno-Bueno G and Palacios J: Epithelial-mesenchymal
transition in breast cancer relates to the basal-like phenotype.
Cancer Res. 68:989–997. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Korpal M, Ell BJ, Buffa FM, Ibrahim T,
Blanco MA, Celià-Terrassa T, Mercatali L, Khan Z, Goodarzi H, Hua
Y, et al: Direct targeting of Sec23a by miR-200s influences cancer
cell secretome and promotes metastatic colonization. Nat Med.
17:1101–1108. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kaimaktchiev V, Terracciano L, Tornillo L,
Spichtin H, Stoios D, Bundi M, Korcheva V, Mirlacher M, Loda M,
Sauter G, et al: The homeobox intestinal differentiation factor
CDX2 is selectively expressed in gastrointestinal adenocarcinomas.
Mod Pathol. 17:1392–1399. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Choi BJ, Kim CJ, Cho YG, Song JH, Kim SY,
Nam SW, Lee SH, Yoo NJ, Lee JY and Park WS: Altered expression of
CDX2 in colorectal cancers. APMIS. 114:50–54. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bakaris S, Cetinkaya A, Ezberci F and
Ekerbicer H: Expression of homeodomain protein CDX2 in colorectal
adenoma and adenocarcinoma. Histol Histopathol. 23:1043–1047.
2008.PubMed/NCBI
|
|
36
|
Pancione M, Remo A, Sabatino L, Zanella C,
Votino C, Fucci A, Di Blasi A, Lepore G, Daniele B, Fenizia F, et
al: Right-sided rhabdoid colorectal tumors might be related to the
serrated pathway. Diagn Pathol. 8:312013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sakuma K, Aoki M and Kannagi R:
Transcription factors c-Myc and CDX2 mediate E-selectin ligand
expression in colon cancer cells undergoing EGF/bFGF-induced
epithelial-mesenchymal transition. Proc Natl Acad Sci USA.
109:7776–7781. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Guo RJ, Suh ER and Lynch JP: The role of
Cdx proteins in intestinal development and cancer. Cancer Biol
Ther. 3:593–601. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang J and Weinberg RA:
Epithelial-mesenchymal transition: At the crossroads of development
and tumor metastasis. Dev Cell. 14:818–829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fidler IJ and Poste G: The 'seed and soil'
hypothesis revisited. Lancet Oncol. 9:8082008. View Article : Google Scholar
|
|
41
|
Brabletz T, Jung A, Reu S, Porzner M,
Hlubek F, Kunz-Schughart LA, Knuechel R and Kirchner T: Variable
beta-catenin expression in colorectal cancers indicates tumor
progression driven by the tumor environment. Proc Natl Acad Sci
USA. 98:10356–10361. 2001. View Article : Google Scholar : PubMed/NCBI
|