Introduction
Multiple sclerosis (MS) is a demyelinating
autoimmune disease of the central nervous system (CNS) in which the
natural repair process, remyelination, is often incomplete
(1,2). Current treatments for MS
predominantly modulate immunological pathways to suppress
inflammatory outbreaks. However, regenerative therapies are not
available.
FTY720, 2-amino-2-[2-(4-octylphenyl) ethyl]
propane-1,3-diol, clinically known as fingolimod
(Gilenya®), is an approved oral immunomodulatory therapy
in relapsing-remitting MS (3,4). The
mechanism of action is proposed to mainly affect lymphocyte
migration. Binding of FTY720-phosphate (FTY720-P) to
sphingosine-1-phosphate receptor 1 (S1P1) on lymphocytes causes
internalization and degradation of the surface receptor (5). Due to this functional antagonism,
infiltration of the CNS is prevented since lymphocytes cannot
respond to S1P gradients and do not egress from lymphoid tissue
(6,7).
As S1P1, 2, 3 and 5 were found to be expressed on
brain resident cells (8–10) and the lipophilic pre-drug FTY720
easily crosses the blood brain barrier (11), it is suggested that FTY720 may also
have direct effects within the CNS. Choi et al demonstrated
that FTY720 exerts its functions in experimental autoimmune
encephalomyelitis (EAE) via action on S1P1 receptors in astrocytes
(12). In vitro, FTY720-P
increases the migration of astrocytes and is involved in the
phosphorylation of extracellular signal-regulated kinase,
Ca2+ signaling, as well as mediation of phospholipase C
and adenylyl cyclase (13,14).
In the present study, the effects of FTY720-P on S1P
receptors as well as the expression of cytokines, chemokines and
growth factors was analyzed in primary murine astrocytes under
inflammatory conditions.
Materials and methods
Preparation and culture of
astrocytes
Astrocytes were prepared from newborn C57BL/6 mouse
brains as previously described (15). C57BL/6 mice were housed under
specific pathogen-free conditions in the central animal facility
(ZTL), Hannover Medical School (Hannover, Germany). All animal care
procedures were performed according to international guidelines on
the use of laboratory animals (16). The experimental procedures were
performed according to the German Animal Welfare Act and approved
by the Local Institutional Animal Care and Research Advisory
Committee of the Hannover Medical School and the Lower Saxony State
Office for Consumer Protection and Food Safety (approval ID nos.
§4-2012/09 and §4-2014/74). Neonatal mice (1–3 days old) were
sacrificed by decapitation and brains were collected. Following
removal of the olfactory bulbs and cerebellum, brains were freed
from meninges and dissociated mechanically and enzymatically (0.1%
trypsin). Cells from two brains were plated on poly-L-lysine
(Sigma-Aldrich, St. Louis, MO, USA) coated tissue flasks (75
cm2; Sarstedt, Nümbrecht, Germany) containing Dulbecco's
modified Eagle's medium (DMEM; Invitrogen Life Technologies,
Karlsruhe, Germany) supplemented with 10% fetal calf serum (FCS)
and 1% penicillin/streptomycin (medium referred to as
MGP+). MGP+ was changed after 24 h and on day
4 and 8. Following removal of loosely attached microglia on day 9
or 10 (shaking for 1–2 h in an orbital shaker) and oligodendrocyte
precursor cells at day 10 or 11 (shaking overnight in an orbital
shaker), the remaining astrocytes were treated with antimitotic
arabinosylcytosine (Ara-C; 100 µM, Sigma-Aldrich) to avoid
the growth of new oligodendrocytes and microglia. Medium containing
Ara-C was then removed after 72 h, cells were washed with
phosphate-buffered saline and harvested with 0.25% trypsin/0.05%
EDTA (PAA Laboratories GmbH, Coelbe, Germany). Astrocytes were
plated at the indicated cell densities for each experiment. These
cultures yielded a purity of ~99% as judged by glial fibrillary
acidic protein (GFAP) immunostaining.
Tumor necrosis factor (TNF)-α,
lipopolysaccharide (LPS) and FTY720-P stimulation
For all experiments, the phosphorylated form of
FTY720 (Cayman Chemicals, Ann Arbor, MI, USA) was reconstituted in
50 mM dimethyl sulfoxide hydrochloric acid (Sigma-Aldrich),
aliquoted and stored at −20°C. The final FTY720-P concentration for
treatment of astrocytes was 1 µM. This was based on previous
studies in vitro (14) and
in EAE rats (11). In our
experiments, FTY720-P was also assessed at concentrations of 0.01
and 0.1 µM (data not shown). However, the strongest effect
on gene expression was found with 1 µM FTY720-P.
To simulate inflammatory conditions, recombinant
murine TNF-α (20 ng/ml; PeproTech, Inc., Rocky Hill, NJ, USA) and
bacterial LPS (100 ng/ml, from Escherichia coli 0111:B4;
Sigma-Aldrich) were used. For the stimulations as well as
co-stimulations, all reagents were diluted in DMEM (Invitrogen Life
Technologies) supplemented with 10% FCS (Merck Millipore,
Darmstadt, Germany) and 1% penicillin/streptomycin to the final
concentrations.
Proliferation assay
To determine the number of astrocytes undergoing
cell division during 24 h of incubation, 1×104 cells
were seeded on uncoated 12 mm glass cover slips (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). After 24 h, astrocytes were
treated with MGP+±1 µM FTY720-P and incubated for
24 h. Dividing nuclei were then labeled with the monoclonal mouse
anti-human KI-67 antibody (1:300; cat. no. 550609; BD Biosciences,
San Jose, CA, USA) and 4′,6-diamidino-2-phenylindole (DAPI;
Invitrogen Life Technologies, Carlsbad, CA, USA) in a final
concentration of 1:1,000. Polyclonal rabbit anti-GFAP (1:300; cat.
no. Z 0334; Dako Denmark A/S, Glostrup, Denmark) antibody was used
as a marker for astrocytes. For quantification, the cover slips
were divided into six optic fields and three images per field were
analyzed in a blinded manner using an Olympus BX61 microscope
(Olympus, Tokyo, Japan). GFAP/KI-67 positive cells were counted and
set in relation to the total astrocyte number. Only cells with
DAPI-labeled nuclei were included in the analysis.
Isolation of RNA and reverse
transcription quantitative polymerase chain reaction (RT-qPCR)
For mRNA measurements, 3×105 astrocytes
were plated per well in 6-well plates (Nalgene Nunc, Rochester, NY,
USA) in MGP+. The medium was changed after 24 h. After 5
days of incubation, cells were treated for 3, 6, 12 or 24 h with
100 ng/ml LPS ± FTY720-P (1 µM) or 20 ng/ml TNF-α ± FTY720-P
or FTY720-P alone. Total RNA was isolated using the RNeasy Mini kit
(Qiagen, Valencia, CA, USA) according to the manufacturer's
instructions and the RNA concentration was measured with the
BioPhotometer plus (Eppendorf, Hamburg, Germany). cDNA was
synthesized using the High Capacity cDNA Reverse Transcription kit
(Applied Biosystems, Foster City, CA, USA). qPCR analysis was
performed using the StepOne™ Real Time PCR System (Invitrogen Life
Technologies) and appropriate TaqMan assays (Applied Biosystems;
see Table I). A negative control
containing PCR amplification mix without the reverse transcribed
cDNA template was included for each PCR plate. The ∆∆Ct method was
used to determine differences in the expression between untreated
and stimulated cells. The gene expression of S1P1, S1P3, S1P5,
interleukin-1β (IL-1β), chemokine (C-C motif) ligand 2 (CCL-2),
CCL-20, chemo-kine (C-X-C motif) ligand 12 (CXCL-12), insulin-like
growth factor (IGF)-1, ciliary neurotrophic factor (CNTF) and glial
cell line-derived neurotrophic factor (GDNF) was quantified against
the housekeeping gene hypoxanthine-guanine phosphoribosyl
transferase.
 | Table IPrimers used for quantitative
polymerase chain reaction. |
Table I
Primers used for quantitative
polymerase chain reaction.
| Gene | Gene expression assay
number |
|---|
| S1P1 | mm00514644_m1 |
| S1P3 | mm04229896_m1 |
| S1P5 | mm01177724_m1 |
| IL-1β | mm01336189_m1 |
| CCL-2 | mm00441242_m1 |
| CCL-20 | mm01268754_m |
| CXCL-12 | mm00445553_m1 |
| IGF-1 | mm00439560_m1 |
| CNTF | mm 00446373_m1 |
| GDNF | mm00599849_m1 |
| HPRT | mm00446968_m1 |
Statistical analysis
All data were plotted using GraphPad Prism version
5.02 (GraphPad Software, Inc., San Diego CA, USA). One-way analysis
of variance was used for statistical analysis followed by Fisher's
test for post hoc comparison. Values are presented as the
arithmetic mean ± standard error of the mean. P<0.05 was
considered to indicate a statistically significant difference.
Results
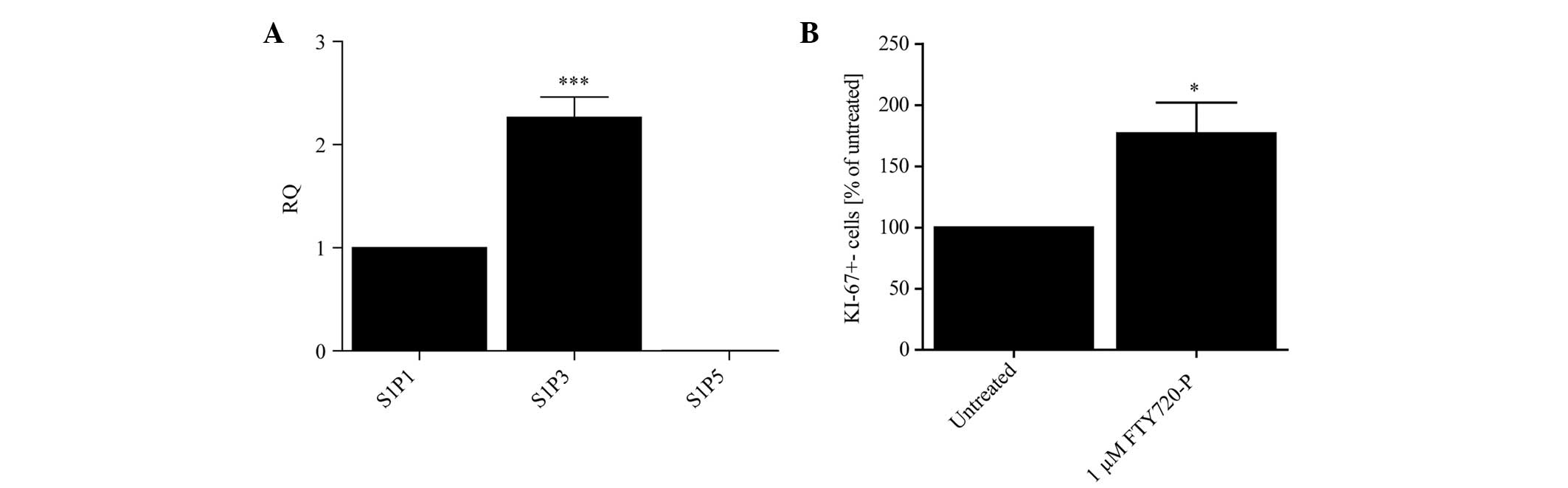
S1P receptors are expressed on astrocytes
and treatment with FTY720-P augments astrocytic proliferation
Initially, the level of mRNA of each S1P receptor
(S1P1, 3 and 5) on primary mouse astrocytes was evaluated under
basal conditions using qPCR. The expression levels in primary
murine astrocytes followed a pattern of S1P3>S1P1>S1P5 in
untreated cells (Fig. 1A). Due to
the fact that the mRNA levels of S1P5 were hardly detectable, this
receptor subtype was omitted from further experiments.
Based on previous studies (14,17,18)
the effect of 24 h treatment with FTY720-P on the proliferation of
cultured astrocytes compared with untreated control cells was
analyzed. Fig. 1B illustrates that
FTY720-P increased the proliferation rate of primary astrocytes as
measured by KI-67 immunostaining.
S1P receptor expression is increased
under pro-inflammatory conditions and is not affected by
FTY720-P
Inflammatory conditions were induced by stimulating
astrocytes with either LPS or the pro-inflammatory cytokine TNF-α
in the presence or absence of FTY720-P. After 3, 6, 12 and 24 h,
the mRNA levels of S1P1 and S1P3 were measured.
As shown in Fig. 2,
the mRNA levels of S1P1 and S1P3 increased significantly following
treatment with TNF-α or LPS compared with the untreated control
cells. In comparison with TNF-α or LPS treatment alone, no
significant differences in the expression of S1P1 or S1P3 were
observed following co-stimulation with LPS and FTY720-P or TNF-α
and FTY720-P (Fig. 2).
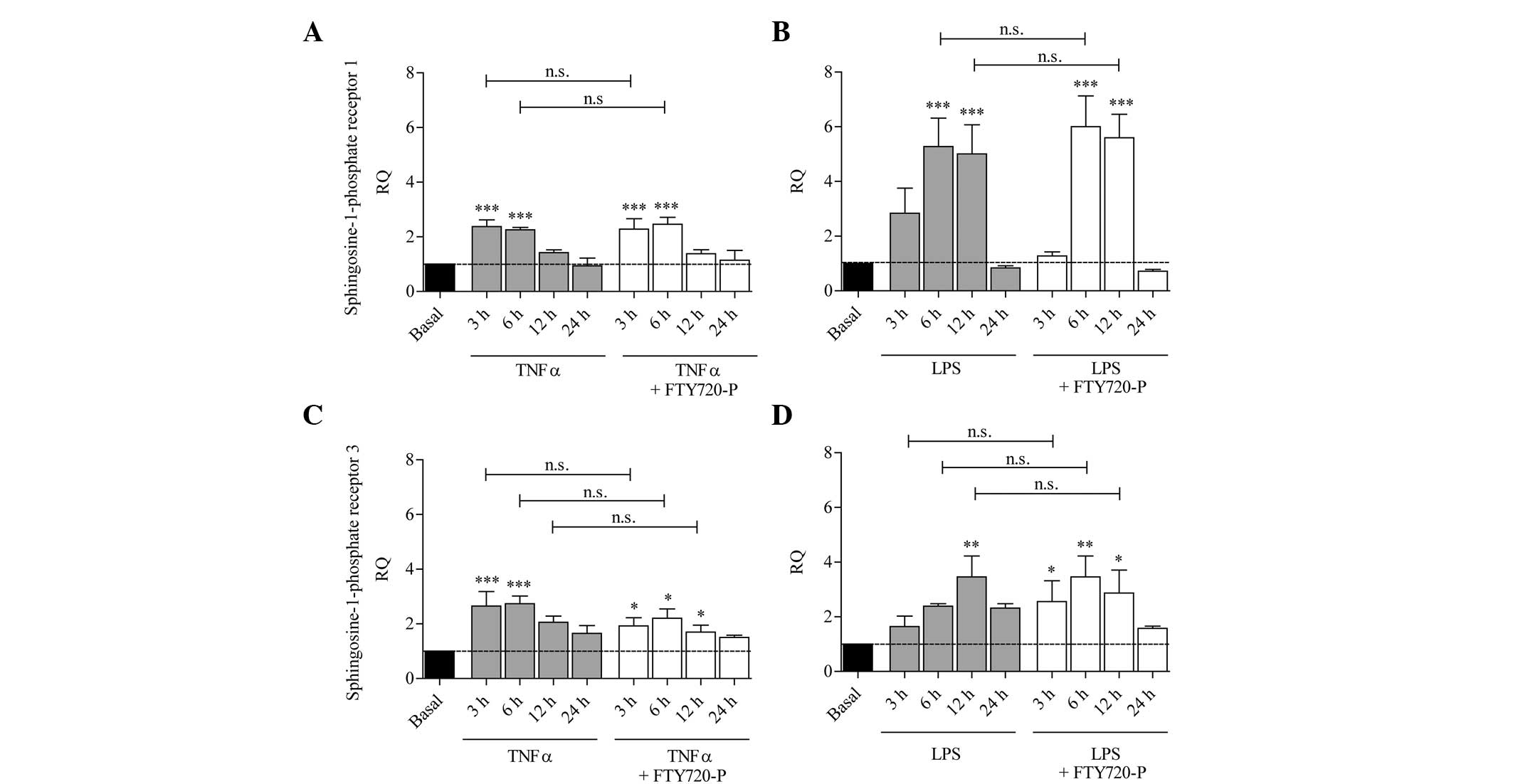 | Figure 2S1P receptor expression is induced
upon stimulation with TNF-α and LPS in cultured murine astrocytes
and is not affected by FTY720-P. The columns represent the RQ of
gene expression following stimulation with (A and C) TNF-α (grey
columns) or (B and D) LPS (grey columns), or following
co-stimulation with TNF-α/LPS and FTY720-P (white columns) for 3,
6, 12 or 24 h. The dashed line represents the basal expression
level in untreated astrocytes. Upon activation with TNF-α or LPS,
mRNA levels of (A and B) S1P1 and (C and D) S1P3 were upregulated
compared with untreated astrocytes (grey columns); this effect was
not altered in the presence of FTY720-P (white columns). Data from
between three and five experiments are presented as the mean ±
standard error of the mean. *P<0.05,
**P<0.005 and ***P<0.001, compared with
the basal expression level (black column). n.s., not significant;
SIP, sphingosine-1-phosphate; TNF-α, tumor necrosis factor-α; LPS,
lipopolysaccharide; RQ, relative quantity; FTY720-P,
FTY720-phosphate. |
Expression of cytokines and chemokines
under pro-inflammatory conditions and FTY720-P
Treatment with TNF-α or LPS induced an increase in
the expression of IL-1β (Fig. 3A and
B), CCL-2 (Fig. 3C and D) and
CXCL-12 (Fig. 4C and D).
Expression of CCL-20 was significantly increased after 3 h
treatment with TNF-α and subsequently decreased, whereas following
treatment with LPS an increased expression was only observed after
24 h treatment (Fig. 4A and
B).
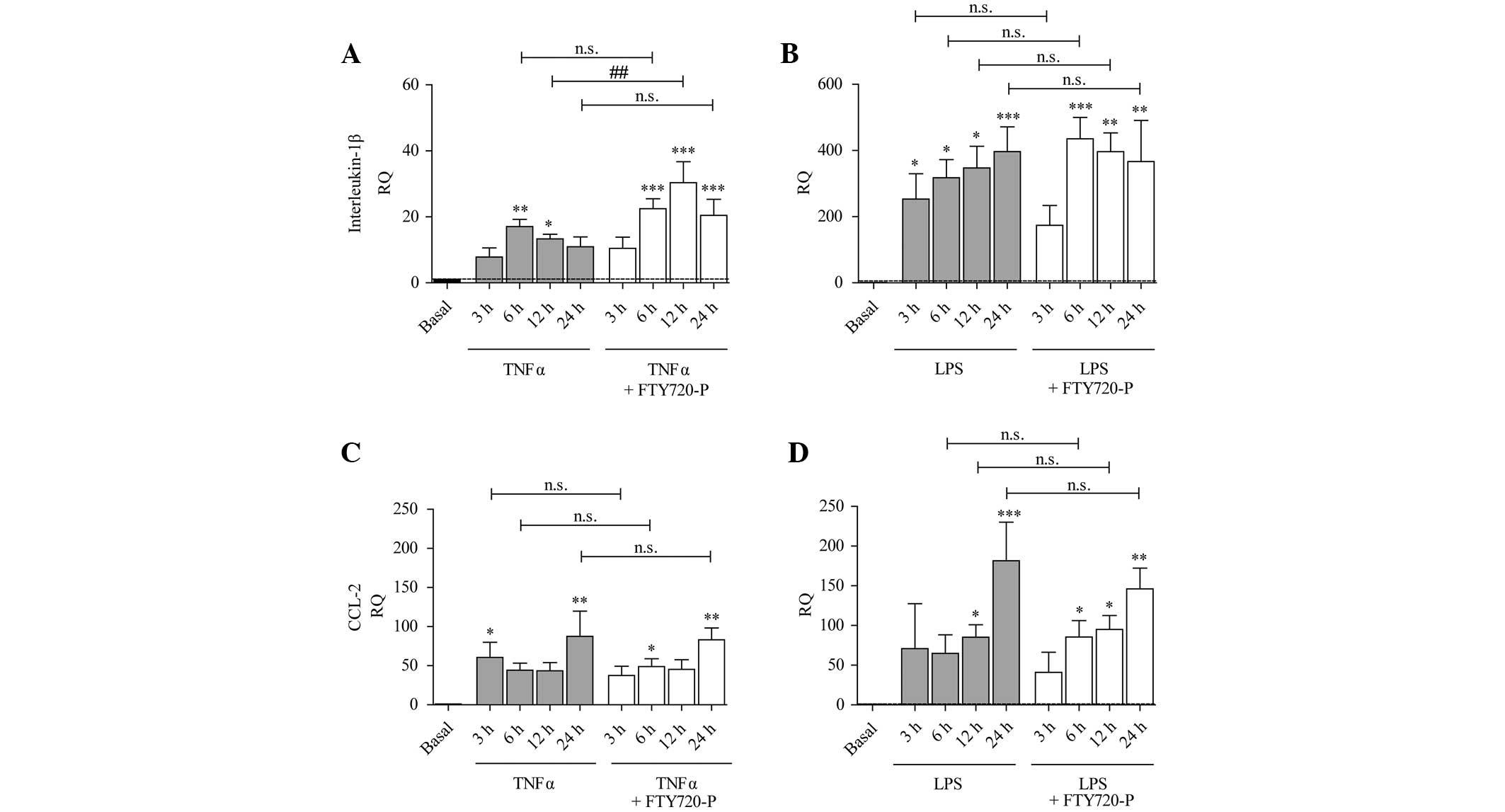 | Figure 3Expression of IL-1β and CCL-2 is
increased under pro-inflammatory conditions and FTY720-P augments
IL-1β expression after 12 h treatment. The columns represent the RQ
of gene expression following stimulation with (A and C) TNF-α (grey
columns) or (B and D) LPS (grey columns), or following
co-stimulation with TNF-α/LPS and FTY720-P (white columns) for 3,
6, 12 or 24 h. The dashed line represents the basal expression
level in untreated astrocytes. TNF-α and LPS treatment induced the
expression of (A and B) IL-1β and (C and D) CCL-2. The presence of
FTY720-P did not alter the expression of (C and D) CCL-2, but
potentiated TNF-α-induced expression of (A) IL-1β (white columns)
after 12 h treatment. Data from between three and five experiments
are presented as the mean ± standard error of the mean.
*P<0.05, **P<0.005 and
***P<0.001, compared with the basal expression level
(black column); ##P<0.005, compared with TNF-α alone. n.s., not
significant; IL, interleukin; CCL-2, chemokine (C-C-motif) ligand
2; RQ, relative quantity; TNF-α, tumor necrosis factor-α; LPS,
lipopolysaccharide; FTY720-P, FTY720-phosphate. |
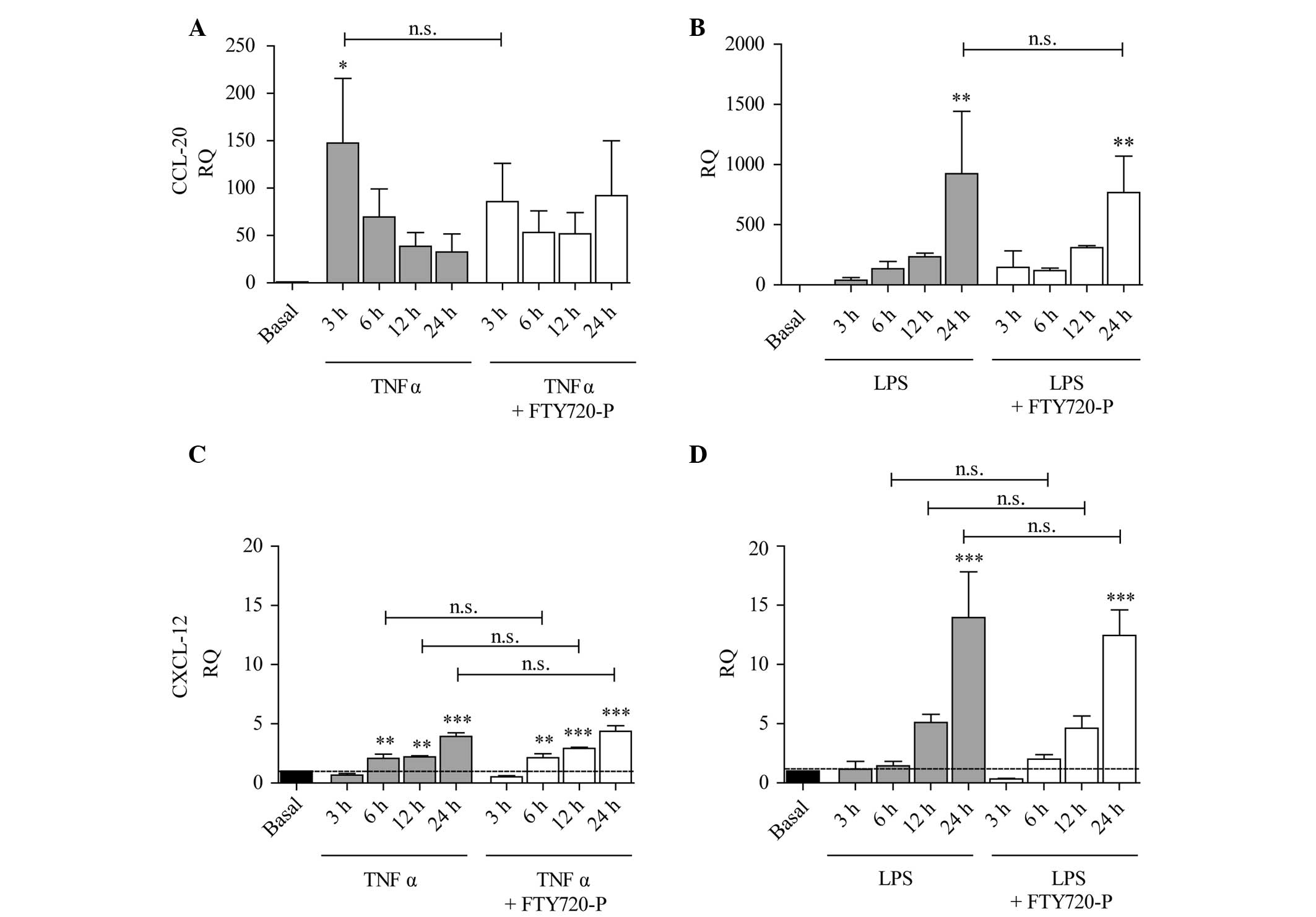 | Figure 4Expression of CCL-20 and CXCL-12 is
increased under pro-inflammatory conditions and is not affected by
FTY720-P. The columns represent the RQ of gene expression following
stimulation with (A and C) TNF-α (grey columns) or (B and D) LPS
(grey columns), or following co-stimulation with TNF-α/LPS and
FTY720-P (white columns) for 3, 6, 12 or 24 h. The dashed line
represents the basal expression level in untreated astrocytes.
Inflammatory stimulation by either TNF-α or LPS significantly
induced the expression of (A and B) CCL-20 and (C and D) CXCL-12.
Treatment with FTY720-P did not have any significant effects on the
inflammation-induced expression of (A and B) CCL-20 (white columns)
or (C and D) CXCL-12. Data from between three and five experiments
are presented as the mean ± standard error of the mean.
*P<0.05, **P<0.005 and
***P<0.001, compared with the basal expression level
(black). n.s., not significant; CCL-20, chemokine (C-C-motif)
ligand 20; CXCL-12, chemokine (C-X-C-motif) ligand 12; RQ, relative
quantity; LPS, lipopolysaccharide; TNF-α, tumor necrosis factor-α;
FTY720-P, FTY720-phosphate. |
Previous studies have suggested that FTY720-P
mediates effects on the inflammation-induced expression and
secretion of different types of cytokines and chemokines (19–21).
In the present study, treatment with FTY720-P did not affect the
inflammation-induced expression of CCL-2, CCL-20 or CXCL-12
(Figs. 3C, D and 4A–D). TNF-α-induced upregulation of IL-1β
was significantly augmented in the presence of FTY720-P only after
12 h (Fig. 3A). This effect was
not observed after 3, 6 or 24 h or following stimulation with LPS
and FTY720-P (Fig. 3B).
Expression of growth factors under
pro-inflammatory conditions and treatment with FTY720-P
The effect of TNF-α and LPS stimulation on the
expression of several growth factors was further evaluated.
The expression of IGF-1 was, similar to CNTF,
partially elevated following LPS treatment, whereas TNF-α evoked a
more prominent induction of gene expression (Fig. 5A–D). In comparison with TNF-α or
LPS treatment alone, FTY720-P did not induce any significant
alterations in the gene expression of IGF-1 or CNTF (Fig. 5A–D).
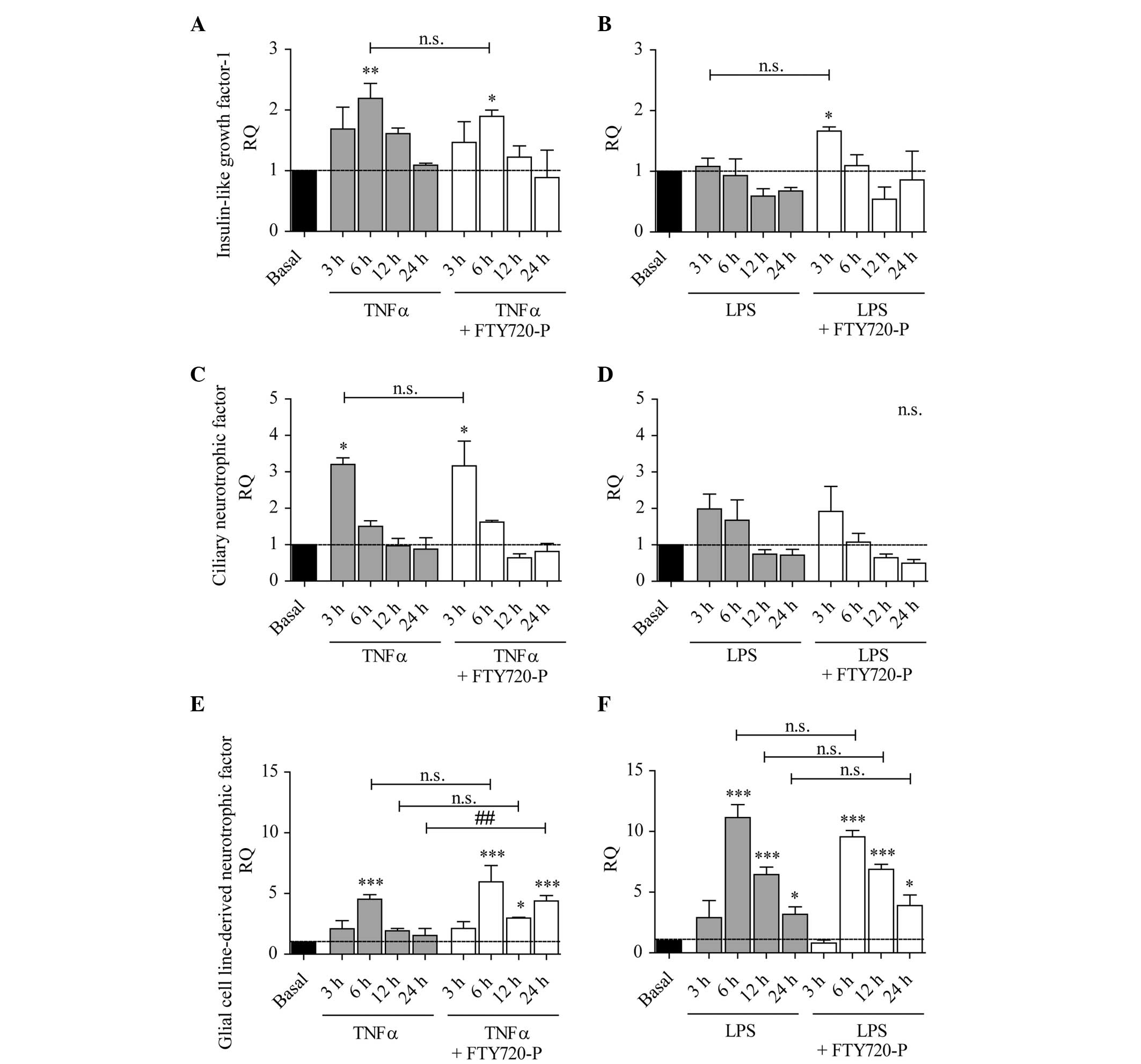 | Figure 5Expression of IGF-1, CNTF and GDNF is
increased under pro-inflammatory conditions and GDNF expression is
potentiated by FTY720-P after 24 h treatment. The columns represent
the RQ of gene expression following stimulation with (A, C and E)
TNF-α (grey columns) or (B, D and F) LPS (grey columns), or
following co-stimulation with TNF-α/LPS and FTY720-P (white
columns) for 3, 6, 12 or 24 h. The dashed line represents the basal
expression level in untreated astrocytes. (A) IGF-1 and (C) CNTF
mRNA levels were increased upon TNF-α stimulation (grey columns),
but not (B and D) LPS stimulation (grey columns). FTY720-P did not
alter the expression of (A-D) IGF-1 or CNTF (white columns). Strong
GDNF upregulation was induced by (E) TNF-α and (F) LPS (grey
columns). (E) The presence of FTY720-P augmented TNF-α-induced
expression of GDNF after 24 h stimulation (white columns). Data
from between three and five experiments are presented as the mean ±
standard error of the mean. *P<0.05,
**P<0.005 and ***P<0.001, compared with
the basal expression level (black column); ##P<0.005,
compared with TNF-α alone n.s., not significant; GDNF, glial cell
line-derived neurotrophic factor; IGF-1, insulin-like growth
factor-1; CNTF, ciliary neurotrophic factor; TNF-α, tumor necrosis
factor-α; LPS, lipopolysaccharide; RQ, relative quantity; FTY720-P,
FTY720-phosphate. |
GDNF gene expression has been demonstrated to be
induced via S1P receptor signaling in astrocytes (22). As illustrated in Fig. 5E and F, it was found that TNF-α and
LPS induced a significant increase in GDNF mRNA levels after 6 h
(TNF-α) and after 12 and 24 h (LPS). However, TNF-α-induced gene
expression decreased with time and mRNA levels returned to basal
levels at 24 h. Following stimulation with TNF-α and FTY720-P the
mRNA level was significantly higher after 24 h as compared with
treatment with TNF-α alone (Fig.
5E). However, this effect was not observed after 3, 6 or 12 h
treatment with TNF-α, nor following treatment with LPS and FTY720-P
(Fig. 5F).
Discussion
In the present study, qPCR was used to investigate
the impact of FTY720-P on inflammation-induced mRNA levels of S1P
receptors as well as selected cytokines, chemokines and growth
factors in primary murine astrocytes.
In our experiments, S1P1 and S1P3 mRNA was
upregulated under inflammatory conditions and FTY720-P did not
alter inflammation-induced increases in the receptors. Therefore,
it was assumed that, although the two receptors are important in
inflammation in astrocytes, FTY720-P is not involved in the
transcriptional regulation of these receptors. Although no
significant alterations in the expression of transforming growth
factor-1β or platelet-derived neurotrophic factor α were detected
following inflammatory stimuli (± FTY720-P) in astrocytes (data not
shown), the gene expression data demonstrated that TNF-α and LPS
upregulated IL-1β, CCL-2, CCL-20, CXCL-12, IGF-1, CNTF and GDNF in
cultured murine astrocytes. However, only small effects of FTY720-P
treatment on the inflammation-induced expression of S1P receptors,
cytokines and growth factors were observed.
Treatment with FTY720-P did not alter the TNF-α- or
LPS-induced increased expression levels of CCL-2, CCL-20 and
CXCL-12. Since Van Doorn et al (20) found a FTY720-mediated limitation of
TNF-α-induced CCL-2 release, it was hypothesized that the different
results could be due to species differences or different FTY720-P
concentrations. Based on studies by Foster et al (11), a relevant concentration was used
that can be achieved in the brain in vivo.
Inflammation-induced IL-1β mRNA levels were
augmented in the presence of FTY720-P in our experiments, although
this effect was only statistically significant after 12 h of
stimulation. Besides exacerbation of inflammation (23,24),
IL-1β is proposed to mediate regenerative functions, including
support of oligodendrocyte survival and remyelination (25,26).
Thus, it is hypothesized that FTY720-P may increase the
regenerative capacity of astrocytes at least during certain time
points.
GDNF was also upregulated under inflammatory
conditions but FTY720-P did not have a major impact on these
inflammation-induced alterations. However, after 24 h treatment
with TNF-α and FTY720-P, the levels of GDNF were increased compared
with TNF-α treatment alone. Accordingly, Yamagata et al
demonstrated that S1P receptor modulation enhanced the mRNA and
protein levels of GDNF within 24 h of incubation (22). As GDNF is a potent mediator of the
survival of different types of neurons (27–29),
the observed potentiation of GDNF expression could represent an
FTY720-P-mediated neuroprotective effect in astrocytes during
inflammatory processes.
In conclusion, the results suggest that FTY720-P is
not likely to be involved in transcriptional modulation of S1P
receptors, IL-1β, CCL-2, CCL-20, CXCL-12, IGF-1, CNTF and GDNF in
cultured murine astrocytes. To a certain extent, FTY720-P may be
important in the potentiation of TNF-α-induced GDNF and IL-1β gene
expression, which possess regenerative capabilities and supports a
protective environment following inflammation.
Acknowledgments
The authors would like to thank Novartis for
financial support and Mrs. I. Cierpka-Leja, Mrs. S. Lang and Mr. A.
Niesel for their excellent technical assistance. This manuscript is
part of a doctoral thesis at the University of Veterinary Medicine
Hannover, which has been handed to the University (Stefanie Janßen,
January 2014). Sections of this manuscript are included in a
doctoral thesis at the Hannover Medical School (Caroline Schlegel,
2014).
References
|
1
|
Goldschmidt T, Antel J, König FB, Brück W
and Kuhlmann T: Remyelination capacity of the MS brain decreases
with disease chronicity. Neurology. 72:1914–1921. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Patrikios P, Stadelmann C, Kutzelnigg A,
Rauschka H, Schmidbauer M, Laursen H, Sorensen PS, Brück W,
Lucchinetti C and Lassmann H: Remyelination is extensive in a
subset of multiple sclerosis patients. Brain. 129:3165–3172. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cohen JA, Barkhof F, Comi G, Hartung HP,
Khatri BO, Montalban X, Pelletier J, Capra R, Gallo P, Izquierdo G,
et al: Oral fingolimod or intramuscular interferon for relapsing
multiple sclerosis. N Engl J Med. 362:402–415. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kappos L, Radue EW, O'Connor P, Polman C,
Hohlfeld R, Calabresi P, Selmaj K, Agoropoulou C, Leyk M,
Zhang-Auberson L and Burtin P; FREEDOMS Study Group: A
placebo-controlled trial of oral fingolimod in relapsing multiple
sclerosis. N Engl J Med. 362:387–401. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oo ML, Thangada S, Wu MT, Liu CH,
Macdonald TL, Lynch KR, Lin CY and Hla T: Immunosuppressive and
anti-angiogenic sphingosine 1-phosphate receptor-1 agonists induce
ubiquiti-nylation and proteasomal degradation of the receptor. J
Biol Chem. 282:9082–9089. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mandala S, Hajdu R, Bergstrom J,
Quackenbush E, Xie J, Milligan J, Thornton R, Shei GJ, Card D,
Keohane C, et al: Alteration of lymphocyte trafficking by
sphingosine-1-phosphate receptor agonists. Science. 296:346–349.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Matloubian M, Lo CG, Cinamon G, Lesneski
MJ, Xu Y, Brinkmann V, Allende ML, Proia RL and Cyster JG:
Lymphocyte egress from thymus and peripheral lymphoid organs is
dependent on S1P receptor 1. Nature. 427:355–360. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chun J, Weiner JA, Fukushima N, Contos JJ,
Zhang G, Kimura Y, Dubin A, Ishii I, Hecht JH, Akita C, et al:
Neurobiology of receptor-mediated lysophospholipid signaling. From
the first lysophospholipid receptor to roles in nervous system
function and development. Ann NY Acad Sci. 905:110–117. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rao TS, Lariosa-Willingham KD, Lin FF,
Palfreyman EL, Yu N, Chun J and Webb M: Pharmacological
characterization of lysophospholipid receptor signal transduction
pathways in rat cerebrocortical astrocytes. Brain Res. 990:182–194.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Spiegel S and Milstien S:
Sphingosine-1-phosphate: An enigmatic signalling lipid. Nat Rev Mol
Cell Biol. 4:397–407. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Foster CA, Howard LM, Schweitzer A,
Persohn E, Hiestand PC, Balatoni B, Reuschel R, Beerli C, Schwartz
M and Billich A: Brain penetration of the oral immunomodulatory
drug FTY720 and its phosphorylation in the central nervous system
during experimental autoimmune encephalomyelitis: Consequences for
mode of action in multiple sclerosis. J Pharmacol Exp Ther.
323:469–475. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Choi JW, Gardell SE, Herr DR, Rivera R,
Lee CW, Noguchi K, Teo ST, Yung YC, Lu M, Kennedy G, et al: FTY720
(fingolimod) efficacy in an animal model of multiple sclerosis
requires astrocyte sphingosine 1-phosphate receptor 1 (S1P1)
modulation. Proc Natl Acad Sci USA. 108:751–756. 2011. View Article : Google Scholar :
|
|
13
|
Mullershausen F, Craveiro LM, Shin Y,
Cortes-Cros M, Bassilana F, Osinde M, Wishart WL, Guerini D,
Thallmair M, Schwab ME, et al: Phosphorylated FTY720 promotes
astrocyte migration through sphingosine-1-phosphate receptors. J
Neurochem. 102:1151–1161. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Osinde M, Mullershausen F and Dev KK:
Phosphorylated FTY720 stimulates ERK phosphorylation in astrocytes
via S1P receptors. Neuropharmacology. 52:1210–1218. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun H, Bénardais K, Stanslowsky N,
Thau-Habermann N, Hensel N, Huang D, Claus P, Dengler R, Stangel M
and Petri S: Therapeutic potential of mesenchymal stromal cells and
MSC conditioned medium in Amyotrophic Lateral Sclerosis (ALS) - in
vitro evidence from primary motor neuron cultures, NSC-34 cells,
astrocytes and microglia. PLoS One. 8:e729262013. View Article : Google Scholar :
|
|
16
|
Nicklas W, Baneux P, Boot R, Decelle T,
Deeny AA, Fumanelli M and Illgen-Wilcke B; FELASA (Federation of
European Laboratory Animal Science Associations Working Group on
Health Monitoring of Rodent and Rabbit Colonies): Recommendations
for the health monitoring of rodent and rabbit colonies in breeding
and experimental units. Lab Anim. 36:20–42. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pébay A, Toutant M, Prémont J, Calvo CF,
Venance L, Cordier J, Glowinski J and Tencé M:
Sphingosine-1-phosphate induces proliferation of astrocytes:
Regulation by intracellular signalling cascades. Eur J Neurosci.
13:2067–2076. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yoshida Y, Nakada M, Sugimoto N, Harada T,
Hayashi Y, Kita D, Uchiyama N, Hayashi Y, Yachie A, Takuwa Y, et
al: Sphingosine-1-phosphate receptor type 1 regulates glioma cell
proliferation and correlates with patient survival. Int J Cancer.
126:2341–2352. 2010.
|
|
19
|
Sheridan GK and Dev KK: S1P1 receptor
subtype inhibits demyelination and regulates chemokine release in
cerebellar slice cultures. Glia. 60:382–392. 2012. View Article : Google Scholar
|
|
20
|
Van Doorn R, Van Horssen J, Verzijl D,
Witte M, Ronken E, Van Het Hof B, Lakeman K, Dijkstra CD, Van Der
Valk P, Reijerkerk A, et al: Sphingosine 1-phosphate receptor 1 and
3 are upregulated in multiple sclerosis lesions. Glia.
58:1465–1476. 2010.PubMed/NCBI
|
|
21
|
Wu C, Leong SY, Moore CS, Cui QL, Gris P,
Bernier LP, Johnson TA, Séguéla P, Kennedy TE, Bar-Or A, et al:
Dual effects of daily FTY720 on human astrocytes in vitro:
Relevance for neuroinflammation. J Neuroinflammation. 10:412013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yamagata K, Tagami M, Torii Y, Takenaga F,
Tsumagari S, Itoh S, Yamori Y and Nara Y: Sphingosine 1-phosphate
induces the production of glial cell line-derived neurotrophic
factor and cellular proliferation in astrocytes. Glia. 41:199–206.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bauer J, Berkenbosch F, Van Dam AM and
Dijkstra CD: Demonstration of interleukin-1 beta in Lewis rat brain
during experimental allergic encephalomyelitis by
immunocytochemistry at the light and ultrastructural level. J
Neuroimmunol. 48:13–21. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Merrill JE: Tumor necrosis factor alpha,
interleukin 1 and related cytokines in brain development: Normal
and pathological. Dev Neurosci. 14:1–10. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Herx LM, Rivest S and Yong VW: Central
nervous system-initiated inflammation and neurotrophism in trauma:
IL-1 beta is required for the production of ciliary neurotrophic
factor. J Immunol. 165:2232–2239. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mason JL, Suzuki K, Chaplin DD and
Matsushima GK: Interleukin-1beta promotes repair of the CNS. J
Neurosci. 21:7046–7052. 2001.PubMed/NCBI
|
|
27
|
Arenas E, Trupp M, Akerud P and Ibáñez CF:
GDNF prevents degeneration and promotes the phenotype of brain
noradrenergic neurons in vivo. Neuron. 15:1465–1473. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Henderson CE, Phillips HS, Pollock RA,
Davies AM, Lemeulle C, Armanini M, Simmons L, Moffet B, Vandlen RA
and Simpson LC; Simmons L, et al: GDNF: A potent survival factor
for motoneurons present in peripheral nerve and muscle. Science.
266:1062–1064. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin LF, Doherty DH, Lile JD, Bektesh S and
Collins F: GDNF: A glial cell line-derived neurotrophic factor for
midbrain dopa-minergic neurons. Science. 260:1130–1132. 1993.
View Article : Google Scholar : PubMed/NCBI
|