Introduction
Pancreatic cancer is an exocrine gland neoplasm of
the pancreas. It is the most prevalent type of malignant tumor of
the digestive system and is the fourth most prevalent of all types
of malignant tumor(1). Due to the
depth and concealed position of the pancreas in the
retroperitoneum, the majority of cases of pancreatic cancer are
diagnosed at late stages with distant metastases. Significant
advances have been made in understanding the biology and
therapeutics of pancreatic cancer, however the 5-year survival rate
remains <6% (1,2). Surgery increases the survival rate,
however, surgery is only suitable for 10–15% of patients as
metastasis is usually already present at diagnosis (3).
Systemic chemotherapy is the major treatment
approach for 85% of patients with advanced pancreatic cancer
(3). Gemcitabine is the standard
chemotherapeutic agent for late stage pancreatic cancer (4), however, its curative effect is
limited. The median survival rate following radical surgery with
adjunct gemcitabine treatment is only 22.1 months (5). In addition, a number of patients with
pancreatic cancer develop resistance to gemcitabine. Gemcitabine,
in combination with fluorouracil, cisplatin, oxaliplatin and
capecitabine, can somewhat improve the survival rates of patients
with advanced disease, however, the median survival rate remains
between 6.5 and 9.4 months (6–10).
Therefore, novel and effective therapeutic strategies are urgently
required for the treatment of advanced pancreatic cancer.
Emodin is an anthraquinone drug, which has been
demonstrated to induce apoptosis and inhibit the growth and
metastasis of various types of malignant tumor. Its low toxicity
has been demonstrated in vivo and in vitro (11–13).
A previous study indicated that emodin may inhibit angiogenesis in
pancreatic cancer (14), however,
the underlying molecular mechanisms remain to be elucidated. In the
present study, the anti-angiogenesis effects of emodin were
investigated in a mouse xenograft pancreatic cancer model. Changes
in the tumor angiogenesis-associated transforming growth factor
(TGF)-β/drosophila mothers against decapentaplegic (Smad) pathways
and in the expression levels of microRNAs (miR), including miR-20b,
miR-155 and miR-210, were examined.
Materials and methods
Reagents
Emodin was purchased from Sigma-Aldrich (St. Louis,
MO, USA), dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich) at
0.2 mmol/l as a stock solution and stored at −20°C. The final
concentration of DMSO was <0.1%. A mouse polyclonal anti-cluster
of differentiation (CD)34 antibody (cat. no. sc-74499), mouse
polyclonal anti-TGFβ receptor (TβR)II antibody (cat. no. sc-17799),
mouse monoclonal anti-Smad4 antibody (cat. no. sc-7966) and goat
polyclonal anti-angiopoietin-like (Angptl)4 antibody (cat. no.
sc-34113) were purchased from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA). A rabbit polyclonal anti-TGF-β1 antibody (cat. no.
ab92486) and rabbit polyclonal anti-TβRI antibody (cat. no.
ab31013) were purchased from Abcam (Cambridge, MA, USA).
TRIzol® reagent was purchased from Invitrogen Life
Technologies (Carlsbad, CA, USA). The RevertAid First Strand cDNA
Synthesis kit was purchased from Fermentas (Burlington, ON,
Canada). The Takara One Step PrimeScript miRNA cDNA Synthesis kit
(Perfect Real Time) and Takara SYBR Premix ExTaq™ II (Perfect Real
Time) were purchased from Takara Bio, Inc. (Otsu, Japan). The study
was approved by the Ethics Committee of First Affiliated Hospital,
Zhejiang University School of Medicine (Hangzhou, China).
Cell line
The SW1990 human pancreatic cancer cell line was
purchased from the American Type Culture Collection (Manassas, VA,
USA) and cultured in RPMI-1640 medium (Invitrogen Life
Technologies), supplemented with 10% heat-incubated fetal bovine
serum (Invitrogen Life Technologies), 100 U/ml penicillin and 100
mg/ml streptomycin (Beyotime Institute of Biotechnology, Shanghai,
China) at 37°C in a humidified 5% CO2 atmosphere. The
cells were passaged at 70–80% confluence.
Animals
Female athymic BALB/c nu/nu mice (4–6-weeks
old, weighing 20–22 g) were purchased from the Shanghai Cancer
Institute for Tumor Implantation (Shanghai, China). The animals
were maintained in a sterile environment maintained at room
temperature (25°C) and 60–65% relative humidity, with ad
libitum provision of water at the Animal Experiment Center of
Zhejiang University School of Medicine (Zheizhang. China).
Orthotopic implantation of pancreatic
cancer and treatment
A total of 2×106 SW1990 cells were
subcutaneously injected into the flanks of donor nude mice. At a
volume of 1 cm3, the subcutaneous tumor was removed
under sterile conditions. The central necrotic tissues in the tumor
were removed and the healthy peripheral tissue from the
subcutaneous tumor was cut into tissue blocks of 1 mm3
for orthotopic transplantation. Recipient nude mice (n=40) were
anesthetized with pelltobarbitalum natricum (50 mg/kg;
Sigma-Aldrich) and opened via a left longitudinal laparotomy. The
spleen and the pancreatic tail were gently exteriorized, and a
tissue pocket in the pancreatic parenchyma was created using
micro-scissors (Santa Cruz Biotechnology, Inc.). A tumor fragment
(~1 mm3) was placed into the tissue pocket, ensuring
that it was entirely surrounded by normal pancreatic tissue.
Following careful relocation of the pancreas and spleen into the
abdominal cavity, the cavity was closed in two layers using 4-0
silk sutures for the muscular layer and nonabsorbable stainless
steel wound clips for the skin.
Following a recovery period of 3 weeks, the in
situ xenograft model of pancreatic cancer was complete and the
mice were randomly divided into four groups, termed the control,
E20, E40 and E80 group, each containing 10 mice. The mice in the
control group were each injected with 0.2 ml 0.9% sodium chloride
(Shanghai Chemical Reagent Plant, Shanghai, China) into the
abdominal cavity. The mice in the E20, E40 and E80 groups were
injected with 20, 40 and 80 mg/kg emodin, respectively, using the
same method as the control (15).
Each group was treated three times each week for 2 weeks. The mice
were euthanized 7 weeks after implantation by cervical dislocation
and the pancreatic tumors were carefully separated and weighed
(scales from Mettler-Toledo International, Inc., Zurich,
Switzerland). The orthotopic tumor tissues were then either stored
in 4% paraformaldehyde (Shanghai Chemical Reagent Plant) for
immunohistochemistry or were frozen in liquid nitrogen.
CD34 immunohistochemistry and calculation
of microvessel density
The tissue sections were cut from the
paraffin-embedded pancreatic cancer tissues, blocked with goat
serum (Zhejiang Tianhang Biological Technology Co., Ltd., Hangzhou,
China) and immunostained following deparaffinization with xylene
for 10 min and rehydration with ethanol for 5 min 3 times.
Immunostaining was performed using primary antibodies specific for
CD34 (1:50) at room temperature for 60 min, followed by staining
with horseradish peroxidase-conjugated secondary antibodies
(1:1,500) at room temperature for 30 min. The slides were developed
in diaminobenzidine (Beyotime Institute of Biotechnology) and
counterstained with hematoxylin (Beyotime Institute of
Biotechnology). The stained slides were dehydrated and mounted in
Permount (Beyotime Institute of Biotechnology) and visualized under
a light microscope (Olympus, Tokyo, Japan). Images were captured
using an attached camera linked to a computer. The slides were
visualized for the identification of clusters of microvessels under
low power (magnification, ×100). Random visual fields (n=5) were
selected for inspection under high power (magnification, ×400). The
number of CD34-positive cells in each visual field was defined as
the microvessel density (16–18).
Detecting the mRNA expression levels of
TGF-β1, TβRI, TβRII, Smad4 and Angptl4 via reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
The total RNA in the tumor tissues was extracted
using TRIzol reagent, according to the manufacturer's instructions,
and quantified by spectrophotometric analysis (RF-5301PC; Shimadzu
Corporation, Kyoto, Japan). The RNA integrity was verified using
agarose gel electrophoresis. cDNA was synthesized using a RevertAid
First Strand cDNA Synthesis kit (Fermentas), a random primer
(β-actin) and 1 mg RNA. The cDNA product was diluted 1:4 into
deionized water. The cDNA, primers specific for TGF-β1, TβRI,
TβRII, Smad4, Angptl4 and β-actin (Table I), and Sybr Green I mix were added
to a 20 µl reaction volume, supplemented with deionized
water, according to the manufacturer's instructions. The PCR was
performed using a Roche real-time PCR system (Roche Diagnostics,
Basel, Switzerland) and the data were analyzed using LightCycler
480 software (Roche Diagnostics). β-actin was used an internal
control. The conditions for the PCR reactions were as follows:
Denaturation at 94°C for 30 sec, annealing at 60°C for 30 sec and
extension at 72°C for 60 sec for up to 40 cycles.
 | Table IPrimers used in the quantitative
polymerase chain reaction. |
Table I
Primers used in the quantitative
polymerase chain reaction.
| Gene | Primer (5′-3′) | Product length
(bp) |
|---|
| TGF-β1 | Forward:
CAATTCCTGGCGATACCTCAG
Reverse: GCACAACTCCGGTGACATCAA | 86 |
| TβRI | Forward:
GCTGTATTGCAGACTTAGGACTG
Reverse: TTTTTGTTCCCACTCTGTGGTT | 90 |
| TβRII | Forward:
AAGATGACCGCTCTGACATCA
Reverse: CTTATAGACCTCAGCAAAGCGAC | 119 |
| Smad4 | Forward:
CCACCAAGTAATCGTGCATCG
Reverse: TGGTAGCATTAGACTCAGATGGG | 76 |
| Angptl4 | Forward:
GGCTCAGTGGACTTCAACCG
Reverse: CCGTGATGCTATGCACCTTCT | 103 |
| β-actin | Forward:
CATTGCCGACAGGATGCAG
Reverse: CTCGTCATACTCCTGCTTGCTG | 169 |
microRNA RT-qPCR
To determine the expression levels of miR, the cDNA
was synthesized using a Takara One Step PrimeScript miRNA cDNA
Synthesis kit (Takara Bio, Inc.), using an miRPrimeScript RT Enzyme
mix (Takara Bio, Inc.) and 1 mg RNA. The cDNA product was diluted
to 1:4 in deionized water and the cDNA, primers specific to miR-20,
miR-155 and miR-210, or the U6B control, and the SYBR Premix Ex
TaqII were added to a 20 µl reaction volume, supplemented
with deionized water, according to the manufacturer's instructions.
The PCR was performed using a LightCycler Real Time PCR 480 system.
The primers used were as follows: miR-20b,
5′-CAAAGUGCUCAUAGUGCAGGUAG-3′, miR-210,
5′-AGCCCCUGCCCACCGCACACUG-3′ and miR-155,
5′-UUAAUGCUAAUCGUGAUAGGGGU-3′.
Western blotting
The total proteins were routinely extracted from the
tumor tissues using radioimmunoprecipitation lysis buffer
(containing 50 mM Tris, pH 7.4; 150 mM NaCl, 1% Triton X-100, 1%
sodium deoxycholate, and 0.1% SDS, sodium orthovanadate, sodium
fluoride, EDTA and leupeptin; Beyotime Institute of Biotechnology)
and the protein concentrations were determined using a
bicinchoninic acid assay (Thermo Fisher Scientific, Waltham, MA,
USA). The proteins were separated on 12% SDS-PAGE gels (Bio-Rad
Laboratories, Inc., Shanghai, China), electrotransferred onto
polyvinylidene fluoride membranes (Invitrogen Life Technologies),
blocked with 5% non-fat milk (Yili, Inc., Inner Mongolia, China)
and subsequently probed with primary antibodies (anti-TGF-β1,
anti-TβRI and anti-GAPDH at a dilution of 1:1,000, anti-Smad4 at a
dilution of 1:800, and anti-TβRII and anti-Angptl4 at a dilution of
1:500) and horseradish peroxidase-conjugated secondary antibody (at
a dilution of 1:10,000). Following washing with Tris-buffered
saline with Tween 20 (Beyotime Institute of Biotechnology), the
bound antibody complexes were analyzed using an enhanced
chemiluminescence reagent (Amersham Pharmacia Biotech., Piscataway,
NJ, USA). A GAPDH antibody (anti-rabbit GAPDH; cat. no. AB-P-R 001;
Hangzhou Goodhere Biotechnology Co., Ltd., Hangzhou, China) was
used as the control.
Statistical analysis
TotalLab 2.1 software (TotalLab, Ltd., Newcastle,
UK) was used to analyze the western blots, and LightCycler 480
software V1.5.0 was used to analyze the RT-qPCR data. The images
presented are representative and data are expressed as the mean ±
standard error of the mean of each group. Differences between
groups of cells or mice were analyzed using analysis of variance
and Student's post-hoc t-test using SPSS 17.0 software (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
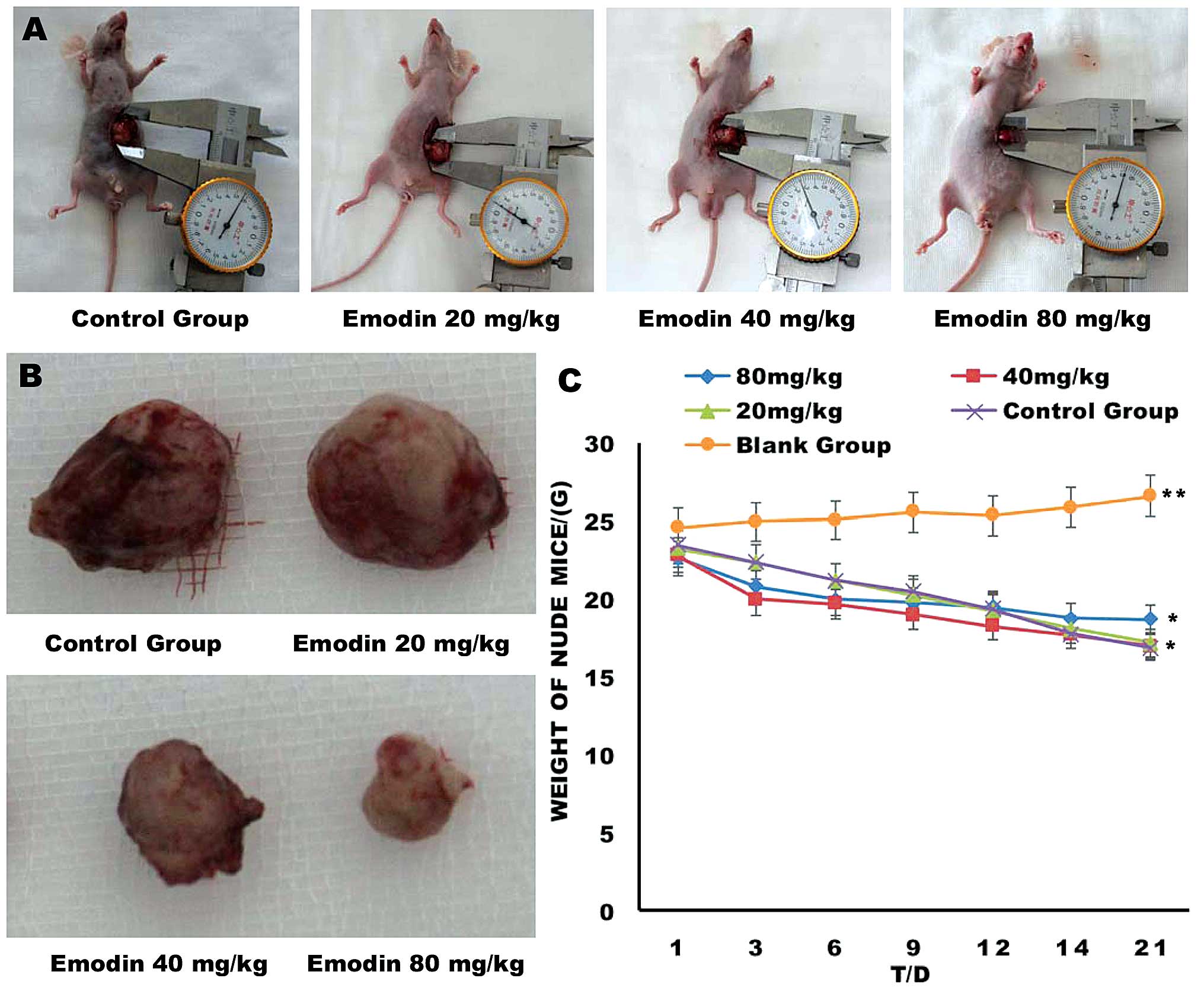
Emodin inhibits the growth of
orthotopically transplanted pancreatic cancer
The tumor weights were measured 7 days after the
final treatment og different doses of emodin (Fig. 1 and Table II). The tumor weights of the E40
and E80 groups were significantly lower compared with those of the
control and E20 groups (P<0.05). No significant difference in
mean tumor weight was observed between the control and E20 groups
(P>0.05). Therefore, tumor growth was effectively inhibited in
the tumor-bearing mice following treatment with 40 mg/kg
emodin.
 | Table IIWeight of orthotopically implanted
pancreatic tumors on day 21 following treatment with emodin. |
Table II
Weight of orthotopically implanted
pancreatic tumors on day 21 following treatment with emodin.
| Group | Tumor weight
(g) | Inhibition
ratea (%) |
|---|
| Control | 1.671±0.289 | 0 |
| E20 | 1.487±0.248 | 11.0 |
| E40 | 1.172±0.205b | 29.8 |
| E80 | 0.741±0.210b | 55.6 |
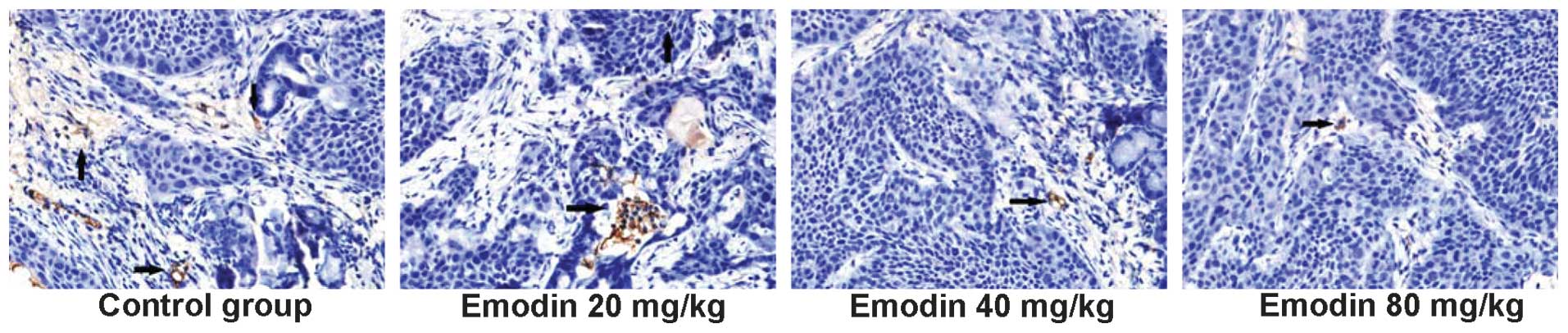
Emodin significantly reduces the
angiogenesis of orthotopically transplanted pancreatic cancer
To determine whether treatment with emodin inhibited
the angiogenesis of pancreatic cancer, the numbers of CD34-positive
cells and the microvessel density were calculated by
immunohistochemistry (Fig. 2 and
Table III). The microvessels
were significantly less dense in the E20, E40 and E80 groups
compared with the control group. Additionally, treatment of
tumor-bearing mice with 20 mg/kg emodin significantly reduced
microvessel density.
 | Table IIIEmodin reduced microvessel density of
orthotopically transplanted pancreatic cancer. |
Table III
Emodin reduced microvessel density of
orthotopically transplanted pancreatic cancer.
| Group | Microvessel
density |
|---|
| Control | 16.5±1.1 |
| E20 | 12.3±1.8a |
| E40 | 5.4±1.6a |
| E80 | 4.8±1.6a |
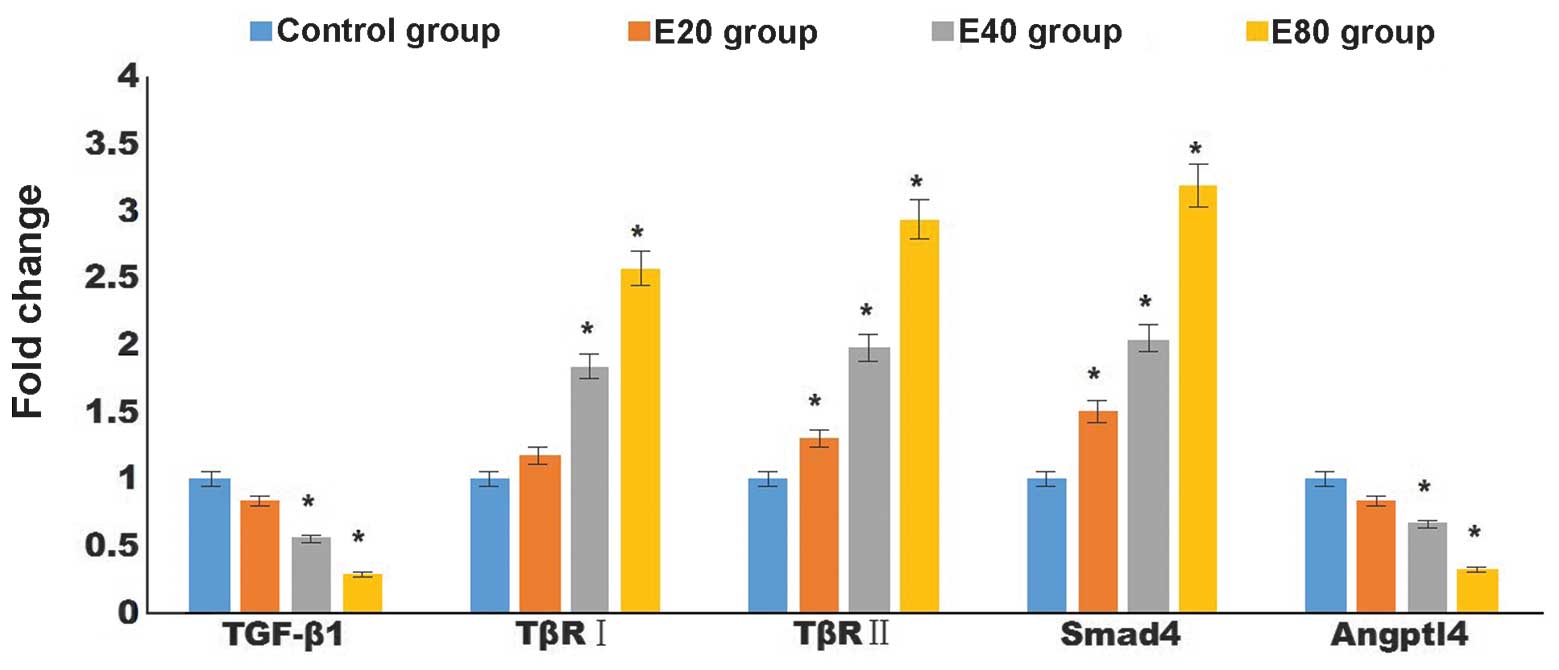
Emodin decreases the mRNA expression
levels of TGF-βI and Angptl4 and increases the mRNA expression
levels of TβRI, TβRII and Smad4
To examine the mechanisms by which emodin inhibits
the angiogenesis of pancreatic cancer, the gene expression levels
of the TGF-βI, TβRI, TβRII, Smad4 and Angptl4
angiogenesis-associated genes were determined. Treatment with
emodin decreased the mRNA expression levels of TGF-β1 and Angptl4
in a dose-dependent manner, and the expression levels were
significantly lower in the E40 and E80 groups compared with those
of the control group (P<0.05). In addition, treatment with
emodin increased the mRNA expression levels of TβRI, TβRII and
Smad4. The expression levels of TβRI were significantly higher in
the E40 and E80 groups compared with the control group (P<0.05)
and the expression levels of TβRII and Smad4 were significantly
higher in the E20, E40 and E80 groups (P<0.05 and Fig. 3).
Emodin decreases the protein expression
levels of TGF-βI and Angptl4, and increases the expression levels
of TβRI, TβRII and Smad4
Consistent with the differences in the mRNA
expression levels, the results of the western blotting demonstrated
that different doses of emodin upregulated the protein expression
levels of TβRII and Smad4 compared with control group in the
pancreatic cancer tissues (P<0.05). Similarly, emodin increased
the protein expression of TβRI compared with the control group with
significant differences between the E40 and E80 group, and the
control group (P<0.05). Treatment with emodin also downregulated
the protein expression levels of TGF-β1 and Angptl4 compared with
the control group in the pancreatic cancer tissues, with
significant differences between the E40 and E80 group, and the
control group (P<0.05; Fig.
4).
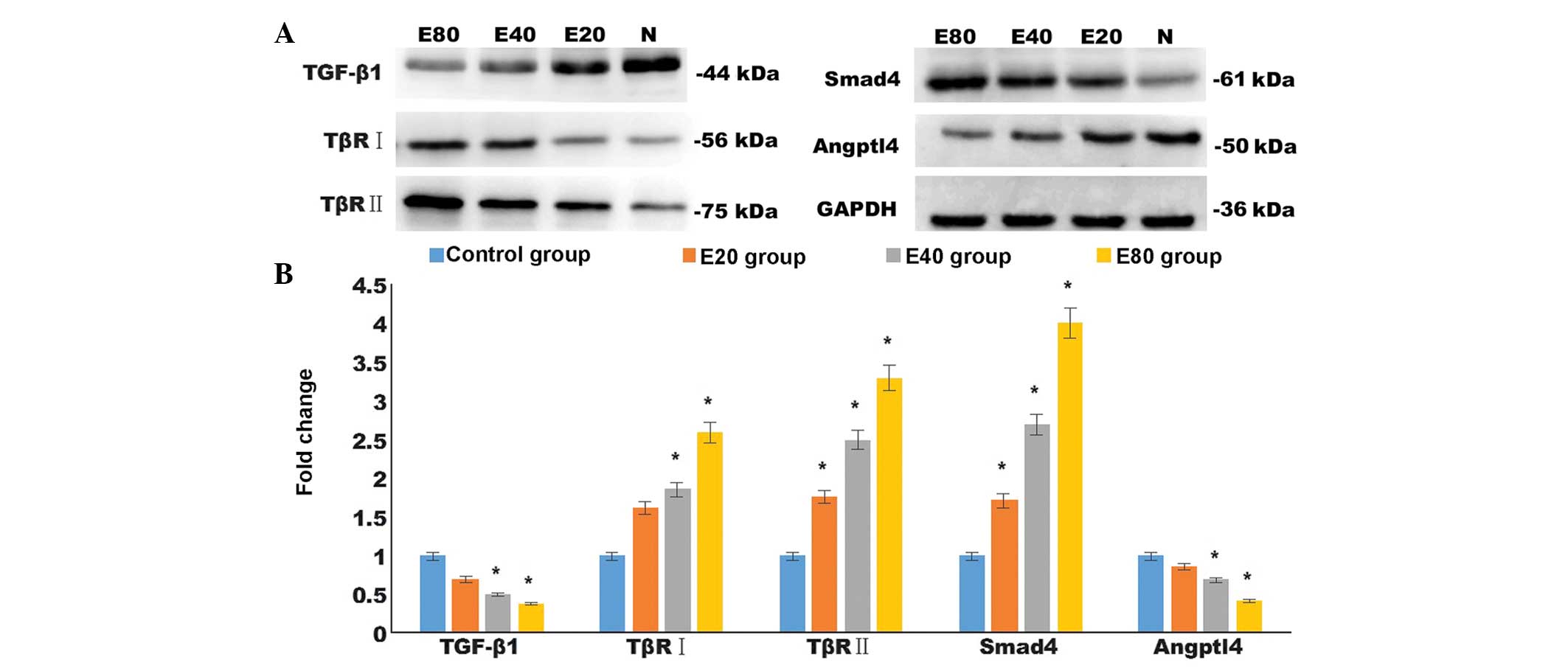 | Figure 4Emodin altered the protein expression
levels of angiogenesis-associated TGF-β1, TβRI, TβRII, Smad4, and
Angptl4. (A) Protein expression levels of TGF-β1, TβRI, TβRII,
Smad4 and Angptl4 in tumor tissues was detected by western
blotting. (B) Quantified data from the western blotting. Data are
expressed as the mean ± standard error of the mean.
*P<0.05, compared with the control group. TGF,
transforming growth factor; TβR, TGF-β receptor; Smad, drosophila
mothers against decapentaplegic; Angptl, angiopoietin-like. |
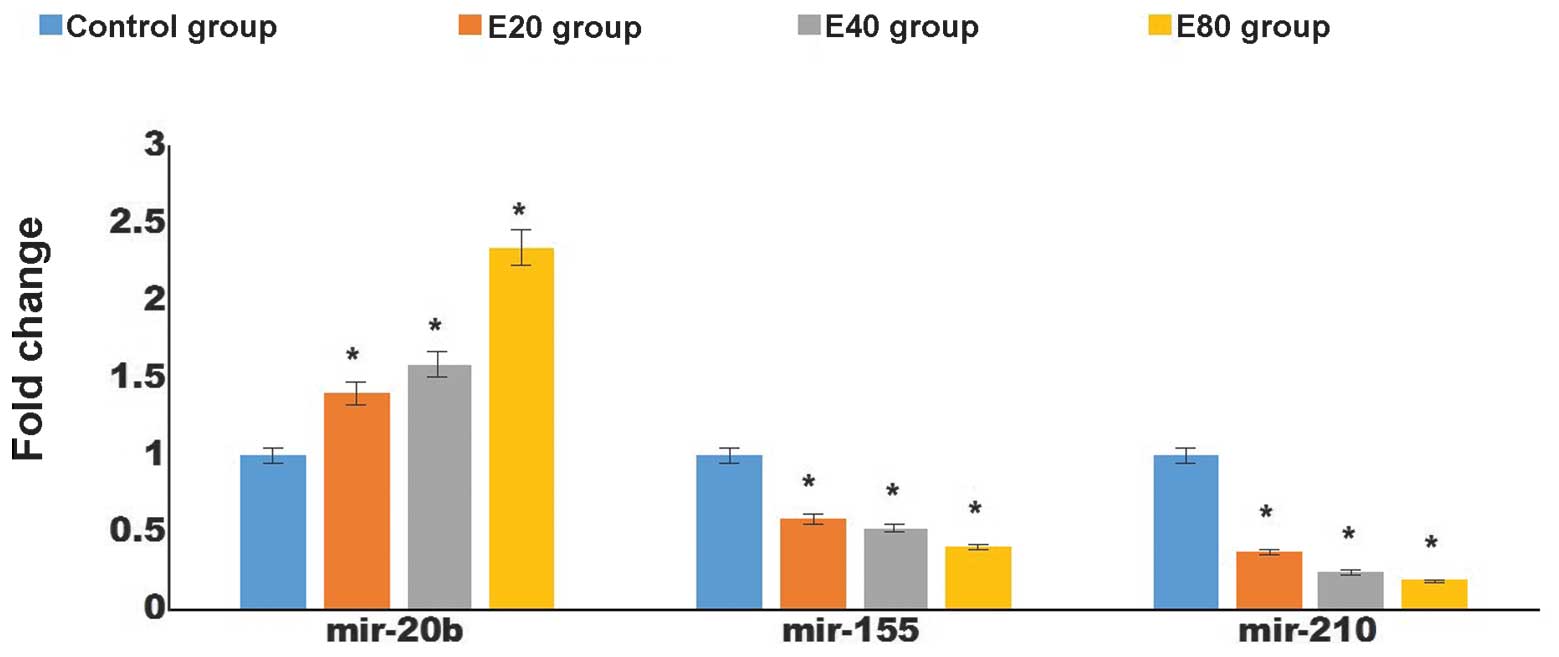
Effect of emodin on the expression levels
of angiogenesis-associated miR-155, miR-210 and miR-20b in
pancreatic cancer tissues
miRNAs have been found to be important in
angiogenesis. To determine whether emodin affects the expression
levels of angiogenesis-associated miRNAs in pancreatic cancer
tissues, the expression levels of miR-155, miR-210 and miR-20b were
assessed by RT-qPCR. The levels of miR-20b in the pancreatic cancer
tissues were higher in the groups treated with emodin (P<0.05),
whereas those of miR-155 and miR-210 were lower compared with the
control group. These changes occurred in a dose-dependent manner
(P<0.05; Fig. 5).
Discussion
The tumor microenvironment is important during
tumori-genesis. Sustained angiogenesis is a hallmark of cancer and
is key to the metastasis of cancer cells. Initial tumor growth
depends on the original blood vascular system, however, when the
tumor is >1–2 mm3, the nutrients supplied by the
existing blood vessels are not sufficient to sustain further tumor
growth, requiring the production of new vessels through the process
of angiogenesis (19). In as early
as in 1971, Folkman (18)
suggested that the growth of tumor cells was dependent on
angiogenesis.
In the present study, treatment with emodin was
observed to inhibit the growth of pancreatic cancer in a
dose-dependent manner. Multiple previous studies have revealed that
emodin can inhibit the proliferation of vascular endothelial cells
(21,22). To assess the anti-angiogenic
properties of emodin in a pancreatic xenograft tumor model, changes
in microvascular density following treatment with emodin were
determined. The microvessel densities of the emodin-treated groups
were significantly lower compared with the control group,
consistent with a previous report that emodin inhibited pancreatic
cancer-associated angiogenesis (14). These data suggested that the
suppression of angiogenesis may be a mechanism by which emodin
inhibits tumor growth.
The TGF-β/Smad pathway is an antitumor signaling
pathway, which is deregulated in several types of cancer (23,24).
In the early stages of tumorigenesis, TGF-β inhibits cell
proliferation, promotes cell differentiation and induces cell
apoptosis and, as tumorigenesis progresses, TGF-β inhibits
immunologic function, increases angio-genesis and promotes invasion
of the tumor (25). TGF-β binds to
and activates TβRII and TβRI, which in turn phosphorylates the Smad
transcription factors. The phosphorylated Smads translocate to the
cell nucleus to regulate a series of gene transcription events
(24). Our previous study
demonstrated that mutations in TβRI and TβRII promoted tumor
angiogenesis and cancer cell proliferation in several types of
cancer, including pancreatic, gall bladder, breast and rectal
cancer (26). The Smad protein
family is the key component of the TGF-β signaling pathway.
Deregulation of the TGF-β/Smad signaling pathways has been observed
in >50% of pancreatic cancer cases, via mutations in the Smad
genes, predominantly Smad4, or in genes of the TGF-β superfamily
and its receptor (27). Similar to
mutations in Kirsten rat sarcoma viral oncogene, tumor protein p53
and cyclin-dependent kinase inhibitor 2A, ~50% of the cases of
pancreatic cancer exhibit mutated Smad4 (28), however, inactivation mutation in
the Smad4 gene usually occurs at a later stage of tumorigenesis. It
has been observed that TGF-β activates the expression of Angptl4 in
tumor cells in the circulation through the Smad signaling pathways
(29). Angptl4 is secreted from
tumor cells, breaks the conjunction of endothelial cells and
subsequently increases capillary permeability (29). The present study revealed that
treatment with emodin downregulated the expression levels of TGF-β1
and Angptl4, and upregulated the expression levels of the TβRI and
TβRII TGF-β1 receptors and Smad4 in the orthotopically implanted
pancreatic tumor mouse models. These results suggested that emodin
may inhibit angiogenesis by inhibiting the expression levels of
TGF-β1 and, therefore, inhibiting the secretion of Angptl4 via
Smad4.
MicroRNAs are small endogenous non-coding
single-chain RNAs. The miRNA gene family occupies ~1% of the human
genome and regulates the expression levels of around one third of
human genes. MicroRNAs can also function as oncogenes or tumor
suppressor genes. It has been demonstrated that the angiogenesis,
which is associated with pancreatic cancer is regulated by
microRNAs (30), and miR-20b,
miR-155 and miR-210 are among the most extensively investigated of
the angiogenesis-associated miRNAs. miR-155 and miR-210 function as
oncogenes in various types of tumor, including breast (31), lung (32) and pancreatic (33) cancer, and clear cell carcinoma of
the kidney (34). In the present
study, treatment with emodin increased the expression of miR-20b
and inhibited the expression levels of miR-155 and miR-210. These
results suggested that treatment with emodin inhibited the growth
of tumors by suppressing tumor-associated angiogenesis.
In conclusion, using an orthotopically transplanted
pancreatic cancer model, it was observed that treatment with emodin
inhibited angiogenesis by regulating the TGF-β/Smad signaling
pathway and the expression levels of miRNA. These results revealed
that emodin inhibits the growth of pancreatic cancer through a
variety of different mechanisms (15), suggesting emodin as a promising
natural drug for the treatment of pancreatic cancer.
Acknowledgments
This study was supported by the Animal Experimental
Center of Zhejiang University School of Medicine (Zheizhang,
China),, the Administration of Traditional Chinese Medicine of
Zhengjing Province, China (no. 2011ZZ010), the Zhejiang Provincial
Science Fund for Distinguished Young Scholars (no. LR12H280001) and
the National Natural Science Foundation of China (nos. 81173606 and
81374020). The authors would like to thank the Research Center at
the Second Affiliated Hospital of Wenzhou Medical University for
technical assistance.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hidalgo M: Pancreatic cancer. N Engl J
Med. 362:1605–1617. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McCracken M, Olsen M, Chen MS Jr, et al:
Cancer incidence, mortality, and associated risk factors among
Asian Americans of Chinese, Filipino, Vietnamese, Korean, and
Japanese ethnicities. CA Cancer J Clin. 57:190–205. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tempero MA, Behrman S, Ben-Josef E, et al:
Pancreatic adenocarcinoma: Clinical practice guidelines in
oncology. J Natl Compr Canc Netw. 3:598–626. 2005.PubMed/NCBI
|
|
5
|
Oettle H, Post S, Neuhaus P, et al:
Adjuvant chemotherapy with gemcitabine vs observation in patients
undergoing curative- intent resection of pancreatic cancer: A
randomized controlled trial. JAMA. 297:267–277. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reni M, Cordio S, Milandri C, et al:
Gemcitabine versus cisplatin, epirubicin, fluorouracil, and
gemcitabine in advanced pancreatic cancer: A randomised controlled
multicentre phase III trial. Lancet Oncol. 6:369–376. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Louvet C, Labianca R, Hammel P, et al:
Gemcitabine in combination with oxaliplatin compared with
gemcitabine alone in locally advanced or metastatic pancreatic
cancer: Results of a GERCOR and GISCAD phase III trial. J Clin
Oncol. 23:3509–3516. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rocha Lima CM, Savarese D, Bruckner H, et
al: Irinotecan plus gemcitabine induces both radiographic and CA
19- 9 tumor marker responses in patients with previously untreated
advanced pancreatic cancer. J Clin Oncol. 20:1182–1191. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Heinemann V, Quietzsch D, Gieseler F, et
al: Randomized phase III trial of gemcitabine plus cisplatin
compared with gemcitabine alone in advanced pancreatic cancer. J
Clin Oncol. 24:3946–3952. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Burtness B, Thomas L, Sipples R, et al:
Phase II trial of weekly docetaxel/irinotecan combination in
advanced pancreatic cancer. Cancer J. 13:257–262. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Su YT, Chang HL, Shyue SK and Hsu SL:
Emodin induces apoptosis in human lung adenocarcinoma cells through
a reactive oxygen species- dependent mitochondrial signaling
pathway. Biochem Pharmacol. 70:229–241. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin SY, Lai WW, Ho CC, et al: Emodin
induces apoptosis of human tongue squamous cancer SCC- 4 cells
through reactive oxygen species and mitochondria- dependent
pathways. Anticancer Res. 29:327–335. 2009.PubMed/NCBI
|
|
13
|
Guo Q, Chen Y, Zhang B, Kang M, Xie Q and
Wu Y: Potentiation of the effect of gemcitabine by emodin in
pancreatic cancer is associated with survivin inhibition. Biochem
Pharmacol. 77:1674–1683. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin SZ, Wei WT, Chen H, Chen KJ, Tong HF,
Wang ZH, Ni ZL, Liu HB, Guo HC and Liu DL: Antitumor activity of
emodin against pancreatic cancer depends on its dual role:
Promotion of apoptosis and suppression of angiogenesis. PLoS One.
7:e421462012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu JX, Zhang JH, Li HH, Lai FJ, Chen KJ,
Chen H, Luo J, Guo HC, Wang ZH and Lin SZ: Emodin induces Panc- 1
cell apoptosis via declining the mitochondrial membrane potential.
Oncol Rep. 28:1991–1996. 2012.PubMed/NCBI
|
|
16
|
Weidner N: Current pathologic methods for
measuring intra-tumoral microvessel density within breast carcinoma
and other solid tumors. Breast Cancer Res Treat. 36:169–180. 1995.
View Article : Google Scholar
|
|
17
|
Kato H, Ishikura H, Kawarada Y, Furuya M,
Kondo S, Kato H and Yoshiki T: Anti- angiogenic treatment for
peritoneal dissemination of pancreas adenocarcinoma: A study using
TNP- 470. Jpn J Cancer Res. 92:67–73. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Folkman J: Fundamental concepts of the
angiogenic process. Curr Mol Med. 3:643–651. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Holash J, Maisonpierre PC, Compton D, et
al: Vessel cooption, regression, and growth in tumors mediated by
angiopoietins and VEGF. Science. 284:1994–1998. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Folkman J: Tumor angiogenesis: Therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kaneshiro T, Morioka T, Inamine M, Kinjo
T, Arakaki J, Chiba I, Sunagawa N, Suzui M and Yoshimi N:
Anthraquinone derivative emodin inhibits tumor- associated
angiogenesis through inhibition of extracellular signal- regulated
kinase 1/2 phosphorylation. Eur J Pharmacol. 553:46–53. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kimura Y, Sumiyoshi M, Taniguchi M and
Baba K: Antitumor and antimetastatic actions of anthrone- C-
glucoside, cassialoin isolated from Cassia garrettiana heartwood in
colon 26- bearing mice. Cancer Sci. 99:2336–2348. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mansour KA, Fritz RC, Jacobs DM and
Vellios F: Pedunculated liposarcoma of the esophagus: A first case
report. J Thorac Cardiovasc Surg. 86:447–450. 1983.PubMed/NCBI
|
|
24
|
Itoh S and ten Dijke P: Negative
regulation of TGF-beta receptor/Smad signal transduction. Curr Opin
Cell Biol. 19:176–184. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Moustakas A and Heldin CH: Signaling
networks guiding epithelial- mesenchymal transitions during
embryogenesis and cancer progression. Cancer Sci. 98:1512–1520.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kang SH, Bang YJ, Im YH, et al:
Transcriptional repression of the transforming growth factor- beta
type I receptor gene by DNA methylation results in the development
of TGF- beta resistance in human gastric cancer. Oncogene.
18:7280–7286. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Miyazono K: TGF- beta/SMAD signaling and
its involvement in tumor progression. Biol Pharm Bull.
23:1125–1130. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jaffee EM, Hruban RH, Canto M and Kern SE:
Focus on pancreas cancer. Cancer Cell. 2:25–28. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Padua D, Zhang XH, Wang Q, Nadal C, Gerald
WL, Gomis RR and Massagué J: TGFbeta primes breast tumors for lung
metastasis seeding through angiopoietin- like 4. Cell. 133:66–77.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Peters BA, Diaz LA, Polyak K, et al:
Contribution of bone marrow- derived endothelial cells to human
tumor vasculature. Nat Med. 11:261–262. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Iorio MV, Ferracin M, Liu CG, et al:
MicroRNA gene expression deregulation in human breast cancer.
Cancer Res. 65:7065–7070. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yanaihara N, Caplen N, Bowman E, Seike M,
Kumamoto K, Yi M, Stephens RM, Okamoto A, Yokota J, Tanaka T, et
al: Unique microRNA molecular profiles in lung cancer diagnosis and
prognosis. Cancer Cell. 9:189–198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee EJ, Gusev Y, Jiang J, Nuovo GJ, Lerner
MR, Frankel WL, Morgan DL, Postier RG, Brackett DJ and Schmittgen
TD: Expression profiling identifies microRNA signature in
pancreatic cancer. Int J Cancer. 120:1046–1054. 2007. View Article : Google Scholar
|
|
34
|
Juan D, Alexe G, Antes T, Liu H,
Madabhushi A, Delisi C, Ganesan S, Bhanot G and Liou LS:
Identification of a microRNA panel for clear- cell kidney cancer.
Urology. 75:835–841. 2010. View Article : Google Scholar
|