Introduction
On an annual basis, sepsis is diagnosed in ~600,000
patients in North America, with a mortality rate ranging between 30
and 50% (1), thereby resulting in
an expensive medical problem. Moreover, sepsis is a leading cause
of mortality among patients in intensive care units (2). Although sepsis can be brought under
control with the use of comprehensive treatment (3), specific therapeutic interventions for
this disease have not been identified, posing a serious threat to
human health.
It is well-known that sepsis can induce systemic
inflammatory response syndrome, frequently leading to multiple
organ failure, including liver injury (4). Although liver dysfunction occurs
frequently in cases of sepsis (5),
the comprehensive understanding of sepsis-associated liver injury
remains limited. The liver may directly function in the development
of inflammation in response to sepsis-induced injury, leading to
the further promotion of sepsis (6), in addition to its vital role in
metabolism. Therefore, liver injury may be positively associated
with sepsis, suggesting that sepsis may be controlled to an extent
through relieving liver injury.
Recently, invading microorganisms have been
identified as a major cause of sepsis (7). Lipopolysaccharide (LPS), a major
component of the outer membrane of Gram-negative bacteria (8), may uncontrollably activate the innate
immune system, resulting in the production of inflammatory
mediators that may be a cause of septic shock (9). This event is caused by activation of
toll-like receptor 4, a conventional pattern-recognition receptor,
which recognizes LPS and results in the triggering of downstream
signaling cascades and production of chemokines, such as tumor
necrosis factor (TNF)-α and interleukin (IL)-10 (10,11).
The latter binds to receptors and then activates a major second
messenger pathway, the Janus activated kinase (JAK)/signal
transducer and activator of transcription (STAT) signaling pathway
(12). Although the continuous
activation of the JAK/STAT pathway contributing to inflammation and
sepsis has been identified (13),
the correlation between the JAK/STAT signaling pathway and
sepsis-associated liver injury remain largely unknown.
MicroRNAs (miRNA), single-stranded noncoding small
RNA molecules ~19–24 nucleotides in length, are posttranscriptional
regulators of gene expression and exhibit their effects by
imperfect base pairing to target mRNAs for degradation or
translational repression (14).
Studies have demonstrated that circulating miRNAs were identified
in the serum and plasma as biomarkers of sepsis (15,16).
Specifically, LPS, an activator of sepsis and the JAK/STAT
signaling pathway, increases the production of miR-155 (17). miR-155 inhibits the negative
feedback loop of the JAK/STAT signaling pathway via
posttranscriptional silencing of suppressor of cytokine signaling 1
(SOCS1) and potentiates the inflammatory signaling of STAT3
(18). Given the important role of
JAK/STAT signaling in sepsis, it is rational to suggest that
miR-155 may be a major contributor to sepsis and inhibition of
miR-155 may suppress sepsis through inactivating JAK/STAT
signaling. However, the role of miR-155 in sepsis-associated liver
injury has not yet been defined. The present study aimed to
investigate the expression of miR-155 in the liver of LPS-exposed
mice and to determined whether inhibition of miR-155 may relieve
sepsis-induced liver injury through inactivating the JAK/STAT
pathway.
Materials and methods
Experimental animals and study
design
A total of 120 male BALB/c mice (weight, 20±2 g)
were obtained from the Department of Animal science of Fudan
University in Shanghai (Shanghai, China). The mice were kept in
cages with a 12 h light-dark cycle and access to dry pellets and
sterile water ad libitum. The mice were randomly divided
into three groups (n=40 per group): Control, LPS and miR-155
inhibitor plus LPS groups. After anesthetizing intraperitoneally
(i.p.) with pentobarbital (0.3 mg kg−1), the control and
LPS groups were injected with sterile saline i.p., and the miR-155
inhibitor plus LPS group was intravenously injected with 80 mg
kg−1 miR-155 inhibitor (Shanghai GenePharma Co., Ltd.,
Shangai, China) for 3 days through the tail vein, twice per day.
Subsequently, the LPS and miR-155 inhibitor plus LPS group were
injected with 20 mg kg−1 LPS (E. coli 0111:B4,
Sigma-Aldrich, St. Louis, MO, USA), and the control group was i.p.
injected with an equal volume of sterile saline at the same time.
Mice in these three groups were sacrificed by decapitation at four
time points, 6, 12, 24 and 48 h after LPS exposure (n=10 per time
point), and livers were removed surgically for the follow-up
experiments. All experiments were conducted in strict accordance
with the recommendations in the Guide for the Care and Use of
Laboratory Animals of the National Institutes of Health. The
present study was approved by the ethics committee of the
Children's Hospital Affiliated to Shanghai Jiaotong University
(Shanghai, China).
Histology and ELISA analyses
Prior to paraffin embedding, livers were fixed in 4%
paraformaldehyde overnight at room temperature and then transferred
to 70% ethanol. Subsequently, organs were embedded and frozen using
liquid nitrogen-cooled isopentane, and then paraffin-embedded
samples were sectioned at 4-μm thickness. For pathological
analysis, paraffin sections were stained with hematoxylin and
eosin. The sections were observed under an optical microscope
(CKX31SF; Olympus Corporation, Tokyo, Japan).
Prior to ELISA analysis, liver samples were
mechanically homogenized in protease-inhibitor (Sigma-Aldrich)
containing phosphate-buffered saline, and the homogenates were
centrifuged at 11,330 × g for 30 min at 4°C. The protein
concentrations of the supernatant fraction was measured according
to a bicinchoninic acid (BCA) protein measurement kit (R&D
Systems, Minneapolis, MN, USA). TNF-α and IL-10 ELISA kits were
purchased from R&D Systems; sheep anti-mouse polyclonal
antibody (1:200) was used to detect TNF-α, and mouse monoclonal
antibody (1:1,000) was used to detect IL-10.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from livers (50 mg) using
TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA)
according to the manufacturer's instructions. First-strand cDNAs
were generated from 3 µg of total RNA using commercially
available kits (Applied Biosystems, Foster City, CA, USA). All
subsequent PCR reactions were performed using the Universal PCR
Master mix (Applied Biosystems). Primers of miR-155, STAT1 and
SOCS1 used for amplification are shown in Table I. Amplification conditions were as
follows: 95°C for 10 min, followed by 40 cycles of 15 sec at 95°C,
1 min at 60°C. The melting curves were acquired by heating the
samples to 95°C for 1 min, cooling to 55°C for 1 min and then
slowly increasing the temperature from 65 to 95°C at a rate of
0.5°C/30 sec. Thermal cycling and fluorescence detection of mRNA
were analyzed by the 7500 real-time PCR system (Applied
Biosystems). To normalize mRNA concentrations, transcriptional
levels of 5S or GAPDH mRNA were identified in parallel for each
sample, and the relative transcriptional level of miR-155 was
adjusted by standardization based on the 5S mRNA levels and, SOCS1
and STAT1 were adjusted by standardization based on the GAPDH mRNA
levels. Samples for each experimental condition were run in
triplicate.
 | Table IGene-specific primers for miR155,
SOCS1, STAT1, GAPDH and 5S in RT-qPCR. |
Table I
Gene-specific primers for miR155,
SOCS1, STAT1, GAPDH and 5S in RT-qPCR.
| Gene | Primer |
|---|
| miR-155 | F:
5′-CGGCGGTTAATGCTAATTGTGAT-3′ |
| R:
5′-GTGCAGGGTCCGAGGT-3′ |
| SOCS1 | F:
5′-TCCGATTACCGGCGCATCACG-3′ |
| R:
5′-CTCCAGCAGCTCGAAAAGGCA-3′ |
| STAT1 | F:
5′-ATTTCTCCTTCTGGCCTTG-3′ |
| R:
5′-AGGAACGTCCCTGGCTG-3′ |
| GAPDH | F:
5′-TGCACCACCAACTGCTTAGC-3′ |
| R:
5′-GCATGGACTGTGGTCATGAG-3′ |
| 5S | F:
5′-TCGTCTGATCTCGGAAGCTA-3′ |
| R:
5′-AAGCCTACAGCACCCGGTAT-3′ |
Statistical analysis
All data were subjected to assessment of the
treatment effects using Student's t-test with SPSS 13.0 software
(SPSS Inc., Chicago, IL, USA). P<0.05 and 0.01 were considered
to indicate a statistically significant difference. The results are
presented as the mean ± standard deviation.
Results
miR-155 inhibitor alleviates the symptoms
of LPS-exposed mice
The ethological changes after LPS treatment in
miR-155 inhibitor-pretreated mice were first surveyed. As expected,
LPS administration lead to fatigue and increased heart rate, and
significantly reduced activity and food intake as compared with the
saline-treated control group. Although mice pretreated with the
miR-155 inhibitor for 3 days also exhibited these symptoms in
contrast to the LPS group, they were less severe. In addition, the
mortality of the LPS group and miR-155 inhibitor plus LPS group was
12.5% and 5%, respectively. In the LPS group, 7.5% of mice died
within 12 h and 5% of mice died between 12 and 24 h. However, 5% of
mice receiving miR-155 inhibitor died within 12 h after LPS
challenge, and no mice died between 12 and 24 h. These results
demonstrate that the miR-155 inhibitor contributes to the reduction
of LPS-induced mortality.
miR-155 inhibitor relieves LPS-induced
liver injury
Next to investigate the effects of the miR-155
inhibitor on liver injury, the histological changes following LPS
treatment in miR-155 inhibitor-pretreated mice were determined. In
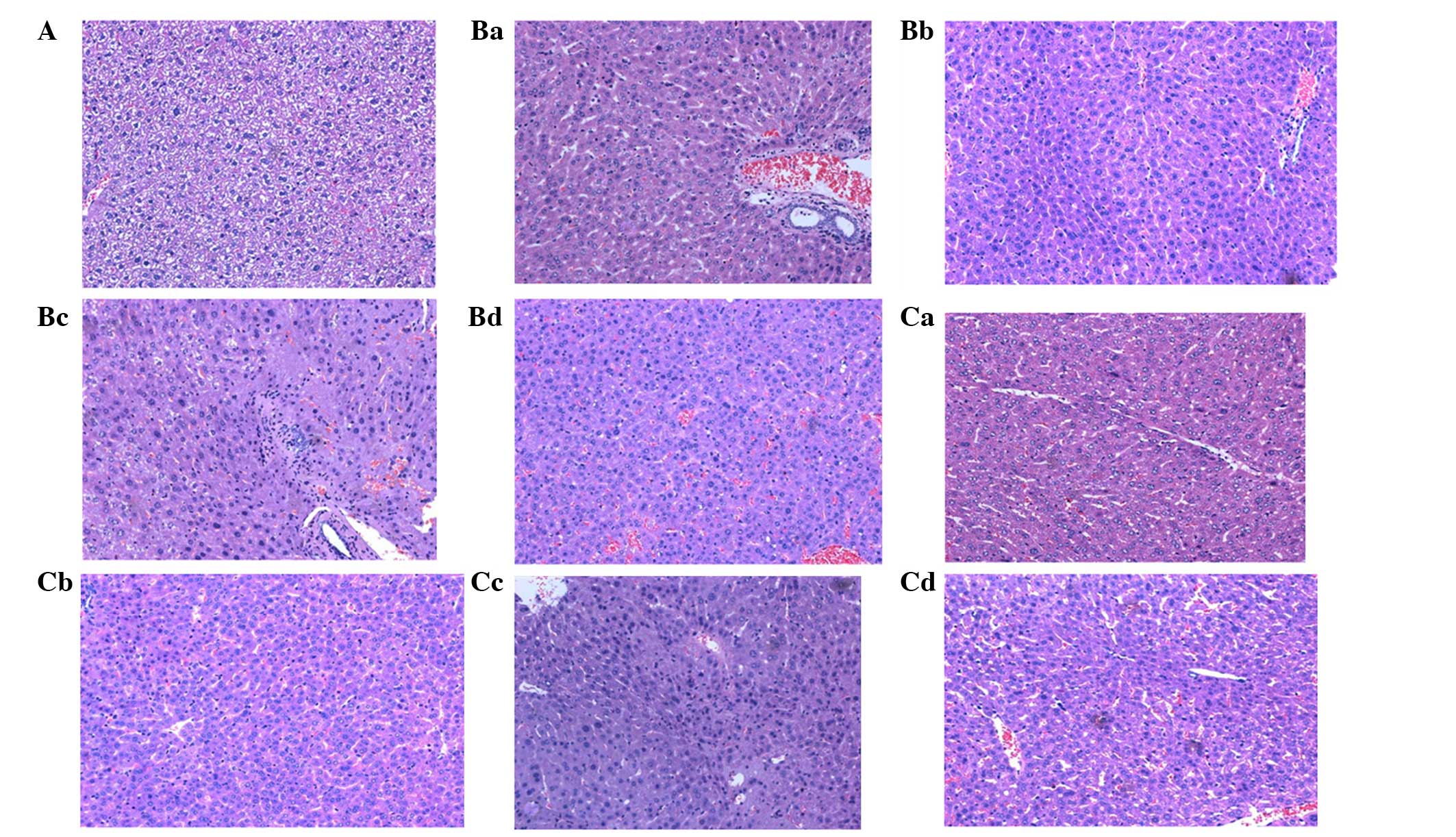
the control group, liver tissue had a normal structure (Fig. 1A). By contrast, when mice were
injected with LPS, characteristics of liver injury, such as
infiltration of inflammatory cells and acidophilic and vacuolar
degeneration, were first observed 6 h after LPS treatment, and the
symptoms became more severe in a time-dependent manner (Fig. 1B). Furthermore, abnormal
characteristics, including karyopyknosis, nodular necrosis,
cytoplasm rarefaction, infiltration of neutrophils into the hepatic
sinusoid and portal area, and disorder of liver structure were
first observed in mice treated with LPS for 24 h and then became
more severe at 48 h. In the miR-155 inhibitor plus LPS group,
miR-155 inhibitor pretreatment markedly relieved LPS-induced
pathological changes in the liver (Fig. 1C). The infiltration of inflammatory
cells was observed at 24 h after LPS treatment, and only slight
infiltration of inflammatory cells and acidophilic degeneration was
observed in the following time-points. Moreover, disorder of liver
structure and nodular necrosis were not observed at any time point.
These data suggest that the miR-155 inhibitor protected mice
against LPS-induced liver injury.
miR-155 inhibitor reduces the expression
of LPS-induced miR-115
To investigate whether expression of miR-155 induced
by LPS can be suppressed by miR-155 inhibitor, expression of
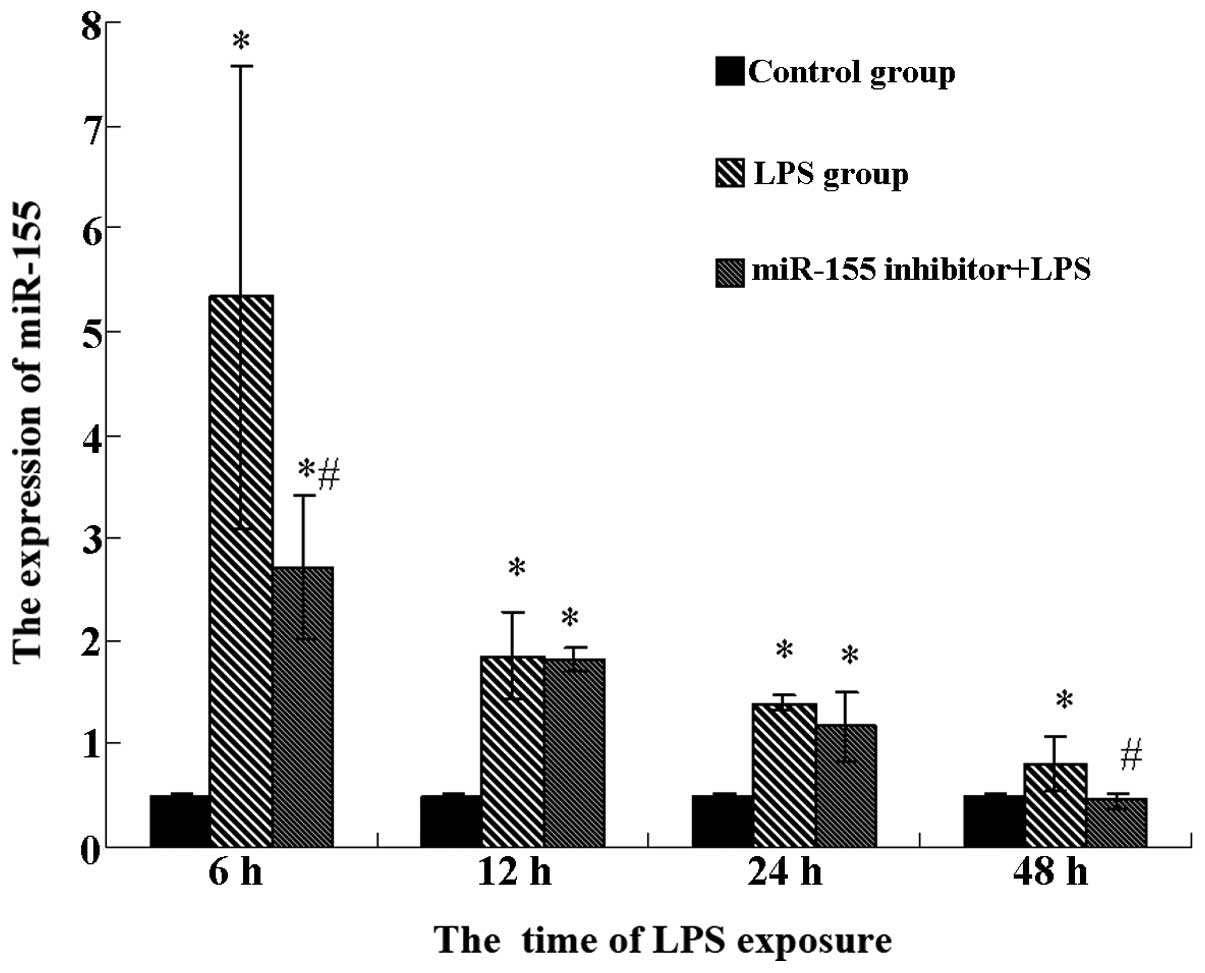
miR-155 in the mouse liver was detected by RT-qPCR, as shown in
Fig. 2. The mouse livers had a low
basal level of miR-155 expression in the control group. The
expression of miR-155 was significantly increased when treated with
LPS for 6 h (P<0.05), which suggests that LPS is able to improve
miR-155 expression in mouse liver, and this effect was inversely
dependent on the time of LPS exposure. miR-155 inhibitor-pretreated
mice incubated with LPS showed significantly lower expression of
miR-155 than saline-pretreated mice incubated with LPS at 6 and 48
h respectively (P<0.05). However, no significant difference was
identified between these two groups at 12 or 24 h. Collectively,
these findings suggest that LPS induces miR-155, which is inhibited
by the miR-155 inhibitor.
miR-155 inhibitor inactivates JAK/STAT1
signaling by elevating SOCS1 expression
It has been described that miR-155 potentiates the
inflammatory signaling of JAK/STAT through targeting SOCS1 protein
(18,19). Therefore, it was primarily assessed
whether the miR-155 inhibitor can regulate the expression of SOCS1
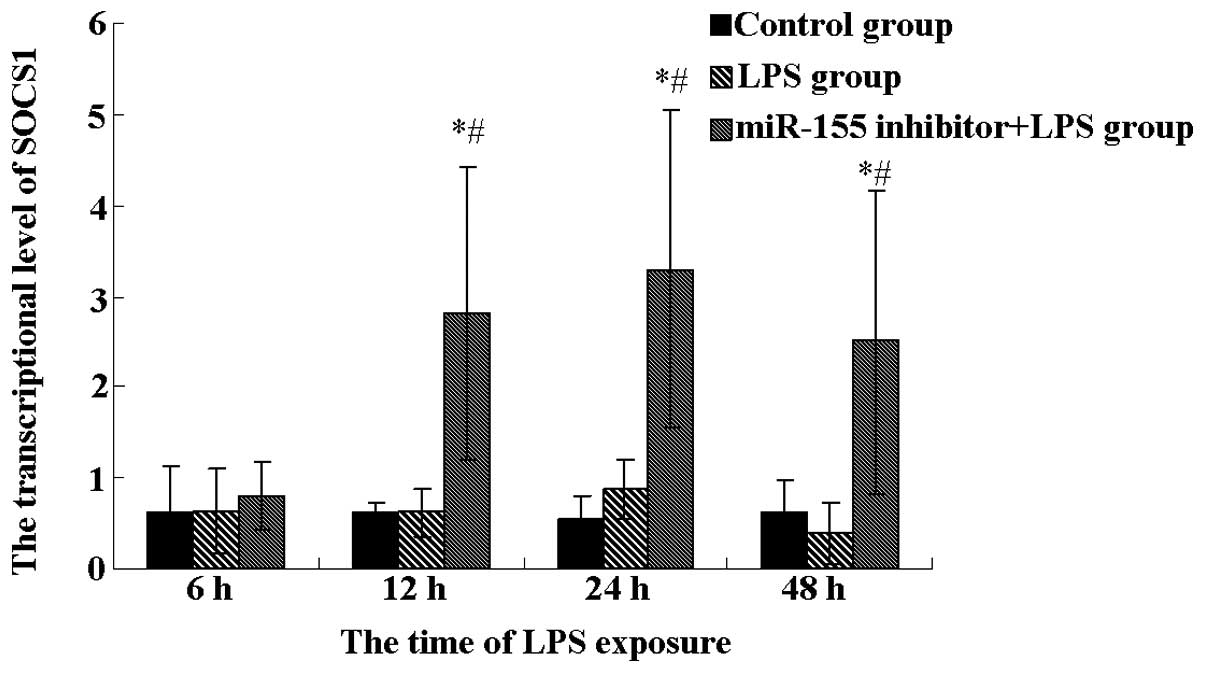
in the mouse liver. As shown in Fig.
3, as expected, the transcriptional level of SOCS1 was detected
to show differential expression in the miR-155 inhibitor plus LPS
group. Although identical expression was found among these three
groups at 6 h, the SOCS1 expression was significantly increased in
the miR-155 inhibitor plus LPS group compared with the control and
LPS groups after 12 h treatment (P<0.05), indicating that the
miR-155 inhibitor enhances the SOCS1 expression.
It was then determined whether SOCS1 expression
enhanced by the miR-155 inhibitor results in the inhibition of
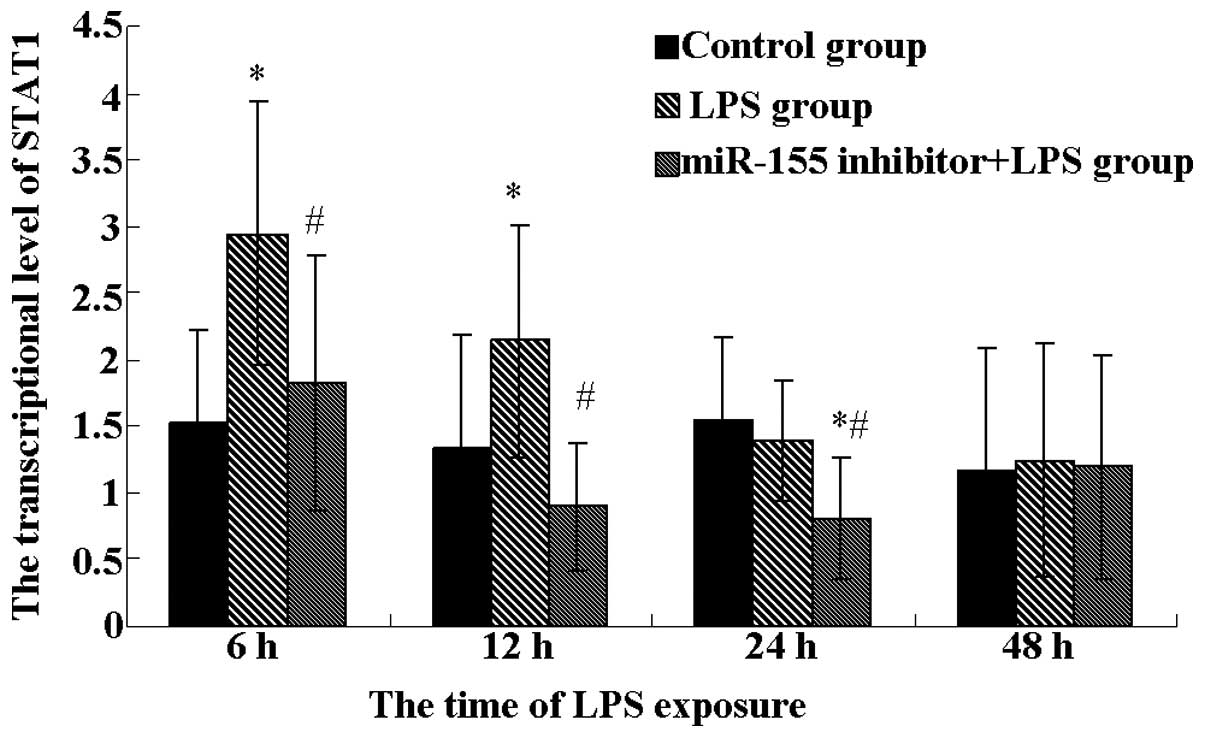
JAK/STAT signaling. STAT1 expression was observed at the mRNA
level, as shown in Fig. 4. Results
showed that the LPS group highly expressed STAT1 at 6 and 12 h,
while the expression of STAT1 was markedly reduced in the miR-155
plus LPS group at the corresponding times (P<0.05).
Additionally, the miR-155 inhibitor plus LPS group also reduced the
expression of STAT1 at 24 h in contrast to the control and LPS
groups (P<0.05), although there was no significant difference
between the control and LPS group. These observations indicate that
the miR-155 inhibitor inactivates JAK/STAT1 signaling by elevating
SOCS1 expression.
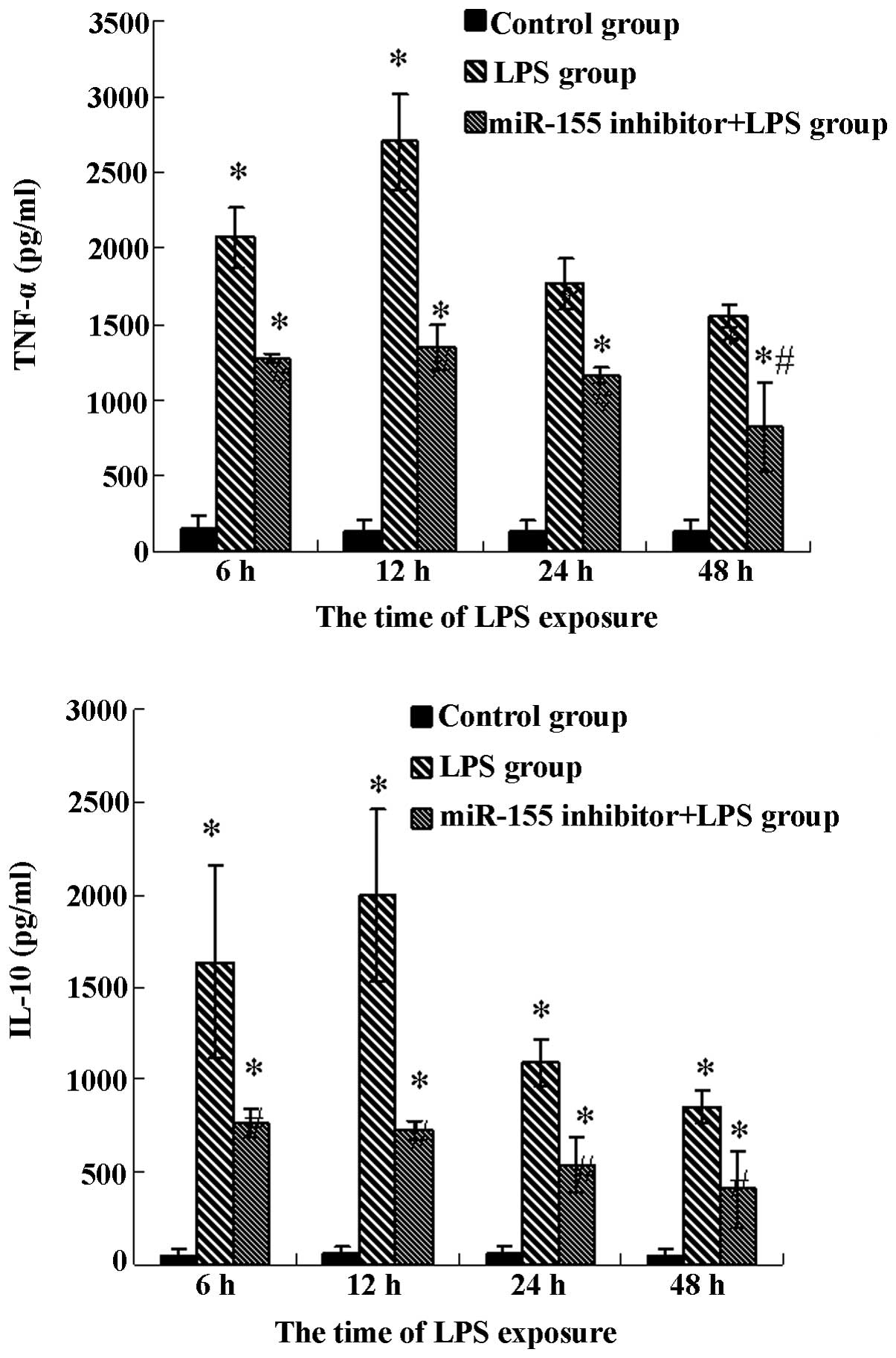
Production of TNF-α and IL-10 are
decreased by the miR-155 inhibitor
Given that LPS results in the production of
cytokines, including TNF-α and IL-10, which are involved in the
process of sepsis, it was then investigated whether the production
of these two cytokines is altered by treatment with LPS in miR-155
inhibitor-pretreated mouse liver. Notably, the TNF-α production was
higher when mice were treated with LPS (P<0.05), however, the
TNF-α production was significantly reduced when miR-155
inhibitor-pretreated mice were treated with LPS (P<0.05,
Fig. 5). Similarly, the production
of IL-10 was significantly increased in the LPS treated group
compared with the saline treated control group (P<0.05), and
this increase can be partly neutralized by pretreatment with the
miR-155 inhibitor (P<0.05). These results reveal that LPS
induces the production of TNF-α and IL-10, which is reversed
partially by the miR-155 inhibitor.
Discussion
In the present study, LPS administration was used to
investigate sepsis-associated liver injury. It was demonstrated
that the miR-155 inhibitor exerts potent anti-inflammatory and
immunomodulatory actions in LPS-exposed mouse liver, as evidenced
by markedly reducing the mortality of LPS-exposed mice and the
significant decreases in liver injury. Previous studies have
demonstrated that overexpression of miRNA let-7e in macrophage
results in sensitivity to LPS in cell culture and animal models
(20), and downregulated miR-125b
upon LPS challenge probably contributes to an increase in the
production and secretion of TNF-α (21). Previous studies, together with the
results from the present study give strong indication that miRNA
maybe central in LPS-induced sepsis and highlights the significance
and relevance of miRNAs, such as miR-155, as potential downstream
biomarkers for therapeutic intervention in sepsis-associated liver
injury.
miR-155, is the most extensively investigated miRNA
in innate immune cells (22), and
mediates the immune response through targeting a number of genes
for translational repression. A previous study demonstrated that
miR-155 most likely directly targets transcripts coding for several
proteins involved in LPS signaling, such as Fas-associated death
domain protein, IκB kinase ε and the receptor (TNFR
superfamily)-interacting serine-threonine kinase 1, thereby
enhancing TNF-α translation (21).
In addition, miR-155 regulates human dendritic cell development and
IL-12 production through targeting Kip1 ubiquitination-promoting
complex 1 and SOCS1, respectively (23). Foxp3-dependent miR-155 confers
competitive fitness to regulatory T cells by inducing SOCS1
downregulation (19). Given the
results in previous studies, administration of the miR-155
inhibitor may lead to the inactivation of above-mentioned genes and
reduced production of cytokines, such as TNF-α. This is consistent
with the current results that miR-155 inhibitor-pretreated mice
induced by LPS not only show the observably upregulated expression
of SOCS1 but also exhibit a significant decrease in the secretion
of TNF-α.
SOCS1, a pivotal downregulating factor for LPS
signal pathways (24), possesses a
negative regulatory role in the JAK/STAT signal cascade (25), thereby acting as an essential
negative regulatory molecule in innate immune responses. Thus, it
was hypothesized that expression of SOCS1 enhanced by the miR-155
inhibitor may inhibit the JAK/STAT signaling pathway. Consistent
with this, follow-up experiments identified a member of the STAT
family, STAT1, which exhibited lower expression levels following
treatment with LPS in miR-155 inhibitor-pretreated mice compared
with treatment with LPS in saline-pretreated mice. It is therefore
rational to conclude that the ability of the miR-155 inhibitor to
protect against LPS-induced liver injury occurs through enhancing
the expression of SOCS1 and in turn inhibiting JAK/STAT
signaling.
Additionally, besides the decrease in the secretion
of TNF-α, IL-10, is also attenuated in miR-155 inhibitor-pretreated
mice induced by LPS. Given that excessive cytokine-mediated
inflammation was hypothesized to be crucial in the development of
other tissue injuries (26),
decreases in the levels of cytokines, such as TNF-α and IL-10, may
alleviate the inflammatory reaction and then ameliorate the
sepsis-associated liver injury.
In conclusion, the present study identified that the
miR-155 inhibitor enhanced the expression of SOCS1 and
significantly relieved the liver injury induced by LPS through
inactivating the JAK/STAT signaling pathway. These findings
emphasize the correlation between miRNA and sepsis-associated liver
injury, and suggest that miR-155 may be a potential target for
treating sepsis-associated liver injury in the future.
Acknowledgments
This study was supported by the Key project of
Shanghai science and technology Committee (grant no. 12411952404).
The authors would like to thank Fenghe (Shanghai) Information
Technology Co. Ltd for their ideas and help, which gave a valuable
added dimension to the research.
References
|
1
|
Starr ME and Saito H: Sepsis in old age:
Review of human and animal studies. Aging Dis. 5:126–136.
2014.PubMed/NCBI
|
|
2
|
Antonelli M, Bonten M, Cecconi M, Chastre
J, Citerio G, Conti G, Curtis JR, Hedenstierna G, Joannidis M,
Macrae D, et al: Year in review in Intensive Care Medicine 2012.
II: Pneumonia and infection, sepsis, coagulation, hemodynamics,
cardiovascular and microcirculation, critical care organization,
imaging, ethics and legal issues. Intensive Care Med. 39:345–364.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bone RC, Balk RA, Cerra FB, Dellinger RP,
Fein AM, Knaus WA, Schein RM and Sibbald WJ: Definitions for sepsis
and organ failure and guidelines for the use of innovative
therapies in sepsis. The ACCP/SCCM Consensus Conference Committee
American College of Chest Physicians/Society of Critical Care
Medicine. Chest. 101:1644–1655. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ring A, Braun JS, Pohl J, Nizet V,
Stremmel W and Shenep JL: Group B streptococcal beta-hemolysin
induces mortality and liver injury in experimental sepsis. J Infect
Dis. 185:1745–1753. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Garvin IP: Remarks on pneumonia biliosa. S
Med Surg. 1:536–544. 1837.
|
|
6
|
Dhainaut JF, Marin N, Mignon A and
Vinsonneau C: Hepatic response to sepsis: Interaction between
coagulation and inflammatory processes. Crit Care Med. 29(7 Suppl):
S42–S47. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bosmann M and Ward PA: The inflammatory
response in sepsis. Trends Immunol. 34:129–136. 2013. View Article : Google Scholar :
|
|
8
|
Rasko DA and Sperandio V: Anti-virulence
strategies to combat bacteria-mediated disease. Nat Rev Drug
Discov. 9:117–128. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kilár A, Dörnyei Á and Kocsis B:
Structural characterization of bacterial lipopolysaccharides with
mass spectrometry and on- and off-line separation techniques. Mass
Spectrom Rev. 32:90–117. 2013. View Article : Google Scholar
|
|
10
|
Takeuchi O and Akira S: Pattern
recognition receptors and inflammation. Cell. 140:805–820. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Saraiva M and O'Garra A: The regulation of
IL-10 production by immune cells. Nat Rev Immunol. 10:170–181.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aaronson DS and Horvath CM: A road map for
those who don't know JAK-STAT. Science. 296:1653–1655. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
O'Shea JJ, Gadina M and Schreiber RD:
Cytokine signaling in 2002: New surprises in the Jak/Stat pathway.
Cell. 109(Suppl): S121–S131. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kutty RK, Nagineni CN, Samuel W,
Vijayasarathy C, Hooks JJ and Redmond TM: Inflammatory cytokines
regulate microRNA-155 expression in human retinal pigment
epithelial cells by activating JAK/STAT pathway. Biochem Biophys
Res Commun. 402:390–395. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang JF, Yu M, Yu G, Bian JJ, Deng XM, Wan
XJ and Zhu KM: Serum miR-146a and miR-223 as potential new
biomarkers for sepsis. Biochem Biophys Res Commun. 394:184–188.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vasilescu C, Rossi S, Shimizu M, Tudor S,
Veronese A, Ferracin M, Nicoloso MS, Barbarotto E, Popa M,
Stanciulea O, et al: MicroRNA fingerprints identify miR-150 as a
plasma prognostic marker in patients with sepsis. PLoS One.
4:e74052009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
O'Connell RM, Taganov KD, Boldin MP, Cheng
G and Baltimore D: MicroRNA-155 is induced during the macrophage
inflammatory response. Proc Natl Acad Sci USA. 104:1604–1609. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang S, Zhang HW, Lu MH, He XH, Li Y, Gu
H, Liu MF and Wang ED: MicroRNA-155 functions as an OncomiR in
breast cancer by targeting the suppressor of cytokine signaling 1
gene. Cancer Res. 70:3119–3127. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu LF, Thai TH, Calado DP, Chaudhry A,
Kubo M, Tanaka K, Loeb GB, Lee H, Yoshimura A, Rajewsky K and
Rudensky AY: Foxp3-dependent microRNA155 confers competitive
fitness to regulatory T cells by targeting SOCS1 protein. Immunity.
30:80–91. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Androulidaki A, Iliopoulos D, Arranz A,
Doxaki C, Schworer S, Zacharioudaki V, Margioris AN, Tsichlis PN
and Tsatsanis C: The kinase Akt1 controls macrophage response to
lipopolysaccharide by regulating microRNAs. Immunity. 31:220–231.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tili E, Michaille JJ, Cimino A, Costinean
S, Dumitru CD, Adair B, Fabbri M, Alder H, Liu CG, Calin GA and
Croce CM: Modulation of miR-155 and miR-125b levels following
lipopolysaccharide/TNF-α stimulation and their possible roles in
regulating the response to endotoxin shock. J Immunol.
179:5082–5089. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang P, Hou J, Lin L, Wang C, Liu X, Li D,
Ma F, Wang Z and Cao X: Inducible microRNA-155 feedback promotes
type I IFN signaling in antiviral innate immunity by targeting
suppressor of cytokine signaling 1. J Immunol. 185:6226–6233. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lu C, Huang X, Zhang X, Roensch K, Cao Q,
Nakayama KI, Blazar BR, Zeng Y and Zhou X: MiR-221 and miR-155
regulate human dendritic cell development, apoptosis and IL-12
production through targeting of p27kip1, KPC1 and SOCS-1. Blood.
117:4293–4303. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nakagawa R, Naka T, Tsutsui H, Fujimoto M,
Kimura A, Abe T, Seki E, Sato S, Takeuchi O, Takeda K, et al:
SOCS-1 participates in negative regulation of LPS responses.
Immunity. 17:677–687. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Croker BA, Kiu H and Nicholson SE: SOCS
regulation of the JAK/STAT signalling pathway. Semin Cell Dev Biol.
19:414–422. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Goodman RB, Pugin J, Lee JS and Matthay
MA: Cytokine-mediated inflammation in acute lung injury. Cytokine
Growth Factor Rev. 14:523–535. 2003. View Article : Google Scholar : PubMed/NCBI
|