Introduction
The role of cholesterol in malignancy has been
recognized for numerous decades. In 1933, Roffo reported that cell
cholesterol levels were associated with carcinogenesis (1). Malignant tumors were also reported to
contain elevated levels of neutral lipids, particularly
phospholipids and cholesteryl ester as compared to those in native,
healthy tissues from which the tumors originated (2). Furthermore, tissues of clear cell
renal cell carcinoma, a representative type of malignant kidney
tumor, contained increased amounts of cholesteryl esters compared
with those in normal kidneys (3).
Cholesteryl ester is synthesized by acyl-CoA:cholesterol
acyltransferase (ACAT), which is a membrane-bound microsomal enzyme
that catalyzes cholesteryl ester formation by using long-chain
fatty acyl-CoA and cholesterol as substrates (4,5). Of
the two human ACAT isoforms, ACAT1 and ACAT2, ACAT1 is expressed in
numerous organs and tissue types, including the brain (6). Previous studies showed that
cholesteryl ester levels in glioma tissues were higher than those
in normal brain tissues (7,8).
Glioma is the most common type of malignant brain tumor and is
derived from glial cells or their precursors. Glioma is classified
as grade I–IV on the basis of its histological anaplasia and the
degree of tumor cell proliferation; the diffuse invasion of brain
tissue by glioma cells results in incomplete surgical resection and
frequent post-surgical tumor recurrence (9). Low-grade glioma occasionally
transforms into an aggressive form, which has a poor prognosis
(10). Therefore, an effective
therapeutic strategy to reduce the proliferation of glioma cells
after surgical resection is necessary.
The present study analyzed human glioblastoma
tissues for ACAT1 expression. To investigate the cancer-promoting
roles of ACAT1 in glioblastoma, the human glioblastoma cell line
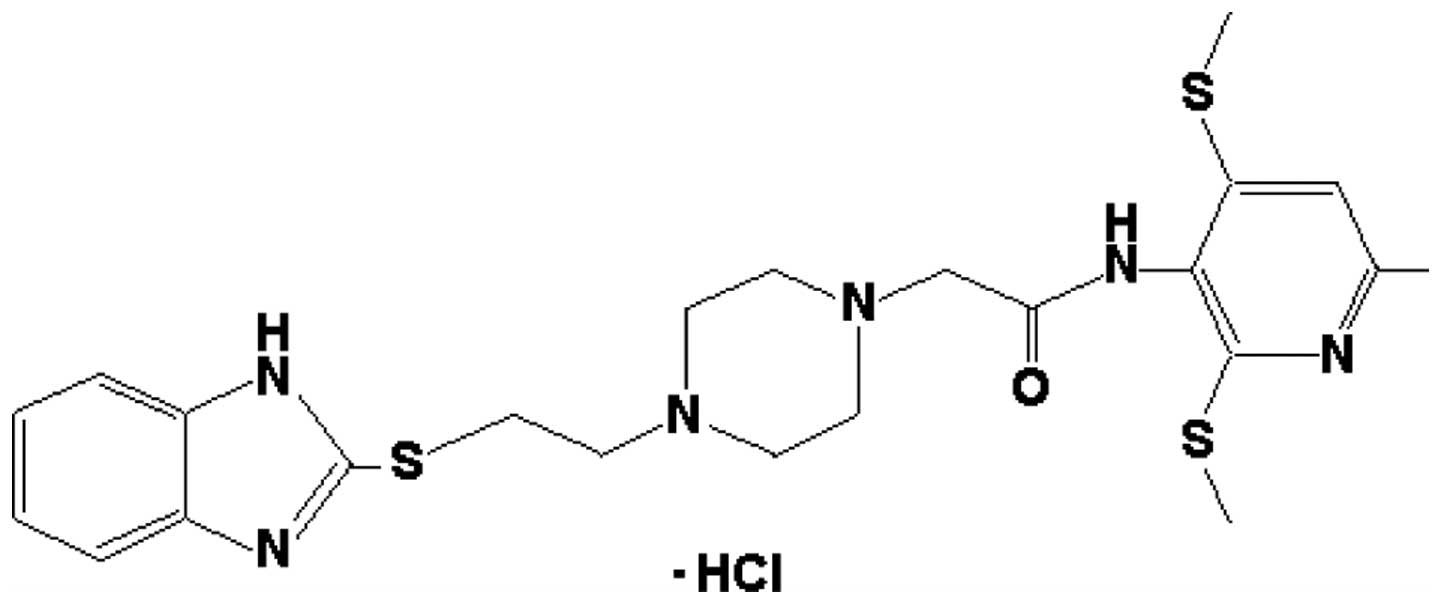
U251-MG was used in order to determine whether K604 (Fig. 1), a specific ACAT1 inhibitor which
does not affect the systemic cholesterol metabolism (11), suppresses the proliferation of
U251-MG cells. Furthermore, to investigate the detailed molecular
mechanism of the activity of ACAT1 in glioma, the effects of K604
on the phosphorylation of Akt and extracellular-signal-regulated
kinase 1/2 (ERK1/2), which have a key role in cell proliferation
and survival (12,13), were assessed. The results of the
present study, as well as those of a previous study (14) supported that ACAT1 may be a
promising therapeutic target for glioblastoma.
Materials and methods
Materials
K604 was kindly provided by Kowa Company Ltd.
(Tokyo, Japan). Rabbit polyclonal anti-actin antibody (cat. no.
A2066), mouse monoclonal anti-phosphorylated ERK1/2 antibody (cat.
no. M8159), SDS, polyacrylamide, bovine serum albumin, saponin,
poly-l-lysine and
dimethyl sulfoxide were purchased from Sigma-Aldrich (St. Louis,
MO, USA). An rabbit polyclonal anti-phosphorylated Akt antibody
(cat, no. 9271), rabbit monoclonal anti-pan Akt antibody (cat. no.
4691) and rabbit monoclonal anti-ERK1/2 antibody (cat. no. 4695)
were purchased from Cell Signaling Technology (Beverly, MA, USA).
Rabbit polyclonal anti-ACAT1 (1:1,000) antibody was prepared as
previously described (15).
Trichloroacetic acid (TCA) was obtained from Wako Pure Chemical
Industries, Ltd. (Osaka, Japan).
Cell culture
U251-MG cells, obtained from American Type Culture
Collection (Manassas, VA, USA) were cultured in Dulbecco's modified
Eagle's medium (Sigma-Aldrich) supplemented with 10%
heat-inactivated fetal bovine serum (Lonza Group Ltd, Basel,
Switzerland), 100 U/ml penicillin (Sigma-Aldrich) and 100 mg/ml
streptomycin (Sigma-Aldrich) at 37°C in an atmosphere containing 5%
CO2.
Immunohistochemistry
The present study was approved by the Ethics
Committee of the Tokushima University Hospital (Tokushima, Japan).
Formalin-fixed, paraffin-embedded specimens of human glioblastoma
tissues were obtained from three patients (70–90 years old) in July
2010, December 2013 and March 2014. No normal brain tissues were
examined. The tumors were classified as grade IV. Tokushima
University Hospital. Informed consent was obtained from the patient
with the presence of a family member as a witness. Paraffin blocks
of tissues were cut into 3-mm slices using a slide microtome (Leica
SM2010 R; Leica Microsystems, Wetzlar, Germany). After the tissue
sections were de-paraffinized in xylene (Wako Pure Chemical
Industries, Ltd.) and re-hydrated in decreasing concentrations of
ethanol (Wako Pure Chemical Industries, Ltd.) they were incubated
with 0.1 M sodium citrate (pH 6.0; Wako Pure Chemical Industries,
Ltd. at 95°C for 20 min for antigen retrieval. Endogenous
peroxidases were blocked with 0.9% hydrogen peroxide (Wako Pure
Chemical Industries, Ltd.), followed by blocking with 10% bovine
serum albumin (Sigma-Aldrich) in phosphate-buffered saline (PBS)
for 30 min. The sections were incubated with rabbit polyclonal
anti-ACAT1 antibody (1:1,000) for 1 h at room temperature. After
removal of primary antibodies by washing the sections for five
times with PBS, the sections were reacted with the secondary
antibody Histofine Simple Stain MAX PO (R) (Nichirei Bioscience,
Inc., Tokyo, Japan) for 30 min. For visualization of the reaction,
a 3,3′-diaminobenzidine H2O2 substrate
(DAB-H2O2; Dako, Glostrup, Denmark) was
applied to the sample. The sections were then counterstained with
Mayer's hematoxylin solution and coverslips were mounted with
Entellan mounting medium (Merck, Kenilworth, NJ, USA).
Immunostained samples were analyzed using a BX51 light microscope
and a DP-25 digital camera (Olympus Corporation, Tokyo, Japan). For
histological examination, de-paraffinized tissue sections were also
stained with hematoxylin and eosin (HE; cat. nos. 8650 and 8659;
Sakura Finetek Japan, Tokyo, Japan).
Immunocytochemistry
U251-MG cells were plated on poly-l-lysine-coated coverslips at
various densities (0.75, 2.25 and 5.25×104
cells/cm2) followed by culture for 12 h. The cells were
fixed with 4% paraformaldehyde (Wako Pure Chemical Industries Ltd.)
for 20 min at room temperature. After the cells were washed with
PBS three times, they were blocked and permeabilized with 10%
normal goat serum (Thermo Fisher Scientific Inc., Waltham, MA, USA)
and 0.05% saponin (Wako Pure Chemical Industries, Ltd.) in PBS at
room temperature for 20 min. The cells were then incubated with the
primary antibody (1:250), for 30 min at room temperature, washed
with PBS, reacted with a secondary antibody [Histofine Simple Stain
MAX PO (R); Nichirei Bioscience, Inc.] and visualized after
incubation with DAB-H2O2 (Dako) solution. The
specimens were mounted with Entellan mounting medium and examined
with a BX51 light microscope and a DP-25 digital camera.
Cell proliferation assay
Cell proliferation was analyzed using an MTT assay
(16). U251-MG cells were plated
at densities of 0.75–6×104 cells/cm2 in a
24-well plate. The cells were cultured for 12 h and then treated
with 1 or 2 mM K604 for 48 h. Cell viability was quantitatively
determined by means of MTT reduction. In brief, MTT (Wako Pure
Chemical Industries, Ltd.) was added to each well to a final
concentration of 0.5 mg/ml. After 3 h of incubation, the medium was
removed and the precipitated formazan crystals were dissolved in
dimethyl sulfoxide (Wako Pure Chemical Industries, Ltd.). An
Infinite M200 microplate reader (Tecan Japan Co., Ltd, Kanagawa,
Japan) was used to measure the absorbance values of the formazan
crystals at 570 nm, with the absorbance at 650 nm then being
subtracted.
Western blot analysis
U251-MG cells were plated in 6-cm dishes (cat. no.
353002; Corning Life Sciences, Flintshire, UK) at various densities
(0.75, 2.25 and 5.25×104 cells/cm2) and
cultured for 12 h. Certain cell samples were treated with K604 (2
or 5 µM) for 24 h. Whole-cell lysates were prepared via TCA
precipitation (17). Briefly, the
cells were washed three times with PBS and were then treated with
10% (w/v) TCA (Wako Pure Chemical Industries, Ltd.) in PBS. After
the samples were incubated on ice for 30 min, they were centrifuged
at 1,000 × g for 5 min at 4°C. The precipitated proteins were
dissolved in SDS-PAGE sample buffer containing 0.125 M Tris-HCl, 4%
(w/v) SDS (Sigma-Aldrich), 20% (v/v) glycerol (Wako Pure Chemical
Industries, Ltd.) and 0.01% (w/v) bromophenol blue (Wako Pure
Chemical Industries, Ltd.) for preparation of cell lysates. The
protein concentration was determined using XL Bradford assay (Apro
Science, Tokushima, Japan) and 10 µg/lane were subjected to
10% SDS-PAGE (Wako Pure Chemical Industries Ltd.) and transferred
onto 0.45-µm Immobilon-P membranes (Millipore, Billerica,
MA, USA). After the membranes were blocked for 1 h in 1% BSA and 3%
nonfat dry milk (BD Biosciences, Franklin Lakes, NJ, USA) in
Tris-buffered saline (TBS) supplemented with 0.1% Tween 20 (TBS-T;
Cell Signaling Technology, Inc.), they were incubated with the
following primary antibodies: Anti-ACAT1 (1:1,000), anti-Akt
(1:1,000), anti-phosphorylated Akt (1:1,000), anti-ERK1/2 antibody
(1:1,000), anti-phosphorylated ERK1/2 (1:1,000) and anti-β-actin
(1:1,000) at room temperature for 1 h. The membranes were then
washed three times with TBS-T, after which they were incubated with
a horseradish peroxidase-labeled anti-rabbit or anti-mouse
secondary antibody (Cell Signaling Technology). After the membranes
were washed with TBS-T, blots were visualized using the enhanced
chemiluminescence reagent ImmunoStar LD (Wako Pure Chemical
Industries, Ltd). Protein expression was normalized to that of
β-actin. The blots were analyzed by using an LAS-3000 luminescent
image analyzer (Fujifilm, Tokyo, Japan) and Image J software
(version 1.47; National Institutes of Health, Bethesda, MD,
USA).
Statistical analysis
Data are expressed as the mean ± standard error of
the mean. Data were analyzed via one-way analysis of variance with
the appropriate control and K604-treated variables, followed by a
non-parametric Dunnett's test, using the statistical package R
(version 3.1.0; available as a free download from http://www.r-project.org). P<0.05 was considered to
indicate a statistically significant difference.
Results
ACAT1 is expressed in human glioblastoma
tissues
To assess the expression of ACAT1 in human glioma,
the present study first evaluated surgically resected glioblastoma
tissues obtained from three patients. According to the World Health
Organization grading system (18),
glioma is classified as a grade-IV brain tumor on the basis of
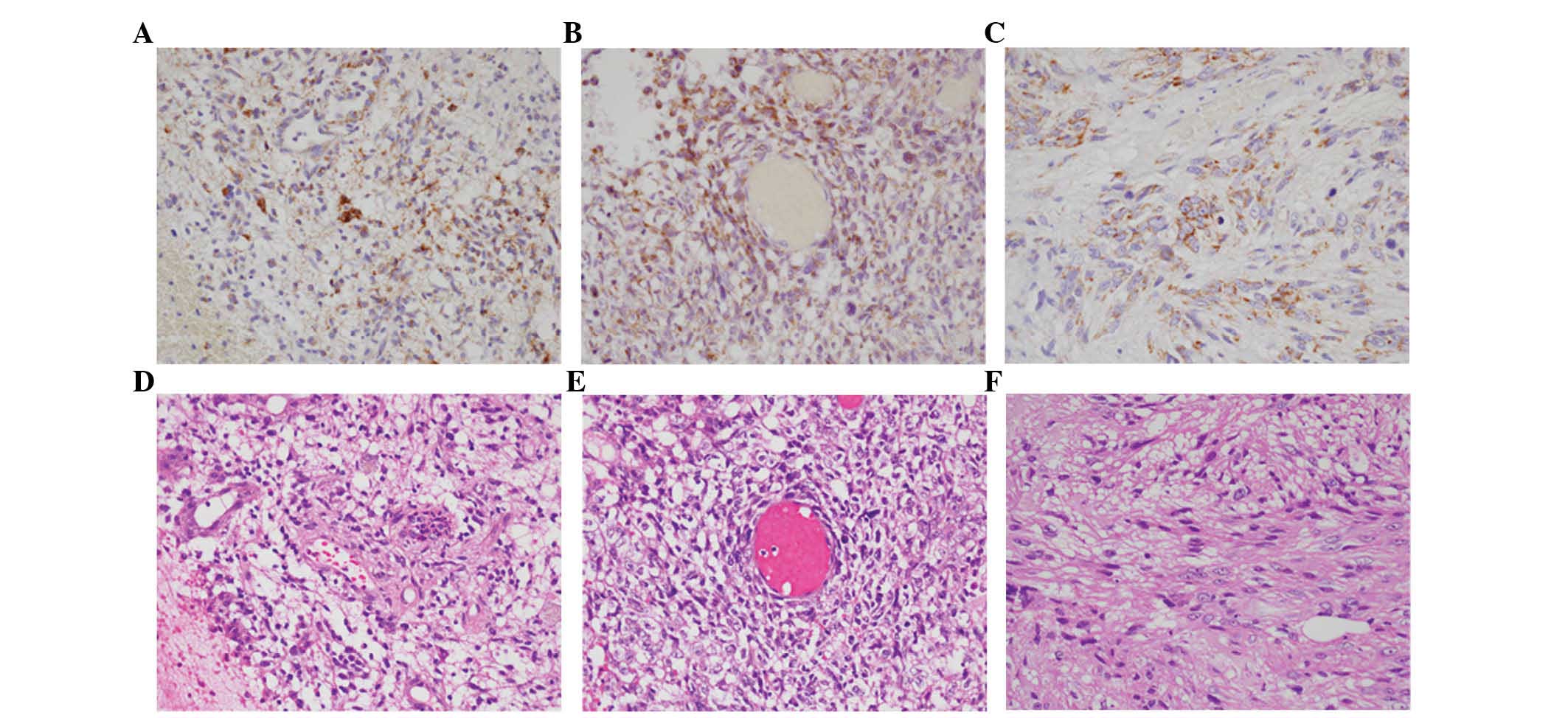
immunohistochemical data. As shown in Fig. 2, abundant ACAT1 expression was
confirmed in all human glioblastoma tissue samples examined
(Fig. 2A–C). Histological analysis
by HE staining confirmed that all tumor specimens were
glioblastoma, as they showed marked cellular anaplasia and high
cell density (Fig. 2D–F).
Expression of ACAT1 in U251-MG cells is
cell density-dependent
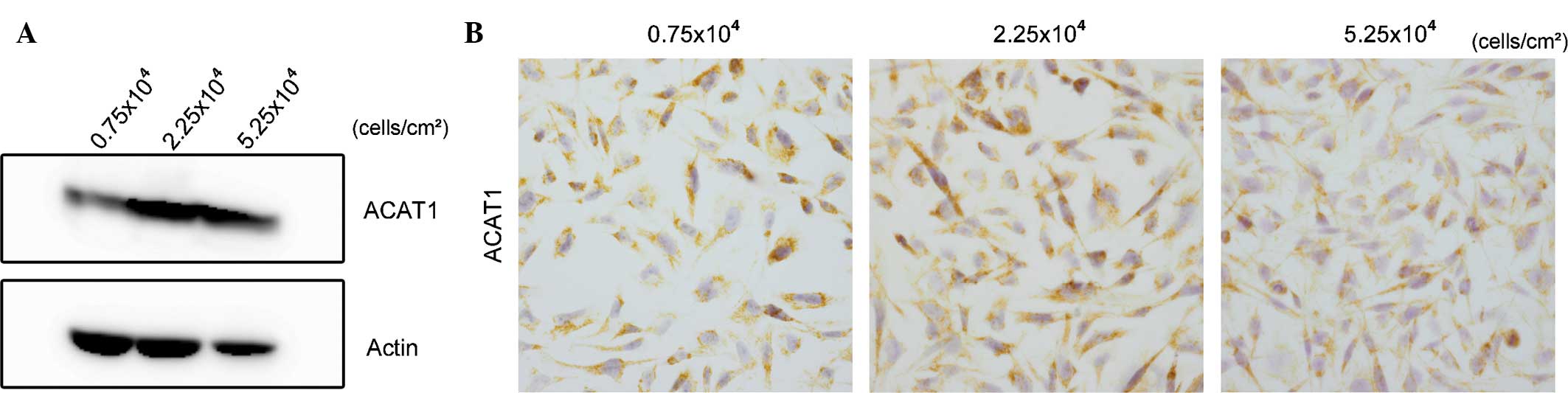
Next, the present study explored ACAT1 expression in
the human glioblastoma cell line U251-MG. Whole-cell lysates of
U251-MG cells plated at various cell densities were prepared by
means of TCA protein precipitation and were subjected to western
blot analysis. As shown in Fig.
3A, the expression levels of ACAT1 were highest at the cell
density of 2.25×104 cells/cm2, the density at
which cells grew logarithmically. Immunocytochemistry confirmed
that positive ACAT1 expression of U251-MG cells was highest at this
cell density (Fig. 3B). These
results suggested that ACAT1 expression in U251-MG cells depended
on their cell growth state, being highest in exponentially
proliferating cells.
ACAT1 inhibitor K604 reduces U251-MG cell
proliferation
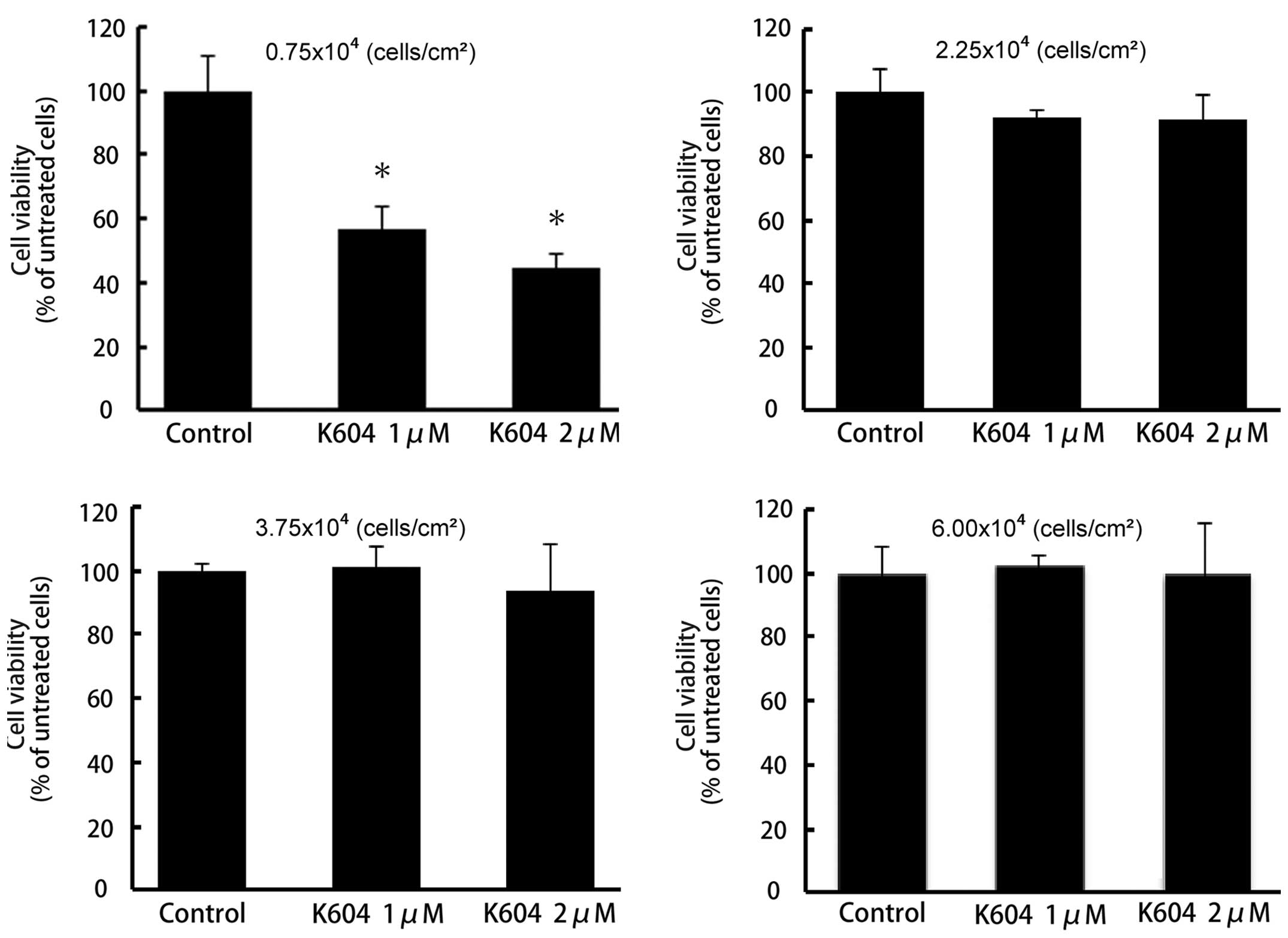
As ACAT1 expression in U251-MG cells depended on the
cell density and proliferative state, the effect of the ACAT1
inhibitor K604 on the proliferation of U251-MG cells was examined.
Of note, proliferation of cells at the low density (plated at
0.75×104 cells/cm2) was significantly
inhibited by K604 treatment (P<0.01) (Fig. 4A). However, K604 had no effect on
the proliferation of U251-MG cells at medium and high cell
densities (2.25–6.0×104 cells/cm2) (Fig. 4B–D).
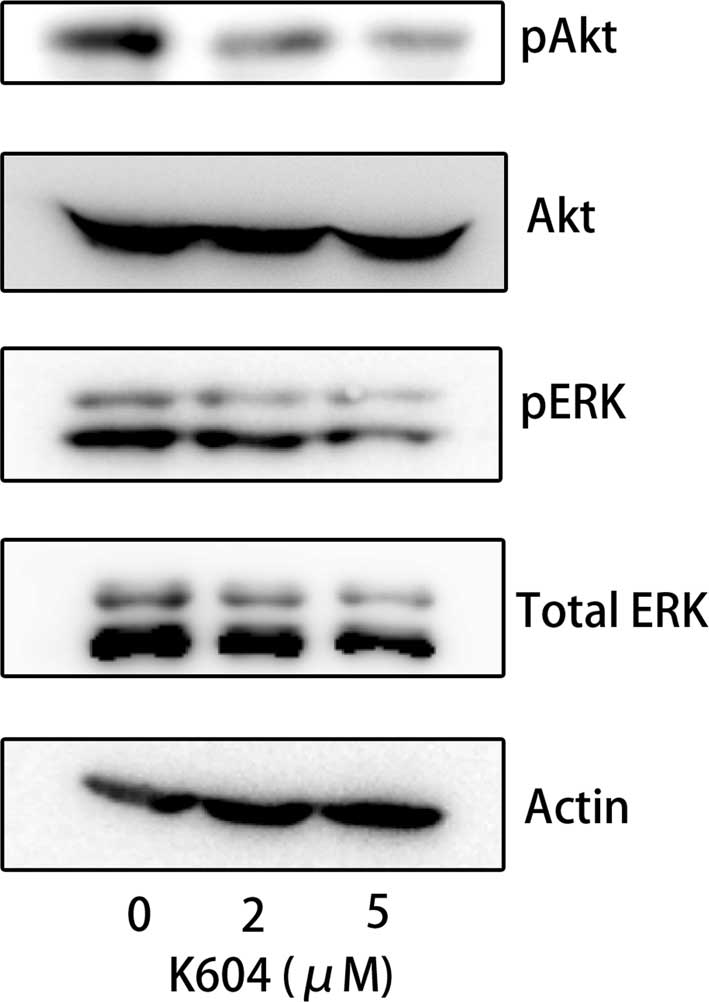
ACAT1 inhibition deactivates ERK1/2 and
Akt in U251-MG cells
To investigate the molecular mechanisms by which the
ACAT1 inhibitor K604 suppressed glioblastoma-cell proliferation,
the present study focused on the effects of K604 on the activation
of ERK1/2 and Akt in U251-MG cells, as these two kinases reportedly
regulate cell survival and proliferation of glioma, and affect the
prognosis of patients with glioma (13,19–21).
As K604 treatment effectively suppressed glioblastoma cell
proliferation at the low cell density (0.75×104
cells/cm2) (Fig. 4),
U251-MG cells plated at this density were treated with 0, 2 or 5
µM K604 for 24 h and subjected to western blot analysis of
the phosphorylation of Akt and ERK1/2. As shown in Fig. 5, K604 inhibited the phosphorylation
of Akt and ERK1/2 in a dose-dependent manner (Fig. 5).
Discussion
The present study showed that the selective ACAT1
inhibitor K604 effectively suppressed the proliferation of U251-MG
glioblastoma cells. ACAT1 expression was highest in giloblastoma
cells at the logarithmic growth phase. Furthermore, treatment with
K604 downregulated the phosphorylation of Akt and ERK1/2. These
results suggested that ACAT1 regulates glioblastoma-cell
proliferation via modification of the Akt and/or the ERK1/2
pathway. A previous study demonstrated that the pharmacological
ACAT inhibitor avasimibe suppressed glioblastoma-cell proliferation
via increased apoptosis and cell cycle arrest (14). Avasimibe, which, in contrast to
K604, inhibits the two ACAT isozymes ACAT1 and ACAT2, reduced the
serum cholesterol levels by preventing apolipo-protein B-containing
lipoprotein synthesis and secretion in minitiature pigs and
transgenic mice (22,23). Furthermore, avasimibe downregulated
ACAT1 expression and cholesterol internalization and augmented
cholesterol efflux in a dose-dependent manner in vitro
(14). By contrast, K604
selectively inhibits ACAT1, and was shown to not affect the serum
cholesterol levels, intracellular ACAT1 expression and cell
cholesterol internalization and/or efflux (11). Therefore, the present study used
the ACAT1-specific inhibitor K604 in order to study the molecular
functions of ACAT1 in glioblastoma cell biology.
A number of studies previously established that Akt
signaling is aberrantly activated in glioma and glioblastoma, which
results in aggressive cell proliferation and a consequent poor
clinical prognosis (20,24,25).
Akt inhibition effectively inhibited the growth of glioblastoma
cells as well as glioblastoma-like stem cells (26). Furthermore, Chakravarti et
al (20) reported that
activation of the phosphatidylinositol 3-kinase-Akt pathway is
significantly correlated with poor prognosis in patients with
glioma (20). The ERK pathway,
however, has been shown to be de-regulated in various human cancer
types (27). A previous study
demonstrated activation of the ERK pathway in human glioblastoma
(20), and glioma cell
proliferation was controlled via ERK1/2 activity (28). All of these results suggested that
activation of Akt and ERK1/2 may be associated with refractory
glioblastoma, which show resistance to clinical treatments due to
radiation resistance and incomplete surgical resection.
De-activation of the Akt and/or ERK1/2 pathway by the specific
ACAT1 inhibitor K604 is a promising therapeutic application for
this malignant tumor.
The molecular mechanisms of the inhibition of Akt
and ERK1/2 phosphorylation by K604 have yet to be elucidated. It is
widely accepted that the activation of Akt and ERK1/2 depends on
the cellular cholesterol levels and the integrated function of
lipid rafts, which are cholesterol-rich microdomains on the cell
membrane (29,30). Protein palmitoylation has a crucial
role in raft localization of the proteins (31), and ACAT inhibition or ablation was
shown to decrease raft localization of the amyloid precursor
protein by reducing its palmitoylation (32). The hyaluronan receptor CD44, which
is the principal molecule that determines the malignant behavior of
glioblastoma (33,34), was shown to be enriched in lipid
rafts and to be reversibly palmitoylated (35,36).
Furthermore, it has been reported that CD44 activated Akt and
ERK1/2 (35) and that CD44
knockdown altered Akt phosphorylation (37,38).
As the U251-MG cells and the glioblastoma tissues examined in the
present study exhibited high expression of CD44 (data not shown),
CD44 may have a crucial role in the growth of glioblastoma via
modification of the Akt and/or ERK1/2 pathway. Alternatively, ACAT1
may affect signal transduction by inducing structural and
functional changes in lipid rafts, as reported by Huang et
al (39).
In conclusion, the present study demonstrated that
K604 inhibited the proliferation of U251-MG cells and the
phosphorylation of Akt and ERK1/2 in U251-MG cells. These results
suggested that suppression of U251-MG-cell proliferation via
pharmacological ACAT1 inhibition may be an attractive therapeutic
strategy for refractory brain tumors. Future studies by our group
will investigate this ACAT1-targeted therapeutic strategy in in
vivo experiments in order to evaluate its potential for
clinical application.
Abbreviations:
|
ACAT
|
acyl-CoA:cholesterol
acyltransferase
|
|
ERK
|
extracellular-signal-regulated
kinase
|
|
HE
|
hematoxylineosin
|
|
DAB-H2O2
|
3,3′-diaminobenzidine
H2O2
|
|
TCA
|
trichloroacetic acid
|
|
TBS
|
tris-buffered saline
|
|
TBS-T
|
TBS supplemented with 0.1% Tween
20
|
|
ANOVA
|
analysis of variance
|
Acknowledgments
The authors would like to thank Ms. Hiroko Akita
(Department of Human Pathology, Institute of Health Biosciences,
University of Tokushima Graduate School) for her excellent
technical assistance. The present study was supported by
Grants-in-Aid for Scientific Research (no. C-23590448) and
Challenging Exploratory Research (no. 26670190 to N.S.) from the
Japan Society for the Promotion of Science (JSPS). The present
study was also supported by the Support Center for Advanced Medical
Sciences, Institute of Health Biosciences, The University of
Tokushima Graduate School.
References
|
1
|
Roffo AH: Heliotropism of cholesterol in
relation to skin cancer. Am J Cancer. 17:42–57. 1933. View Article : Google Scholar
|
|
2
|
Yasuda M and Bloor WR: Lipid content of
tumors. J Clin Invest. 11:677–682. 1932. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gebhard RL, Clayman RV, Prigge WF,
Figenshau R, Staley NA, Reesey C and Bear A: Abnormal cholesterol
metabolism in renal clear cell carcinoma. J Lipid Res.
28:1177–1184. 1987.PubMed/NCBI
|
|
4
|
Rudel LL, Lee RG and Cockman TL: Acyl
coenzyme A: Cholesterol acyltransferase types 1 and 2: Structure
and function in atherosclerosis. Curr Opin Lipidol. 12:121–127.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chang TY, Chang CC and Cheng D:
Acyl-coenzyme A: Cholesterol acyltransferase. Annu Rev Biochem.
66:613–638. 1997. View Article : Google Scholar
|
|
6
|
Sakashita N, Miyazaki A, Takeya M,
Horiuchi S, Chang CC, Chang TY and Takahashi K: Localization of
human acyl-coenzyme a: Cholesterol acyltransferase-1 (ACAT-1) in
macrophages and in various tissues. Am J Pathol. 156:227–236. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nygren C, von Holst H, Månsson JE and
Fredman P: Increased levels of cholesterol esters in glioma tissue
and surrounding areas of human brain. Br J Neurosurg. 11:216–220.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fredman P, von Holst H, Collins VP, Ammar
A, Dellheden B, Wahren B, Granholm L and Svennerholm L: Potential
ganglioside antigens associated with human gliomas. Neurol Res.
8:123–126. 1986.PubMed/NCBI
|
|
9
|
Kaba SE and Kyritsis AP: Recognition and
management of gliomas. Drugs. 53:235–244. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maher EA, Furnari FB, Bachoo RM, Rowitch
DH, Louis DN, Cavenee WK and DePinho RA: Malignant glioma: genetics
and biology of a grave matter. Genes Dev. 15:1311–1333. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ikenoya M, Yoshinaka Y, Kobayashi H,
Kawamine K, Shibuya K, Sato F, Sawanobori K, Watanabe T and
Miyazaki A: A selective ACAT-1 inhibitor, K-604, suppresses fatty
streak lesions in fat-fed hamsters without affecting plasma
cholesterol levels. Atherosclerosis. 191:290–297. 2007. View Article : Google Scholar
|
|
12
|
García-Regalado A, Guzmán-Hernández ML,
Ramírez-Rangel I, Robles-Molina E, Balla T, Vázquez-Prado J and
Reyes-Cruz G: G protein-coupled receptor-promoted trafficking of
Gbeta1gamma2 leads to AKT activation at endosomes via a mechanism
mediated by Gbeta1gamma2-Rab11a interaction. Mol Biol Cell.
19:4188–4200. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang W and Liu HT: MAPK signal pathways
in the regulation of cell proliferation in mammalian cells. Cell
Res. 12:9–18. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bemlih S, Poirier MD and El Andaloussi A:
Acyl-coenzyme A: cholesterol acyltransferase inhibitor Avasimibe
affect survival and proliferation of glioma tumor cell lines.
Cancer Biol Ther. 9:1025–1032. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chang CC, Chen J, Thomas MA, Cheng D, Del
Priore VA, Newton RS, Pape ME and Chang TY: Regulation and
immunolocalization of acyl-coenzyme A: Cholesterol acyltransferase
in mammalian cells as studied with specific antibodies. J Biol
Chem. 270:29532–29540. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: Application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Niikura Y, Nonaka T and Imajoh-Ohmi S:
Monitoring of caspase-8/FLICE processing and activation upon Fas
stimulation with novel antibodies directed against a cleavage site
for caspase-8 and its substrate, FLICE-like inhibitory protein
(FLIP). J Biochem. 132:53–62. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lawlor MA and Alessi DR: PKB/Akt: A key
mediator of cell proliferation, survival and insulin responses? J
Cell Sci. 114:2903–2910. 2001.PubMed/NCBI
|
|
20
|
Chakravarti A, Zhai G, Suzuki Y, Sarkesh
S, Black PM, Muzikansky A and Loeffler JS: The prognostic
significance of phosphatidylinositol 3-kinase pathway activation in
human gliomas. J Clin Oncol. 22:1926–1933. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lopez-Gines C, Gil-Benso R, Benito R, Mata
M, Pereda J, Sastre J, Roldan P, Gonzalez-Darder J and
Cerdá-Nicolás M: The activation of ERK1/2 MAP kinases in
glioblastoma pathobiology and its relationship with EGFR
amplification. Neuropathology. 28:507–515. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Burnett JR, Wilcox LJ, Telford DE,
Kleinstiver SJ, Barrett PH, Newton RS and Huff MW: Inhibition of
ACAT by avasimibe decreases both VLDL and LDL apolipoprotein B
production in miniature pigs. J Lipid Res. 40:1317–1327.
1999.PubMed/NCBI
|
|
23
|
Delsing DJ, Offerman EH, van Duyvenvoorde
W, van Der Boom H, de Wit EC, Gijbels MJ, van Der Laarse A, Jukema
JW, Havekes LM and Princen HM: Acyl-CoA:Cholesterol acyltransferase
inhibitor avasimibe reduces atherosclerosis in addition to its
cholesterol-lowering effect in ApoE*3-Leiden mice.
Circulation. 103:1778–1786. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cheng CK, Fan QW and Weiss WA: PI3K
signaling in glioma-animal models and therapeutic challenges. Brain
Pathol. 19:112–120. 2009. View Article : Google Scholar :
|
|
25
|
Robinson JP, Vanbrocklin MW, McKinney AJ,
Gach HM and Holmen SL: Akt signaling is required for glioblastoma
maintenance in vivo. Am J Cancer Res. 1:155–167. 2011.PubMed/NCBI
|
|
26
|
Gallia GL, Tyler BM, Hann CL, Siu IM,
Giranda VL, Vescovi AL, Brem H and Riggins GJ: Inhibition of Akt
inhibits growth of glioblastoma and glioblastoma stem-like cells.
Mol Cancer Ther. 8:386–393. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Reddy KB, Nabha SM and Atanaskova N: Role
of MAP kinase in tumor progression and invasion. Cancer Metastasis
Rev. 22:395–403. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen D, Zuo D, Luan C, Liu M, Na M, Ran L,
Sun Y, Persson A, Englund E and Salford LG: Glioma cell
proliferation controlled by ERK activity-dependent surface
expression of PDGFRA. PLoS One. 9:e872812014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhuang L, Lin J, Lu ML, Solomon KR and
Freeman MR: Cholesterol-rich lipid rafts mediate akt-regulated
survival in prostate cancer cells. Cancer Res. 62:2227–2231.
2002.PubMed/NCBI
|
|
30
|
Ringerike T, Blystad FD, Levy FO, Madshus
IH and Stang E: Cholesterol is important in control of EGF receptor
kinase activity but EGF receptors are not concentrated in caveolae.
J Cell Sci. 115:1331–1340. 2002.PubMed/NCBI
|
|
31
|
Linder ME and Deschenes RJ:
Palmitoylation: Policing protein stability and traffic. Nat Rev Mol
Cell Biol. 8:74–84. 2007. View
Article : Google Scholar
|
|
32
|
Bhattacharyya R, Barren C and Kovacs DM:
Palmitoylation of amyloid precursor protein regulates amyloidogenic
processing in lipid rafts. J Neurosci. 33:11169–11183. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Knüpfer MM, Poppenborg H, Hotfilder M,
Kühnel K, Wolff JE and Domula M: CD44 expression and hyaluronic
acid binding of malignant glioma cells. Clin Exp Metastasis.
17:71–76. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wei KC, Huang CY, Chen PY, Feng LY, Wu TW,
Chen SM, Tsai HC, Lu YJ, Tsang NM, Tseng CK, et al: Evaluation of
the prognostic value of CD44 in glioblastoma multiforme. Anticancer
Res. 30:253–259. 2010.PubMed/NCBI
|
|
35
|
Ponta H, Sherman L and Herrlich PA: CD44:
From adhesion molecules to signalling regulators. Nat Rev Mol Cell
Biol. 4:33–45. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Oliferenko S, Paiha K, Harder T, Gerke V,
Schwärzler C, Schwarz H, Beug H, Günthert U and Huber LA: Analysis
of CD44-containing lipid rafts: Recruitment of annexin II and
stabilization by the actin cytoskeleton. J Cell Biol. 146:843–854.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Subramaniam V, Vincent IR, Gardner H, Chan
E, Dhamko H and Jothy S: CD44 regulates cell migration in human
colon cancer cells via Lyn kinase and AKT phosphorylation. Exp Mol
Pathol. 83:207–215. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Park YS, Huh JW, Lee JH and Kim HR: shRNA
against CD44 inhibits cell proliferation, invasion and migration
and promotes apoptosis of colon carcinoma cells. Oncol Rep.
27:339–346. 2012.
|
|
39
|
Huang LH, Gui J, Artinger E, Craig R,
Berwin BL, Ernst PA, Chang CC and Chang TY: Acat1 gene ablation in
mice increases hematopoietic progenitor cell proliferation in bone
marrow and causes leukocytosis. Arterioscler Thromb Vasc Biol.
33:2081–2087. 2013. View Article : Google Scholar : PubMed/NCBI
|