Introduction
Traditional herbal medicine is considered to be one
of the most important complementary or alternative medicines in the
majority of countries, and has been increasingly accepted
worldwide. Despite substantial advances in modern scientific
medicine, traditional medicine remains the primary form of
treatment, which is readily available to the majority of
individuals in several countries (1). Traditional herbal medicines usually
contain a number of compounds, which affect multiple targets
(2,3). The combination of multiple drugs is
considered to maximize therapeutic efficacy by facilitating
synergistic actions and preventing potential adverse effects
(2). Dangkwisoo-san (DS) is a
herbal formula, which has been traditionally used for the treatment
of pain and blood stagnation caused by physical trauma in Korea
(4). DS contains constituents of
nine species of herbal plants, including Angelicae gigantis Radix,
Paeoniae Radix, Linderae Radix, Sappan Lignum, Cyperi Rhizoma,
Carthami Flos, Persicae Semen, Cinnamomi Cortex and Glycyrrhizae
Radix et Rhizoma, which have various pharmacological effects on the
body (5,6). However, no investigations regarding
the effects of DS on gastrointestinal (GI) motility have been
previously performed, to the best of our knowledge.
The interstitial cells of Cajal (ICCs) are the
pacemaker cells of the GI system and have multifunctional roles.
ICCs generate rhythmic oscillations in membrane potential, termed
slow waves (7–9). The loss of ICCs is implicated in
various motility disorders, which indicates that ICCs are important
in the regulation of GI motility (10). In addition, endogenous agents,
including neurotransmitters, hormones and paracrine substances,
modulate GI tract motility by affecting ICCs. Thus, in
investigating GI motility, ICCs are the major area of interest and,
at present, several novel drugs are being developed in the area of
GI motility utilizing ICCs. Therefore, the present study
investigated whether DS affects the pacemaker potentials of
cultured ICCs and characterized the CCK receptor subtypes
involved.
Materials and methods
Ethics
Animal care and experiments were conducted in
accordance with the guidelines issued by the ethics committee of
Pusan National University (Busan, Republic of Korea; Approval no.
PNU-2014-0725) and the National Institutes of Health Guide for the
Care and Use of Laboratory Animals.
Preparation of cells and cell
cultures
Male and female BALB/c mice [age, 3–7 days; n=75
(male: 56%; female: 44%) obtained from Samtako Bio Korea Inc.
(Osan, Korea)] were anesthetized with 99% ether (Sigma-Aldrich, St.
Louis, MO, USA) and sacrificed by cervical dislocation. The small
intestines in the region between 1 cm below the pyloric ring and
the cecum were removed and opened along the mesenteric border. The
luminal contents were removed by washing with Krebs-Ringer
bicarbonate solution (Sigma-Aldrich). The tissues were pinned to
the base of a Sylgard dish and the was mucosa removed by sharp
dissection. Small tissue strips (0.2×0.2 inches) of the intestinal
muscle, consisting of circular and longitudinal muscle, were
equilibrated in Ca2+-free Hanks' solution (containing
5.36 mmol/l KCL, 125 mmol/l NaCl, 0.34 mmol/l NaOH, 0.44 mmol/l
Na2HCO3, 10 mmol/l glucose, 2.9 mmol/l
sucrose and 11 mmol/l HEPES; Sigma-Aldrich) for 30 min.
Subsequently, the cells (density, 85%) were dispersed using an
enzyme solution containing 1.3 mg/ml collagenase (Worthington
Biochemical Co., Lakewood, NJ, USA), 2 mg/ml bovine serum albumin
(Sigma-Aldrich), 2 mg/ml trypsin inhibitor (Sigma-Aldrich) and 0.27
mg/ml ATP (Sigma-Aldrich). The cells were plated onto sterile glass
coverslips coated with murine collagen (2.5 μg/ml, BD
Biosciences, Franklin Lakes, NJ, USA) in a 35-mm culture dish and
then cultured at 37°C in a 95% O2, 50 ml/l
CO2 incubator in a smooth muscle growth medium
(Clonetics Corp., San Diego, CA, USA) supplemented with 2%
antibiotics/antimycotics (Gibco Life Technologies, Grand Island,
NY, USA) and murine stem cell factor (SCF; 5 ng/ml; Sigma-Aldrich).
The ICCs were identified immunologically by incubation with an
anti-c-kit antibody (cat. no. 12-1172; phycoerythrin-conjugated rat
anti-mouse c-kit monoclonal antibody; eBioscience, San Diego, CA,
USA) at a dilution of 1:50 for 20 min (11). The ICCs were morphologically
distinct from other cell types in the culture, enabling the
identification of the cells using phase contrast microscopy (IX-71;
Olympus Corporation, Tokyo, Japan) once they had been verified with
the anti c-kit antibody.
Patch-clamp experiments
The whole-cell patch-clamp configuration was used to
record membrane potentials (current clamp) from the cultured ICCs.
An axopatch ID (Axon Instruments, Inc., Foster City, CA, USA) was
used to amplify membrane currents and potentials. The command pulse
was applied using an IBM-compatible personal computer and pClamp
software (version 6.1; Axon Instruments, Inc.). The data obtained
were filtered at 5 kHz and viewed on an HM507 oscilloscope (Hameg
Instruments GmbH, Melrose, MA, USA), a computer monitor and using a
pen recorder (Gould 2200; Gould, Valley View, OH, USA). The results
were analyzed using pClamp and Origin (version 6.0) software
(MicroCal, Northampton, MA, USA). All experiments were performed at
30–32°C.
Fura-2-acetoxymethyl ester (Fura-2-AM)
loading and measurement of intracellular free calcium ion
concentration [Ca2+]i
The cultured ICC clusters were loaded with 5
μmol/l of the acetoxymethyl ester form of fura-2 (Molecular
ProbesLife Technologies, Carlsbad, CA, USA), diluted from a 1
mmol/l stock in dimethyl sulfoxide (DMSO; Sigma-Aldrich), in normal
medium for 20 min at 37°C. The recording of
[Ca2+]i was performed using a
microfluorometric system consisting of an inverted fluorescence
microscope (Diaphot 300; Nikon Corporation, Tokyo, Japan) with a
dry-type fluorescence objective lens (40X; numerical aperture
0.85), a photomultiplier tube (type R 1527; Hamamatsu, Shizuoka,
Japan), and a PTI-Deltascan illuminator (Photon Technology
International, Inc., Edison, NJ, USA). The cells were superfused at
a flow rate of 1.5 ml/min. Light was provided by a 75-W xenon lamp
(UXL-75XE; Ushio, Japan). To control the excitation frequency, a
chopper wheel was used to alternate the light path to
monochromators (340 and 380 nm) with a frequency of 5 or 10 Hz. A
short-pass dichroic mirror passed an emission light of <570 nm
onto the photomultiplier tube, and the intensity was measured at
510 nm. A mechanical image mask was placed in the emission path to
limit measurement to a single cell. Data acquisition and control of
light application were performed using computer software (Felix
version 1.1; Photon Technology International, Inc.). Due to
uncertainties in calibrating the fura-2 signals in intact cells, no
calibration of [Ca2+]i was performed;
instead, all results are reported as changes in the 340 nm/380 nm
signal ratio.
Solutions and drugs
The physiological salt solution used to bathe cells
(Na+-Tyrode) contained 5 mmol/l KCl, 135 mmol/l NaCl, 2
mmol/l CaCl2, 10 mmol/l glucose, 1.2 mmol/l
MgCl2 and 10 mmol/l HEPES, and was adjusted to pH 7.4
with NaOH. The pipette solution contained 140 mmol/l KCl, 5 mmol/l
MgCl2, 2.7 mmol/l K2ATP, 0.1 mmol/l NaGTP,
2.5 mmol/l creatine phosphate disodium, 5 mmol/l HEPES and 0.1
mmol/l EGTA, adjusted to pH 7.2 with KOH. To evaluate the effect of
guanosine 5′-[β-thio] diphosphate (GDP-β-S; Sigma-Aldrich) on ICCs,
GDP-β-S was included in the pipette solution. DS is composed of
nine species of herbal plants, each of which were purchased from
Kwangmyungdang Natural Pharmaceutical Co. (Ulsan, Korea). The
constituents and formula of DS is described in Table I. A total of 60 g DS was boiled in
1 liter of distilled water in a Herb Extractor (DW-290; Daewoong
Pharmaceutical Co., Ltd., Seoul, Korea) for 2 h, yielding a final
200 ml volume containing the DS extract. The supernatant was
harvested in sterile conditions by centrifu-gation (110 × g at 4°C
for 3 min) and lyophilized through evaporation at −80°C, yielding a
final quantity of 4.6 g. The lyophilized DS extract was dissolved
in 500 μl sterile phosphate-buffered saline prior to
administration to the cells. The water extract of DS (voucher no.
MH2014-0001) was deposited at the Division of Longevity and
Biofunctional Medicine, School of Korean Medicine, Pusan National
University (Pusan, Korea). All other drugs were obtained from
Sigma-Aldrich. The drug treatments were dissolved in distilled
water and added to bath solution to produce the desired
concentrations, just prior to use. The addition of these chemicals
to the bath solution did not alter the pH of the solution.
4-Diphenylacetoxy-N-methyl-piperidine methiodide [4-DAMP; 10
μM (Sigma-Aldrich)] and 5 μM thapsigargin
(Sigma-Aldrich) were dissolved in DMSO for a 50 mmol/l stock
solution and added to the bathing solution on the day of the
experiment. The final concentration of DMSO in the bath solution
remained <0.1%, and it was confirmed that this concentration of
DMSO did not affect the results recorded. In addition, 25 μl
methoctramine (Sigma-Aldrich) was dissolved in distilled water for
a 50 mmol/l stock solution and added to the bathing solution on the
day of the experiment.
 | Table IComposition of Dangkwisoo-san. |
Table I
Composition of Dangkwisoo-san.
| Scientific
name | Herbal name | Quantity (g) |
|---|
| Angelica
gigas Nakai | Angelicae gigantis
Radix | 5.625 |
| Paeonia
lactiflora Pall | Paeoniae Radix | 3.750 |
| Lindera
strichnifolia Fern'andez-Villar | Linderae Radix | 3.750 |
| Caesalpinia
sappan L. | Sappan Lignum | 3.750 |
| Cyperus
rotundus L. | Cyperi Rhizoma | 3.750 |
| Carthamus
tinctorious L. | Carthami Flos | 3.000 |
| Prunus
persica Batsch | Persicae Semen | 2.655 |
| Cinnamomum
cassia | Presl Cinnamomi
Cortex | 2.250 |
| Glycyrrhiza
uralensis Fisch | Glycyrrhizae Radix
et Rhizoma | 1.875 |
Statistical analysis
All data are expressed as the mean ± standard error
of the mean. Student's t-test for unpaired data was used to compare
the control and experimental groups. Origin statistical software
(version 6.0) was used to perform statistical analyses (MicroCal)
and P<0.05 was considered to indicate a statistically
significant difference.
Results
Effect of DS on pacemaker potentials in
cultured ICCs
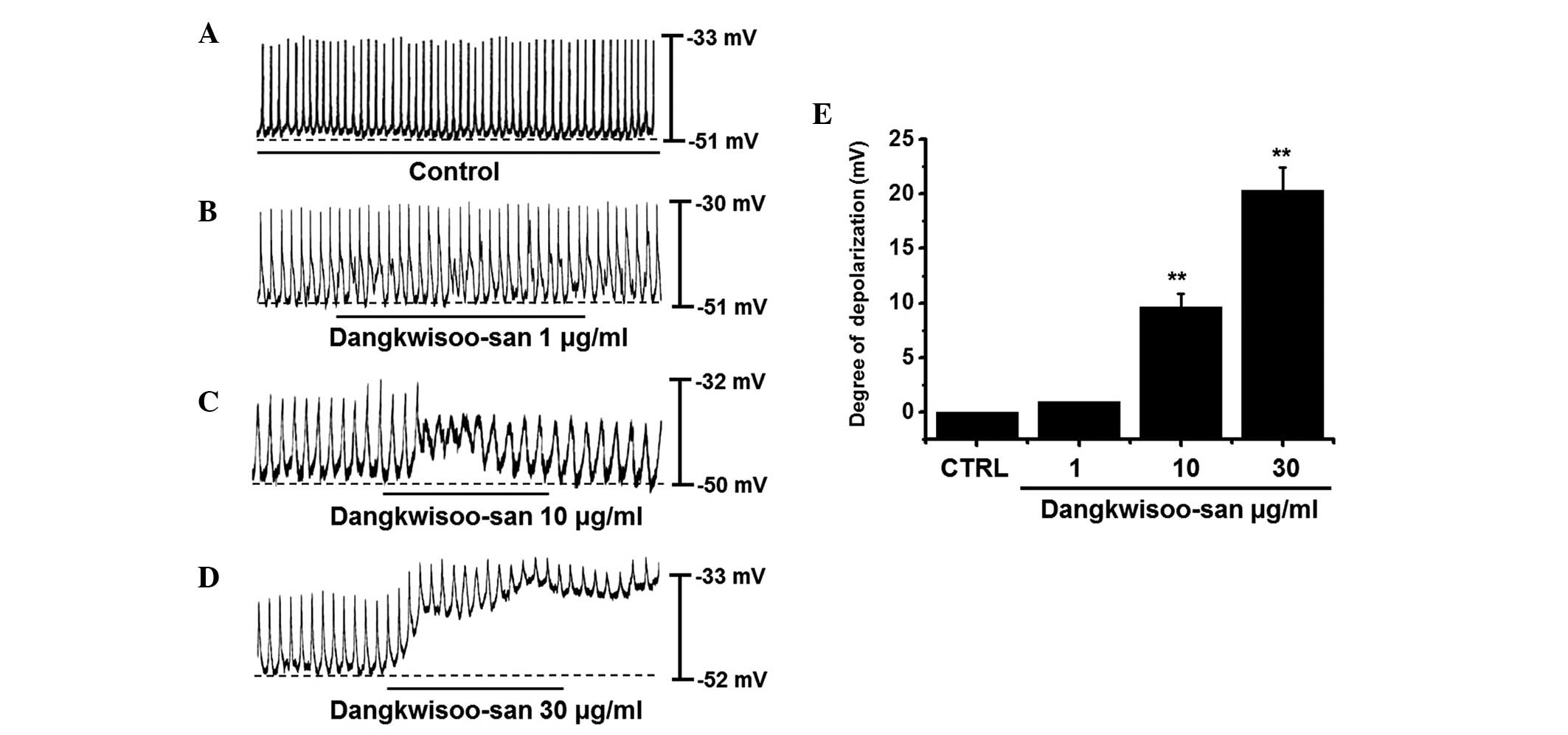
Initially, the effects of DS on pacemaker potentials
were examined. Recordings from cultured ICCs under current clamp
mode (I=0) revealed spontaneous pacemaker potentials. The resting
membrane potential was −51.4±2.6 mV and the amplitude was 20.2±2.3
mV. In the presence of DS (1–30 μg/ml), the membrane
potentials were depolarized to 1.0±0.1 mV at 1 μg/ml,
9.7±1.3 mV at 10 μg/ml and 20.5±2.2 mV at 30 μg/ml
(Fig. 1A–D). The summarized values
and bar graph of the DS effects on pacemaker potentials are shown
in Fig. 1E (n=6). These results
suggested that DS had a pacemaker depolarization effect on the
ICCs.
Identification of DS receptor subtypes in
cultured ICCs
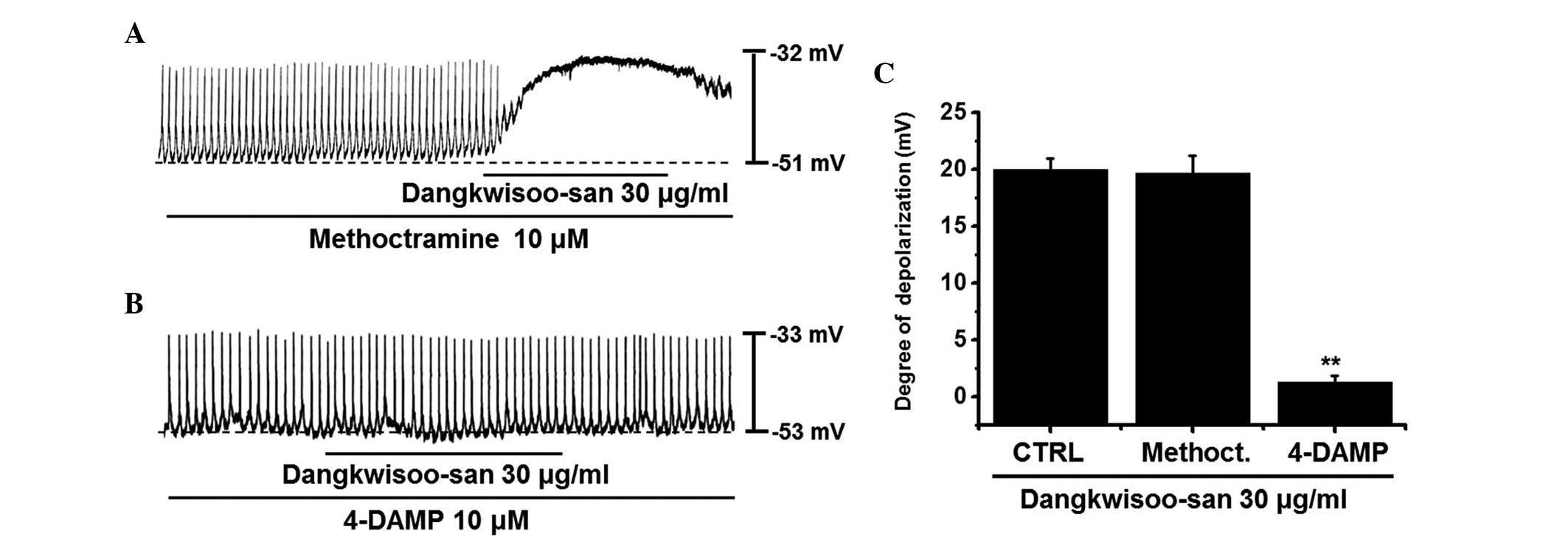
To investigate the association between DS and its
receptors, muscarinic receptors were investigated as they are known
to mediate the membrane depolarization and excitatory junction
potential in the GI tract (12,13).
In the GI tract, isolated ICCs express the M2 and
M3 subtypes of the muscarinic receptors (14). To identify the muscarinic receptor
subtypes involved in the effects of DS, the ICCs were pretreated
with muscarinic receptor antagonists and then treated with DS.
Methoctramine, a muscarinic M2 receptor antagonist, and
4-DAMP, a muscarinic M3 receptor antagonist, were used
for pretreatment at a concentration of 10 μM for 5 min and
DS was added. Treatment with methoctramine or 4-DAMP had no effect
on pacemaker potentials. Pretreatment with methoctramine did not
inhibit the effect of DS (Fig.
2A), and the membrane depolarization produced in the presence
of methoctramine by DS was 19.6±2.1 mV (n=5). However, following
pre-treatment with 4-DAMP, membrane depolarization by DS was found
to be inhibited (Fig. 2B), and the
membrane depolarization produced in the presence of 4-DAMP by DS
was 1.3±0.5 mV (n=6; Fig. 2C).
These results suggested that DS had an effect on the ICCs through
the M3 receptor.
Involvement of G proteins in DS-induced
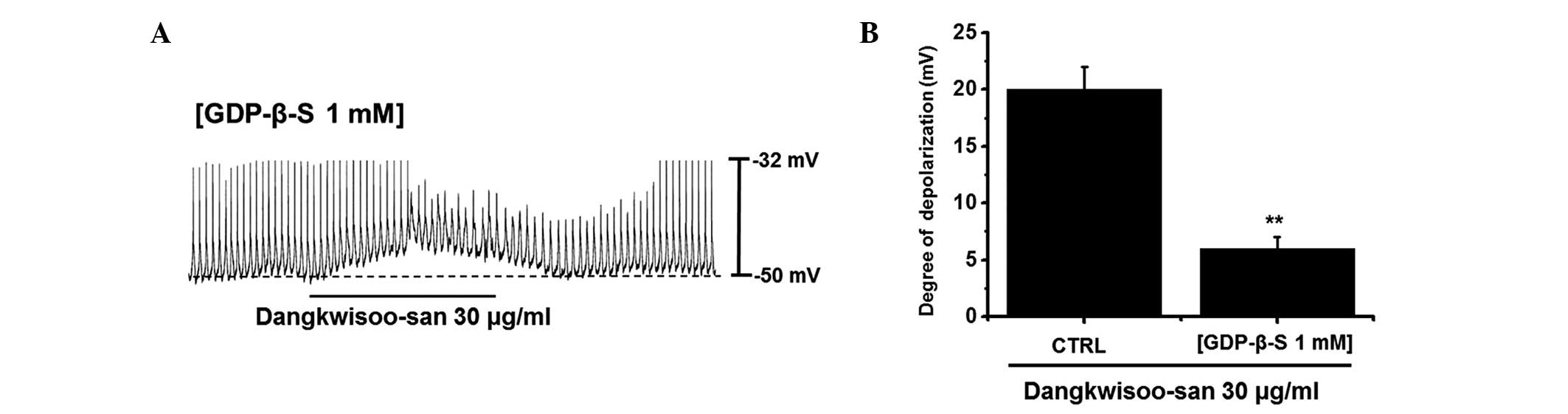
depolarizations of pacemaker potentials in cultured ICCs
The effects of GDP-β-S, a non-hydrolysable guanosine
5′-diphosphate analogue, which permanently inactivates G-protein
binding proteins (15,16) were examined to determine whether
G-proteins are involved in the effects of DS on cultured ICCs. DS
(30 μg/ml) induced membrane depolarizations in ICCs
(Fig. 1). However, when GDP-β-S (1
mM) was in the pipette solution, DS (30 μg/ml) induced the
membrane depolarizations only marginally (Fig. 3A). The membrane depolarizations
induced by DS were significantly affected by the presence of
GDP-β-S (1 mM) in the pipette solution (n=5; Fig. 3B). These results suggested that
G-proteins are involved in the DS-induced pacemaker depolarizations
in ICCs.
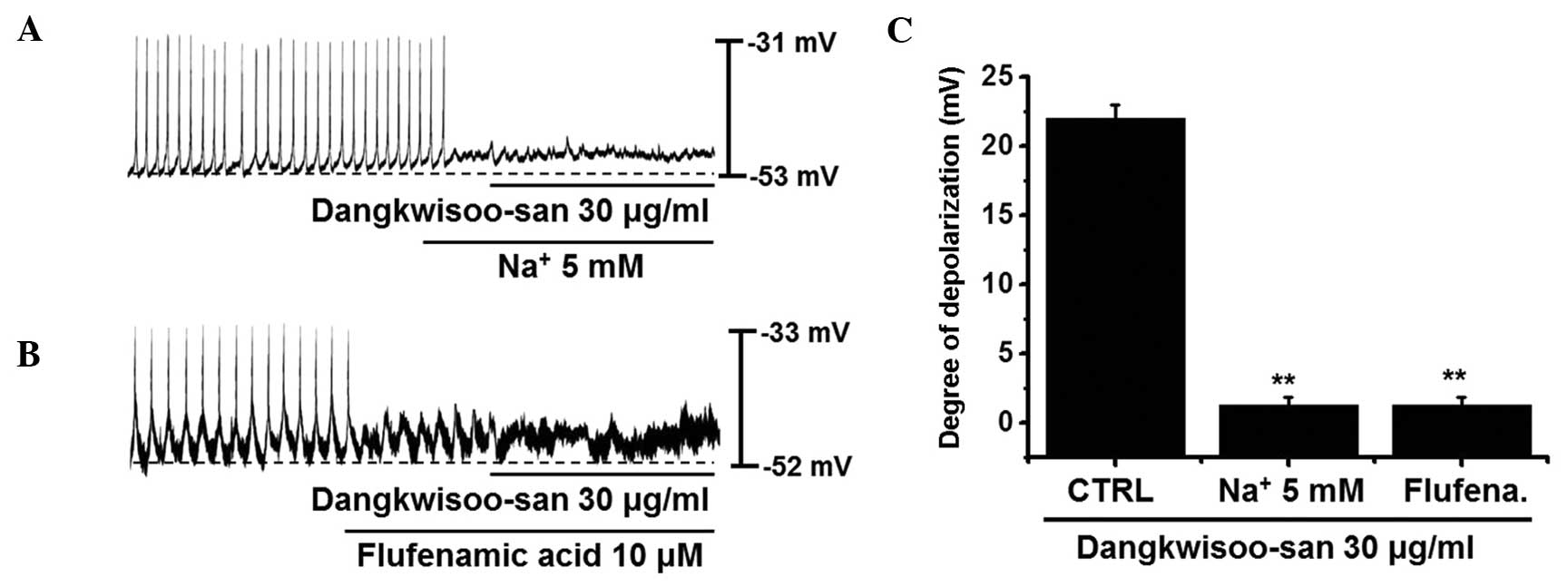
Effects of low external Na+
concentration or nonselective cation channel blocker on DS-induced
depolarizations in pacemaker potentials in cultured ICCs
To determine the characteristics of the pacemaker
depolarizations induced by DS, a low external Na+
concentration solution and a nonselective cation channel blocker
were assessed. External Na+ was substituted for by the
same concentrations of N-methyl-D-glucamine. In the presence
of an external Na+ 5 mM solution, pacemaker potentials
were eradicated. Under these conditions, DS (30 μg/ml) did
not induce pacemaker depolarizations (Fig. 4A). In the external Na+ 5
mM solution, the pacemaker depolarizations produced by DS were
1.4±0.4 mV, which was significantly different, compared with the
normal control solution (n=6; Fig.
4). In the presence of flufenamic acid (10 μM), a
nonselective cation channel blocker, the pacemaker potentials were
eradicated. Additionally, in these conditions, DS did not induce
pacemaker depolarizations (Fig.
4B). Following pretreatment with flufenamic acid, the pacemaker
depolarizations produced by DS were 1.3±0.6 mV, which was
significantly different, compared with the normal control solution
(n=5; Fig. 4C). These results
suggested that external Na+ and nonselective cation
channels are involved in DS-induced depolarizations in pacemaker
potentials in cultured ICCs.
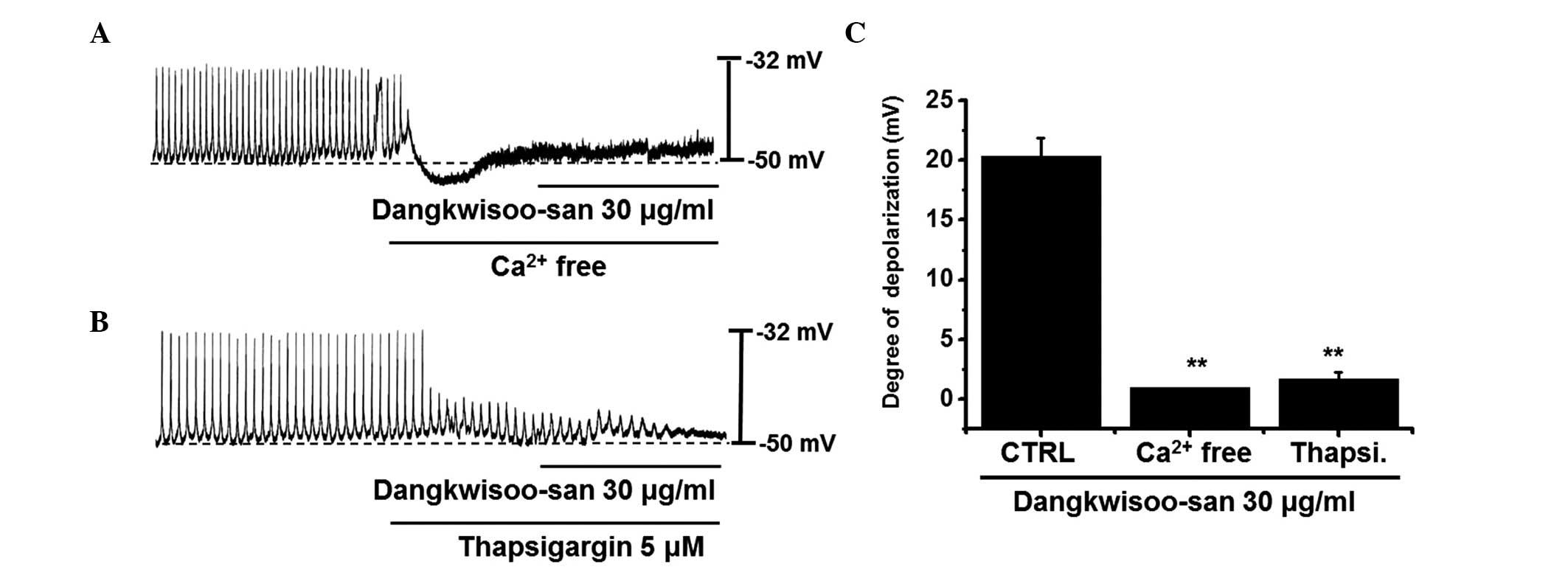
Effects of external Ca2+-free
solution and Ca2+-ATPase inhibitors of endoplasmic
reticulum on DS-induced depolarizations on pacemaker potentials in
cultured ICCs
To investigate the role of external Ca2+
or internal Ca2+, DS was applied under external
Ca2+-free conditions and in the presence of
thapsigargin, a Ca2+-ATPase inhibitor of endoplasmic
reticulum. In external Ca2+-free solution, pacemaker
potentials were completely eradicated. In this condition, DS had no
effect on pacemaker potentials (Fig.
5A). These effects were significantly different, compared with
those of DS in the normal Ca2+ solution (n=6; Fig. 5). In addition, pretreatment with
thapsigargin (5 μM) suppressed the pacemaker potentials and,
in this condition, DS had no effect on pacemaker potentials
(Fig. 5B). In the presence of
thapsigargin, the effects were significantly different, compared
with DS in the absence of thapsigargin (n=6; Fig. 5C). These results suggested that
external Ca2+ or internal Ca2+ regulations
are important in modulating pacemaker potentials in cultured
ICCs.
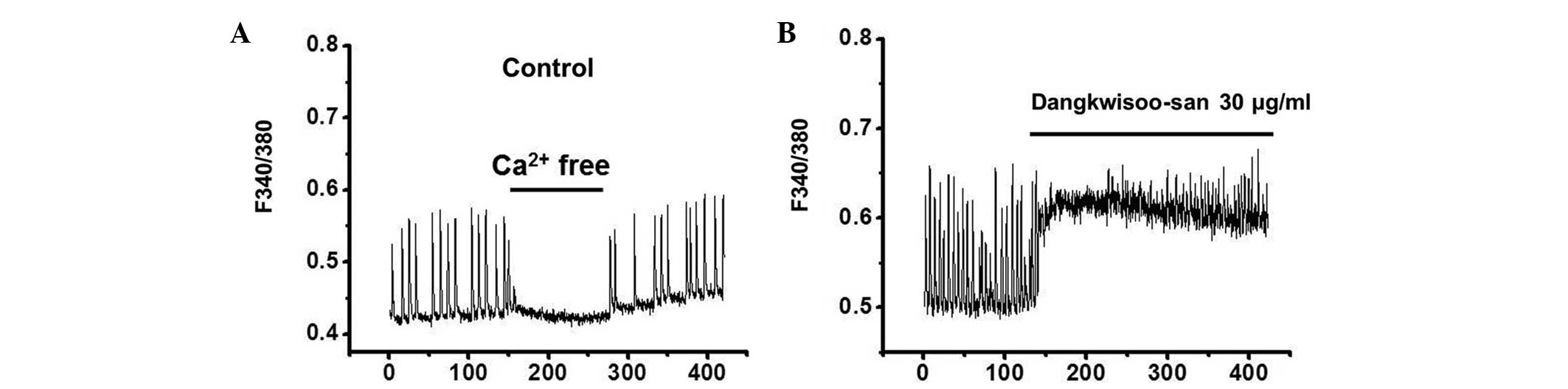
Response of [Ca
2+]i to DS
To investigate the effects of DS on
[Ca2+]i oscillations, spontaneous
[Ca2+]i oscillations were measured in ICCs
clusters. This was due to the fact that
[Ca2+]i oscillations in ICCs are primarily
responsible for GI pacemaker activity (17). Spontaneous
[Ca2+]i oscillations were observed in ICC
clusters treated with 5 μM fura-2-AM. Fig. 6 shows the changes in the 340 nm/380
nm signal ratio. In normal conditions, spontaneous
[Ca2+]i oscillations were induced (Fig. 6A). In the presence of DS (30
μg/ml), the [Ca2+]i in ICCs was
increased (Fig. 6B). These results
suggested that DS increased the [Ca2+]i in
ICCs.
Discussion
In the present study, ICCs were used to investigate
the association between DS and GI motility. DS has not been
previously used to treat GI motility diseases, and this is the
first study, to the best of our knowledge, regarding the potential
effects of DS on ICCs. Due to the central role of ICCs in GI
motility, loss of these cells is detrimental in disorders,
including inflammatory bowel disease, chronic idiopathic intestinal
pseudo-obstruction, intestinal obstruction with hypertrophy,
achalasia, Hirschsprung disease, juvenile pyloric stenosis,
juvenile intestinal obstruction and anorectal malformation
(10). Therefore, investigation
into the biology of ICCs provides novel opportunities to develop
drugs with the ability to regulate GI motility.
Acetylcholine depolarizes the membrane potential of
slow waves and leads to contraction of gastrointestinal smooth
muscle (18). Therefore,
muscarinic receptors are important in the regulation of GI
motility. Muscarinic receptors are composed of five subtypes,
M1–M5. However, GI smooth muscles express the
M2 and M3 subtypes of the muscarinic
receptors. M2 receptors are more widely distributed than
M3 receptors, in the ratio of 80% M2 to 20%
M3 (19). In molecular
studies, mRNA of M2 and M3 receptors were
detected using reverse-transcription polymerase chain reaction from
isolated ICCs (14). In the
present study, 4-DAMP, a muscarinic M3 receptor
antagonist, inhibited DS-induced pacemaker depolarizations, whereas
methoctramine, a muscarinic M2 receptor antagonist, did
not. Thus, it appears that DS modulated pacemaker potentials
through muscarinic M3 receptor-mediated pathways in the
ICCs of the mouse small intestine (Fig. 2).
Generally, DS has been traditionally used in Korea
for the treatment of pain and blood stagnation caused by physical
trauma (4). In the present study,
however, it was found that DS modulated GI motility using ICCs. DS
produced pacemaker depolarizations in current clamp mode (Fig. 1). 4-DAMP, a muscarinic
M3 receptor antagonist, inhibited DS-induced pacemaker
depolarizations, whereas methoctramine, a muscarinic M2
receptor antagonist, did not (Fig.
2). When GDP-β-S (1 mM) was included in the pipette solution,
DS induced pacemaker depolarizations marginally (Fig. 3). Low Na+ solution
externally inhibited the DS-induced pacemaker depolarizations.
Additionally, the nonselective cation channel blocker, flufenamic
acid, inhibited the DS-induced pacemaker depolarizations (Fig. 4). Pretreatment with
Ca2+-free solution and thapsigargin, a
Ca2+-ATPase inhibitor in the endoplasmic reticulum,
suppressed the DS-induced pacemaker depolarizations (Fig. 5). In addition,
[Ca2+]i analysis revealed that DS increased
[Ca2+]i (Fig.
6). These results suggested that DS may affect GI motility via
the modulation of pacemaker potentials through muscarinic
M3 receptor activation by a G protein-dependent external
and internal Ca2+ regulation and external Na+
in ICCs.
In conclusion, the present data suggested that DS
may have an ability to modulate the pacemaker potentials in ICCs.
In future investigations, the active compounds within DS and their
mechanism of action require detailed investigation.
Acknowledgments
This study was supported by a National Research
Foundation of Korea Grant funded by the Korean government (grant
no. 2014R1A5A2009936).
References
|
1
|
Jiang M, Yang J, Zhang C, Liu B, Chan K,
Cao H and Lu A: Clinical studies with traditional Chinese medicine
in the past decade and future research and development. Planta Med.
76:2048–2064. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Qiu J: 'Back to the future' for Chinese
herbal medicines. Nat Rev Drug Discov. 6:506–507. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang L, Zhou GB, Liu P, Song JH, Liang Y,
Yan XJ, Xu F, Wang BS, Mao JH, Shen ZX, et al: Dissection
ofmechanisms of Chinese medicinal formula Realgar-Indigo naturalis
as an effective treatment for promyelocytic leukemia. Proc Natl
Acad Sci USA. 105:4826–4831. 2008. View Article : Google Scholar
|
|
4
|
Kim JH, Park SH, Kim YW, Ha JM, Bae SS,
Lee GS, Cho SI, Choi BT and Shin HK: The traditional herbal
medicine, Dangkwisoo-San, prevents cerebral ischemic injury through
nitric oxide-dependent mechanisms. Evid Based Complement Alternat
Med. 2011:7183022011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li R, Guo M, Zhang G, Xu X and Li Q:
Nicotiflorin reduces cerebral ischemic damage and upregulates
endothelial nitric oxide synthase in primarily cultured rat
cerebral blood vessel endothelial cells. J Ethnopharmacol.
107:143–150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang N, Minatoguchi S, Arai M, Uno Y,
Hashimoto K, Xue-Hai C, Fukuda K, Akao S, Takemura G and Fujiwara
H: Lindera strychnifolia is protective against post-ischemic
myocardial dysfunction through scavenging hydroxyl radicals and
opening the mitochondrial KATP channels in isolated rat hearts. Am
J Chin Med. 32:587–598. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ward SM, Burns AJ, Torihashi S and Sanders
KM: Mutation of the proto-oncogene c-kit blocks development of
interstitial cells and electrical rhythmicity in murine intestine.
J Physiol. 480:91–97. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huizinga JD, Thuneberg L, Klüppel M,
Malysz J, Mikkelsen HB and Bernstein A: W/kit gene required for
interstitial cells of Cajal and for intestinal pacemaker activity.
Nature. 373:347–349. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sanders KM: A case for interstitial cells
of Cajal as pacemakers and mediators of neurotransmission in the
gastrointestinal tract. Gastroenterology. 111:492–515. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim BJ, Lim HH, Yang DK, Jun JY, Chang IY,
Park CS, So I, Stanfield PR and Kim KW: Melastatin-type transient
receptor potential channel 7 is required for intestinal pacemaking
activity. Gastroenterology. 129:1504–1517. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Goto K, Matsuoka S and Noma A: Two types
of spontaneous depolarizations in the interstitial cells freshly
prepared from the murine small intestine. J Physiol. 559:411–422.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huizinga JD, Chang G, Diamant NE and
El-Sharkawy TY: Electrophysiological basis of excitation of canine
colonic circular muscle by cholinergic agents and substance P. J
Pharmacol Exp Ther. 231:692–699. 1984.PubMed/NCBI
|
|
13
|
Inoue R and Chen S: Physiology of
muscarinic receptor operated nonselective cation channels in
guinea-pig ileal smooth muscle. Exs. 66:261–268. 1993.
|
|
14
|
Epperson A, Hatton WJ, Callaghan B,
Doherty P, Walker RL, Sanders KM, Ward SM and Horowitz B: Molecular
markers expressed in cultured and freshly isolated interstitial
cells of Cajal. Am J Physiol Cell Physiol. 279:C529–C539.
2000.PubMed/NCBI
|
|
15
|
Komori S, Kawai M, Takewaki T and Ohashi
H: GTP-binding protein involvement in membrane currents evoked by
carbachol and histamine in guinea-pig ileal muscle. J Physiol.
450:105–126. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ogata R, Inoue Y, Nakano H, Ito Y and
Kitamura K: Oestradiol-induced relaxation of rabbit basilar artery
by inhibition of voltage-dependent Ca channels through GTP-binding
protein. Br J Pharmacol. 117:351–359. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aoyama M, Yamada A, Wang J, Ohya S,
Furuzono S, Goto T, Hotta S, Ito Y, Matsubara T, Shimokata K, et
al: Requirement of ryanodine receptors for pacemaker
Ca2+ activity in ICC and HEK293 cells. J Cell Sci.
117:2813–2825. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hirst GD, Dickens EJ and Edwards FR:
Pacemaker shift in the gastric antrum of guinea-pigs produced by
excitatory vagal stimulation involves intramuscular interstitial
cells. J Physiol. 541:917–928. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sanders KM: G protein-coupled receptors in
gastrointestinal physiology. IV. Neural regulation of
gastrointestinal smooth muscle. Am J Physiol. 275:G1–G7.
1998.PubMed/NCBI
|